Abstract
We evaluated results of temozolomide (TMZ) therapy in six patients, aged 34–78 years, presenting aggressive pituitary tumors. In all the patients tested O6-methylguanine-DNA methyltransferase (MGMT) immunoexpression in surgical specimens was absent. Patients received temozolomide 140–320 mg/day for 5 days monthly for at least 3 months. In two patients minimum time for evaluation could not be reached because of death in a 76-year-old man with a malignant prolactinoma and of severe neutro-thrombopenia in a 47-year-old woman with nonfunctioning pituitary adenoma. In two patients (a 34-year-old acromegalic woman and a 39-year-old woman with Nelson's syndrome) no response was observed after 4 and 6 months, respectively, and the treatment was stopped. Conversely, two 52- and 42-year-old women with Cushing's disease had long-term total clinical and radiological remissions which persisted after stopping temozolomide. We conclude that TMZ therapy may be of variable efficacy depending on—until now—incompletely understood factors. Cooperative work on a greater number of cases of aggressive pituitary tumors should be crucial to establish the indications, doses, and duration of temozolomide administration.
1. Introduction
Aggressive pituitary tumors are invasive macroadenomas refractory to surgical and medical treatments, showing tendency to continuous growth and implicating a bad vital prognosis [1]. Until some years ago, no therapies were efficacious in treating that kind of tumors. First publications of treatment with the alkylating agent temozolomide (TMZ) appeared in 2006 [2, 3] and since then, variable responses to this drug have been reported in a limited number of cases with tumor volume reduction and control of the disease in some of them. We present here our experience with the use of temozolomide in six patients with different variants of aggressive pituitary tumors.
2. Patients and Methods
Six patients with intention-to-treat with TMZ, presenting different types of aggressive pituitary tumors, were evaluated. They were 5 women aged 34–52 and one 78-year-old man. They all presented macroadenomas (more than 10 mm) with cavernous sinus invasion, two of them with third par palsies and one with bitemporal hemianopsia. The only male patient had pituitary carcinoma (malignant prolactinoma) with an isolated parietal metastasis which was first biopsied and then surgically excised. All patients had had unsuccessful previous pituitary surgery (from 1 to 5 times), radiotherapy in 3, and conventional drug treatment in 4 of them, aimed at controlling hyperfunction and/or tumor volume (Table 1). The definition of aggressive pituitary tumor was based on clinical grounds (invasive macroadenomas refractory to surgical and medical treatments, showing tendency to continuous growth) as previously stated. We use the denomination pituitary carcinoma when extrapituitary presence of tumor (metastasis) is found. Temozolomide was administered as oral pills in variable doses, from 140 to 320 mg/day for 5 days every month, for at least 3 months before evaluating results. TMZ administration was preceded by the oral intake of ondansetron, as antiemetic prevention. Hematologic and liver function tests were performed before each cycle of therapy. Results of treatment were evaluated by monthly clinical examination and pituitary MRI after at least 3 months of therapy; computerized visual field examination and routine hormone tests were also made, when indicated.
Table 1.
Main clinical traits of 6 patients with intention-to-treat with temozolomide.
| Patient | Sex | Age | Tumor type | Number of previous surgeries | RxT | Previous drug therapy |
|---|---|---|---|---|---|---|
| JB | M | 78 | PRL Ca | 1 | Yes | CAB |
| SA | F | 47 | CNFPA | 3 | No | CAB |
| LC | F | 34 | GH-oma | 2 | No | CAB, SSAs |
| DDO | F | 39 | NS | 2 | Yes | None |
| CM | F | 42 | CD | 1 | No | None |
| GM | F | 52 | CD | 5 | Yes | KNZ |
PRLCa: prolactin carcinoma; GH-oma: somatotropinoma; CNFPA: clinically nonfunctioning pituitary adenoma; NS: Nelson' syndrome; CD: Cushing's disease; RxT: radiotherapy; CAB: cabergoline; SSAs: somatostatin analogs; KNZ: ketoconazole.
For determinations of MGMT and marker of cell proliferation Ki67 on pathological specimens, all blocks were formalin buffer fixed and paraffin embedded. Cuts of 3-4 microns were made and stained with hematoxylin and eosin. Immunohistochemical determinations for adenohypophyseal hormones GH, FSH, LH, and TSH were made by using rabbit polyclonal Cell Marque (http://www.cellmarque.com/) antibodies whereas for PRL and ACTH, rabbit polyclonal DAKO (http://www.dako.com/) antibodies were employed. Ki67 and O6-methylguanine-DNA methyltransferase Ab-1 (MGMT) were measured by using mouse monoclonal antibodies from Thermo Scientific (http://www.thermoscientific.com/) in a 1 : 20 dilution. Immunostaining for MGMT was considered negative when lower than 10%.
3. Results
Figure 1 shows a MGMT-negative macrocorticotropinoma study of patient GM as compared to a MGMT-positive glioblastoma. Table 2 shows the results of MGMT and Ki67 immunohistochemistry, individual doses administered, length of therapy, and clinical outcome in the six patients.
Figure 1.
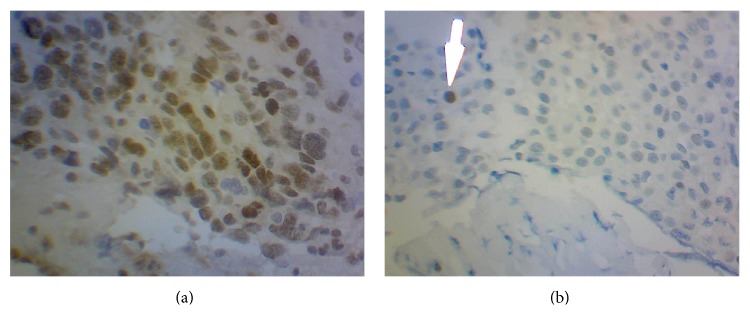
The upper panel (a) shows a diffuse positive MGMT control (glioblastoma). The lower panel (b) corresponds to a negative MGMT immunostaining of macrocorticotropinoma in patient GM.
Table 2.
Results of MGMT and Ki67 immunohistochemistry, doses, length of therapy, and clinical outcome in 6 patients with intention-to-treat with TMZ.
| Patient | Tumor type | MGMT | Ki67 | TMZ mg/d | Months | Outcome |
|---|---|---|---|---|---|---|
| JB | PRL Ca | (−) | 10% | 140 | 1 | Death |
| SA | CNFPA | ND | 2% | 150 | 1 | Failure |
| LC | GH-oma | (−) | 3% | 320 | 4 | Failure |
| DDO | Nelson's | (−) | 1% | 240 | 6 | Failure |
| CM | CD | (−) | 6% | 250 | 13 | Remission |
| GM | CD | (−) | 4% | 180 | 29 | Remission |
Drug therapy effect could not be evaluated in patients JB and SA because they failed to complete a 3-month treatment. JB had a malignant prolactinoma with brain metastases which deceased after the first administration of TMZ and SA developed severe thrombocytopenia and neutropenia after the first cycle of therapy. In two more patients TMZ was stopped after 4 (LC) and 6 (DDO) months of treatment, because it was considered ineffective in reducing tumor size. The two last patients having macrocorticotropinoma and Cushing's disease have been reported in detail elsewhere [4]. They showed clinical response after just 3-4 cycles of administration of TMZ with remission of ocular signs, normalization of cortisol alterations, and significant shrinkage (more than 50%) of the tumors, which completely disappeared one year later and, most interestingly, long time (19–30 months) after stopping therapy the patients remained well with no signs of tumor relapse [4].
4. Discussion
Frequency of pituitary tumors appears to be higher than previously suspected, as high as 1 in 1000 of the general population [5, 6]. They are usually benign and in most cases controlled by surgery, radiation, or medical treatments. In 2004 the World Health Organization defined as “atypical” those tumors exhibiting a MIB-1 (Ki-67) proliferative index >3%, strong p53 immunoreactivity, and increased mitotic activity [7]. They make up 15% of resected pituitary tumors [8]. Up to 45% of macroadenomas show signs of invasion of the sphenoid or cavernous sinus [9]. The concept of “aggressive” pituitary tumors represents a clinical appreciation to designate tumors that may recur quickly after surgery, grow into the cavernous sinus or skull base, and show resistance to the usual therapeutic means. The name pituitary carcinoma is reserved for those tumors with neural or extraneural metastases which make up less than 1% of the totality of pituitary tumors. It has to be emphasized that they do not show histological differences with other aggressive tumors save for the existence of metastases [10].
So called silent pituitary adenomas are tumors, mainly gonadotrope, corticotrope, and somatotrope, having an aggressive behavior, with frequent recurrences which made up 9% in 100 samples studied retrospectively [11]. They can be classified as “silent,” with immunohistochemical evidence but no biochemical or clinical evidence of hormone excess, or “clinically silent” with immunohistochemical and biochemical evidence but no clinical evidence of hormone excess.
Temozolomide is an alkylating drug which has been used mainly in the treatment of glioblastoma multiforme but also for colorectal cancer and melanoma [12–14]. This drug has been used for the treatment of pituitary carcinoma and aggressive adenoma from the year 2006 onwards [2, 3]. Its mechanism of action is through sticking an alkyl group to DNA bases, principally guanine, which induces methylation. Subsequently, it provokes the fragmentation of DNA by repairing enzymes in its attempt to replace the alkylated bases [15]. Up to now, around 105 pituitary tumors treated with TMZ have been reported in the literature with variable results (Table 3) [4, 16–53]. More than half (~60%) were aggressive adenomas, the remaining being pituitary carcinomas. Most were functioning tumors, especially corticotropinomas and prolactinomas (~80%). Global efficacy of TMZ therapy oscillated between 55% for aggressive adenomas and 58% for pituitary carcinomas, but it has to be underlined that criteria for efficacy were quite diverse, going from variable reduction to “stabilization” in tumoral size. It has to be remarked that in none of the reported cases a sustained disappearance of tumor after stopping TMZ was described. As far as aggressive macrocorticotropinomas are concerned, we were able to find 37 published cases silent or with overt hypercortisolism. Once again, criteria employed to evaluate response were quite diverse. In just one of those cases [20], the tumor disappeared under treatment but if the patient was treated with a CAPTEM schema (capecitabine plus temozolomide) we cannot know which one of the two drugs was more effective.
Table 3.
Literature update on aggressive pituitary adenomas and carcinomas.
| Author, year [reference] |
Sex/age | Tumor type | Ki67 (%) | MGMT | TMZ (mg/m2) & schedule (no. cycles) | MRI (% shrinkage) | Clinical outcome |
|---|---|---|---|---|---|---|---|
| Thearle et al., 2011 [16] | M/50 | ACTH SA Ad → Ca → NS | 31 | NA | 200 × 5/28 + CAP (4) | Reduced (75) | Death |
|
| |||||||
| Dillard et al., 2011 [17] | M/56 | ACTH Ad | 5-6 | NA | 150–200 × 5/28 (4) | Reduced (60) | CR |
|
| |||||||
| Annamalai et al., 2012 [18] | M/65 | ACTH Ca | 5–15 | Low | 200 × 5/28 (15) | PR of METS | “Remained well” |
|
| |||||||
| Moshkin et al., 2011 [19] | M/46 | ACTH SA Ad → Ca | 1–5 | (+) | 200 × 5/28 (16) | No change | Progression |
|
| |||||||
| Zacharia et al., 2014 [20] | M/50 | ACTH Ad | <5 | (−) | 150, 5/28 + CAP (30) | SD | PR |
| F/46 | ACTH Ad | 15–20 | (−) | 150, 5/28 + CAP (32) | CR | CR | |
| M/44 | ACTH Ad | <5 | (−) | 150, 5/28 (45) + CAP + A-SST | CR | CR | |
|
| |||||||
| Raverot et al., 2010 [21] | M/31 | ACTH Ca → NS | 20 | 50 (+) | 150–200 × 5/28 | No change | NA |
| M/49 | ACTH Ad | 20 | <1 | 150–200 × 5/28 | No change | NA | |
| M/38 | ACTH Ca | 10 | 30 (+) | 150–200 × 5/28 | SR | “Significant response” | |
| F/42 | ACTH Ad | 0.5 | 0 | 150–200 × 5/28 | SR | “Significant response” | |
| M/32 | PRL Ca | NA | NA | 150–200 × 5/28 (24) | Reduced (60), disappearance of METS | NA | |
| M/52 | PRL Ad | 0.5 | 30 | 150–200 × 5/28 (8) | No change | NA | |
| M/54 | PRL Ca | 1 | 0 | 150–200 × 5/28 (5) | No change | NA | |
| F/30 | PRL Ca | 10 | 100 | 150–200 × 5/28 (3) | No change | NA | |
|
| |||||||
| Bush et al., 2010 [22] | NA | Null cell Ad | <3 | — | 75 × 21/7 (10) | Reduced (20) | Stable |
| NA | ACTH Ad | 18 | <10 | 75 × 21/28 (11) | Reduced (80) | “Improved” | |
| NA | NF Ad | <3 | 10–50 | 75 × 21/(13) | SD | SD | |
| NA | Null cell Ad | 6 | >50 | 75 × 21/7 (10) | SD | SD | |
| NA | PRL Ad | NA | <10 | 75 × 21/7 (11) | Reduced (80) | “Improved” | |
| NA | Null cell Ca | >20 | >50 | 75 × 21/7 (2) | SD × 2 months | NA | |
| NA | Null cell Ca | >20 | <10 | 75 × 21/7 (7) | Progression | Death | |
|
| |||||||
| Hirohata et al., 2013 [23] | M/59 | NF Ca | 74.6 | (+) | 150–200 × 5/28 (5) | PR | NA |
| F/42 | ACTH Ca | 3.4 | (−) | 150–200 × 5/28 (7) | PR | NA | |
| F/60 | PRL Ca | 18.7 | (−) | 150–200 × 5/28 (13) | CR | NA | |
| M/23 | NF Ca | 2.5 | (+) | 150–200 × 5/28 (7) | SD | NA | |
| F/53 | ACTH Ca (Crooke cell) | 2.0 | (+) | 150–200 × 5/28 (20) | CR | NA | |
| F/60 | PRL Ca | 27.8 | (+) | 150–200 × 5/28 (12) | PR | NA | |
| M/57 | ACTH Ca | 10 | (+) | 150–200 × 5/28 (8) | SD | NA | |
| F/73 | NF Ca | 5.6 | (−) | 150–200 × 5/28 (22) | PR | NA | |
| M/60 | PRL Ca | 40.2 | (−) | 150–200 × 5/28 (24) | PR | NA | |
| F/61 | NF Ca | 12.2 | (+) | 75 × 6 weeks + RT | Progression | NA | |
| F/66 | PRL Ad | 9.4 | (−) | 75 × 6 weeks + RT | CR | NA | |
| F/49 | PRL Ad | 3.9 | (−) | NA (3) | Progression | NA | |
| F/45 | ACTH Ad (Crooke cell) | 46.8 | (+) | 150–200 × 5/28 (11) | PR | NA | |
|
| |||||||
| Losa et al., 2010 [24] | M/64 | ACTH Ad | NA | NA | 150–200 × 5/28 | Progression | Death |
| M/52 | ACTH Cd | 1 | (−) | 150–200 × 5/28 | “Response” | Required GC therapy | |
| F/55 | ACTH Ad → NS | 5 | (−) | 150–200 × 5/28 | SD | NA | |
| F/53 | ACTH Ad | 2.5 | (+) | 150–200 × 5/28 | Progression | No change | |
| M/62 | PRL Ad | 9 | (−) | 150–200 × 5/28 | SD | NA | |
| F/57 | PRL Ad | NA | Noninformative | 150–200 × 5/28 | “Response” | “Improved” | |
|
| |||||||
| Moyes et al., 2009 [25] | F/64 | ACTH Ad → NS | “High” | (−) | 200 × 5/28 (6) | “Marked shrinkage” | “Improved” |
|
| |||||||
| Takeshita et al., 2009 [26] | F/46 | ACTH Ca → NS | ~3 | <5 (−) | 150–200 × 5/28 (23) | CR tumor + METS | Required GC therapy |
|
| |||||||
| Curtò et al., 2010 [27] | M/42 | ACTH Ca | 2–18 | <5 (−) | 150–200 × 5/28 (17) | Reduced (>90) | “Improved” |
|
| |||||||
| Mohammed et al., 2009 [28] | F/43 | ACTH Ad | NA | (−) | 150–200 × 5/28 (12) | PR | “Improved” |
| M/60 | ACTH Ca → NS | NA | (+) | 150–200 × 5/28 (12) | PR | “Improved” | |
|
| |||||||
| Bode et al., 2010 [29] | NA | ACTH Ca → NS | NA | NA | 150 × 5/28 | PR | NA |
|
| |||||||
| Jouanneau et al., 2012 [30] | NA | SA → Ca | NA | NA | 200 × 5/28 | NR | NA |
|
| |||||||
| Asimakopoulou et al., 2014 [31] | F/55 | ACTH Ad (Crooke cell) | 1 | NA | 150–200 × 5/28 | CR | CR |
|
| |||||||
| Bengtsson et al., 2015 [32] | F/71 | ACTH Ad | 50 | 90 | 150–200 × 5/28 | SD | NA |
| F/31 | GH Ad | 7 | 9–100 | 150–200 × 5/28 (6) | Reduced (50) | Regrowth after TMZ stop | |
| F/13 | GH Ad | 5 | 95 | 150–200 × 5/28 | NA | NA | |
| M/33 | PRL-GH Ad | 23 | 10 | 150–200 × 5/28 (3) | Reduced (35) | SD 25 months after TMZ | |
| M/22 | PRL Ad | 8 | 90 | 150–200 × 5/28 (15) | Reduced (25) | Death | |
| M/34 | PRL Ad | 6 | 9–100 | 150–200 × 5/28 (4) | Stable 40 m after TMZ | PR | |
| M/45 | PRL Ad | 2 | 100 | 150–200 × 5/28 (5) | Progression | PR | |
| M/55 | PRL Ad | 10 | 20 | 150–200 × 5/28 (11) | Reduced (66) | Death | |
| M/60 | PRL Ad | 2 | 9 | 150–200 + CAB (21) | Reduced (80) | Death | |
| M/68 | PRL Ad | NA | 150–200 × 5/28 | Progression | Death | ||
| M/23 | PRL Ad | 41 | 100 | 150–200 × 5/28 (4) | Progression | Death | |
| M/22 | NF Ad | 2 | 9 | 150–200 × 5/28 (12) | Reduced (55) | SD 69 m after TMZ | |
| M/45 | NF Ad | 2 | 100 | 150–200 × 5/28 (18) | Reduced (28) | NA | |
| F/52 | NF Ad | 10 | 90 | 150–200 × 5/28 (5) | Progression | Death | |
| M/59 | NF Ad | 10 | 90 | 150–200 × 5/28 (6) | Progression | Death | |
| M/57 | NF Ad | 3.3 | 95 | 150–200 × 5/28 | Progression | Death | |
| M/51 | ACTH Ca | 80 | 0–60 | 150–200 × 5/28 | NA | Death | |
| M/62 | ACTH Ca (NS) | 10 | 95 | 150–200 × 5/28 | NA | Lost to follow-up | |
| M/70 | ACTH Ca | 70 | 9 | 150–200 × 5/28 | NA | NA | |
| M/46 | GH Ca | 60 | 90 | 150–200 × 5/28 | NA | Death | |
| F/40 | GH Ca | 20 | 9 | 150–200 × 5/28 | CR | CR after 48 months | |
| F/49 | PRL-GH Ca | 5 | 9 | 150–200 × 5/28 | CR | CR after 91 months | |
| F/32 | PRL Ca | 20 | 50 | 150–200 × 5/28 | NA | Death | |
| F/59 | PRL Ca | 10 | NA | 150–200 × 5/28 | NA | PR | |
|
| |||||||
| Vieira Neto et al., 2013 [33] | F/54 | GH S Ca | 2.6 | 68 | 150–200 × 5/28 | SD | NA |
|
| |||||||
| Morokuma et al., 2012 [34] | M/58 | NF Ca/NEM-1 | 7.6 | (−) | 75/d × 42 days; then 192 × 5/28 + RT (20) | “Visibly declined” | “Improved” |
|
| |||||||
| Zhong et al., 2014 [35] | F/30 | NF Ad | 20 | NA | 200/d × 5/4 consecutive weeks/2 months + RT (4) | CR | NA |
|
| |||||||
| Syro et al., 2009 [36] | M/70 | Gn Ad | 2–6 | 30–>50 | 200 × 5/28 (6) | “Minor reduction” and intratumoral necrosis | Death |
|
| |||||||
| Hagen et al., 2009 [37] | F/48 | PRL Ad to mixed PRL-GH Ad to Ca | 5 | (−) | 150–200 + CAB/STT-A | Reduced (62) | “Improved” |
| M/60 | PRL Ad | ~2 | (−) | 150–200 + CAB | Reduced (80) | “Improved” | |
| M/20 | NF Ad | ~2 | Few (+) | 150–200 | Reduced (55) | “Improved” | |
|
| |||||||
| Mendola et al., 2014 [38] | M/58 | NS Ca | 10 | NA | 160 × 5/28 (1) | No | No change |
|
| |||||||
| Strowd et al., 2015 [39] | F/44 | PRL Ad | NA | NA | 150–200 × 5/28 (3 months) | “Reduction in tumor size” | PR |
|
| |||||||
| Ceccato et al., 2015 [40] | F/67 | NF Ad | <3 | NA | 150–200 × 5/28 | Progression | No change |
| F/39 | GH Ad | <3 | NA | 150–200 × 5/28 | Progression | No change | |
| M/40 | NF Ad | <3 | NA | 150–200 × 5/28 | Decreased (49) | NA | |
| M/32 | ACTH Ad | <3 | NA | 150–200 × 5/28 | Decreased (63) | No change | |
| M/47 | NF Ad → ACTH | <3 | NA | 150–200 × 5/28 + pasireotide | Decreased (21) | No change | |
|
| |||||||
| Philippon et al., 2012 [41] | M/41 | PRL Ca/MEN-1 | NA | NA | 200 × 5/28 (24) | Decreased (62) | “Improved” |
|
| |||||||
| Fadul et al., 2006 [42] | M/38 | NF Ca | 1 | NA | 200 × 5/23 (12) | PR | PR |
| M/26 | PRL Ca | 10 | 200 × 5/23 (10) | PR | PR | ||
|
| |||||||
| Kovacs et al., 2007 [43] | M/46 | PRL Ca | 40–60 | NA | 200 × 5/28 (7) | “Shrinkage” | “Improved” |
|
| |||||||
| Cornell et al., 2013 [44] | M/40 | ACTH Ad | 5–7 | NA | 200 × 5/28 (3) | Progression | No change |
|
| |||||||
| Phillips et al., 2012 [45] | M/25 | PRL Ad | 23 | NA | 350 × 5 (1) | No change | Death |
|
| |||||||
| Rotondo et al., 2012 [46] | F/49 | Crooke cell Ad | 5–8 | (−) | 85 p.o daily + SRT | NA | NA |
|
| |||||||
| Arnold et al., 2012 [47] | F/61 | ACTH Ca | NA | NA | NA (12) | “Resolved” | PR |
|
| |||||||
| Morin et al., 2012 [48] | M/22 | GH Ad | 3-4 | NA | 200 × 5/28 (5) | No change | Increased signs |
|
| |||||||
| Whitelaw et al., 2012 [49] | M/34 | PRL Ad | 15 | (−) | 200 × 5/28 (6) | “Dramatic reduction” | “Significant improvement” |
| M/32 | PRL Ad | 8 | (−) | 200 × 5/28 (6) | “Substantial reduction” | “Significant improvement” | |
| M/13 | PRL Ad | 4 | (−) | 200 × 5/28 (12) | PR | PR | |
|
| |||||||
| Ersen et al., 2012 [50] | NA | Gn Ad | NA | Two zones: (−) and (+), 60% | 200 × 5/28 (14) | SD | “Clinical improvement” |
|
| |||||||
| Scheithauer et al., 2012 [51] | F/13 months | Pituitary blastoma | NA | Varied from 40 to 60% | 100 × 5/28 (12 + 6) | Progression | NA |
|
| |||||||
| Ortiz et al., 2012 [52] | M/38 | ACTH Ad → Ca | NA | High | 200 × 5/28 (8) | No change | Progression |
|
| |||||||
| Batisse et al., 2013 [53] | M/47 | GH Ad | (−) | High | 200 × 5/28 (3) | Progression | No significant response |
|
| |||||||
| Bruno et al., 2015 [4] | F/52 | ACTH Ad | 6 | (−) | 150–200 × 5/28 (29) | CR | CR |
| F/42 | ACTH Ad | 4 | (−) | 150–200 × 5/28 (12) | CR | CR | |
Ad: adenoma; Ca: carcinoma; SA: silent adenoma; NS: Nelson's syndrome; NF: nonfunctioning; PRL: lactotrope; ACTH: corticotrope; GH: somatotrope; Gn: gonadotrope; MRI: magnetic resonance imaging; NA: not available; RT: radiotherapy; CR: complete response; PR: partial response; SR: “significant” reduction; METS: metastases; SD: stable disease; CAB: cabergoline; STT-A: somatostatin agonist; CAP: capecitabine; →: change; (og): ongoing.
Doses of temozolomide usually recommended in neurology are adapted to body surface and oscillate from 150 to 200 mg/m2 [54]. Doses employed in our patients were variable, but generally lower than recommended. It has to be underlined that dose amount was mostly determined by availability following individual medical coverage. Interestingly, patients MC and GM who had total remission received fixed doses of 250 mg/d and 180 mg/d, respectively, while, if adapted to body surface area, those figures should have been 291–388 mg/d for MC and 273–364 mg/d for GM. The role that the DNA repairing systems may play in the effectiveness of temozolomide is controversial, especially concerning MGMT. This enzyme can reverse methylation of the guanine residues, thus antagonizing the effect of the drug. It has been reported that a low expression or the absence of this enzyme strongly correlates with the response to TMZ [15]. This has been challenged by other authors, who failed to find such a correlation [21, 23]. It has been proposed that the preservation of another enzyme system, MSH6 (DNA mismatch repair protein), correlated better with the response to TMZ than the absence of MGMT [23, 54].
In our series, the five patients in whom we were able to measure MGMT failed to show a significant expression (less than 5%); two of them having aggressive corticotropinoma had excellent clinical responses to temozolomide. Nevertheless, this does not enable us to extrapolate any conclusions at this respect, since two other MGMT-negative patients who completed the minimum period of treatment failed to show a response.
For a more rational use of TMZ several points deserve clarification: What should be the starting and maintenance doses? How can efficacy be defined? How long should the treatment be given? How big is the mutagenic risk? What is the recurrence risk after stopping TMZ? What is the probability of relapse with resistance to TMZ after stopping a successful therapy?
In conclusion, although less common, clinically aggressive pituitary tumors are not at all exceptional and pose special therapeutic challenges because surgery and radiotherapy are frequently useless and usual drug therapy is of variable and unpredictable efficacy. So called “silent” tumors appear to be particularly aggressive and, although less frequent, invasive corticotropinomas may present a difficult challenge as well, since besides local complications, they put life at risk because of the metabolic consequences of excess cortisol secretion. Temozolomide may be a salvage drug in selected cases, mainly in prolactinoma and corticotrope tumors. Cooperative work on a greater number of cases of aggressive pituitary tumors should be of the outmost importance to establish the indications, doses, and duration of temozolomide administration.
Acknowledgments
The authors are deeply indebted to Drs. Mariano Volpacchio and Santiago Rossi, from the Centro de Diagnóstico Dr. Enrique Rossi, Buenos Aires, who generously provided gratuitous control MRI in patient MC.
Disclosure
This paper was presented in part at The Endocrine Society Annual Meeting, Phoenix, AZ, 2013.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Colao A., Grasso L. F. S., Pivonello R., Lombardi G. Therapy of aggressive pituitary tumors. Expert Opinion on Pharmacotherapy. 2011;12(10):1561–1570. doi: 10.1517/14656566.2011.568478. [DOI] [PubMed] [Google Scholar]
- 2.Lim S., Shahinian H., Maya M. M., Yong W., Heaney A. P. Temozolomide: a novel treatment for pituitary carcinoma. The Lancet Oncology. 2006;7(6):518–520. doi: 10.1016/s1470-2045(06)70728-8. [DOI] [PubMed] [Google Scholar]
- 3.Syro L. V., Uribe H., Penagos L. C., et al. Antitumour effects of temozolomide in a man with a large, invasive prolactin-producing pituitary neoplasm. Clinical Endocrinology. 2006;65(4):552–553. doi: 10.1111/j.1365-2265.2006.02653.x. [DOI] [PubMed] [Google Scholar]
- 4.Bruno O. D., Juárez-Allen L., Christiansen S. B., Danilowicz K. Long-lasting complete remission after therapy with temozolomide in two patients with macrocorticotropinoma causing Cushing's disease. Clinical Endocrinology. 2015 doi: 10.1111/cen.12727. [DOI] [PubMed] [Google Scholar]
- 5.Daly A. F., Rixhon M., Adam C., Dempegioti A., Tichomirowa M. A., Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liège, Belgium. Journal of Clinical Endocrinology and Metabolism. 2006;91(12):4769–4775. doi: 10.1210/jc.2006-1668. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez A., Karavitaki N., Wass J. A. H. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK) Clinical Endocrinology. 2010;72(3):377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd R. J., Kovacs K., Young W. F., Jr., et al. Tumours of the pituitary gland. In: DeLellis R. A., Lloyd R. V., Heitz P. U., editors. Pathology and Genetics of Endocrine Tumours. Lyon, France: International Agency for Research and Cancer (IARC); 2004. pp. 9–48. [Google Scholar]
- 8.Zada G., Woodmansee W. W., Ramkissoon S., Amadio J., Nose V., Laws E. R., Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. Journal of Neurosurgery. 2011;114(2):336–344. doi: 10.3171/2010.8.jns10290. [DOI] [PubMed] [Google Scholar]
- 9.Meij B. P., Lopes M.-B. S., Ellegala D. B., Alden T. D., Laws E. R., Jr. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. Journal of Neurosurgery. 2002;96(2):195–208. doi: 10.3171/jns.2002.96.2.0195. [DOI] [PubMed] [Google Scholar]
- 10.Heaney A. P. Clinical review: pituitary carcinoma: difficult diagnosis and treatment. Journal of Clinical Endocrinology and Metabolism. 2011;96(12):3649–3660. doi: 10.1210/jc.2011-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade A. N., Baccon J., Grady M. S., Judy K. D., O'Rourke D. M., Snyder P. J. Clinically silent somatotroph adenomas are common. European Journal of Endocrinology. 2011;165(1):39–44. doi: 10.1530/eje-11-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart M. G., Garside R., Rogers G., Stein K., Grant R. Temozolomide for high grade glioma. The Cochrane Database of Systematic Reviews. 2013;(4) doi: 10.1002/14651858.CD007415.pub2.CD007415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietrantonio F., Perrone F., De Braud F., et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Annals of Oncology. 2014;25(2):404–408. doi: 10.1093/annonc/mdt547. [DOI] [PubMed] [Google Scholar]
- 14.Quirt I., Verma S., Petrella T., Bak K., Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12(9):1114–1123. doi: 10.1634/theoncologist.12-9-1114. [DOI] [PubMed] [Google Scholar]
- 15.Mccormack A. I., Wass J. A. H., Grossman A. B. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. European Journal of Clinical Investigation. 2011;41(10):1133–1148. doi: 10.1111/j.1365-2362.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 16.Thearle M. S., Freda P. U., Bruce J. N., Isaacson S. R., Lee Y., Fine R. L. Temozolomide (Temodar) and capecitabine (Xeloda) treatment of an aggressive corticotroph pituitary tumor. Pituitary. 2011;14(4):418–424. doi: 10.1007/s11102-009-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillard T. H., Gultekin S. H., Delashaw J. B., Jr., Yedinak C. G., Neuwelt E. A., Fleseriu M. Temozolomide for corticotroph pituitary adenomas refractory to standard therapy. Pituitary. 2011;14(1):80–91. doi: 10.1007/s11102-010-0264-1. [DOI] [PubMed] [Google Scholar]
- 18.Annamalai A. K., Dean A. F., Kandasamy N., et al. Temozolomide responsiveness in aggressive corticotroph tumours: a case report and review of the literature. Pituitary. 2012;15(3):276–287. doi: 10.1007/s11102-011-0363-7. [DOI] [PubMed] [Google Scholar]
- 19.Moshkin O., Syro L. V., Scheithauer B. W., et al. Aggressive silent corticotroph adenoma progressing to pituitary carcinoma. The role of temozolomide therapy. Hormones. 2011;10(2):162–167. doi: 10.14310/horm.2002.1307. [DOI] [PubMed] [Google Scholar]
- 20.Zacharia B. E., Gulati A. P., Bruce J. N., et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery. 2014;74(4):E447–E455. doi: 10.1227/neu.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 21.Raverot G., Sturm N., De Fraipont F., et al. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. Journal of Clinical Endocrinology and Metabolism. 2010;95(10):4592–4599. doi: 10.1210/jc.2010-0644. [DOI] [PubMed] [Google Scholar]
- 22.Bush Z. M., Longtine J. A., Cunningham T., et al. Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O6-methylguanine methyltransferase (MGMT) promoter methylation and expression. Journal of Clinical Endocrinology and Metabolism. 2010;95(11):E280–E290. doi: 10.1210/jc.2010-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirohata T., Asano K., Ogawa Y., et al. DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan society for hypothalamic and pituitary tumors. The Journal of Clinical Endocrinology & Metabolism. 2013;98(3):1130–1136. doi: 10.1210/jc.2012-2924. [DOI] [PubMed] [Google Scholar]
- 24.Losa M., Mazza E., Terreni M. R., et al. Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. European Journal of Endocrinology. 2010;163(6):843–851. doi: 10.1530/eje-10-0629. [DOI] [PubMed] [Google Scholar]
- 25.Moyes V. J., Alusi G., Sabin H. I., et al. Treatment of Nelson's syndrome with temozolomide. European Journal of Endocrinology. 2009;160(1):115–119. doi: 10.1530/eje-08-0557. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita A., Inoshita N., Taguchi M., et al. High incidence of low O6-methylguanine DNA methyltransferase expression in invasive macroadenomas of Cushing's disease. European Journal of Endocrinology. 2009;161(4):553–559. doi: 10.1530/EJE-09-0414. [DOI] [PubMed] [Google Scholar]
- 27.Curtò L., Torre M. L., Ferraù F., et al. Temozolomide-induced shrinkage of a pituitary carcinoma causing cushing's disease—report of a case and literature review. TheScientificWorldJournal. 2010;10:2132–2138. doi: 10.1100/tsw.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed S., Kovacs K., Mason W., Smyth H., Cusimano M. D. Use of temozolomide in aggressive pituitary tumors: case report. Neurosurgery. 2009;64(4):E773–E774. doi: 10.1227/01.neu.0000339115.12803.4e. [DOI] [PubMed] [Google Scholar]
- 29.Bode H., Seiz M., Lammert A., et al. SOM230 (Pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Experimental and Clinical Endocrinology and Diabetes. 2010;118(10):760–763. doi: 10.1055/s-0030-1253419. [DOI] [PubMed] [Google Scholar]
- 30.Jouanneau E., Wierinckx A., Ducray F., et al. New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary. 2012;15(1):37–43. doi: 10.1007/s11102-011-0341-0. [DOI] [PubMed] [Google Scholar]
- 31.Asimakopoulou A., Tzanela M., Koletti A., Kontogeorgos G., Tsagarakis S. Long-term remission in an aggressive Crooke cell adenoma of the pituitary, 18 months after discontinuation of treatment with temozolomide. Clinical Case Reports. 2014;2(1):1–3. doi: 10.1002/ccr3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bengtsson D., Schrøder H. D., Andersen M., et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. The Journal of Clinical Endocrinology & Metabolism. 2015;100(4):1689–1698. doi: 10.1210/jc.2014-4350. [DOI] [PubMed] [Google Scholar]
- 33.Neto L., Chimelli L., Pereira P. D. M., et al. The role of temozolomide in the treatment of a patient with a pure silent pituitary somatotroph carcinoma. Endocrine Practice. 2013;19(6):e145–e149. doi: 10.4158/ep12400.cr. [DOI] [PubMed] [Google Scholar]
- 34.Morokuma H., Ando T., Hayashida T., et al. A case of nonfunctioning pituitary carcinoma that responded to temozolomide treatment. Case Reports in Endocrinology. 2012;2012:5. doi: 10.1155/2012/645914.645914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong C., Yin S., Zhou P., Jiang S. Pituitary atypical adenoma or carcinoma sensitive to temozolomide combined with radiation therapy: a case report of early identification and management. Turkish Neurosurgery. 2014;24(6):963–966. doi: 10.5137/1019-5149.jtn.9629-13.1. [DOI] [PubMed] [Google Scholar]
- 36.Syro L. V., Scheithauer B. W., Ortiz L. D., et al. Effect of Temozolomide in a patient with recurring oncocytic gonadotrophic pituitary adenoma. Hormones. 2009;8(4):303–306. doi: 10.14310/horm.2002.1247. [DOI] [PubMed] [Google Scholar]
- 37.Hagen C., Schroeder H. D., Hansen S., Hagen C., Andersen M. Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. European Journal of Endocrinology. 2009;161(4):631–637. doi: 10.1530/EJE-09-0389. [DOI] [PubMed] [Google Scholar]
- 38.Mendola M., Passeri E., Ambrosi B., Corbetta S. Multiple cerebral hemorrhagic foci from metastases during temozolomide treatment in a patient with corticotroph pituitary carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2014;99(8):2623–2624. doi: 10.1210/jc.2014-1183. [DOI] [PubMed] [Google Scholar]
- 39.Strowd R. E., Salvatori R., Laterra J. J. Temozolomide retreatment in a recurrent prolactin-secreting pituitary adenoma: Hormonal and radiographic response. Journal of Oncology Pharmacy Practice. 2015 doi: 10.1177/1078155215569556. [DOI] [PubMed] [Google Scholar]
- 40.Ceccato F., Lombardi G., Manara R., et al. Temozolomide and pasireotide treatment for aggressive pituitary adenoma: expertise at a tertiary care center. Journal of Neuro-Oncology. 2015;122(1):189–196. doi: 10.1007/s11060-014-1702-0. [DOI] [PubMed] [Google Scholar]
- 41.Philippon M., Morange I., Barrie M., et al. Long-term control of a MEN1 prolactin secreting pituitary carcinoma after temozolomide treatment. Annales d'Endocrinologie. 2012;73(3):225–229. doi: 10.1016/j.ando.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Fadul C. E., Kominsky A. L., Meyer L. P., et al. Long-term response of pituitary carcinoma to temozolomide. Report of two cases. Journal of Neurosurgery. 2006;105(4):621–626. doi: 10.3171/jns.2006.105.4.621. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs K., Horvath E., Syro L. V., et al. Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Human Pathology. 2007;38(1):185–189. doi: 10.1016/j.humpath.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Cornell R. F., Kelly D. F., Bordo G., et al. Chemotherapy-induced regression of an adrenocorticotropin-secreting pituitary carcinoma accompanied by secondary adrenal insufficiency. Case Reports in Endocrinology. 2013;2013:10. doi: 10.1155/2013/675298.675298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips J., East H. E., French S. E., et al. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones. 2012;11(4):477–482. doi: 10.14310/horm.2002.1380. [DOI] [PubMed] [Google Scholar]
- 46.Rotondo F., Cusimano M., Scheithauer B. W., Coire C., Horvath E., Kovacs K. Atypical, invasive, recurring Crooke cell adenoma of the pituitary. Hormones. 2012;11(1):94–100. doi: 10.1007/BF03401542. [DOI] [PubMed] [Google Scholar]
- 47.Arnold P. M., Ratnasingam D., O'Neil M. F., Johnson P. L. Pituitary carcinoma recurrent to the lumbar intradural extramedullary space: case report. The Journal of Spinal Cord Medicine. 2012;35(2):118–121. doi: 10.1179/2045772311y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morin E., Berthelet F., Weisnagel J., Bidlingmaier M., Serri O. Failure of temozolomide and conventional doses of pegvisomant to attain biochemical control in a severe case of acromegaly. Pituitary. 2012;15(1):97–100. doi: 10.1007/s11102-010-0232-9. [DOI] [PubMed] [Google Scholar]
- 49.Whitelaw B. C., Dworakowska D., Thomas N. W., et al. Temozolomide in the management of dopamine agonist-resistant prolactinomas. Clinical Endocrinology. 2012;76(6):877–886. doi: 10.1111/j.1365-2265.2012.04373.x. [DOI] [PubMed] [Google Scholar]
- 50.Ersen A., Syro L. V., Penagos L., et al. Non-uniform response to temozolomide therapy in a pituitary gonadotroph adenoma. The Canadian Journal of Neurological Sciences. 2012;39(5):683–685. doi: 10.1017/s0317167100018242. [DOI] [PubMed] [Google Scholar]
- 51.Scheithauer B. W., Horvath E., Abel T. W., et al. Pituitary blastoma: a unique embryonal tumor. Pituitary. 2012;15(3):365–373. doi: 10.1007/s11102-011-0328-x. [DOI] [PubMed] [Google Scholar]
- 52.Ortiz L. D., Syro L. V., Scheithauer B. W., et al. Anti-VEGF therapy in pituitary carcinoma. Pituitary. 2012;15(3):445–449. doi: 10.1007/s11102-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 53.Batisse M., Raverot G., Maqdasy S., et al. Aggressive silent GH pituitary tumor resistant to multiple treatments, including temozolomide. Cancer Investigation. 2013;31(3):190–196. doi: 10.3109/07357907.2013.775293. [DOI] [PubMed] [Google Scholar]
- 54.Matsuno A., Murakami M., Hoya K., et al. Molecular status of pituitary carcinoma and atypical adenoma that contributes the effectiveness of temozolomide. Medical Molecular Morphology. 2014;47(1):1–7. doi: 10.1007/s00795-013-0050-z. [DOI] [PubMed] [Google Scholar]


