Abstract
Upper limb lymphedema can be an unfortunate sequela following the oncologic treatment of breast cancer. The surgical treatment of lymphedema has had a recent renewed clinical interest paralleling innovative descriptions of surgical techniques and imaging modalities. In addition, an improved understanding of the physiology and pathophysiology of lymphedema has allowed improved translation to the clinical condition. Various surgical options exist to decrease the symptom-burden of upper limb lymphedema, including vascularized lymph node (VLN) transfer, lymphovenous bypass (LVB), liposuction, lymphatic grafting, and excisional procedures. Modern imaging techniques help to improve the consistency and accuracy of these surgical treatment options. A multi-modal treatment plan utilizing non-operative and surgical therapies has the potential to improve various factors related to overall patient quality of life. This review details all of the current operative treatment strategies and modern imaging modalities used in the treatment of lymphedema.
Keywords: Lymphatic mapping, lymphedema, lymphatic surgery, microsurgery, lymphovenous bypass (LVB), vascularized lymph node (VLN) transfer, liposuction, lymphatic grafting
Introduction
Lymphedema is a condition characterized by persistent edema related to lymphatic injury or disease. Over time, chronic lymphedema leads to fat deposition and subsequent fibrosis of the surrounding tissues (1). Lymphedema is classified into primary and secondary types. Primary lymphedema is of congenital (genetic, developmental abnormalities) or idiopathic origin. Secondary lymphedema occurs following injury to lymphatic structures, often following infection surgery, and radiation (2,3). Worldwide Wuchereria bancrofti, a parasitic infection, is the leading cause of lymphedema. It is estimated that between 140 and 250 million people are affected by this condition around the world. However, in western and industrialized societies, breast cancer treatment involving lymphadenectomy and/or radiation to the regional lymphatic system is the major source of clinical lymphedema (4,5). Lymphedema has been reported to occur within days and up to 30 years after breast cancer treatment (6). In addition, 80% of patients experience onset of symptoms within 3 years of surgery as the remainder of patients have a 1% incidence of lymphedema each year (7).
The incidence of breast cancer-related lymphedema (BCRL) varies from 6-49% following axillary lymph node dissection and between 2-7% in patients after sentinel lymph node biopsy (1,3,5,8-11). Although many patients will experience mild symptoms in the early stages of disease, chronic lymphedema is a progressive disease that significantly decreases patients’ quality of life, with known consequences related to a woman’s physical, psychological, and emotional well-being (12). Mainstay treatment algorithms focus on non-surgical modalities of treatment, including comprehensive physiotherapy involving multilayer compression wrapping, manual drainage techniques, and various exercises. Modern lymphedema care is slowly incorporating surgical interventions into multimodal treatment plans in treating patients with BCRL (12).
Pathophysiology of lymphedema
The lymphatic system has multiple functions including transport of lipids, regulation of body fluid homeostasis, and immune cell trafficking (2,4,8). The lymphatic structural components act in concert to achieve a unidirectional egress of lymph in the normally functioning lymphangion (functional unit of the lymphatic system). The pathophysiologic process begins when there is an accumulation of interstitial fluid at the lymphatic capillary level resulting in a net fluid efflux. Venous capillaries reabsorb 90% of the fluid in the interstitium, while the remaining fluid is transported to the blood by the lymphatics as lymph (2). This occurs at the level of lymphatic capillaries through a semi-permeable endothelial membrane facilitating physiologic uptake of fluid and macromolecules. As fluid moves toward lymphatic pre-collectors (containing valves) and collecting ducts, phenotypic changes occur within the ultrastructure of the lymph vessels with a resultant increasing smooth muscle cells (SMC) (13). The extracellular structural environment prevents valve incompetence and lymph stasis or reflux. This is also in part due to a synchronized pump mechanism that has been described to propel lymph from lymphangion to lymphangion. Under normal conditions, the same volume of efferent lymph is transported from the interstitium as the volume of afferent lymph transported back to the blood stream through the major lymphatic drainage pathways and through the nodal circulation. Any lymphatic dysfunction resulting in reduction of lymph transport capacity causes an imbalance of intraluminal volume resulting in increases in intraluminal pressure. Persistent lymphatic hypertension leads to histological changes such as SMC hypertrophy, extracellular remodeling, reduction in valve competence, bi-directional luminal flow, and pathologic lymphatic ectasia (14). Early impairment of lymphodynamics can have downstream effects that perpetuate lymphatic dysfunction and ultimately overwhelm the lymphatic system resulting in regurgitant lymphatic fluid into the subdermal lymphatics (dermal backflow) and the interstitial compartment. These processes result in progressive fluid accumulation and extremity swelling.
Because the aforementioned pathophysiology of the lymphatic system is time dependent, the clinical manifestation of lymphedema are similarly time dependent. Typically early lymphedema is amenable to compression physiotherapy, but with time the chronic fluid compartments will lead to fat deposition. As disease progresses, skin fibrosis and hyperkeratosis will develop. This is commonly associated with an immunologic impairment often manifesting as recurrent cellulitis or dermatolymphangioadenitis (DLA) attacks. Additionally, immune cells such as CD4+, Th2 cells are also implicated in promoting a pro-fibrotic environment through cytokine release (15-17). The ability to reduce infection, restore lymphatic flow, reduced extremity circumference, improve patient quality of life, and slow the progression of fibrosis are all associated with the goals of novel surgical techniques, which is why a thorough understanding of the pathophysiology aids the surgeon in interpreting lymphatic mapping and patient symptoms to select ideal candidates for surgery.
Lymphatic imaging and mapping
Lymphoscintigraphy
Lymphoscintigraphy or isotopic lymphoscintigraphy, is an objective and reliable non-invasive imaging modality used to diagnose extremity lymphedema, characterize its severity, and assess post-therapeutic results (18). This imaging modality involves an intradermal injection of radiolabeled colloid in the distal aspect of the edematous limb and subsequent imaging of the lymphatic vasculature (2,19,20). The study provides information regarding both lymphatic anatomy as well as lymphatic function (21). Typical abnormalities seen in patients with lymphedema include absent or delayed radiotracer transport, cutaneous flare, dermal infiltration or backflow, and poorly visualized lymphatic collectors and lymph nodes (22) (Figure 1).
Figure 1.
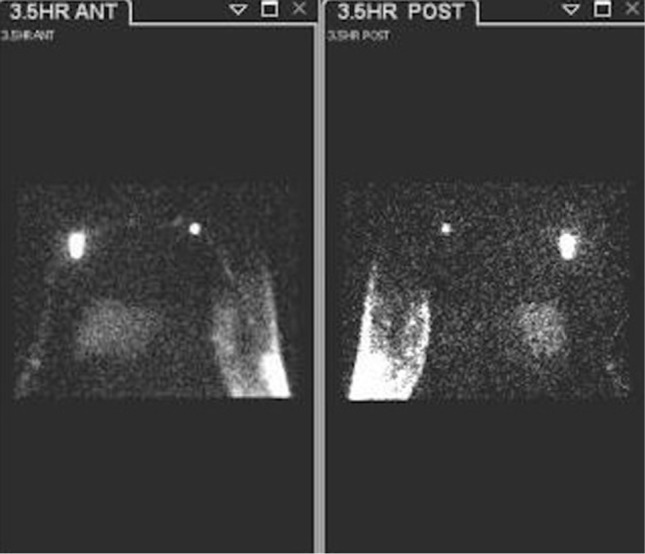
A 59-year-old female with a history of left mastectomy and axillary nodal dissection 8 years prior to the lymphoscintigraphic evaluation shown. Dermal backflow on the left side can be appreciated at 3.5 h after injection in the forearm, while right side lymphatic transport is normal. A lymphatic vessel draining into an axillary node can be seen indicating delayed clearance and partial obstruction.
According to previous studies (23,24), baseline lymphoscintigraphy can be useful to predict long term response to complex decongestive therapy (CDT) in patients with early stage unilateral limb lymphedema. Both qualitative and quantitative lymphoscintigraphy can be used to assess the severity of disease. Qualitative lymphangiographic scoring typically involves the visual interpretation of lymphoscintigraphy and the presence of lymphatic trunks, caliber of trunks, visualization of lymph nodes, collateralization of lymphatics, dermal back flow, and subjective delay in uptake of radiotracer. Quantitative lymphoscintigraphy may vary in methodology amongst groups. However, quantification typically focuses on lymphatic tracer uptake through time of initial and delayed uptake at the injection site, clearance time from the injection site, clearance times from anatomic limb, detectable radioactive residual radiolabelled colloid, and various other calculations. Qualitative lymphoscintigraphy alone can provide a reliable diagnosis (25), but it has been shown to have lower diagnostic value than a combination of quantitative and qualitative lymphoscintigraphy (19). Several recent studies have used quantitative lymphoscintigraphy to assess the severity of lymphatic insufficiency in BCRL, as well as the outcomes following treatment in patients with lower-limb lymphedema (26,27).
Currently, lymphoscintigraphy is considered the gold-standard imaging modality for the diagnosis of patients with lymphedema and for evaluation of lymphatic disorders in the swollen extremity (28,29). Lymphoscintigraphy can detect delayed tracer transport even in mild lymphedema without morphological abnormalities and is useful to evaluate the functional lymph flow in patients following physiologic surgery for lymphedema.
Magnetic resonance angiography and lymphangiography (MRL) and computed tomography (CT)
MRL has been used as an aid in the clinical diagnosis of lymphatic disorders since 1990 (30,31). MRL has a number of potential advantages compared with lymphoscintigraphy, including higher spatial resolution enabling depiction of lymphatic channels, higher temporal resolution, production of three-dimensional (3D) images, higher signal-to-noise ratio, fewer artifacts, assessing the thickness of the underlying tissues and the absence of exposure to ionizing radiation (32). In recent years, a number of different contrast agents have also been developed and tested in MR lymphangiography for imaging of the lymphatic system (33-35). A common agent used is the extracellular, water soluble paramagnetic Gd-BOPTA (Gadolium dimeglumine) (36). The advantages of using MRL are the capability to map the morphologic architecture of the affected lymphatic system while simultaneously analyzing the lymphatic vessels and nodes in a dynamic fashion (36-38). This technique has been shown to be safe and feasible and with minimal complications (37). Most of these agents are injected into the dermis for the staging of malignant lymph nodes or to show lymphatic drainage patterns.
CT imaging is a valuable imaging modality for many disease entities including lymphedema. In the setting of lymphedema, CT imaging has been used to aid in the diagnosis of unilateral extremity swelling, as other common sources of swelling, including deep venous thrombosis (DVT), lipedema, lymphedema, cellulitis, hematomas, and Baker’s cyst rupture, can be detected with this imaging modality. In addition, CT scans are useful for assessing skin thickening, subcutaneous swelling, and calculating limb volume measurements. However, as an imaging modality used to evaluate lymphedema, it is not considered a first-line choice due to concerns for radiation exposure and less diagnostic and prognostic precision (39).
Ultrasonography (US)
U/S is a non-invasive, low cost, non-radiating technique that is routinely used to assess edema and thickness of limbs. With a high-resolution ultrasonographic imaging, there are some reports that support its use in differentiating lymphedema from lipedema (40). Conventional U/S creates images based on differences in reflection and diffraction of ultra-high frequency sound waves. To be useful for lymphatic imaging, contrast enhancers (microbubbles) consisting of gaseous cores enclosed in lipid or polymer shells are injected, allowing visualization of lymph nodes as the microbubbles are disrupted by the applied acoustic waves. Lymph nodes are able to be targeted using this method because microbubbles are phagocytosed by macrophages and subsequently transported to reside in selected lymph nodes (41-43). Furthermore, U/S can be crucial in the pre-operative planning of vascularized lymph node (VLN) transfer. Duplex US has been found to be valuable for understanding the exact location and number of lymph nodes present in a given donor site, the size and caliber of the vascular pedicle, the flap thickness of the flap, and the associated structural anatomy (44).
Near-infrared (NIR) fluorescence imaging, indocyanine green (ICG)
Fluorescence imaging is an optical technique in which incident photons excite molecules in tissue, which then emit light (usually at a longer wavelength) as the electrons return to the ground state (41). ICG is a tracer that is injected in the dermis and visualized with the NIR technology. When injected intravenously, ICG does not contain any active metabolites, which facilitates rapid processing and excretion into bile without secondary effects (45). High-performance optics and NIR detectors are able to visualize relatively high resolution images up to several centimeters into soft tissues (46). This technique evaluates the lymphatic channels in real time. In addition to detecting lymph flow abnormalities, this technique has been shown to be safe (nontoxic/nonionizing). Also, the tracer has a short half-life which allows for repetitive application, making it a convenient, minimally invasive, and suitable method for preoperative, intraoperative, and postoperative lymphatic channel evaluation (47,48). In addition, it is easy to use, has a high-noise-signal ratio, separating the target from the background, very sensitive even with small concentrations of the ICG, it is a low cost, user-friendly technology (45). The use of ICG imaging technology has rapidly expanded and although procedural protocols may differ slightly, this novel technology now permeates into multiple surgical specialties. Intravenous administration serves as a useful tool in cerebral angiography, coronary angiography, assessment of peripheral artery disease and vascular graft patency, perfusion prior to transplantation of solid organs, evaluation of sentinel lymph node biopsy and lymphadenectomy during oncologic resections, aids in flap monitoring, and is used to delineate biliary and hepatic anatomy during general surgery procedures. Specifically related to its application in lymphedema, ICG injection into the dermis is able to delineate the morphologically of the lymphatic system and provide a real-time functional analysis of the lymphatic channels and nodes. As a result, ICG lymphography is the most clinically implemented imaging tool used to evaluate severity of disease and monitor surgical outcomes in primary and secondary lymphedema (49-51). In addition, ICG lymphography has been able to demonstrate the efficacy of manual lymphatic drainage therapy in increasing lymph flow and to detect early signs of lymphatic dysfunction in breast cancer survivors (41,52,53).
Clinical lymphedema
Lymphedema is a chronic condition of the lymphatic system in which there is interstitial accumulation of protein-rich fluid and subsequent inflammation, adipose tissue hypertrophy, and fibrosis (2). In addition to inflammation, slowed lymphatic flow has also been shown to incite lipogenesis and fat deposition and later leading to increased fibrocyte activation and connective tissue overgrowth. Affected patients develop progressively firmer subcutaneous tissue as fibrosis ensues, in addition to hypertrophy of adipose tissue. These pathologic changes manifest initially as swelling of the affected limb or region, described as soft and pitting, but later progress to a more firm and fibrotic state. As this condition gets worse, it can cause physical, emotional, and social distress to any patient (1,4,54,55).
Lymphedema is diagnosed by history, physical examination, and physiologic measures. In more advanced stages, the clinical presentation is very evident. However, if a patient presents in an early stage, this scenario can be more challenging since there are many causes of limb swelling. Physical examination features classically unique to lymphedema include peau d’ orange changes of the skin, indicating cutaneous and subcutaneous fibrosis (56), and a positive Stemer sign (the inability to grasp the skin of the dorsum of the second digit). Documentation and diagnosis of lymphedema has classically been made through circumferential measurements or volumetric documentation comparing the patient’s affected and unaffected limb (>2 cm limb difference or a volume differential of greater than 200 cc). Crucial to the diagnosis is a thorough understanding of a patient’s previous treatment history, including surgery and radiation therapy. Non-invasive methods that can be used during a patient’s clinical examination include bioelectric impedance analysis (57,58), tonometry (59), and perometry (60). Bioimpedance technologies are commonly used in body composition analysis and allow for a more direct measure of differences in edema volume, versus simple measure of differences in limb volume that do not take specific tissue compartment changes into account (61,62).
The differential diagnosis of lymphedema is broad and includes systemic causes of edema, such as cardiac failure, renal failure, malignancy, and protein losing conditions, and local etiologies, including lipedema, deep vein thrombosis, chronic venous insufficiency, myxedema, cyclical, and idiopathic edema.
There are several classification scales for lymphedema. However, the most commonly accepted is based on the International Society of Lymphology (ISL) (63).
Stage 0: a subclinical state where swelling is not evident despite impaired lymph transport. This stage may exist for months or years before edema becomes evident.
Stage I: this represents early onset of the condition where there is accumulation of tissue fluid that subsides with limb elevation. The edema may be pitting at this stage.
Stage II (early): limb elevation alone rarely reduces swelling and pitting manifest.
Stage II (late): there may or may not be pitting as tissue fibrosis is more evident
Stage III: the tissue is hard (fibrotic) and pitting is absent. Skin changes such as thickening are seen.
Severity:
Mild: <20% excess limb volume;
Moderate: 20-40% excess limb volume;
Severe: >40% excess limb volume.
Surgical treatment of lymphedema
Although the gold standard for treatment of lymphedema is considered physiotherapy, termed complete decongestive therapy (CDT), surgical therapy has gained momentum in recent years. The burden of massive fibrosis may hamper the benefits of microsurgical procedures, which is why excisional surgery may be of use in cases dominated by excess subcutaneous tissue and skin. In addition, liposuction techniques have been described as a valuable method to remove subcutaneous tissues with the potential for sustained limb volume reduction. Overall, the surgical treatment for lymphedema may be divided into two groups: excision procedures and physiologic procedures. Recent and future clinical experiences have devised combination and staged procedures using multiple modalities to achieve a desirable outcome.
Excisional surgery
First described in 1912, the Charles procedure is a debulking surgery used to remove skin and subcutaneous tissue, leaving the deep fascia intact. Split-thickness skin grafts are used to cover raw areas, and many times, grafted skin can be obtained from the excised areas (64). This procedure is reserved for patients with late stage lymphedema historically termed ‘elephantiasis’. Indications for performing an extensive surgery as the Charles procedure are focused on functional disability and recurrent infections or cellulitis events.
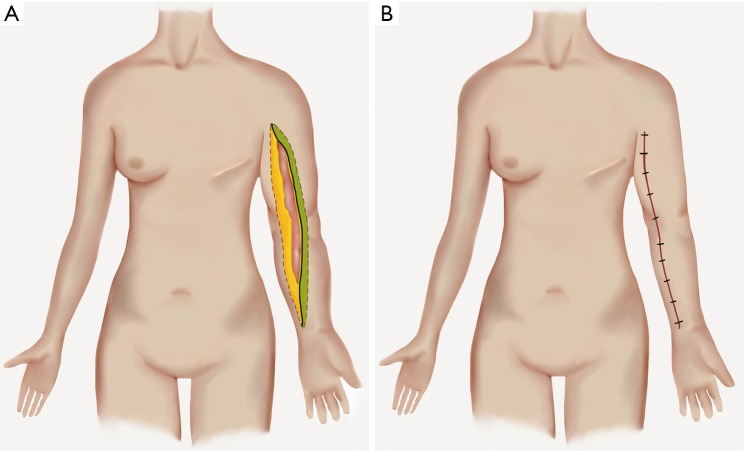
Shortly thereafter, Sistrunk described another method for debulking lymphedematous tissue in 1918, known as the modified Kondoleon procedure or Thompson procedure (65,66). The procedure involves a lateral elliptical, partial excision of skin, subcutaneous structures, and the deep fascia along the lower extremity. The exposed muscle is then covered by local flaps. This procedure was reserved for end stage lymphedema marked by hyperkeratosis. Conceptually, Sistrunk described the potential to reconnect the superficial lymphatics with deep lymphatics to restore lymphatic function, but he emphasized the success of the surgery was dependent on the excision of diseased portions of the superficial system (67). In the upper limb, a medial ellipse of skin and subcutaneous tissue may be excised along the length of the extremity or where excess tissue exists (Figure 2A,B). Careful dissection around superficial venous structures and adjacent lymphatics can help to preserve remaining lymphatic drainage. The outcomes of such procedures have been poorly studied and indications for such excisional procedures are limited in upper limb lymphedema.
Figure 2.
(A) The medial elliptical excision pattern is shown; (B) undermining of the lateral flap (in green) will allow for appropriate closure of the wound following excision.
Another option for removal of subcutaneous tissue includes the use of liposuction for the treatment of lymphedema. In clinical stages dominated by fatty infiltration and fibrosis, liposuction allows for selective removal of these tissues with preservation of the overlying skin. When excessive fibrosis exists, liposuction may be technically challenging due to the resistance to suctioning from the fibrotic tissue. This volume reducing technique may be used in conjunction with CDT to maintain specific limb volumes. Short-term outcomes have shown a reduction of 61-101% with long-term outcomes after 4- and 15-year showing persistent reduction in limb circumference along with improvements in patient quality of life metrics (68-72). The major limitation to long-term success following liposuction techniques is strict adherence to lifelong CDT and compression therapy. Despite encouraging results, liposuction techniques do not reverse or slow the pathophysiologic process of the lymphatic system. As surgeons become familiarized with both excisional and physiologic procedures (described below), combination or staged procedures are likely to increase in frequency around the world with improved and sustainable results.
Physiologic surgery
Physiologic surgery describes a constellation of sophisticated microsurgical procedures to treat lymphedema using common microsurgical techniques. Although numerous techniques have been historically described, only few techniques are currently being used and these include lymphaticovenous anastomosis (LVA), VLN transfers, and lymphatic grafting. Advances in microsurgical technique within the last 20 years have largely propelled the field to boast safe and efficacious outcomes following these procedures.
Lymphovenous bypass (LVB)
LVB surgery was described in the 1960s to provide a physiologic shunt for accumulated intraluminal lymphatic fluid to drain to the venous system via a microsurgical anastomosis (73). Early experience promoted the use of this technique in the treatment of lymphedema, but later studies found only temporary relief of symptoms (74,75). Since that early experience, advances in technology and surgical technique have allowed surgeons to change their approach to LVB surgery. Imaging techniques, particularly lymphodynamic evaluation with ICG, have allowed clinicians to assess lymphatic vessel patency and function. Better visualization prior to surgery has allowed for more reliable planning, technical execution, and likely improved long-term patency.
Current techniques for LVB utilize either subdermal lymphatics or the deeper epifascial system. The use of subdermal lymphatics, termed LVA, has been championed by Koshima using supermicrosurgical techniques (0.3-0.8 mm) to create a physiologic shunt (76-78). This procedure takes advantage of the highly complaint subdermal lymphatic system, which is responsible for a majority of regurgitant lymphatic fluid seen in dermal backflow. In addition, subdermal and subcutaneous venules are used as recipient veins and have little/no blackflow, which will create a favorable gradient following LVB. Reported outcomes using this technique have been favorable for populations with earlier staged disease (79,80).
LVB techniques utilizing deeper lymphatic collectors and pre-collectors are of larger caliber without the need for specialized instrumentation. In these cases, particular attention must be paid to the directionality of flow in these larger lymphatic vessels. ICG dynamic lymphography may be used to show shadows of larger/deeper lymphatics to help guide the surgeon to identify these structures. Flow directionality may help to stratify surgical technique to end-to-end or side-to-end techniques for LVB (Figure 3). In addition to considerations for the lymphatic vessel, flow characteristics of the chosen vein or venule must be identified to prevent venous blood regurgitation into the lymphatic system, creating an unfavorable gradient.
Figure 3.
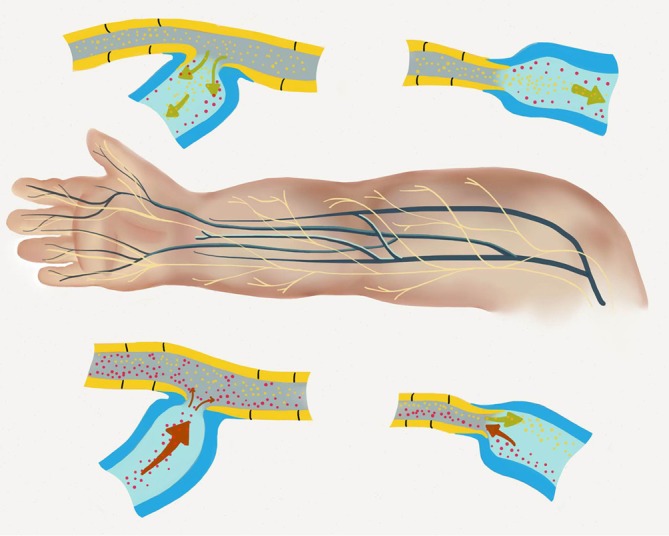
Lymphovenous bypass configurations are shown. End-end and side-end techniques are utilized and dependent on the flow-directionality of both the lymphatic and venous systems.
VLN transfer
VLN transfers have greatly increased in popularity recently. This method of reconstruction uses common microsurgical techniques to transfer lymph nodes to either the axilla or distally in the arm/forearm to restore lymphatic flow. This physiologic reconstructive technique relies on both the intrinsic nodal blood circulation, and lymphangiogenesis/lymphatic sprouting to provide a method to drain excess lymphatic fluid into the venous circulation.
Selection of the VLN transfer donor site, upper limb recipient site, and selecting an optimal patient that may benefit from VLN transfer requires multiple considerations. Multiple donor sites include the groin, submental, and supraclavicular regions, where selective lymph nodes from these regions may be incorporated into a free flap and harvested as a VLN flap.
The groin VLN flap has been critically examined as recent reports of donor site morbidity have been published related to groin VLN flap harvest (81,82). Multiple lymph node chains exist in the groin region and selective harvest of draining nodes from the lower abdomen minimize the risk of inducing lower limb lymphedema (Figure 4). Reverse lymphatic mapping has been recently described as a method to visualize and identify both lower limb draining nodes and lower abdominal lymph nodal drainage patterns to avoid less surgically induced lymphedema complications (83). Lymph nodes in the superficial transverse chain may be harvested based off of either the superficial circumflex iliac artery (SCIA) and superficial circumflex iliac vein (SCIV) or the superficial inferior epigastric artery (SIEA) and superficial inferior epigastric vein (SIEV). In a recent imaging evaluation, Dayan et al. found that the epicenter of these lymph nodes was located one-third the distance lateral from the pubic tubercle to the anterior-superior iliac spine and 3.1 cm below this line (inguinal ligament). The superficial nodes were typically located within the bifurcation of the SCIV and SIEV (67%), while a lesser incidence medial to the SIEV (19%) and inferior to the SCIV (14%) (83).
Figure 4.
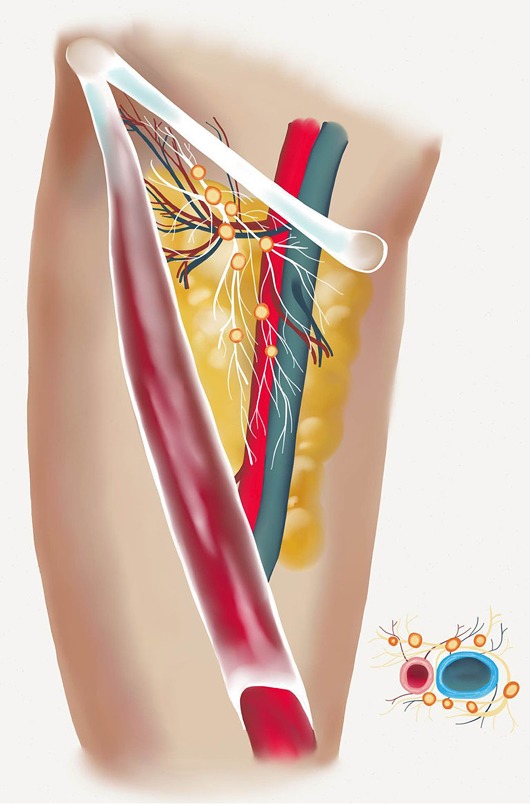
Regional anatomy of the groin is shown. Superior and lateral chain lymph nodes can be appreciated. These nodes are nourished by the superficial circumflex vessels and/or the superficial inferior epigastric vessels and are located between the inguinal ligament and the groin crease.
The submental VLN flap has been described as an alternative lymph node flap. Level 1a and 1b lymph nodes are harvested based on the submental artery and vein. This perfusing artery emanates from the facial artery approximately 1 cm below the angle of the mandible and travels anteriorly toward the mandibular symphysis. This flap has the advantage of providing a high quantity of lymph nodes (approximately 4 nodes per side) at a remote site from the extremities, which minimizes any risk of developing iatrogenic lymphedema. In addition, the flap size is small, allowing for a smaller recipient site (44,84).
The supraclavicular VLN flap has also been described as another option for VLNs. Harvest of level V lymph nodes in the posterior triangle of the neck is possible based off of the supraclavicular vessels (Figures 5,6). The transverse cervical artery and vein in addition to the external jugular vein are commonly harvested with this flap. The right neck is the preferred site for harvest given the left-side location of the main thoracic duct. Avoiding injury to these large lymphatic channels is of paramount importance as to avoid iatrogenic lymphedema (85,86).
Figure 5.
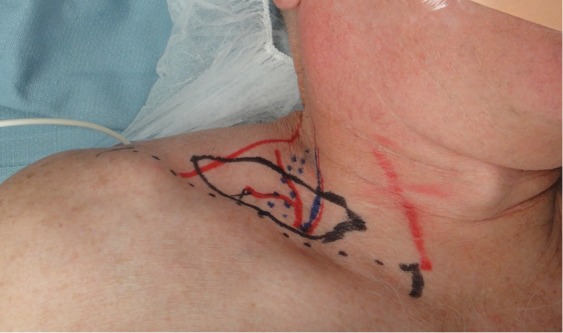
The right supraclavicular VLN flap is shown marked. The transverse cervical vessels serve as the main pedicle as the supraclavicular vessel emerges from this main vessel. Standardized markings utilizing common landmarks will ensure a consistency in flap elevation.
Figure 6.
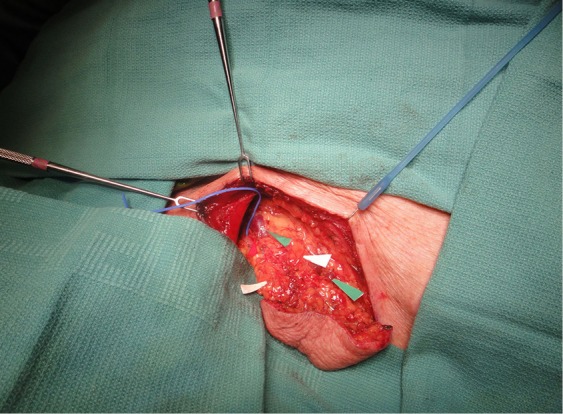
Green arrows indicate available venous drainage options. Lymph nodes are identified by white arrows and can be palpated within the deep portion of the flap.
The choice of recipient site has become a recent topic of debate. Anatomic and distal placement of VLN transfers has been advocated as the preferred choice by authors worldwide. Advocates for anatomic placement in the axilla cite that replacement of functioning nodes into the previous site of axillary node removal will better restore lymphatic flow through lymphatic regeneration, lymphangiogenesis, and axillary scar release (87,88). On the other hand, advocates for distal transfers at the level of the wrist or elbow cite the mechanism of action related to the nodal blood circulation and intrinsic lymphovenous connections creating a local lymphovenous shunt powered by the arterial and venous anastomosis. Long-term mechanism of action related to distal VLN transfers have proven the existence of local lymphatic fluid decompression through these connections (49). Likely, a combination of these two theories provides relief from the symptoms of lymphedema.
Lymphatic grafting
One of the earliest methods of physiologic lymphedema surgery related to the process of providing lymphatic vessels to either bridge an area of obstruction or bypass the region. Sir Harold Gillies provided early descriptions of his famous “waltzing” flap containing lymphatic wicks to bridge either pelvic lymphatic obstruction in lower limb lymphedema or axillary obstruction in upper limb lymphedema (89). Further evaluation of this concept led Baumeister and Siuda to describe and popularize a method of lymphatic grafting to re-establish lymphatic flow in an affected limb (Figure 7). In an early evaluation of 37 patients with BCRL, the authors found a majority of patients had volumetric limb measurement improvements up through 3 years of follow-up evaluation. In addition, functional studies indicated significantly improved lymphatic transport indices and decreased episodes of cellulitis (90). Recently, free lymphatic grafts have been used with reported successful outcomes. In order to improve upper limb lymphedema, Felmerer et al. used free lymphatic grafts in 7 patients isolated from the ventromedial thigh. Two or three lymphatic collectors can be identified superficial to the deep fascia and were harvested at lengths of up to 30 cm. These free grafts were anastomosed to ascending lymphatics of the upper limb to a central drainage location in the neck. MRL was used to identify functioning lymphatic structures to aid in surgical identification and dissection. Favorable outcomes were reported with most patients (6 of 7 patients) eliminating the dependence on compression and/or lymphatic drainage physiotherapy (91).
Figure 7.
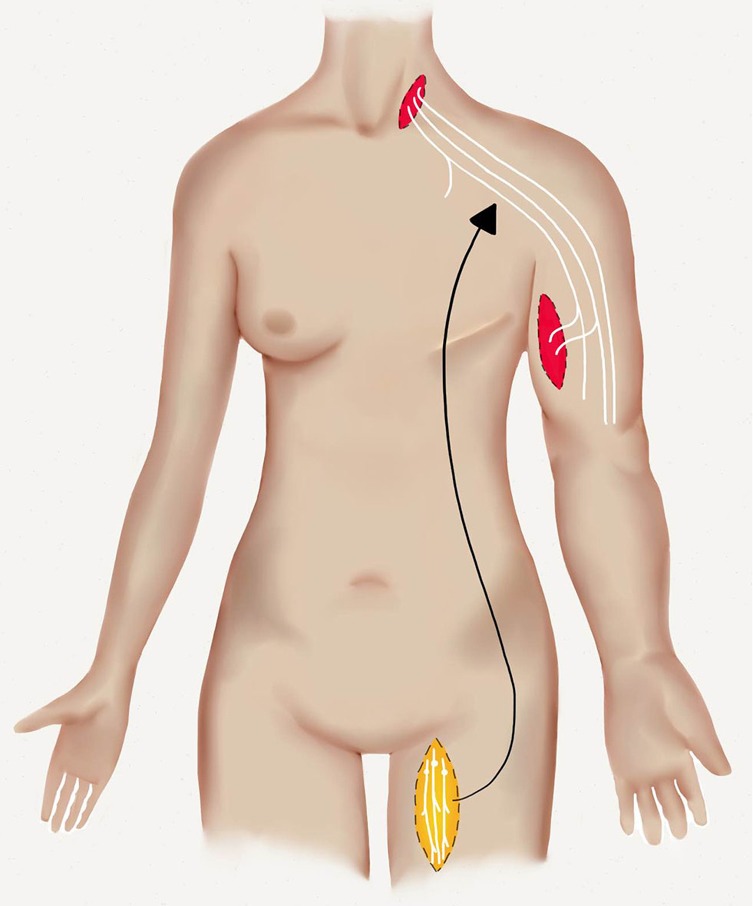
Lymphatic grafts are harvested from the medial aspect of the thigh. Two to three are harvested and are used to bypass an axillary obstruction in upper limb lymphedema.
Outcomes of lymphedema treatment
With increasing and growing options related to the surgical treatment of lymphedema, improved understanding of outcomes assessment is necessary in order to critically evaluate an optimal treatment modality. Currently, objective and subjective outcomes parameters are used to determine efficacy of treatment. The most common objective outcomes used include circumference limb measurements, volumetric limb measurements, and rate of treated cellulitis episodes. Information related to these outcomes are routinely measured and compared given specific treatment algorithms. In addition to these objective outcomes, subjective outcomes assessment has become increasingly important. Variations in limb measurements exist given the dynamic nature of swelling in lymphedema patients. Global patient quality of life and functional assessment may represent an ideal outcomes assessment method, which would allow more accurate tracking of longitudinal outcomes. Validated questionnaires exist for evaluating symptoms related to lymphedema. Condition-specific questionnaires, such as the LYMQOL (92) and the ULL-27 (93), provide a comprehensive assessment of multiple domains that contribute to overall quality of life. A recent study prospectively evaluating patients who underwent VLN transfer for upper limb lymphedema, found that all QoL domains as measured by the LYMQOL validated questionnaire, improved as soon as 1-6 months following VLN transfer, which closely mirrored improvements in limb circumference improvements (94). Ongoing studies related to this aspect of lymphedema care will help to gain an understanding of the utility of these surgical procedures.
Conclusions
Comprehensive lymphedema care encompasses a full spectrum of evaluation and work-up, imaging interpretation, and non-surgical and surgical interventions. A management team focused on optimizing care of this subset of patients will maximize both limb circumference reduction and improvements in quality of life. Novel surgical therapies offer unique solutions and can be implemented individually or in combination with other therapeutic modalities. As the understanding of these surgical therapies improves, surgical decision-making will becoming increasingly enhanced to optimize objective and subjective outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Paskett ED, Naughton MJ, McCoy TP, et al. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev 2007;16:775-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464-72. [DOI] [PubMed] [Google Scholar]
- 3.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 2006;13:491-500. [DOI] [PubMed] [Google Scholar]
- 4.Ugur S, Arıcı C, Yaprak M, et al. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol 2013;11:72-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesvold IL, Dahl AA, Løkkevik E, et al. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol 2008;47:835-42. [DOI] [PubMed] [Google Scholar]
- 6.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer 2007;110:1868-74. [DOI] [PubMed] [Google Scholar]
- 7.Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001;92:1368-77. [DOI] [PubMed] [Google Scholar]
- 8.Johansen S, Fosså K, Nesvold IL, et al. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol 2014;53:521-9. [DOI] [PubMed] [Google Scholar]
- 9.Nesvold IL, Fosså SD, Holm I, et al. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 2010;49:347-53. [DOI] [PubMed] [Google Scholar]
- 10.Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol 2006;15:153-65. [DOI] [PubMed] [Google Scholar]
- 11.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [DOI] [PubMed] [Google Scholar]
- 12.Taghian NR, Miller CL, Jammallo LS, et al. Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol 2014;92:227-34. [DOI] [PubMed] [Google Scholar]
- 13.Jila A, Kim H, Nguyen VP, et al. Lymphangiogenesis following obstruction of large postnodal lymphatics in sheep. Microvasc Res 2007;73:214-23. [DOI] [PubMed] [Google Scholar]
- 14.Faul JL, Berry GJ, Colby TV, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 2000;161:1037-46. [DOI] [PubMed] [Google Scholar]
- 15.Mehrara BJ, Avraham T, Soares M, et al. p21cip/WAF is a key regulator of long-term radiation damage in mesenchyme-derived tissues. FASEB J 2010;24:4877-88. [DOI] [PubMed] [Google Scholar]
- 16.Avraham T, Yan A, Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol 2010;299:C589-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampell JC, Yan A, Elhadad S, et al. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 2012;7:e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 2003;44:43-57. [PubMed] [Google Scholar]
- 19.Weissleder H, Weissleder R. Lymphedema: evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 1988;167:729-35. [DOI] [PubMed] [Google Scholar]
- 20.Witte M, McNeill G, Crandall C, et al. Whole body lymphangioscintigraphy in ferrets chronically infected with Brugia malayi. Lymphology 1988;21:251-7. [PubMed] [Google Scholar]
- 21.Vaqueiro M, Gloviczki P, Fisher J, et al. Lymphoscintigraphy in lymphedema: an aid to microsurgery. J Nucl Med 1986;27:1125-30. [PubMed] [Google Scholar]
- 22.Witte CL, Witte MH, Unger EC, et al. Advances in imaging of lymph flow disorders. Radiographics 2000;20:1697-719. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH, Choi JY, Lee JY, et al. Lymphscintigraphy predicts response to complex physical therapy in patients with early stage extremity lymphedema. Lymphology 2007;40:172-6. [PubMed] [Google Scholar]
- 24.Kleinhans E, Baumeister RG, Hahn D, et al. Evaluation of transport kinetics in lymphoscintigraphy: follow-up study in patients with transplanted lymphatic vessels. Eur J Nucl Med 1985;10:349-52. [DOI] [PubMed] [Google Scholar]
- 25.Partsch H. Practical aspects of indirect lymphography and lymphoscintigraphy. Lymphat Res Biol 2003;1:71-3; discussion 73-4. [DOI] [PubMed] [Google Scholar]
- 26.Szuba A, Strauss W, Sirsikar SP, et al. Quantitative radionuclide lymphoscintigraphy predicts outcome of manual lymphatic therapy in breast cancer-related lymphedema of the upper extremity. Nucl Med Commun 2002;23:1171-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim YB, Hwang JH, Kim TW, et al. Would complex decongestive therapy reveal long term effect and lymphoscintigraphy predict the outcome of lower-limb lymphedema related to gynecologic cancer treatment? Gynecol Oncol 2012;127:638-42. [DOI] [PubMed] [Google Scholar]
- 28.Gloviczki P, Calcagno D, Schirger A, et al. Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg 1989;9:683-9; discussion 690. [DOI] [PubMed] [Google Scholar]
- 29.Williams WH, Witte CL, Witte MH, et al. Radionuclide lymphangioscintigraphy in the evaluation of peripheral lymphedema. Clin Nucl Med 2000;25:451-64. [DOI] [PubMed] [Google Scholar]
- 30.Liu NF, Wang CG. The role of magnetic resonance imaging in diagnosis of peripheral lymphatic disorders. Lymphology 1998;31:119-27. [PubMed] [Google Scholar]
- 31.Liu N, Wang C, Sun M. Noncontrast three-dimensional magnetic resonance imaging vs lymphoscintigraphy in the evaluation of lymph circulation disorders: A comparative study. J Vasc Surg 2005;41:69-75. [DOI] [PubMed] [Google Scholar]
- 32.Zhou GX, Chen X, Zhang JH, et al. MR lymphangiography at 3.0 Tesla to assess the function of inguinal lymph node in low extremity lymphedema. J Magn Reson Imaging 2014;40:1430-6. [DOI] [PubMed] [Google Scholar]
- 33.Barrett T, Choyke PL, Kobayashi H. Imaging of the lymphatic system: new horizons. Contrast Media Mol Imaging 2006;1:230-45. [DOI] [PubMed] [Google Scholar]
- 34.Bellin MF, Lebleu L, Meric JB. Evaluation of retroperitoneal and pelvic lymph node metastases with MRI and MR lymphangiography. Abdom Imaging 2003;28:155-63. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Kawamoto S, Brechbiel MW, et al. Detection of lymph node involvement in hematologic malignancies using micromagnetic resonance lymphangiography with a gadolinum-labeled dendrimer nanoparticle. Neoplasia 2005;7:984-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu N, Zhang Y. Magnetic Resonance Lymphangiography for the Study of Lymphatic System in Lymphedema. J Reconstr Microsurg 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Lohrmann C, Bartholomä JP, Foeldi E, et al. Magnetic resonance lymphangiography in Klippel-Trénaunay syndrome. Br J Radiol 2007;80:e188-92. [DOI] [PubMed] [Google Scholar]
- 38.White RD, Weir-McCall JR, Budak MJ, et al. Contrast-enhanced magnetic resonance lymphography in the assessment of lower limb lymphoedema. Clin Radiol 2014;69:e435-44. [DOI] [PubMed] [Google Scholar]
- 39.Shin SU, Lee W, Park EA, et al. Comparison of characteristic CT findings of lymphedema, cellulitis, and generalized edema in lower leg swelling. Int J Cardiovasc Imaging 2013;29 Suppl 2:135-43. [DOI] [PubMed] [Google Scholar]
- 40.Naouri M, Samimi M, Atlan M, et al. High-resolution cutaneous ultrasonography to differentiate lipoedema from lymphoedema. Br J Dermatol 2010;163:296-301. [DOI] [PubMed] [Google Scholar]
- 41.Munn LL, Padera TP. Imaging the lymphatic system. Microvasc Res 2014;96:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abe H, Schacht D, Kulkarni K, et al. Accuracy of axillary lymph node staging in breast cancer patients: an observer-performance study comparison of MRI and ultrasound. Acad Radiol 2013;20:1399-404. [DOI] [PubMed] [Google Scholar]
- 43.Kebudi A, Calişkan C, Yetkin G, et al. The role of pre-operative B mode ultrasound in the evaluation of the axillary lymph node metastases in the initial staging of breast carcinoma. Acta Chir Belg 2005;105:511-4. [DOI] [PubMed] [Google Scholar]
- 44.Patel KM, Chu SY, Huang JJ, et al. Preplanning vascularized lymph node transfer with duplex ultrasonography: an evaluation of 3 donor sites. Plast Reconstr Surg Glob Open 2014;2:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [DOI] [PMC free article] [PubMed]
- 46.Weiler M, Kassis T, Dixon JB. Sensitivity analysis of near-infrared functional lymphatic imaging. J Biomed Opt 2012;17:066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;127:1979-86. [DOI] [PubMed] [Google Scholar]
- 48.Mihara M, Hara H, Araki J, et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One 2012;7:e38182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel KM, Lin CY, Cheng MH. From theory to evidence: long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg 2015;31:26-30. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Yoshimatsu H, Koshima I. Navigation lymphatic supermicrosurgery for iatrogenic lymphorrhea: supermicrosurgical lymphaticolymphatic anastomosis and lymphaticovenular anastomosis under indocyanine green lymphography navigation. J Plast Reconstr Aesthet Surg 2014;67:1573-9. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Narushima M, Yoshimatsu H, et al. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann Plast Surg 2014;73:706-9. [DOI] [PubMed] [Google Scholar]
- 52.Tan IC, Maus EA, Rasmussen JC, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil 2011;92:756-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008;112:2809-19. [DOI] [PubMed] [Google Scholar]
- 54.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009;27:390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meneses KD, McNees MP. Upper extremity lymphedema after treatment for breast cancer: a review of the literature. Ostomy Wound Manage 2007;53:16-29. [PubMed] [Google Scholar]
- 56.Rockson SG. Lymphedema. Am J Med 2001;110:288-95. [DOI] [PubMed] [Google Scholar]
- 57.Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol 2006;4:51-56. [DOI] [PubMed] [Google Scholar]
- 58.Cornish B. Bioimpedance analysis: scientific background. Lymphat Res Biol 2006;4:47-50. [DOI] [PubMed] [Google Scholar]
- 59.Bagheri S, Ohlin K, Olsson G, et al. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymphat Res Biol 2005;3:66-80. [DOI] [PubMed] [Google Scholar]
- 60.Moseley A, Piller N, Carati C. Combined opto-electronic perometry and bioimpedance to measure objectively the effectiveness of a new treatment intervention for chronic secondary leg lymphedema. Lymphology 2002;35:136-43. [PubMed] [Google Scholar]
- 61.Watanabe R, Miura A, Inoue K, et al. Evaluation of leg edema using a multifrequency impedance meter in patients with lymphatic obstruction. Lymphology 1989;22:85-92. [PubMed] [Google Scholar]
- 62.Mikes DM, Cha BA, Dym CL, et al. Bioelectrical impedance analysis revisited. Lymphology 1999;32:157-65. [PubMed] [Google Scholar]
- 63.International Society of Lymphology . The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013;46:1-11. [PubMed] [Google Scholar]
- 64.Charles H. Elephantiasis of the leg. In: Latham A, English TC. editors. A system of treatment. Vol. 3. London, Churchill; 1912. [Google Scholar]
- 65.Sistrunk WE. Modification of the operation for elephantiasis. J Amer JAMA 1918;71:800-3. [Google Scholar]
- 66.Savage RC. The surgical management of lymphedema. Surg Gynecol Obstet 1984;159:501-8. [PubMed] [Google Scholar]
- 67.Sistrunk WE. Contribution to plastic surgery: removal of scars by stages; an open operation for extensive laceration of the anal sphincter; the kondoleon operation for elephantiasis. Ann Surg 1927;85:185-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greene AK, Maclellan R. Management of lymphedema with suction-assisted lipectomy. Plast Reconstr Surg 2014;134:36. [Google Scholar]
- 69.Peled AW, Slavin SA, Brorson H. Long-term Outcome After Surgical Treatment of Lipedema. Ann Plast Surg 2012;68:303-7. [DOI] [PubMed] [Google Scholar]
- 70.Damstra RJ, Voesten HG, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg 2009;96:859-64. [DOI] [PubMed] [Google Scholar]
- 71.Brorson H, Ohlin K, Olsson G, et al. Controlled compression and liposuction treatment for lower extremity lymphedema. Lymphology 2008;41:52-63. [PubMed] [Google Scholar]
- 72.Brorson H, Ohlin K, Olsson G, et al. Quality of life following liposuction and conservative treatment of arm lymphedema. Lymphology 2006;39:8-25. [PubMed] [Google Scholar]
- 73.Yamamoto T, Koshima I. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2014;133:887e-8e. [DOI] [PubMed] [Google Scholar]
- 74.Puckett CL, Jacobs GR, Hurvitz JS, et al. Evaluation of lymphovenous anastomoses in obstructive lymphedema. Plast Reconstr Surg 1980;66:116-20. [DOI] [PubMed] [Google Scholar]
- 75.Gloviczki P, Hollier LH, Nora FE, et al. The natural history of microsurgical lymphovenous anastomoses: an experimental study. J Vasc Surg 1986;4:148-56. [PubMed] [Google Scholar]
- 76.Yamamoto T, Yamamoto N, Yamashita M, et al. Establishment of supermicrosurgical lymphaticovenular anastomosis model in rat. Microsurgery 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 77.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [DOI] [PubMed] [Google Scholar]
- 78.Koshima I, Nanba Y, Tsutsui T, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg 2003;19:209-15. [DOI] [PubMed] [Google Scholar]
- 79.Nagase T, Gonda K, Inoue K, et al. Treatment of lymphedema with lymphaticovenular anastomoses. Int J Clin Oncol 2005;10:304-10. [DOI] [PubMed] [Google Scholar]
- 80.Chim H, Drolet B, Duffy K, et al. Vascular anomalies and lymphedema. Plast Reconstr Surg 2010;126:55e-69e. [DOI] [PubMed] [Google Scholar]
- 81.Pons G, Masia J, Loschi P, et al. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J Plast Reconstr Aesthet Surg 2014;67:119-23. [DOI] [PubMed] [Google Scholar]
- 82.Viitanen TP, Mäki MT, Seppänen MP, et al. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130:1246-53. [DOI] [PubMed] [Google Scholar]
- 83.Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [DOI] [PubMed] [Google Scholar]
- 84.Cheng MH, Lin CY, Patel K. A prospective assessment of anatomic variability of the submental vascularized lymph node flap. Plast Reconstr Surg 2014;134:33. [DOI] [PubMed] [Google Scholar]
- 85.Yeo MS, Lim SY, Kiranantawat K, et al. A comparison of vascularized cervical lymph node transfer with and without modified Charles’ procedure for the treatment of lower limb lymphedema. Plast Reconstr Surg 2014;134:171e-2e. [DOI] [PubMed] [Google Scholar]
- 86.Raju A, Chang DW. Vascularized Lymph Node Transfer for Treatment of Lymphedema: A Comprehensive Literature Review. Ann Surg 2015;261:1013-23. [DOI] [PubMed] [Google Scholar]
- 87.Becker C, Vasile JV, Levine JL, et al. Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012;39:385-98. [DOI] [PubMed] [Google Scholar]
- 88.Viitanen TP, Visuri MT, Hartiala P, et al. Lymphatic vessel function and lymphatic growth factor secretion after microvascular lymph node transfer in lymphedema patients. Plast Reconstr Surg Glob Open 2013;1:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gillies H, Millard DR Jr. eds. The Principles and Art of Plastic Surgery. London: Butterworth & Co. (Publishers) Ltd., 1957. [Google Scholar]
- 90.Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg 1990;85:64-74; discussion 75-6. [DOI] [PubMed] [Google Scholar]
- 91.Felmerer G, Sattler T, Lohrmann C, et al. Treatment of various secondary lymphedemas by microsurgical lymph vessel transplantation. Microsurgery 2012;32:171-7. [DOI] [PubMed] [Google Scholar]
- 92.Loudon A, Barnett T, Piller N, et al. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med 2014;14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv 2013;7:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel KM, Lin CY, Cheng MH. A Prospective Evaluation of Lymphedema-Specific Quality-of-Life Outcomes Following Vascularized Lymph Node Transfer. Ann Surg Oncol 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]