Abstract
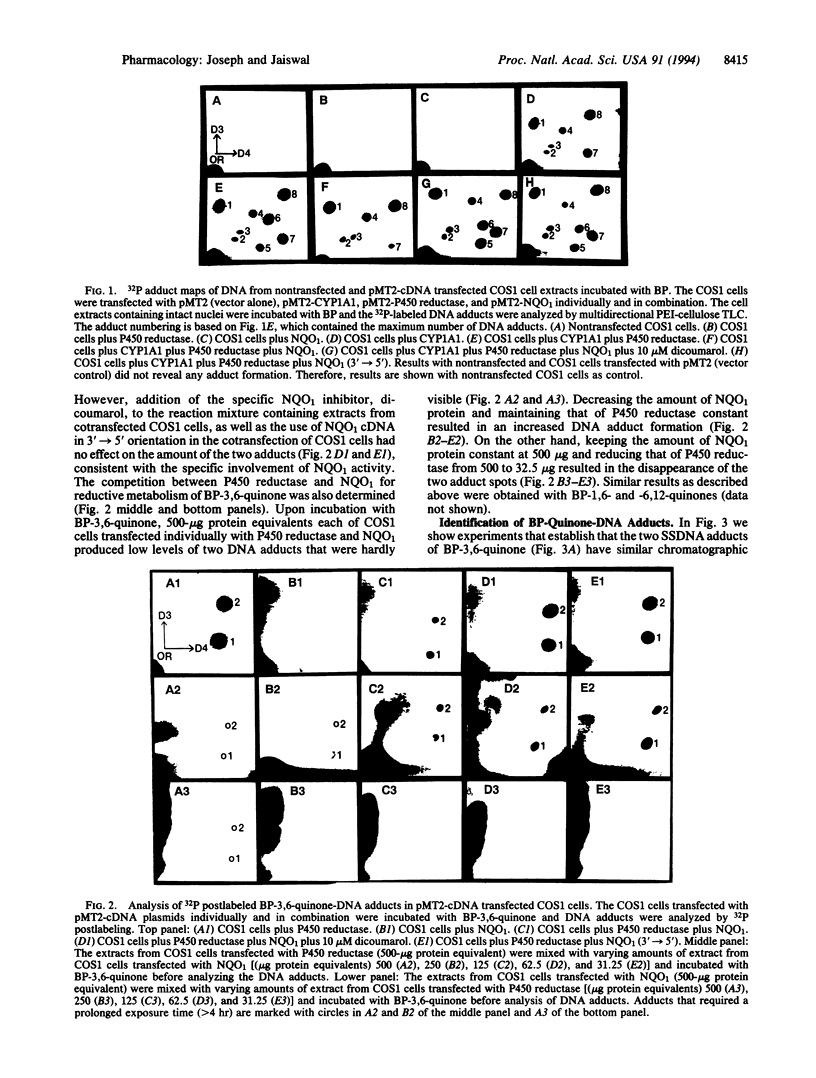
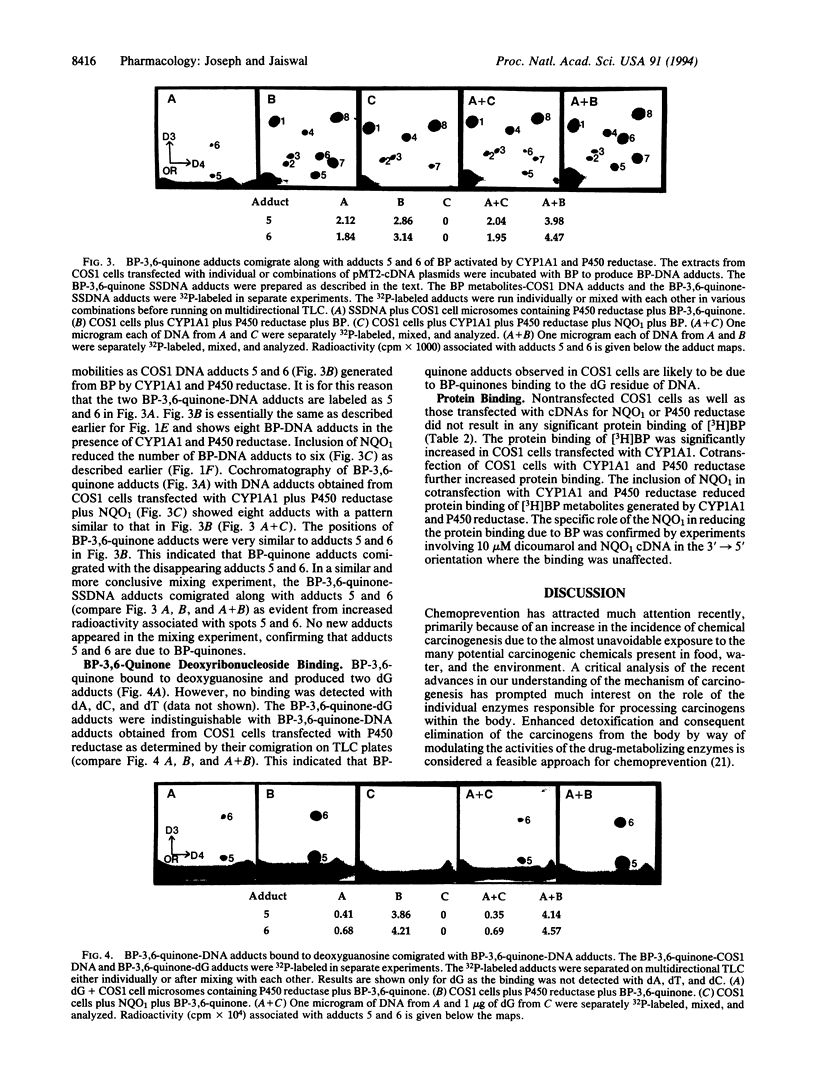
Monkey kidney COS1 cells transiently transfected with plasmids pMT2-cytochrome P450 1A1 (CYP1A1), pMT2-cytochrome P450 reductase (P450 reductase), and pMT2-NAD(P)H:quinone oxidoreductase1 (NQO1 or DT diaphorase), individually or in combination, expressed significantly elevated levels of the respective enzyme(s). The transfected cells were homogenized to break cell membranes without affecting the nuclei and incubated with benzo[a]pyrene (BP) to determine the role of cDNA-encoded enzymes in metabolic activation and/or detoxification of BP. These studies were performed by measuring the capacity of the transfected cells to form DNA adducts as determined by 32P postlabeling and protein adduct detection. Cotransfection of the COS1 cells with cDNAs encoding CYP1A1 and P450 reductase resulted in eight distinct BP-DNA adducts. Inclusion of cDNA encoding NQO1 along with CYP1A1 and P450 reductase in transfection reduced the number of DNA adducts to six. The two lost DNA adducts were specifically eliminated due to the presence of cDNA-derived NQO1 activity. Subsequent experiments with BP-1,6-quinone, BP-3,6-quinone, and BP-6,12-quinone identified these two adducts as those of BP quinones. In an in vitro system, BP-3,6-quinone produced two adducts with deoxyguanosine (dG) but not with dA, dC, and dT. Furthermore, the positions of BP-3,6-quinone-dG adducts on TLC plate correspond to those that are prevented by cDNA-derived NQO1, thus identifying these adducts as BP quinones of dG. In addition, NQO1 reduced the amount of protein-BP adducts generated by CYP1A1 and P450 reductase into transfected COS1 cells. These results show that semiquinones can directly bind to DNA and demonstrate that NQO1 activity can specifically reduce the binding of quinone metabolites of BP generated by CYP1A1 and P450 reductase to DNA and protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach A. C., Gupta R. C. Human biomonitoring and the 32P-postlabeling assay. Carcinogenesis. 1992 Jul;13(7):1053–1074. doi: 10.1093/carcin/13.7.1053. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chesis P. L., Levin D. E., Smith M. T., Ernster L., Ames B. N. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci U S A. 1986 Feb;83(3):787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., McBride O. W., Adesnik M., Nebert D. W. Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16. J Biol Chem. 1988 Sep 25;263(27):13572–13578. [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Katiyar S. K., Agarwal R., Zaim M. T., Mukhtar H. Protection against N-nitrosodiethylamine and benzo[a]pyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea. Carcinogenesis. 1993 May;14(5):849–855. doi: 10.1093/carcin/14.5.849. [DOI] [PubMed] [Google Scholar]
- Lind C. Formation of benzo[a]pyrene-3,6-quinol mono- and diglucuronides in rat liver microsomes. Arch Biochem Biophys. 1985 Jul;240(1):226–235. doi: 10.1016/0003-9861(85)90027-x. [DOI] [PubMed] [Google Scholar]
- Lorentzen R. J., Lesko S. A., McDonald K., Ts'o P. O. Toxicity of metabolic benzo(a)pyrenediones to cultured cells and the dependence upon molecular oxygen. Cancer Res. 1979 Aug;39(8):3194–3198. [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- Monks T. J., Hanzlik R. P., Cohen G. M., Ross D., Graham D. G. Quinone chemistry and toxicity. Toxicol Appl Pharmacol. 1992 Jan;112(1):2–16. doi: 10.1016/0041-008x(92)90273-u. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Powis G., Svingen B. A., Appel P. Quinone-stimulated superoxide formation by subcellular fractions, isolated hepatocytes, and other cells. Mol Pharmacol. 1981 Sep;20(2):387–394. [PubMed] [Google Scholar]
- Pulkrabek P., Leffler S., Grunberger D., Weinstein I. B. Modification of deoxyribonucleic acid by a diol epoxide of benzo[a]pyrene. Relation to deoxyribonucleic acid structure and conformation and effects on transfectional activity. Biochemistry. 1979 Nov 13;18(23):5128–5134. doi: 10.1021/bi00590a016. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Shaw P. M., Reiss A., Adesnik M., Nebert D. W., Schembri J., Jaiswal A. K. The human dioxin-inducible NAD(P)H: quinone oxidoreductase cDNA-encoded protein expressed in COS-1 cells is identical to diaphorase 4. Eur J Biochem. 1991 Jan 1;195(1):171–176. doi: 10.1111/j.1432-1033.1991.tb15691.x. [DOI] [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S., Aoyama T., McBride O. W., Hardwick J. P., Gelboin H. V., Gonzalez F. J. Human NADPH-P450 oxidoreductase: complementary DNA cloning, sequence and vaccinia virus-mediated expression and localization of the CYPOR gene to chromosome 7. Mol Pharmacol. 1989 Jul;36(1):83–88. [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]



