Abstract
Epidemiological evidence suggests a role for vitamin D in type 2 diabetes prevention. We investigated the effects of vitamin D3 supplementation on glucose metabolism and inflammation in subjects with prediabetes. A 5-month randomized, double-blind, placebo-controlled intervention with three arms (placebo, 40 μg/d, or 80 μg/d vitamin D3) was carried out among sixty-eight overweight (BMI 25–35) and aging (≥60 years) subjects from Finland, with serum 25-hydroxyvitamin D3 [25(OH)D3] < 75 nmol/L and either impaired fasting glucose or impaired glucose tolerance. Analyses included 66 subjects who completed the trial. Glucose metabolism was evaluated by fasting and 2-hour oral glucose tolerance test-derived indices and glycated hemoglobin. Inflammation was evaluated by high-sensitive C-reactive protein and five cytokines. Although a dose-dependent increase in serum 25(OH)D3 over the supplementation period was observed (P trend < 0.001), there were no other statistically significant differences in changes in the 13 glucose homeostasis indicators between the study groups other than increase in the 120 min glucose concentration (P trend = 0.021) and a decreasing trend both in 30 min plasma insulin (P trend = 0.030) and glycated hemoglobin (P trend = 0.024) concentrations. A borderline statistically significant decreasing trend in interleukin-1 receptor antagonist concentration was observed (P = 0.070). Vitamin D3 supplementation does not improve glucose metabolism in ageing subjects with prediabetes but may have modest anti-inflammatory effects.
1. Introduction
There is a lot of epidemiological evidence to suggest that vitamin D has a role in glucose homeostasis, especially in maintaining normal glucose homeostasis and protecting against type 2 diabetes [1, 2].
However, controlled supplementation trials with vitamin D or its analogues have not been able to produce similar results. In a recent systematic review and meta-analysis of 15 trials lasting from two months to 7 years, no significant improvements were seen in glucose homeostasis when all trials were included [3]. The results from the more recent trials have been inconsistent. Two trials in subjects with impaired glucose tolerance (IGT) did find a positive effect on glucose homeostasis with vitamin D3 supplementation [4–7], whereas three trials in subjects with IGT and one in subjects with normal glucose tolerance found no effects [8–10].
Insulin resistance, metabolic syndrome, and type 2 diabetes are associated with a systemic, chronic inflammation, where, for example, circulating levels of proinflammatory cytokines and C-reactive protein are elevated [11, 12]. Although vitamin D has immunomodulatory and anti-inflammatory properties, especially based on experimental studies [13], bodies of evidence from human studies in the prediabetic state assessing the role of moderate to large dose supplementation of vitamin D in inflammation and glucose metabolism are still relatively few.
As more evidence is clearly needed, we decided to study the placebo-controlled effect of 5-month 40 μg/d (1,600 IU) or 80 μg/d (3,200 IU) oral vitamin D3 supplementation on glucose homeostasis in an eastern Finnish population with either impaired fasting glucose (IFG) or IGT during winter time when serum vitamin D3 concentrations drop without supplementation. Furthermore, we assessed the anti-inflammatory effect of the supplementation by measurements of serum high-sensitive C-reactive protein (hsCRP), plasma soluble tumor necrosis factor receptor type II (psTNFrII), plasma interleukin-6 (pIL-6), plasma interleukin-1 receptor antagonist (pIL-1RA), and plasma interleukin-1 beta (pIL-1β) concentrations.
2. Materials and Methods
2.1. Subject Selection
The Glucose Metabolism Effects of Vitamin D Supplementation in Prediabetes (VitDmet) study was a 5-month randomized, double-blind, placebo-controlled supplementation trial that was conducted in winter 2011/2012 in eastern Finland. The primary aim was to assess the effects of vitamin D3 supplementation on glucose homeostasis in subjects with IFG or IGT. For inclusion the subjects needed to be ≥60 years of age with evidence of disturbed glucose homeostasis, that is, either IFG (fasting plasma glucose concentration 5.6 to 6.9 mmol/L) or IGT (oral glucose tolerance test (OGTT) 120 min plasma glucose concentration 7.8 to 11.1 mmol/L), but not type 2 diabetes (fasting plasma glucose concentration ≥ 7.0 mmol/L or OGTT 120 min plasma glucose concentration ≥ 11.1 mmol/L), and to be overweight (BMI > 25), but not severely obese (BMI < 35). Furthermore, subjects needed to have their serum 25(OH)D3 concentration <75 nmol/L. Exclusion criteria were any disease that could be affected by vitamin D, such as sarcoidosis, or a condition that could affect vitamin D metabolism, such as kidney disease.
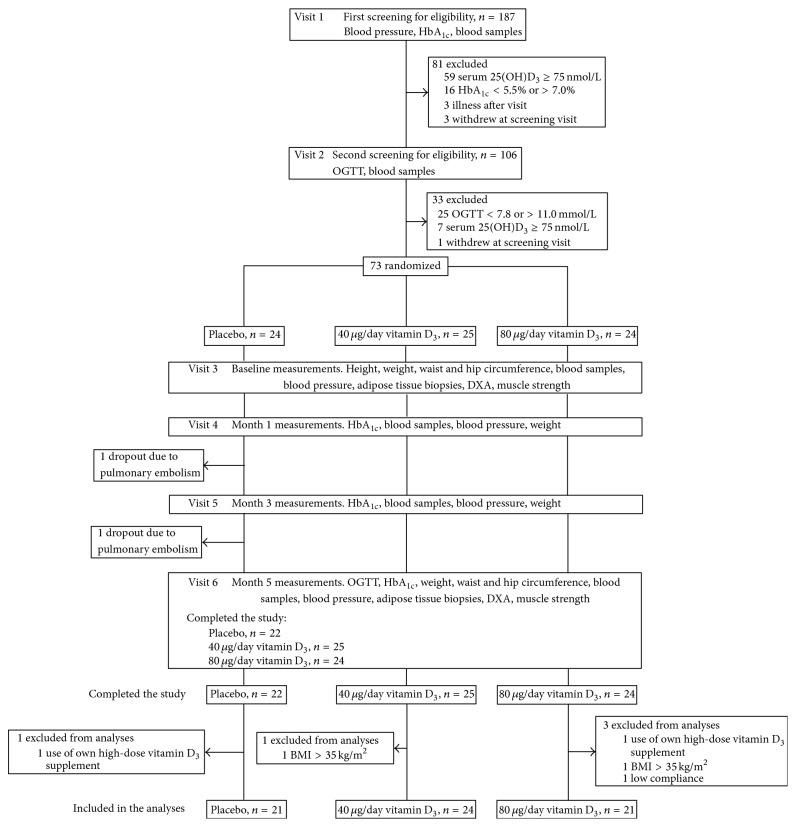
A two-stage screening was used, as shown in Figure 1. The adult voluntary subjects were recruited by advertisements in regional newspapers and after a prescreening on the phone for inclusion and exclusion criteria (self-reported elevated blood glucose, age, height, weight, and diseases) were invited for the first screening at the study clinic (visit 1). A written informed consent was collected, HbA1c was measured with a point-of-care device, the subjects were interviewed, and their self-administered questionnaires were checked. If the subject's HbA1c was 5.5–7.0%, fasting venous blood samples were drawn and analyzed for plasma glucose and serum 25(OH)D3 concentrations. Those fulfilling the inclusion criteria were scheduled for the OGTT (visit 2), after which those fulfilling all criteria were randomized to receive either placebo, 40 μg/d, or 80 μg/d vitamin D3 daily. Each dose comprised a combination of placebo and 20 μg tablets, two in the morning and two in the evening. Randomization was carried out by a statistician, in the order of entry after visit 2. Study nurse distributed the containers according to the statistician-generated list and neither the nurse nor a participant knew the allocation group. All tablets and containers were physically identical, the allocation arm code hidden in the serial number on the container label. The average time between visit 2 and the beginning of the supplementation was approximately one week. During the 5-month supplementation period between October 2011 and April 2012, the subjects visited the study site three times: 1 and 3 months after the start of the supplementation and at the end of the study (Figure 1).
Figure 1.
Flow diagram of the study. 25(OH)D3: 25-hydroxyvitamin D3; BMI: body mass index; OGTT: oral glucose tolerance test.
We aimed to recruit 102 participants, 34 into each of the three supplementation groups, but due to lower than expected public interest to participate and generally higher than anticipated serum 25(OH)D3 concentrations in the base population, at closing of the recruitment window we had 73 randomized subjects in the three supplementation arms (63 men and 10 women). According to power computations, to reach 80% power (i.e., β = 0.20) at α = 0.05, with a difference of 1 SD to 0.75 SD in the effect measure between the groups, there needed to be 16 to 28 subjects per group. 1 SD equals an effect size of 10% to 50% in most of the outcome parameters.
The Research Ethics Committee of the Northern Savo Hospital District has approved the study protocol. All subjects gave a written informed consent to participate in the study. The study is registered in the ClinicalTrials.gov (identification code: NCT01479933).
2.2. Measurements
Serum 25(OH)D3 concentration was measured from venous blood samples by a high performance liquid chromatography with coulometric electrode array (HPLC-CEAD), as described [14]. The interassay CV% of the method is <8.0% and the intra-assay CV% is <7.3%. Plasma glucose concentration was assayed by the photometric hexokinase method (KoneLab 20XT, Thermo Fischer Scientific, Vantaa, Finland) and plasma insulin concentration by the chemiluminescence assay (DiaSorin Liaison, DiaSorin, Dietzenbach, Germany). A two-hour OGTT was carried out with a 75-gram glucose load. Plasma glucose and plasma insulin concentrations were measured at three time points: at start (i.e., fasting), at 30 min, and at 120 min. Homeostatic modeling assessment (HOMA2) indices were computed according to the nonlinear function presented by Wallace and coworkers [15]. The insulinogenic index (IGI) was computed as follows: IGI = (30 min insulin − 0 min insulin)/(30 min glucose − 0 min glucose) in the OGTT, as described by Phillips and coworkers [16], and the insulin sensitivity index (ISI) was computed as follows: ISI = 10000/Sqr[(fasting glucose × fasting insulin) × (mean glucose × mean insulin)], as described by Matsuda and DeFronzo [17]. HbA1c was measured with a point-of-care device from a capillary blood sample as instructed by the manufacturer (DCA Vantage, Siemens Healthcare Diagnostics, Deerfield, Illinois, USA). Serum liver enzymes gamma-glutamyl transpeptidase (sGGT) and alanine aminotransferase (sALAT) were determined with enzymatic photometric IFCC methods, kidney function marker serum creatinine (sCREA) with an enzymatic photometric test, and serum total calcium (sCA) with colorimetric Arsenazo III test at site with a clinical chemistry analyzer (Konelab 20XT, Thermo Fisher Scientific, Vantaa, Finland). Serum parathyroid hormone (sPTH) and serum thyroid stimulating hormone (sTSH) were assayed by chemiluminescence methods (DiaSorin Liaison, DiaSorin, Dietzenbach, Germany). Total blood count (TBC) and inflammatory cytokines hsCRP, psTNFrII, pIL-6, pIL-1RA, and pIL-1β were measured in Eastern Finland Laboratory Centre, Kuopio, Finland. HsCRP was measured with Roche Cobas 6000 automated analyser. Plasma IL-6, IL-1RA, soluble TNFRII, and IL-1β were measured with ELISA from R&D Systems (Minneapolis, MN, USA).
Body weight was measured to 0.1 kg with subjects wearing light underwear, with a recently calibrated Vetek TI-1200 scale (Vetek, Sweden), and height in a Frankfurt position to 0.5 cm by a wall-mounted device (KaWe, Germany). Body mass index (BMI) was computed as weight/height squared (kg/m2). Blood pressure was measured after a 5 min rest from the upper arm at a supine position, using a recently calibrated semiautomatic device (OMRON Intellisense M7, OMRON Healthcare, Netherlands). Systolic and diastolic blood pressure values were computed as the mean of the last two of three measurements.
Participant compliance in taking supplements as instructed was assessed by counting the study supplements not consumed at the last study visit.
2.3. Statistical Analysis
From the statistical analyses we excluded two subjects who had an increase in their BMI to >35 kg/m2 between the randomization and the start of the supplementation (visit 3), two subjects who reported at the end of the study that they had started to use a high-dose (50 μg/day and 125 μg/day) vitamin D3 supplement in addition to the study supplements during the study period, and one subject due to low compliance. The subject with the low compliance in the 80 μg/day group had used only 17% of the study tablets. After the exclusions, the number of subjects in the analyses with the baseline data was 68 (Table 1) and in the analyses with the change variables 66 (Table 2), after the two dropouts (Figure 1) were excluded.
Table 1.
Characteristics of the study population (n = 68) at the start of the supplementation period.
| Variable | Mean or median | Standard deviation or interquartile range |
|---|---|---|
| Age (years)a | 65.7 | 7.0 |
| 25(OH)D3 (nmol/L)b | 57.0 | 11.0 |
| HbA1c (mmol/mol)b | 40 | 3 |
| HbA1c (%)b | 5.8 | 2.5 |
| Fasting glucose (nmol/L)a | 6.1 | 0.7 |
| 120 min glucose (nmol/L)a | 6.1 | 2.5 |
| Fasting insulin (mU/L)b | 12.0 | 6.0 |
| 120 min insulin (mU/L)c | 61.9 | 62.3 |
| HOMA2 %betab | 82.2 | 24.1 |
| HOMA2 %ISa | 70.2 | 39.2 |
| HOMA2 %IRb | 1.6 | 0.8 |
| Waist circumference (cm)b | 106 | 9 |
| Body mass index (kg/m2)b | 29.4 | 2.7 |
| Systolic BP (mmHg)a | 144 | 30 |
| Diastolic BP (mmHg)b | 92 | 10 |
| Serum PTH (pg/ml)a | 42.6 | 12.7 |
| Serum calcium (mM)b | 2.33 | 0.07 |
| Serum creatinine (µmol/L)a | 71.7 | 14.5 |
| Serum ALAT (U/L)a | 27.5 | 17.6 |
| Serum GGT (U/L)a | 29.4 | 23.7 |
| Serum TSH (mU/L)a | 2.6 | 1.7 |
| Serum HDL cholesterol (mmol/L)b | 1.4 | 0.4 |
| Serum LDL cholesterol (mmol/L)b | 3.2 | 0.9 |
| Serum triglycerides (mmol/L)a | 1.3 | 0.7 |
| Serum ApoA1 (g/L)b,c | 1.6 | 0.2 |
| Serum ApoB (g/L)b,d | 1.0 | 0.2 |
| Serum adiponectin (µg/mL)a,c | 4.8 | 3.3 |
| Serum C-reactive protein (mg/L)a,c | 1.2 | 1.5 |
| Plasma TNF receptor II (pg/mL)a | 2240 | 480 |
| Plasma IL-6 (pg/mL)a | 1.3 | 0.8 |
| Plasma IL-1 beta (pg/mL)a | 0 | 0 |
| Plasma IL-1 receptor antagonist (pg/mL)a | 200 | 95 |
aMedian and interquartile range; bmean and standard deviation;c n = 66; d n = 65.
25(OH)D3: 25-hydroxyvitamin D3; ALAT: alanine aminotransferase; Apo: apoprotein; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; HOMA: homeostatic modeling assessment; IL: interleukin; IR: insulin resistance; IS: insulin sensitivity; LDL: low-density lipoprotein; PTH: parathyroid hormone; TNF: tumor necrosis factor; TSH: thyroid-stimulating hormone.
Table 2.
Changes over time in the outcome parameters by supplementation group (n = 66).
| Parameter | Placebo (n = 21) | 40 μg/d (n = 24) | 80 μg/d (n = 21) | P K-W | P M-WU d | P J-T |
|---|---|---|---|---|---|---|
| Serum 25(OH)D3 (nmol/L)a | 4.1 (17.3) | 27.7 (17.2)c | 45.0 (23.4)c | <0.001 | <0.001, <0.001 | <0.001 |
| Serum PTH (pg/mL)b | 5.6 (7.4)c | −2.6 (10.8) | −6.4 (9.6)c | <0.001 | 0.003, <0.001 | <0.001 |
| Serum calcium (mM)a | −0.06 (0.08)c | −0.04 (0.07)c | −0.03 (0.08) | 0.367 | — | 0.082 |
| HbA1c (mmol/mol)a | −1.8 (1.2)c | −1.2 (2.0)c | −1.0 (2.0)c | 0.130 | — | 0.024 |
| HbA1c (%)b | −0.2 (0.2)c | −0.1 (0.2)c | −0.1 (0.2)c | 0.231 | — | 0.061 |
| Fasting glucose (nmol/L)a | −0.1 (0.4) | −0.1 (0.3)c | −0.2 (0.5)c | 0.483 | — | 0.104 |
| 30 min glucose (nmol/L)a,e | −0.4 (1.1) | −0.2 (1.0) | −0.3 (1.4) | 0.811 | — | 0.380 |
| 120 min glucose (nmol/L)a | −0.5 (1.6) | 0.7 (1.7) | 0.5 (1.8) | 0.039 | 0.011, 0.021 | 0.021 |
| Fasting insulin (mU/L)b | 0.3 (6.1) | 0.6 (2.0) | −0.1 (5.3) | 0.690 | — | 0.241 |
| 30 min insulin (mU/L)b | −0.8 (29.6) | −0.8 (35.1) | −7.7 (31.7) | 0.142 | — | 0.030 |
| 120 min insulin (mU/L)b,e | 1.0 (34.7) | 10.5 (33.8) | 1.4 (60.6) | 0.584 | — | 0.430 |
| HOMA2 %betaa | 6.2 (24.5) | 8.0 (14.5)c | 6.6 (17.3) | 0.605 | — | 0.300 |
| HOMA2 %ISa | −2.9 (28.8) | −6.0 (23.7) | 2.0 (20.6) | 0.554 | — | 0.287 |
| HOMA2 %IRa | 0.13 (0.71) | 0.09 (0.33) | 0.03 (0.99) | 0.714 | — | 0.243 |
| Insulin sensitivity indexa | −0.1 (1.2) | −0.4 (1.4) | 0.3 (1.5) | 0.624 | — | 0.398 |
| Insulinogenic indexb,e | 1.5 (9.2) | 0.1 (8.4) | −2.8 (7.3)c | 0.306 | — | 0.063 |
| Waist circumference (cm)a,e | 0.02 (3.0) | 0.1 (2.3) | 0.5 (1.9) | 0.741 | — | 0.311 |
| Body mass index (kg/m2)a | 0.25 (0.86) | 0.34 (0.55)c | 0.30 (0.58)c | 0.973 | — | 0.404 |
| Serum ALAT (U/L)b | 0.3 (10.1) | 1.6 (8.7) | 0.07 (7.5) | 0.345 | — | 0.505 |
| Serum GGT (U/L)b | −0.5 (14.2) | 0.9 (7.3) | −1.0 (8.6) | 0.462 | — | 0.211 |
| Serum adiponectin (µg/mL)b | −0.3 (1.6) | −0.1 (1.7) | −0.1 (1.1) | 0.997 | — | 0.497 |
| Serum C-reactive protein (mg/L)b | −0.01 (2.6) | −0.1 (1.4) | −0.6 (1.0)c | 0.328 | — | 0.122 |
| Plasma TNF receptor II (pg/mL)b | 46.5 (387.5) | −15.8 (265.1) | 42.5 (312.7) | 0.635 | — | 0.235 |
| Plasma IL-6 (pg/mL)b | −0.2 (0.6) | 0.04 (0.7) | −0.1 (0.6) | 0.371 | — | 0.380 |
| Plasma IL-1 beta (pg/mL)b | na | na | na | (0.775) | — | (0.287) |
| Plasma IL-1 receptor antagonist (pg/mL)b | 19.2 (85.1) | 2.7 (43.2) | −8.9 (52.6) | 0.383 | — | 0.070 |
aValues are mean (standard deviation).
bValues are median (interquartile range).
cWilcoxon Signed Rank test for the change within group, P WSR < 0.05.
dMann-Whitney U P value for the difference is computed in the following order: placebo and 40 µg/d, placebo and 80 µg/d.
e n = 65.
25(OH)D3: 25-hydroxyvitamin D3; ALAT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HOMA: homeostatic modeling assessment; IL: interleukin; IR: insulin resistance; IS: insulin sensitivity; J-T: Jonckheere-Terpstra; K-W: Kruskal-Wallis; M-WU: Mann-Whitney U; PTH: parathyroid hormone.
Glucose homeostasis was assessed by fasting glucose, insulin, and HbA1c measurements and OGTT glucose and insulin measurements. The derived variables to be tested were the change in the chosen variables over the supplementation period. These were as follows: serum HbA1c (concentration and %), fasting, 30 min and 120 min glucose and insulin concentrations, HOMA2 insulin resistance (HOMA2-IR), insulin sensitivity (HOMA2-IS) and beta cell function (HOMA2-B%), IGI, and ISI. Inflammatory effects were assessed by analyzing the change in the hsCRP, psTNFrII, pIL-6, pIL-1RA, and pIL-1β concentrations over the supplementation period. Physiological and safety indicators were changes in the 25(OH)D3, sPTH, sCA, sCREA, sGGT, sALAT, and sTSH.
As a large proportion of the variables did not follow the normal distribution, we used the Kruskal-Wallis test (P K-W) to test the intergroup differences, Mann-Whitney U test (P M-WU) to test the pairwise intergroup differences (placebo versus 40 μg/d and placebo versus 80 μg/d), and the Jonckheere-Terpstra test (P J-T) for trend across the groups. Related-Samples Wilcoxon Signed Rank test (P WSR) was used to study the intragroup change over time. A two-sided P < 0.05 was considered statistically significant for the Kruskal-Wallis test, a 1-sided P < 0.05 for the Jonckheere-Terpstra test, and a two-sided P < 0.025 for the Mann-Whitney U test (i.e., with the Bonferroni correction). All statistical analyses were computed with the SPSS Statistical Package for Windows, version 21.0 (Armonk, NY: IBM Corp.).
The supplementation effect analyses were conducted per protocol; as for the dropouts there were no data available at the end of the study to compute the change variables.
3. Results
3.1. Randomization
Despite the moderately small sample size, the randomization yielded reasonably identical groups with regard to vitamin D and glucose metabolism-related parameters.
3.2. Subject Characteristics
Characteristics of the 68 study subjects at baseline are presented in Table 1. The median age of the subjects at entry was 65.7 (interquartile range 62.7 to 69.7) years, and the median serum 25(OH)D3 concentration was 57.2 (interquartile range 47.2 to 67.2) nmol/L. The arithmetic mean 25(OH)D3 concentration was 57.0 nmol/L with a standard deviation of 11.0 nmol/L. One of the 68 subjects had serum 25(OH)D3 concentration under 30 nmol/L and 18 subjects had concentration below 50 nmol/L.
Of the 68 subjects that entered the supplementation period, 66 completed the study (Figure 1).
3.3. Serum 25(OH)D3 and PTH
There was a marked dose-dependent increase in the average serum 25(OH)D3 concentration in the three study groups, from 55.3 to 59.0 nmol/L, from 57.7 to 85.4 nmol/L, and from 58.1 to 103.1 nmol/L, in the placebo, 40 μg/d, and 80 μg/d groups, respectively (mean ranks for change 17.1, 35.4, and 47.8, resp., P K-W < 0.001; see Table 2). At baseline, one of the subjects in the 80 μg/day group had serum 25(OH)D3 >75 nmol/L. At the end of the study, 3/21 (14%) subjects in the placebo group, 16/24 (67%) subjects in the 40 μg/d group, and 18/21 (86%) subjects in the 80 μg/d group had serum 25(OH)D3 > 75 nmol/L. Concomitant with the increase in the serum 25(OH)D3 concentrations, there was a dose-dependent decrease in the serum PTH concentrations (Table 2).
3.4. Glucose Metabolism
Changes in the tested glucose homeostasis parameters over the supplementation period are given in Table 2. The only statistically significant effect between the groups was an increase in the 120 min plasma glucose concentration, that is, opposite to expected (P K-W = 0.039, P J-T = 0.021), although only the pairwise group difference for the placebo versus 40 μg/d was statistically significant after Bonferroni correction (P = 0.022), and a decreasing trend in the HbA1c concentration (P J-T = 0.024) and in the 30 min insulin concentration (P J-T = 0.030). Borderline statistically significant trend was observed in the IGI (P J-T = 0.063).
3.5. Inflammation
In the tested inflammation markers, shown in Table 2, the only borderline statistically significant finding was a decreasing trend in the plasma IL-1RA concentration (P J-T = 0.070). There were detectable concentrations of pIL-1β only for four subjects at entry and for two subjects at the end of the study and therefore the P values cannot be considered reliable.
3.6. Safety
No statistically significant changes were observed in sCA (Table 2) or in the parameters of liver or kidney function or in the TBC (data not shown). No statistically significant differences between groups were observed in changes in waist circumference or BMI, in blood pressure, or in circulating lipids (data not shown).
3.7. Sensitivity Analyses
We repeated the analyses by including also those with BMI > 35 at the start of the supplementation period, those who started their own high-dose vitamin D supplementation during the trial, or the one with low compliance (n = 71). The results were generally similar to those with the exclusions, except for the P for trend for IL1-Ra, which was statistically significant (P J-T = 0.027).
4. Discussion
Our study showed that oral supplementation with daily 40 μg or 80 μg doses of vitamin D3 for five months markedly increases the circulating concentrations of 25(OH)D3, even over the winter months that usually are associated with a decrease in the 25(OH)D3 in this population [18]. We also showed that the 80 μg dose is more potent than the 40 μg dose in increasing serum 25(OH)D3 and that a considerably large proportion of the subjects in both supplementation groups reached serum 25(OH)D3 concentrations above 75 nmol/L. Furthermore, we showed that the supplementation also resulted in a decrease in serum PTH concentration but did not induce a rise in serum total calcium concentration. Vitamin D3 supplementation, even at 80 μg per day, was also well tolerated and safe.
The primary outcome of the study, body glucose homeostasis, remained for the most part practically unchanged, thus speaking against the role of vitamin D3 as an important regulator of glucose homeostasis in an ageing Finnish population with prediabetes. Among the 13 glucose homeostasis indices analyzed, the 120 min plasma glucose was the only index that was statistically significant also in the pairwise analyses. We believe that other changes observed (30 min insulin, HbA1c) may be related to random fluctuation of glucose metabolism in individuals with impaired glucose metabolism. Among the interventional studies on glucose homeostasis indicators in populations with prediabetes, an open-label study by Nazarian et al. showed an improvement in insulin sensitivity in 11 IFG subjects after a 4-week 10,000 IU/d (250 μg/d) vitamin D3 intervention [4]. In an earlier post hoc analysis by Pittas et al. among the 92 subjects with IFG at baseline [19], there was a lower rise in fasting plasma glucose concentration and lower increase in HOMA-IR in 3 years in the group taking 700 IU (17.5 μg) vitamin D3 plus 500 mg calcium citrate daily as compared with subjects taking placebo. Glucose homeostasis was also improved in the study by Mitri et al., where 16-week vitamin D3 supplementation of 2,000 IU/d (50 μg/d) improved β-cell function and attenuated the rise in HbA1c [5]. In an open-label study by Dutta et al. in subjects with IGT or IFG, vitamin D3 supplementation of 60,000 IU (1500 μg) once per week for 8 weeks and then monthly along with 1250 mg of calcium carbonate/d resulted in lower fasting blood glucose and improvement in insulin resistance during an average follow-up of 28 months, when compared with subjects taking only calcium carbonate [6]. In a recent placebo-controlled study by Gagnon et al., vitamin D3 upplementation for six months with a dose aimed at increasing the serum 25(OH)D3 to ≥75 nmol/L (2000–6000 IU/d, 50–150 μg/d) improved insulin sensitivity in subjects with prediabetes, but not in subjects without prediabetes [7]. In contrast, in a study by Harris et al. in 89 overweight or obese African American subjects, 12-week supplementation with 4,000 IU/d (100 μg/d) of vitamin D3, compared with placebo, led to reduced insulin sensitivity and increased insulin secretion [8]. In the 12-month supplementation trial by Davidson et al. in 117 Latino and African American subjects in California with hypovitaminosis D3 (i.e., serum 25(OH)D3 concentration <30 ng/mL (75 nmol/L)), the average weekly dose of 88,865 IU (2,222 μg, or about 317 μg/day) of vitamin D3 had no effect on insulin secretion, insulin sensitivity, or development of type 2 diabetes, as compared with placebo [9]. Our results support these null findings on glucose homeostasis in Finns, a Caucasian population at northern latitudes, even though we have earlier observed vitamin D3 sufficiency to associate with the lower risk of type 2 diabetes in an observational study of the same source population [20]. The observed increase in the 120 min plasma glucose is somewhat unexpected, but one explanation may simply be a chance finding due to a largish number of tests that were carried out. The increase was larger in the 40 μg group than in the 80 μg group, which supports that the finding occurred by chance as we believe is the case with other variables we monitored, that is, 30 min insulin and HbA1c.
Taken together, the evidence from the observational studies supports the association between low vitamin D status and impaired glucose metabolism, but the results from the experimental studies have not found improvements in glucose metabolism with vitamin D supplementation. A recent Mendelian randomisation study that looked into the association between body vitamin D status and glucose homeostasis and type 2 diabetes with four SNPs and four glycaemic traits showed an association in two SNPs near genes related to 25(OH)D synthesis with fasting insulin, but not in any of the other eleven tests conducted [21]. However, if a limitation is to be pointed out, many of the trials have been conducted in subjects with healthy glucose homeostasis or in subjects with diabetes, and only few have been carried out in subjects with prediabetes, the subjects that would probably show any effect on glucose homeostasis most easily. Therefore, well-designed randomized trials with large sample sizes and in people with insufficient vitamin D status may be needed to elucidate the role of vitamin D supplementation in glucose homeostasis and in prevention of type 2 diabetes.
We found supplemental vitamin D3 to exert some anti-inflammatory action, when a borderline statistically significant decrease in the IL-1RA concentration was found. IL-1RA is regarded as one of the most sensitive markers of inflammation, as it is readily secreted by many cell types and plasma levels are high enough to be reliably measured. Elevated values of IL-1RA have also been shown to predict the onset of type 2 diabetes [22–24]. The results from previous studies of the anti-inflammatory effects of vitamin D supplementation have been inconsistent. Some studies have found vitamin D supplementation to improve the levels of certain inflammatory markers, such as IL-6, IL-10, CRP, or TNF-α [6, 10, 25–27], whereas others have found no effects on any of the studied markers [19, 28–30]. However, only a few studies have been conducted in subjects with prediabetes [6, 19, 31] and, to our knowledge, no study thus far has investigated the impact of vitamin D supplementation on IL-1RA.
Our study was conducted during October to April, the period of low UVB light exposure from the sun in Finland, to allow as unbiased supplemental vitamin D effect as possible. Strengths of the study were the homogenous study population and use of two different, sufficiently large vitamin D3 doses, which enabled studying possible dose response. A limitation of the study was the unbalanced gender distribution, especially the low number of females. Another limitation is the lower than planned total number of study subjects, which was due to the need to close recruitment to allow a sufficiently long supplementation during the low UVB exposure period, and the higher than anticipated average 25(OH)D3 concentration of the study population at the start of the study. However, the fairly consistent results of our study do not suggest a marked change in observed effects even if some more subjects would have been included, or if intention-to-treat analysis had been carried out instead of per-protocol analysis, given that per-protocol tends to exaggerate the treatment effects, if anything.
5. Conclusions
In conclusion, our study does not support the role of winter-time relatively high-dose vitamin D3 supplementation as a means to improve glucose homeostasis in a general ageing population with prediabetes but suggests a modest anti-inflammatory effect.
Acknowledgments
The study has been funded by the University of Eastern Finland, the Academy of Finland (no. 137826 to Sari Voutilainen and no. 131593 to Vanessa D. F. de Mello), the Juho Vainio Foundation, the Finnish Foundation for Cardiovascular Research, and the Finnish Diabetes Research Foundation.
Conflict of Interests
The authors have no potential conflict of interests.
References
- 1.Khan H., Kunutsor S., Franco O. H., Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proceedings of the Nutrition Society. 2013;72(1):89–97. doi: 10.1017/s0029665112002765. [DOI] [PubMed] [Google Scholar]
- 2.Forouhi N. G., Ye Z., Rickard A. P., et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of Prospective studies. Diabetologia. 2012;55(8):2173–2182. doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 3.George P. S., Pearson E. R., Witham M. D. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabetic Medicine. 2012;29(8):e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 4.Nazarian S., Peter J. V. St., Boston R. C., Jones S. A., Mariash C. N. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Translational Research. 2011;158(5):276–281. doi: 10.1016/j.trsl.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitri J., Dawson-Hughes B., Hu F. B., Pittas A. G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. The American Journal of Clinical Nutrition. 2011;94(2):486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta D., Mondal S. A., Choudhuri S., et al. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Research and Clinical Practice. 2014;103(3):e18–e23. doi: 10.1016/j.diabres.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon C., Daly R. M., Carpentier A., et al. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and beta-cell function in multi-ethnic vitamin D-deficient adults at risk for Type 2 diabetes: a Pilot Randomized, Placebo-Controlled Trial. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109607.e109607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris S. S., Pittas A. G., Palermo N. J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes, Obesity and Metabolism. 2012;14(9):789–794. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 9.Davidson M. B., Duran P., Lee M. L., Friedman T. C. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36(2):260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beilfuss J., Berg V., Sneve M., Jorde R., Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60(3):870–874. doi: 10.1016/j.cyto.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Fève B., Bastard J.-P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nature Reviews Endocrinology. 2009;5(6):305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 12.Elks C. M., Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Current Hypertension Reports. 2010;12(2):99–104. doi: 10.1007/s11906-010-0096-4. [DOI] [PubMed] [Google Scholar]
- 13.Chagas C. E. A., Borges M. C., Martini L. A., Rogero M. M. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4(1):52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurmi T., Tuomainen T.-P., Virtanen J., Mursu J., Voutilainen S. High-performance liquid chromatography and coulometric electrode array detector in serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 analyses. Analytical Biochemistry. 2013;435(1):1–9. doi: 10.1016/j.ab.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Wallace T. M., Levy J. C., Matthews D. R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 16.Phillips D. I., Clark P. M., Hales C. N., Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabetic Medicine. 1994;11(3):286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M., DeFronzo R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Virtanen J. K., Nurmi T., Voutilainen S., Mursu J., Tuomainen T.-P. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. European Journal of Nutrition. 2011;50(5):305–312. doi: 10.1007/s00394-010-0138-3. [DOI] [PubMed] [Google Scholar]
- 19.Pittas A. G., Harris S. S., Stark P. C., Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 20.Hurskainen A.-R., Virtanen J. K., Tuomainen T.-P., Nurmi T., Voutilainen S. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes/Metabolism Research and Reviews. 2012;28(5):418–423. doi: 10.1002/dmrr.2286. [DOI] [PubMed] [Google Scholar]
- 21.Ye Z., Sharp S. J., Burgess S., et al. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. The Lancet Diabetes & Endocrinology. 2015;3(1):35–42. doi: 10.1016/s2213-8587(14)70184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmenniemi U., Ruotsalainen E., Pihlajamäki J., et al. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110(25):3842–3848. doi: 10.1161/01.CIR.0000150391.38660.9B. [DOI] [PubMed] [Google Scholar]
- 23.Herder C., Brunner E. J., Rathmann W., et al. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the whitehall II study. Diabetes Care. 2009;32(3):421–423. doi: 10.2337/dc08-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carstensen M., Herder C., Kivimäki M., et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: whitehall II prospective cohort study. Diabetes. 2010;59(5):1222–1227. doi: 10.2337/db09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zittermann A., Frisch S., Berthold H. K., et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. The American Journal of Clinical Nutrition. 2009;89(5):1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 26.Matias P. J., Jorge C., Ferreira C., et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clinical Journal of the American Society of Nephrology. 2010;5(5):905–911. doi: 10.2215/cjn.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucharles S., Barberato S. H., Stinghen A. E. M., et al. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. Journal of Renal Nutrition. 2012;22(2):284–291. doi: 10.1053/j.jrn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkman M. P., Sorva A. J., Tilvis R. S. C-reactive protein and fibrinogen of bedridden older patients in a six-month vitamin D supplementation trial. Journal of Nutrition, Health and Aging. 2009;13(5):435–439. doi: 10.1007/s12603-009-0080-3. [DOI] [PubMed] [Google Scholar]
- 29.von Hurst P. R., Stonehouse W., Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103(4):549–555. doi: 10.1017/s0007114509992017. [DOI] [PubMed] [Google Scholar]
- 30.Jorde R., Sneve M., Torjesen P. A., Figenschau Y., Goslash;ransson L. G., Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50(2):175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez J. A., Zughaier S. M., Law J., et al. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. European Journal of Clinical Nutrition. 2013;67(3):264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]