Abstract
Background
Cuff pressure (Pcuff) control is mandatory to avoid leakage of oral secretions passing the tracheal tube and tracheal ischemia. The aim of the present trial was to determine the efficacy of a mechanical device (PressureEasy®) in the continuous control of Pcuff in patients intubated with polyvinyl chloride (PVC)-cuffed tracheal tubes, compared with routine care using a manometer.
Methods
This is a prospective, randomized, controlled, cross-over study. All patients requiring intubation with a predicted duration of mechanical ventilation ≥48 h were eligible. Eighteen patients randomly received continuous control of Pcuff with PressureEasy® device for 24 h, followed by discontinuous control (every 4 h) with a manual manometer for 24 h, or vice versa. Pcuff and airway pressure were continuously recorded. Pcuff target was 25 cmH2O during the two periods.
Results
The percentage of time spent with Pcuff 20–30 cmH2O (median (IQR) 34 % (17–57) versus 50 % (35–64), p = 0.184) and the percentage of time spent with Pcuff <20 cmH2O (23 % (5–63) versus 43 % (16–60), p = 0.5) were similar during continuous control of Pcuff and routine care, respectively. However, the percentage of time spent with Pcuff >30 cmH2O was significantly higher during continuous control compared with routine care of tracheal cuff (26 % (14–39) versus 7 % (1–18), p = 0.002). No significant difference was found in Pcuff (25 (18–28) versus 21 (18–26), p = 0.17), mean airway pressure (14 (10–17) versus 14 (11–16), p = 0.679), or coefficient of variation of Pcuff (19 % (11–26) versus 20 % (11–25), p = 0.679) during continuous control compared with routine care of tracheal cuff, respectively.
Conclusions
PressureEasy® did not demonstrate a better control of Pcuff between 20 and 30 cmH2O, compared with routine care using a manometer. Moreover, the device use resulted in significantly higher time spent with overinflation of tracheal cuff, which might increase the risk for tracheal ischemic lesions.
Trial registration
Clinicaltrial.gov: NCT02109003
Keywords: Cuff pressure, Control, Tracheal ischemia, Microaspiration, Mechanical ventilation
Background
Intubation is an invasive procedure that is still performed in a large proportion of critically ill patients [1]. Some long-term intubation-related complications are caused by inappropriate cuff pressure (Pcuff), and include microaspiration, and tracheal ischemic lesions [2, 3]. Microaspiration of contaminated oropharyngeal secretions is the main route of entry for bacteria into the lower respiratory tract [4, 5]. Colonization of the lower respiratory tract could progress into ventilator-associated pneumonia (VAP) when local or general defense mechanisms are altered in intubated critically ill patients [6]. VAP is the most frequent infection acquired in the ICU and is associated with high morbidity and mortality, especially in patients with comorbidities [7]. Tracheal ischemic injury is also associated with high morbidity, especially when routine care for tracheal cuff is not adequately provided [8–11].
Based on international recommendations, Pcuff should be kept between 20 and 30 cmH2O using a manometer [12, 13]. However, several recent studies suggested that a noncontinuous control of Pcuff using a manometer is not effective in intubated critically ill patients [14]. Further, previous studies did not identify modifiable risk factors for overinflation or underinflation of tracheal cuff [15–18].
Recently, several devices allowing continuous control of Pcuff were evaluated [19–22]. These devices are classified into pneumatic (i.e., does not require power supply, but a single-use 200-ml cylindrical cuff) and electronic devices (requiring power supply). Two prospective randomized controlled animal and human studies first validated the use of a pneumatic device [19, 20]. A noncommercially available device has also been validated and proved to be efficient in continuously controlling Pcuff in ICU patients [22]. However, other commercially available automated devices were only validated in in vitro studies [23, 24]. Recent data suggest that these devices interfere with the self-sealing characteristics of high-volume low-pressure (HVLP) tracheal cuffs [23]. Further, these electronic devices have been shown to be less efficient than the pneumatic device in continuous control of Pcuff, because of rapid correction of overinflation episodes [21].
A new mechanical device with an improved design aiming at continuously controlling Pcuff has been commercialized (PressureEasy®, Smiths medical). The advantages of using such device are its small size and lower cost compared with other devices. However, to our knowledge, no study has evaluated the efficiency of this device in controlling Pcuff. Therefore, we conducted this prospective randomized cross-over study to determine the efficiency of this device in continuously controlling Pcuff. Our hypothesis was that this mechanical device would allow significant reduction of time spent with underinflation or overinflation of Pcuff, compared with routine care, using a manual manometer.
Methods
Ethical aspects
This prospective randomized controlled study was performed in a 16-bed ICU of the teaching hospital of Sabadell (Spain), from April 2014 to July 2014. The institutional review board of the Parc Tauli University Hospital approved the study. Written consent was obtained from the patients or from their next of kin (ClinicalTrials.gov identifier: NCT02109003 http://clinicaltrials.gov/show/NCT02109003).
Inclusion and exclusion criteria
All patients older than 18 years who were intubated with a predicted duration of mechanical ventilation ≥48 h were eligible for the study. Patients were excluded if they (1) were admitted to the ICU with a previous tracheostomy, (2) were enrolled in another study that might influence this study results, or (3) were intubated and mechanically ventilated >48 h at the time of randomization.
Randomization
Patients were randomly assigned to receive continuous control of Pcuff with the mechanical device (PressureEasy®) (Fig. 1) for 24 h, followed by discontinuous control (every 4 h) with a manual manometer (Hi-Lo Hand Pressure Gauge, Covidien, TM, Malinckrodt TM) for 24 h; or discontinuous control of Pcuff followed by continuous control of Pcuff. The target of Pcuff was 25 cmH2O during the two periods. Randomization was performed using a computer-generated random assignment list in balanced blocs of four. Treatment assignments were contained in sealed opaque envelopes sequentially numbered.
Fig. 1.
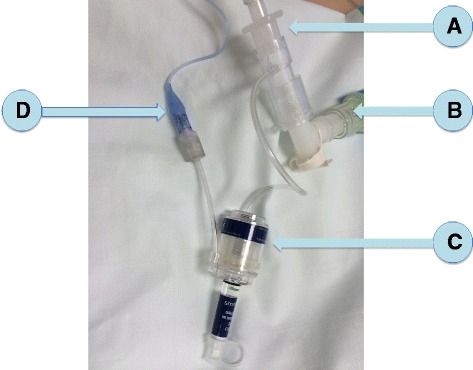
The PressureEasy® device. a Tracheal tube, (b) ventilator circuit, (c) PressureEasy®, and (d) external tracheal cuff
PressureEasy® is a single-patient use device, designed to continuously monitor tracheal Pcuff. Its indicator window signals that Pcuff is maintained between 20 and 30 cmH2O. In addition, the airway pressure auto-feedback feature boosts Pcuff to ensure proper sealing when high pressures are used during ventilation. A pressure feedback line is designed to eliminate cuff leaks at peak inspiratory pressure.
Outcome measurement
In order to determine the percentage of patients with underinflation and overinflation of tracheal cuff and the duration of these episodes, the Pcuff and the airway pressure were continuously recorded at a digitizing frequency of 100 Hz for 48 h (Physiotrace®; Estaris, Lille, France) [25], including 24 h of continuous control of Pcuff using the mechanical device and 24 h of manual control of Pcuff using the manometer. The connection between the pressure transducer and the tracheal cuff was identical in the two study periods, with a three-way stopcock of which the third port was either connected to the mechanical device or closed and connected to the manometer every 4 h (Fig. 2). A second pressure transducer was connected to the heat and moisture exchanger, in order to record respiratory pressure. Pressure transducers were connected to a laptop, in order to continuously transfer pressure-time curves and mean values. Connections were checked every 4 h. The engineer who analyzed the data (JDJ), using the same program, was blinded to the randomization order.
Fig. 2.
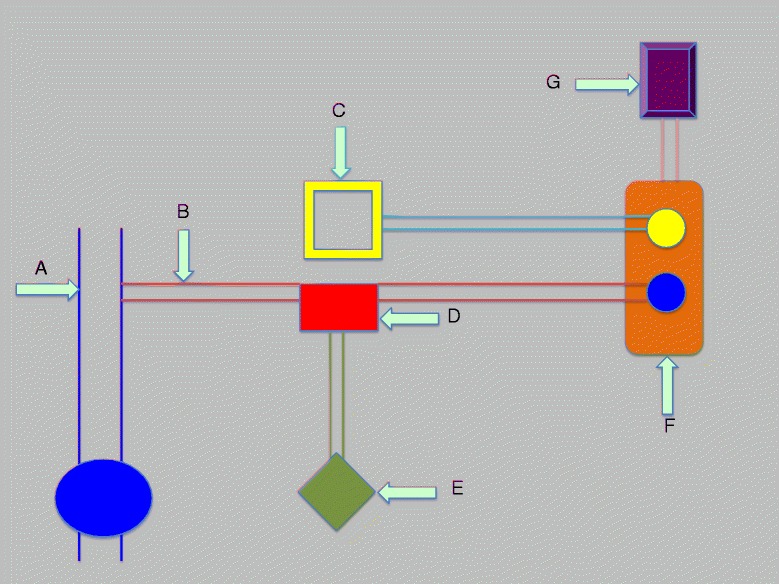
Description of connections between pressure transducers, ventilator, and tracheal tube. a Tracheal tube, (b) connection between external cuff and the three-way stopcock, (c) ventilator, (d) three-way stopcock, (e) PressureEasy® device or manometer, (f) pressure transducers connected to the ventilator and to tracheal cuff, and (g) laptop
Study population
All the patients were intubated with a high-volume low-pressure PVC-cuffed tracheal tube with continuous subglottic secretion drainage (TaperGuard Evac, Covidien, Mallinckrodt) and were included within the first 48 h of intubation. Tracheal tube size was 8 and 7.5 in men and women, respectively. During the manometer period, nurses adjusted Pcuff every 4 h. Tracheal suctioning was performed six times a day or more frequently if clinically indicated.
Other definitions
Underinflation of tracheal cuff was defined as Pcuff <20 cmH20 for >5 min over the 24-h period of recording. Overinflation of tracheal cuff was defined as Pcuff >30 cmH20 for >5 min over the 24-h period of recording [14].
The primary objective was to determine the efficiency of the mechanical device in reducing the percentage of time spent with underinflation or overinflation of tracheal cuff, compared with routine care using a manometer. The secondary objective was to determine the efficiency of the mechanical device in reducing the percentage of patients with underinflation or overinflation of tracheal cuff, compared with routine care.
The coefficient of variation of Pcuff was calculated as standard deviation/mean Pcuff × 100.
Statistical analyses
Sample size calculation
Based on unpublished preliminary results, the mean percentage of time spent with underinflation or overinflation of tracheal cuff was 30 % (SD 20 %) in patients intubated with a PVC-cuffed tracheal tube receiving routine care of Pcuff using a manual manometer. The expected mean percentage of time with underinflation or overinflation of tracheal cuff using the mechanical device was 10 % (expected difference of 20 %). Considering a power of 90 % and an alpha risk of 5 %, the inclusion of 22 patients was required in a parallel-group design. However, when we took into account the cross-over design, the number of patients to include was 18.
Result analysis
All analyses were performed in an intention-to-treat manner. Distribution of quantitative variables was tested using the Shapiro-Wilk test. Normally and nonnormally distributed variables were expressed as mean ± SD and median (25th, 75th interquartile), respectively. The difference between the two groups was considered as significant if p < 0.05. McNemar and Wilcoxon tests were used to compare qualitative and quantitative variables between the two 24-h periods, respectively.
We calculated the median (25th, 75th interquartile) value of mean airway pressure in all patients and considered patients with airway pressure >75th interquartile as patients with high airway pressure. In order to determine the impact of high airway pressure on the efficiency of the mechanical device, we repeated all statistical analyses after exclusion of patients with high airway pressure.
Results
Patient characteristics
Eighteen patients were included in this study. Their mean age was 62 ± 12 years. At ICU admission, SAPS II and SOFA scores were 51 ± 14 and 7.7 ± 3.1, respectively. Duration of mechanical ventilation and ICU stay were 25 ± 18 days and 33 ± 21 days, respectively. No significant difference was found between the two study periods regarding the percentage of patients receiving sedation or neuromuscular-blocking agents. Glasgow Coma score, SOFA score, and PEEP were also similar during the two periods (Table 1). All patients received assist-control ventilation during the recording period. No significant difference was found in mean airway pressure between the two study periods (Table 2.)
Table 1.
Patient characteristics during the 48 h following randomization
| Variables | Continuous control of P cuff | Routine care | p values |
|---|---|---|---|
| n = 18 | n = 18 | ||
| SOFA score | 8.8 ± 3.4 | 9 ± 3.7 | 0.458 |
| Sedation | 14 (77) | 14 (77) | >0.999 |
| Neuromuscular-blocking agent use | 1 (5) | 0 (0) | >0.999 |
| Glasgow coma scorea | 7.5 ± 3 | 9 ± 3.7 | 0.137 |
| PEEP | 6.7 ± 1.9 | 6.6 ± 1.9 | 0.157 |
Data are frequencies (%) or mean ± SD
SOFA sequential organ failure assessment, PEEP positive end-expiratory pressure
aThe verbal response was evaluated as in intubated patients: 1 no answer, 2 seems able to give simple responses, and 5 seems able to speak
Table 2.
Impact of the mechanical device on tracheal cuff pressure
| Variables | Continuous control of P cuff | Routine care | p values |
|---|---|---|---|
| n = 18 | n = 18 | ||
| Mean airway pressure, cmH2O | 14 (10–17) | 14 (11–16) | 0.679 |
| Mean P cuff, cmH2O | 25 (18, 28) | 21 (18, 26) | 0.17 |
| Coefficient of variation of P cuff, % | 19 (11, 26) | 20 (12, 25) | 0.67 |
| P cuff 20–30 cmH2O | |||
| Yesa | 18 (100) | 18 (100) | NA |
| % of timeb | 34 (17, 57) | 50 (35, 64) | 0.184 |
| P cuff <20, cmH2O | |||
| Yesa | 17 (94) | 18 (100) | >0.999 |
| % of timeb | 23 (5, 63) | 43 (16, 60) | 0.528 |
| P cuff >30, cmH2O | |||
| Yesa | 18 (100) | 16 (89) | 0.500 |
| % of timeb | 26 (14, 40) | 7 (1, 18) | 0.002 |
Data are frequencies (%) or median (interquartile range)
P cuff cuff pressure, NA not applicable
aYes indicates the number of patients with at least one P cuff 20–30, >20, or >30 cmH2O
b% of time indicates all the time spent with P cuff 20–30, >20, or >30 cmH2O reported to the total recording time during each study period
Impact of the mechanical device on Pcuff
No significant difference was found in mean Pcuff, percentage of time spent with Pcuff between 20 and 30 cmH2O, percentage of time spent with Pcuff <20 cmH2O, or coefficient of variation of Pcuff. The percentage of time spent with Pcuff >30 cmH2O was significantly higher during continuous control compared with routine care of tracheal cuff (Table 2). No significant difference was found in the percentage of patients with Pcuff 20–30 cmH2O, Pcuff <20 cmH2O, or Pcuff >30 cmH2O during continuous control of Pcuff and routine care (Table 2). No significant difference was found in the number of underinflation episodes between continuous control and routine care periods (median (IQR) 0 (0, 5) versus 2 (0, 7), p = 0.733).
Subgroup analysis
After exclusion of the four patients with high mean airway pressure (>17 cmH2O), no significant difference was found in mean Pcuff (26 (18, 28) versus 22 (19, 27) cmH2O, p = 0.177), percentage of time spent with Pcuff between 20 and 30 cmH2O (50 (24, 58) versus 53 (24, 71), p = 0.124), percentage of time spent with Pcuff <20 cmH2O (16 (2, 51) versus 35 (9, 51), p = 0.3), or coefficient of variation of Pcuff (15 (10, 22) versus 16 (10, 24), p = 0.363). The percentage of time spent with Pcuff >30 cmH2O was still significantly higher during continuous control compared with routine care of tracheal cuff (31 (18, 42) versus 8 (3, 20), p = 0.006).
Discussion
The results of our study suggest that PressureEasy® was not more efficient in maintaining Pcuff within the recommended range (20–30 cmH2O) than routine care of tracheal cuff using a manometer every 4 h. No significant difference was found between continuous control of Pcuff using the mechanical device and routine care regarding Pcuff, percentage of time spent with underinflation of tracheal cuff, and coefficient of variation of Pcuff. However, the percentage of time spent with overinflation of tracheal cuff was significantly higher during continuous control compared with routine care.
One potential explanation for this result is the high frequency of Pcuff control using the manometer, i.e., every 4 h, during routine care. A recent prospective study performed in a cohort of 102 patients receiving invasive mechanical ventilation and manual control of Pcuff using a manometer every 8 h found a lower percentage of time (18 %) spent within the target range [14]. However, this explanation is unlikely because our results are in agreement with those of another recent trial, in which Pcuff was controlled using a manometer every 8 h [13]. Further, previous studies showed that each time a manometer is connected to the tracheal cuff, a sudden drop of Pcuff is observed [22, 26]. This is probably related to transient underinflation of tracheal cuff and might promote microaspiration of contaminated secretions [27]. Therefore, controlling Pcuff more frequently in order to maintain it within the target range could probably not be recommended.
In spite of strictly applying instructions for user of the mechanical device, the percentage of time spent within the target range of Pcuff was only 34 %. Previous studies using different devices to continuously control Pcuff reported better performances and a percentage within the targeted range >90 % [19, 20, 22, 28]. Another potential explanation for the low efficiency of the device is the mechanism of Pcuff control. This device uses the respiratory flow to inflate tracheal cuff during inspiration. Therefore, it is dependent on respiratory flow and airway pressure that may widely vary in critically ill patients. In addition, the key principle is probably an equilibrium between airway pressure and Pcuff, which means that the time constant of the system might play a role in the higher Pcuff [29, 30]. Further, the exact Pcuff target, using the mechanical device, could not be precisely determined or modified. In order to test the hypothesis that our results could be explained by the bad performance of the device in patients with high airway pressures, we repeated all statistical analyses after exclusion of patients with the highest mean airway pressure but found similar results. This might be explained by the fact that high airway pressure could have occurred during only a short period of the total recording time and the relatively small number of included patients.
To our knowledge, our study is the first to evaluate the PressureEasy® mechanical device, and its results suggest that the device should not be used in critically ill patients. Our study also raises the important question of why medical devices such as tracheal tubes or Pcuff controllers could obtain the Communauté Européenne (CE) mark and be used in critically ill patients without any published clinical data proving their efficiency. Nevertheless, clinical evaluation before CE mark could be very complex and might reduce the development of new technologies.
No significant difference was found in coefficient of variation of Pcuff between the two study periods. However, Pcuff variation in study patients was relatively small and had probably no clinical impact, except in those patients with Pcuff around 20 or 30 cmH2O.
Some limitations of our study should be acknowledged. First, this was a single-center study. All study patients received assist-control ventilation, and a high proportion of them were sedated. Therefore, our results may not be generalized to patients in other ICUs, especially to nonsedated patients receiving pressure support ventilation. Second, the number of studied patients was relatively small. However, Pcuff was continuously recorded for 48 h. In addition, the number of included patients was calculated based on our hypothesis before starting the study. Third, because of the study design, namely the fact that every patient was its own control, we could not evaluate complications related to underinflation or overinflation of tracheal cuff. However, we think that this design is probably optimal as a first step in order to validate the device, before performing larger studies evaluating its impact on complications. Our study design allowed adjustment of patient-related confounding factors, such as tracheal anatomy, tracheal tube size, and airway pressure. Fourth, we excluded patients who could not be included during the first 48 h of their invasive mechanical ventilation. This exclusion criterion was selected because duration of intubation was identified as a risk factor for underinflation of tracheal cuff [14]. Fifth, we did not collect Pcuff values obtained manually during the routine care period and did not evaluate the relationship between manometer connection and any drop in Pcuff. However, several previous well-designed and performed studies have clearly confirmed this relationship [22, 26]. Finally, Glascow Coma score was used to evaluate consciousness in study patients. However, a sedation score would have been more appropriate in sedated patients.
Conclusions
The PressureEasy® device did not demonstrate a better control of Pcuff within the target range (20–30 cmH2O), compared with routine care using a manometer every 4 h. Moreover, the percentage of time spent with overinflation of tracheal cuff was more frequent using this device compared with routine care, which might increase the risk for tracheal ischemic lesions. Therefore, the use of this device could not be recommended in critically ill patients. Further large studies are required to confirm our results.
Abbreviations
- CE
Communauté Européene
- HPLV
high-volume low-pressure
- Pcuff
cuff pressure
- PEP
positive expiratory pressure
- SAPS
simplified acute physiology score
- SOFA
sequential organ failure assessment
- PVC
polyvinyl chloride
Footnotes
Competing interests
SN is a member of the advisory board in Covidien. The other authors declare that they have no competing interests.
Authors’ contributions
SN, JV, AA, and IML designed the study. AR and PS included the patients, performed the pressure recording, and collected the data. JDJ performed the data analyses. SN and IML coordinated the study. SN performed the statistical analysis and drafted the manuscript. All authors participated in the data interpretation and read and approved the final manuscript.
Contributor Information
Saad Nseir, Email: s-nseir@chru-lille.fr.
Andrey Rodriguez, Email: alrodriguez@tauli.cat.
Paula Saludes, Email: psaludes@tauli.cat.
Julien De Jonckheere, Email: Julien.DEJONCKHEERE@CHRU-LILLE.FR.
Jordi Valles, Email: jvalles@tauli.cat.
Antonio Artigas, Email: aartigas@tauli.cat.
Ignacio Martin-Loeches, Email: DRMARTINLOECHES@GMAIL.COM.
References
- 1.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 2.Touat L, Fournier C, Ramon P, Salleron J, Durocher A, Nseir S. Intubation-related tracheal ischemic lesions: incidence, risk factors, and outcome. Intensive Care Med. 2013;39:575–82. doi: 10.1007/s00134-012-2750-6. [DOI] [PubMed] [Google Scholar]
- 3.Nseir S, Zerimech F, Jaillette E, Artru F, Balduyck M. Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets. 2011;11:413–23. doi: 10.2174/187152611796504827. [DOI] [PubMed] [Google Scholar]
- 4.Blot SI, Poelaert J, Kollef M. How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis. 2014;14:119. doi: 10.1186/1471-2334-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuret P, Philippon B, Fabre X, Kaaki M. Effect of tracheal suctioning on aspiration past the tracheal tube cuff in mechanically ventilated patients. Ann Intensive Care. 2012;2:45. doi: 10.1186/2110-5820-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135:521–8. doi: 10.1378/chest.08-1617. [DOI] [PubMed] [Google Scholar]
- 7.Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2014;31:34–48. doi: 10.1007/s00134-014-3564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hameed AA, Mohamed H, Al-Mansoori M. Acquired tracheoesophageal fistula due to high intracuff pressure. Ann Thorac Med. 2008;3:23–5. doi: 10.4103/1817-1737.37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J. 1984;288:965–8. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combes X, Schauvliege F, Peyrouset O, Motamed C, Kirov K, Dhonneur G, et al. Intracuff pressure and tracheal morbidity: influence of filling with saline during nitrous oxide anesthesia. Anesthesiology. 2001;95:1120–4. doi: 10.1097/00000542-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Jaillette E, Martin-Loeches I, Artigas A, Nseir S. Optimal care and design of the tracheal cuff in the critically ill patient. Ann Intensive Care. 2014;4:7. doi: 10.1186/2110-5820-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005, 171:388–416 [DOI] [PubMed]
- 13.Nseir S, Zerimech F, De Jonckheere J, Alves I, Balduyck M, Durocher A. Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med. 2010;36:1156–63. doi: 10.1007/s00134-010-1892-7. [DOI] [PubMed] [Google Scholar]
- 14.Nseir S, Brisson H, Marquette C-H, Chaud P, Di Pompeo C, Diarra M, et al. Variations in endotracheal cuff pressure in intubated critically ill patients: prevalence and risk factors. Eur J Anaesthesiol. 2009;26:229–34. doi: 10.1097/EJA.0b013e3283222b6e. [DOI] [PubMed] [Google Scholar]
- 15.Lizy C, Swinnen W, Labeau S, Poelaert J, Vogelaers D, Vandewoude K, et al. Cuff pressure of endotracheal tubes after changes in body position in critically ill patients treated with mechanical ventilation. Am J Crit Care. 2014;23:e1–8. doi: 10.4037/ajcc2014489. [DOI] [PubMed] [Google Scholar]
- 16.Sole ML, Su X, Talbert S, Penoyer DA, Kalita S, Jimenez E, et al. Evaluation of an intervention to maintain endotracheal tube cuff pressure within therapeutic range. Am J Crit Care. 2011;20:109–17. doi: 10.4037/ajcc2011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubes D, Klein AA, Lips M, Rulisek J, Kopecky P, Blaha J, et al. The effect of adjusting tracheal tube cuff pressure during deep hypothermic circulatory arrest: a randomised trial. Eur J Anaesthesiol. 2014;31:452–6. doi: 10.1097/EJA.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 18.Servin SO, Barreto G, Martins LC, Moreira MM, Meirelles L, Neto JAC, et al. Atraumatic endotracheal tube for mechanical ventilation. Rev Bras Anestesiol. 2011;61:311–9. doi: 10.1016/S0034-7094(11)70037-X. [DOI] [PubMed] [Google Scholar]
- 19.Duguet A, D’Amico L, Biondi G, Prodanovic H, Gonzalez-Bermejo J, Similowski T. Control of tracheal cuff pressure: a pilot study using a pneumatic device. Intensive Care Med. 2007;33:128–32. doi: 10.1007/s00134-006-0417-x. [DOI] [PubMed] [Google Scholar]
- 20.Nseir S, Duguet A, Copin M-C, De Jonckheere J, Zhang M, Similowski T, et al. Continuous control of endotracheal cuff pressure and tracheal wall damage: a randomized controlled animal study. Crit Care. 2007;11:R109. doi: 10.1186/cc6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisson H, Bouhemad B, Lu Q, ROUBY JJ. Comparison of two automated endotracheal tube cuff pressure regulators in intubated critically ill patients. (abstract) Intensive Care Med. 2011;37:296. [Google Scholar]
- 22.Farré R, Rotger M, Ferre M, Torres A, Navajas D. Automatic regulation of the cuff pressure in endotracheally-intubated patients. Eur Respir J. 2002;20:1010–3. doi: 10.1183/09031936.02.02692001. [DOI] [PubMed] [Google Scholar]
- 23.Weiss M, Doell C, Koepfer N, Madjdpour C, Woitzek K, Bernet V. Rapid pressure compensation by automated cuff pressure controllers worsens sealing in tracheal tubes. Br J Anaesth. 2009;102:273–8. doi: 10.1093/bja/aen355. [DOI] [PubMed] [Google Scholar]
- 24.Chenelle CT, Oto J, Sulemanji D, Fisher DF, Kacmarek RM. Evaluation of an automated endotracheal tube cuff controller during simulated mechanical ventilation. Respir Care. 2015;60:183–90. doi: 10.4187/respcare.03387. [DOI] [PubMed] [Google Scholar]
- 25.De Jonckheere J, Logier R, Dassonneville A, Delmar G, Vasseur C. PhysioTrace: an efficient toolkit for biomedical signal processing. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2005;7:6739–41. doi: 10.1109/IEMBS.2005.1616051. [DOI] [PubMed] [Google Scholar]
- 26.Memela ME, Gopalan PD. Variations in endotracheal tube cuff pressure: is 8-hourly monitoring enough? South African J Crit Care. 2014;30:35–40. [Google Scholar]
- 27.Nseir S, Zerimech F, Fournier C, Lubret R, Ramon P, Durocher A, et al. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med. 2011;184:1041–7. doi: 10.1164/rccm.201104-0630OC. [DOI] [PubMed] [Google Scholar]
- 28.Jaillette E, Zerimech F, De Jonckheere J, Makris D, Balduyck M, Durocher A, et al. Efficiency of a pneumatic device in controlling cuff pressure of polyurethane-cuffed tracheal tubes: a randomized controlled study. BMC Anesthesiol. 2013;13:50. doi: 10.1186/1471-2253-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolzan DW, Gomes WJ, Peixoto TCA, Faresin SM, Carvalho AC, De Paola AAV, et al. Clinical use of the volume-time curve for endotracheal tube cuff management. Respir Care. 2014;59:1628–35. doi: 10.4187/respcare.02683. [DOI] [PubMed] [Google Scholar]
- 30.Richard J-CM, Mercat A. Tracheal cuff management as part of a lung-protective strategy. Respir Care. 2014;59:1810–1. doi: 10.4187/respcare.03745. [DOI] [PubMed] [Google Scholar]


