Abstract
Introduction
Nutraceuticals (NUT) are forms of compounds with biological activity commonly used to improve health in dosage largely exceeding those obtainable in food.
Aim
We compared, in a double blind randomized cross-over trial, the effects of two NUT combinations on the control of glico-lipidic metabolism in patients with hypercholesterolemia not on statins.
Methods
At study start patients were given dietary counseling and received placebo for 2 weeks. After this run-in period, patients were randomized: (1) Combination A [Policosanol, Red yeast rice (Monakolin K 3 mg), Berberine 500 mg, Astaxantine, Folic Acid and Coenzyme Q10] for 4 weeks followed by 4 weeks of Combination B [Red yeast rice (Monakolin K 3.3 mg), Berberine 531.25 mg and leaf extract of Morus alba]; (2) Combination B for 4 weeks followed by 4 weeks of Combination A.
Results
Combination B reduced LDL cholesterol below 130 mg/dl in 56.5 % of the patients, and Cambination A only in 21.7 % of them (p ≤ 0.027). Both treatments reduced plasma levels of triglycerides, total and LDL cholesterol and increased HDL cholesterol (all p < 0.03). Total and LDL cholesterol reduction was more pronounced in patients taking Combination B (p < 0.005). Combination B reduced also glycated hemoglobin, fasting glucose and insulin plasma levels as well as HOMA index (p < 0.005).
Conclusions
An increased content of Berberin and Monacolin K and the addition of Morus alba extract improves the effect on plasma cholesterol and on glucose metabolism of the NUT Combination. These effects may allow the speculation of a more marked improvement in cardiovascular prognosis.
Keywords: Nutraceuticals, Hypercholesterolemia, Insulin sensitivity
Introduction
International guidelines for treatment of dyslipidemia suggest the use of nutraceuticals (NUT) as first line choice for the treatment of patients with hypercholesterolemia and at low or moderate cardiovascular risk [1], since in randomized trials it has been demonstrated that some combinations of NUT are able to significantly improve the lipid profile and, consequently, the cardiovascular prognosis [2].
However, there are two issues that still deserve investigation: (1) the observation that the percentage of patients reaching the target level of serum LDL with NUT treatment is still low; (2) the knowledge that despite the marked reduction in serum LDL concentration obtained in large interventional trials, the percentage of patients who experience a cardiovascular event remains elevated so that it has been defined the “forgotten majority” [3].
With regard to NUT cholesterol lowering treatment, it seems reasonable to hypothesize that to overcome the problem of the low percentage of subjects reaching the therapeutic target, it may be useful the use of NUT combinations which include more components in order to recruit several different mechanisms able to improve the lipid profile.
In order to organize a strategy to reduce the “forgotten majority” it is relevant to consider that dyslipidemia is just one of the determinants of cardiovascular prognosis, while, according to the philosophy of the global cardiovascular risk, a combined activity on more than one risk factor may be required to reduce the probability to develop acute cardiovascular events [3].
In particular, Khaw et al. [4] demonstrated in a large population without known diabetes that the risk for cardiovascular disease and total mortality is associated with glycated hemoglobin (HbA1c) concentrations and increases continuously through the sample distribution.
Furthermore, it has been reported that fasting glucose, fasting insulin and insulin sensitivity, as expressed by Homeostasis Model Assessment Insulin Resistance (HOMA) index, were associated with incident cardiovascular disease in individuals without diabetes [5].
Recently, Wang et al. [6], have reported that Morus alba, mulberry fruit, used to treat and prevent diabetes in traditional oriental medicine, exerts an α-glucosidase inhibitory and antioxidant activity in vitro, which may reduce postprandial glucose peak, thus improving HbA1c concentration.
We planned the present study to compare in a double blind randomized trial the effects of two NUT combinations on the control of serum LDL levels (percentage of subjects reaching the target therapeutic level) and glucose metabolism and endothelial function in patients with hypercholesterolemia not requiring statins or statin intolerant.
Methods
This was a monocentric, 8-week, randomized, double-blind, cross-over, placebo-controlled study during which all subjects assumed placebo for 2 weeks and subsequently NUT Combination A or Combination B according to a cross-over randomized double blind design, so that all patients received both NUT Combinations. All determinations were performed at the end of each of the three study steps.
The primary endpoint was the percentage of patients obtaining normalization of LDL plasma levels (LDL <130 mg/dl). Secondary end points were the changes in total, HDL and LDL cholesterol, triglycerides, Hb1Ac, fasting glucose and insulin plasma levels, improvement of insulin sensitivity index (HOMA index) and endothelial-dependent dilation (FMD) in relation to the administration of each NUT Combination.
The study was conducted in accordance with the guidelines of the declaration of Helsinki, and the study protocol was approved by the Ethic Committee of the Federico II University of Naples. Written informed consent was obtained from each subject.
The inclusion criteria were hypercholesterolemia not requiring statins or in statin intolerant subjects, age between 18 and 70 years.
We excluded from the study subjects with intolerance to NUT compounds, pregnant women and women planning to conceive, patients treated with lipid lowering drugs during the previous 6 weeks and patients with severe hypertriglyceridemia (>500 mg/dl).
Protocol
After the screening visit and recruitment, patients were given dietary counseling, according to their clinical conditions and received placebo for 2 weeks. At the end of this run-in period, patients were randomized into two arms assigned to receive: (1) Combination A for 4 weeks followed by a 4 week treatment with Combination B; (2) Combination B for 4 weeks followed by 4 week treatment with Combination A. After all study section blood pressure (BP), heart rate, waist girth, lipid and glucose profile, plasma levels of insulin and HbA1c and FMD were assessed.
Measurements and Definitions
Fasting plasma glucose, lipids, insulin and HbA1c were measured by standard methods. Waist circumference was measured at each visit as midway between the lowest rib and the iliac crest using an anthropometric tape. Systolic and diastolic BP were measured by standard sphygmomanometer after 5 min in the supine position, according to the guidelines of the European Society of Hypertension/European Society of Cardiology [7].
Nut Combinations
The Combination A contains: Policosanol (10 mg), Red yeast rice (200 mg; 3 mg monacolin K), Berberine (500 mg), Astaxantine (0.5 mg), Folic Acid (200 mcg) and Coenzyme Q10 (2 mg). This combination is approved in Italy for the control of dyslipidemia (Armolipid Plus®, RottapharmSpA).
The Combination B contains: Berberine (531.25 mg), Red yeast rice powder (220 mg; 3.3 mg monacolin K) and leaf extract of Morus alba (200 mg). This product has recently been approved in Italy (LopiGLIK™, Akademy Pharma).
Endothelial Function
Endothelial function was assessed with the flow-mediated dilation test (FMD) by digital pulse amplitude, using a fingertip PAT as previously described [8]. The system (Itamar Medical Ltd., Caesarea, Israel) consists of a finger probe to assess digital volume changes accompanying pulse waves. All subjects included in the study were maintained without smoking, alcohol or caffeine from the night before the evaluation. Reactive hyperemia was measured as a PAT reactive hyperaemic index (RHI) calculated as the ratio of the average amplitude of the PAT signal over a 1-min time interval starting 1-min after cuff deflation divided by the average amplitude of the PAT signal of a 3.5-min time period before cuff inflation (baseline) and normalized to the RHI of the control arm. Endothelial dysfunction was defined as a RHI ≤1.68 calculated by the mean value minus 2 standard deviations of 20 healthy asymptomatic control individuals without history of cardiovascular disease and without major cardiovascular risk factors.
Statistical Analysis
Sample power was calculated based on a target LDL cholesterol levels <130 mg/dL, with a power of 80 % and an α-error = 5 % with a predicted success in subjects taking the experimental medications of 90 %, compared to 40 % of the control group, adjusting for cross-over. According to this calculation, 22 patients are required to have 80 % chance of detecting a significant increase in the primary outcome measure from 40 % in the control group to 90 % in the experimental group [9].
Data were analyzed using SPSS (version 22.0; SPSS, Chicago, Illinois, USA) and expressed as mean ± SD. All variables deviating from normal distribution were log transformed before parametric statistics were calculated. Descriptive statistics were performed using ANOVA or χ2-distribution, with Monte Carlo simulation to generate exact p values. The paired t test was used to calculate the significance of difference from baseline in each arm and inter group. The Sidak correction was applied for multiple comparisons. The null hypothesis was rejected for two-tailed a value less than 0.025.
Results
Twenty three patients were enrolled in the study (mean age 59.48 ± 6.3; 52 % women).
Baseline demographic and clinical characteristics of the study population were reported in the Table 1. No patient was taking any drug except the study treatment, in particular, although average systolic BP levels were slightly above the normal thresholds, hypertensive patients were at low-to-moderate global cardiovascular risk profile and consequently, according to current ESC/ESH guidelines, they did not require any pharmacological therapies before life-style changes. No significant difference in weight, waist circumference, systolic and diastolic BP were recorded during the study (data not shown). No adverse event was reported.
Table 1.
Baseline characteristics of the study population (each value represents mean ± SD)
| N = 23 | |
|---|---|
| Age (year) | 59.5 ± 6.3 |
| Gender (M/W %) | 48/52 |
| SBP (mmHg) | 142.5 ± 14.1 |
| DBP (mmHg) | 85.5 ± 10.9 |
| Waist circumference (cm) | 95.4 ± 9.4 |
| Total cholesterol (mg/dl) | 246.1 ± 15.1 |
| HDL cholesterol (mg/dl) | 47.4 ± 10.8 |
| LDL cholesterol (mg/dl) | 175.7 ± 13.4 |
| Triglycerides (mg/dl) | 114.9 ± 41.6 |
| Fasting plasma glucose (mg/dl) | 92.0 ± 7.53 |
| BMI (Kg/m2) | 26.8 ± 3.6 |
| HbA1c ( %) | 5.6 ± 0.4 |
The comparison between the percentages of subjects in whom LDL cholesterol was reduced below 130 mg/dl at the end of each of the two active treatment periods shows a statistically significant difference with a larger number of individuals with a normal value of plasma LDL cholesterol with Combination B as compared to Combination A (56.5 vs 21.7 %, χ2 = 0.027) (Fig. 1).
Fig. 1.
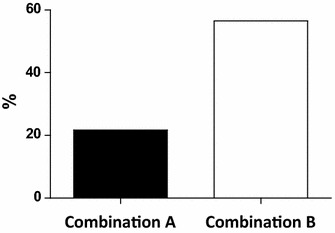
Percentage of subjects with LDL <130 mg/dl at the end of active treatment (N = 23; χ2 = 0.027)
Table 2 shows lipid profile at baseline and after treatment with Combination A and Combination B. Both treatments induce a significant reduction of plasma levels of triglycerides, total and LDL cholesterol and an increase of HDL cholesterol (all p < 0.03). Combination B therapy showed a further advantage in the reduction of total and LDL cholesterol as compared to Combination A (both p < 0.005). For all these parameters we calculated the percentage change obtained by both Combinations vs baseline. Combination B decreased the values of total and LDL cholesterol more than Combination A (p = 0.004 and p = 0.005, respectively). No significant difference was recorded in the percentage changes of triglycerides and HDL cholesterol observed with the two NUT Combinations (Fig. 2).
Table 2.
Lipid profile at the end of the three study periods (each value represents mean ± SD; N = 23)
| Baseline | NUT A | NUT B | P < NUT A vs baseline | P < NUT B vs baseline | P < NUT B vs NUT A | |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | 246.1 ± 15.1 | 213.6 ± 18.7 | 197.9 ± 28.4 | 0.0001 | 0.0001 | 0.008 |
| HDL cholesterol (mg/dl) | 47.4 ± 10.8 | 51.8 ± 13.0 | 52.5 ± 13 | 0.03 | 0.02 | NS |
| LDL cholesterol (mg/dl) | 175.7 ± 13.4 | 142.5 ± 17.1 | 126.8 ± 27.8 | 0.0001 | 0.0001 | 0.01 |
| Triglycerides (mg/dl) | 114.9 ± 41.6 | 96.2 ± 43.9 | 93.3 ± 38.4 | 0.0001 | 0.0001 | NS |
Fig. 2.
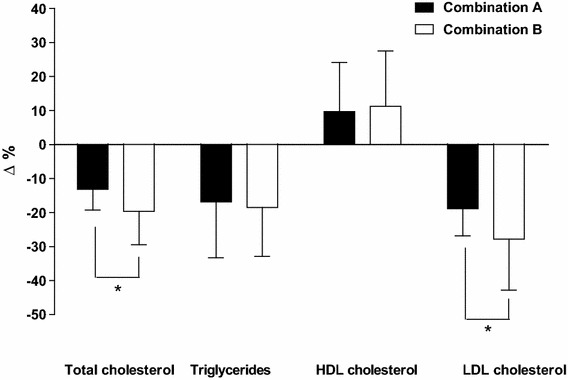
Percentage changes in lipid profile (each bar represent mean ± ST; N = 23; *p < 0.005)
When the parameters of glucose metabolism were examined, there was a significant reduction for serum fasting glucose, insulin and HbA1c only after treatment with the NUT Combination B (Table 3). As a consequence, there was also a significant difference between these values and the corresponding ones recorded at the end of the NUT Combination A treatment (Table 3). None of the patients experienced a clinically evident hypoglycemia.
Table 3.
Glucose metabolismat the end of the three study periods (each value represents mean ± SD; N = 23)
| Baseline | NUT A | NUT B | P < NUT A vs baseline | P < NUT B vs baseline | P < NUT B vs NUT A | |
|---|---|---|---|---|---|---|
| Fasting plasma glucose (mg/dl) | 92.00 ± 7.5 | 93.65 ± 13.7 | 84.35 ± 7.7 | NS | 0.0001 | 0.0001 |
| Insulin (µU/ml) | 10.1 ± 6.8 | 10.2 ± 6.6 | 7.9 ± 5.5 | NS | 0.006 | 0.02 |
| HOMA index | 2.33 ± 1.7 | 2.45 ± 1.7 | 1.66 ± 1.1 | NS | 0.006 | 0.002 |
| HbA1c (mmol/mol) | 38.00 ± 4.2 | 37.85 ± 4.1 | 37.22 ± 4.1 | NS | 0.002 | 0.03 |
We calculated the HOMA index, according to the formula by Matthews [10], to evaluate the effects of two NUT Combinations on insulin resistance. Only Combination B induced an improvement in insulin sensitivity reaching a value which was statistically significantly different from baseline and from NUT Combination A (p = 0.006 vs baseline and p = 0.002 vs Combination A) (Table 3).
No statistically significant difference was detectable among the absolute values of FMD recorded throughout the study (RHI baseline = 2.04 ± 0.8; RHI NUT A = 1.99 ± 0.6; RHI NUT B = 2.02 ± 0.5)
Discussion
This randomized, cross-over, double-blind study confirms that 4 weeks of treatment with a NUT Combination approved in Italy for the control of dyslipidemia (Armolipid Plus®, RottapharmSpA), is able to improve the lipid profile in patients with hypercholesterolemia not requiring statins or statin intolerant [2, 11].
However, the addition of the extract of Morus alba, NUT with an α-glucosidase inhibitory and antioxidant activity, potentiates the cholesterol lowering effect of the NUT combination. In particular, reasonably an account of a more complete action on plasma lipids, this NUT Combination was able to normalize serum LDL concentration in about fifty percent of the study population. This higher percentage of favorable therapeutic responses resulted in a more marked reduction in the mean level of plasma total and LDL cholesterol levels obtained with this NUT Combination as compared to the other active treatment.
The decision to add Morus alba to the NUT combination was mainly based on the observation that this NUT is able to exert an antioxidant and α-glucosidase inhibitory activity, which may stand for, at least in part, the high hypoglycemic capacity of Morus alba extract that accounts for its diabetic prevention activity [6]. However, studies in vitro and in vivo have also reported a favorable effect of this NUT on lipid profile[12], thus it is not surprising that it may be able to potentiate the reduction in total and LDL cholesterol exerted by previous NUT Combinations.
In addition, the results of our study lend further support to the evidence that Morus alba improves glucose metabolism [6] and, consequently, to the possibility that a combination with this NUT, may act on the global cardiovascular risk also trough mechanisms different from a cholesterol lowering effect. Since a continuous relationship between HbA1c and cardiovascular disease as well as all-cause mortality has been reported both in men and women without diabetes, we used HbA1c to assess whether there was an effect of the NUT Combination including Morus alba on glucose metabolism which may improve cardiovascular prognosis. A reduction in HbA1c was observed, which was statistically significant but small in magnitude. However, it is well accepted that Hb1Ac is a reliable index of the behavior of glycaemia in the preceding 3 months [13, 14]. Thus, even a small reduction in Hb1Ac induced by a 4 week treatment may indicate a clinically relevant change in glucose metabolism. The simultaneous reduction in fasting glucose and insulin is consistent with the reduction of HbA1c and may be explained by the improvement of insulin sensitivity observed during treatment with the NUT-Morus alba combination.
The lack of improvement in insulin sensitivity during the treatment with the NUT without Morus alba is in contrast with a previous report [11], in which this effect was documented. However, in that study most patients were overweight or frankly obese (mean BMI 28 ± 3.8 kg/m2) and were studied over a longer follow-up. The absence of data on changes in body weight did not rule out the possibility that the improvement in insulin sensitivity could be due to weight loss. Another difference from the Affuso’s study is that we failed to detect improvement in FMD related to NUTS. On the contrary, in the previous report [11] it was described a statistically significant increase in FMD in patients treated with the NUT combination without Morus alba. Indeed, the analysis of the individual responses of our study population clearly shows a difference in the effect of treatment on FMD between subjects showing normal or impaired FMD at baseline. The percentage change reported by Affuso is similar in magnitude to what we observed with both treatments in subjects with impaired FMD at baseline. Therefore, it seems possible to speculate that in our study we failed to achieve a statistically significant change in FMD on account of a larger heterogeneity of our population.
Although our results do not clarify the mechanisms underlying the favorable effect of NUT Combination including Morus alba on glucose metabolism, the observation of a reduction in fasting plasma glucose concentration seems to rule out the possibility that it may be mediated by the inhibition of α-glucosidase, which interferes with glucose absorption, and to suggest that it may represent a possible consequence of improved insulin sensitivity on hepatic gluconeogenesis. Finally it has to be considered that, though alpha-glucosidase inhibitors act mostly on post-prandial glycaemia, chronic treatment with these drugs has been shown to reduce also fasting blood glucose in clinical trials [15].
Limitations
The results of this study should be interpreted in light of several limitations.
Despite the study results showed a significant improvement of lipid and glycemic profile, the limited number of patients requires confirmation in a larger population
The limited follow up time reduces the possibility to generalize the data observed on HbA1c levels reduction, which requires a longer period of observation to evaluate the variations.
However, these preliminary results are very encouraging and could be considered hypothesis generating for a larger trial.
Finally, since available literature data demonstrate the positive effects of berberine on glycemic control, both in diabetic and dysglicemic patients [16, 17], it is also to consider the possibility that the small sample study population may account for the lack of changes on glucose profile during treatment with NUT Combination A.
References
- 1.European Association for Cardiovascular P, Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 2.Izzo R, de Simone G, Giudice R, Chinali M, Trimarco V, De Luca N, et al. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J Hypertens. 2010;28(7):1482–1487. doi: 10.1097/HJH.0b013e3283395208. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–1228. doi: 10.1016/j.jacc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7(12):e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Xiang L, Wang C, Tang C, He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One. 2013;8(7):e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 8.Gargiulo P, Marciano C, Savarese G, D’Amore C, Paolillo S, Esposito G, et al. Endothelial dysfunction in type 2 diabetic patients with normal coronary arteries: a digital reactive hyperemia study. Int J Cardiol. 2013;165(1):67–71. doi: 10.1016/j.ijcard.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 9.Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921–1986. doi: 10.1002/sim.1783. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Affuso F, Ruvolo A, Micillo F, Sacca L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20(9):656–661. doi: 10.1016/j.numecd.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol. 2010;48(8–9):2374–2379. doi: 10.1016/j.fct.2010.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciano C, Galderisi M, Gargiulo P, Acampa W, D’Amore C, Esposito R, et al. Effects of type 2 diabetes mellitus on coronary microvascular function and myocardial perfusion in patients without obstructive coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39(7):1199–1206. doi: 10.1007/s00259-012-2117-9. [DOI] [PubMed] [Google Scholar]
- 15.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005(2):CD003639. [DOI] [PMC free article] [PubMed]
- 16.Derosa G, Romano D, D’Angelo A, Maffioli P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis. 2015;239(1):87–92. doi: 10.1016/j.atherosclerosis.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Di Pierro F, Putignano P, Villanova N, Montesi L, Moscatiello S, Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharmacol. 2013;5:167–174. doi: 10.2147/CPAA.S54308. [DOI] [PMC free article] [PubMed] [Google Scholar]


