Abstract
Purpose
Men receiving androgen-deprivation therapy (ADT) for prostate cancer may be at risk for cognitive impairment; however, evidence is mixed in the existing literature. Our study examined the impact of ADT on impaired cognitive performance and explored potential demographic and genetic predictors of impaired performance.
Patients and Methods
Patients with prostate cancer were assessed before or within 21 days of starting ADT (n = 58) and 6 and 12 months later. Age- and education-matched patients with prostate cancer treated with prostatectomy only (n = 84) and men without prostate cancer (n = 88) were assessed at similar intervals. Participants provided baseline blood samples for genotyping. Mean-level cognitive performance was compared using mixed models; cognitive impairment was compared using generalized estimating equations.
Results
ADT recipients demonstrated higher rates of impaired cognitive performance over time relative to all controls (P = .01). Groups did not differ at baseline (P > .05); however, ADT recipients were more likely to demonstrate impaired performance within 6 and 12 months (P for both comparisons < .05). Baseline age, cognitive reserve, depressive symptoms, fatigue, and hot flash interference did not moderate the impact of ADT on impaired cognitive performance (P for all comparisons ≥ .09). In exploratory genetic analyses, GNB3 single-nucleotide polymorphism rs1047776 was associated with increased rates of impaired performance over time in the ADT group (P < .001).
Conclusion
Men treated with ADT were more likely to demonstrate impaired cognitive performance within 6 months after starting ADT relative to matched controls and to continue to do so within 12 months after starting ADT. If confirmed, findings may have implications for patient education regarding the risks and benefits of ADT.
INTRODUCTION
A growing body of evidence suggests many patients experience cognitive problems as a consequence of cancer treatment.1 However, important gaps remain in this area. One is the paucity of studies on patients with prostate cancer, the most common cancer in US men.2 Concern rests primarily with the 44% of patients with prostate cancer who undergo androgen-deprivation therapy (ADT).3 In addition to producing adverse effects that can interfere with cognitive functioning (eg, fatigue and depressive symptoms),4,5 ADT may directly affect cognitive functioning, as suggested by research showing lower testosterone levels are associated with worse cognitive functioning in healthy older men.6
The few studies of cognitive functioning among patients with prostate cancer undergoing ADT have yielded mixed results. Although some have found evidence of cognitive problems,7 others have not.8,9 A recent meta-analysis of 14 studies concluded that patients with prostate cancer undergoing ADT performed worse than controls in one of seven cognitive domains for which effect sizes could be calculated (ie, visuomotor ability).10 However, most studies had important methodologic limitations, including use of cross-sectional designs, absence of comparison groups, short follow-up periods, and/or limited consideration of clinical significance.10 In addition, there has been little effort to identify moderators of the impact of ADT on cognitive functioning. Research in patients with cancer receiving chemotherapy has indicated that differences in cognitive functioning among patients with similar treatment histories may be partially explained by certain genetic variants11,12 and extent of cognitive reserve (ie, innate and developed cognitive capacities).13 Research in other populations has suggested that depressive symptoms, fatigue, and hot flashes may contribute additional risk of cognitive impairment.14–17
We sought to advance understanding of the impact of ADT on cognitive functioning by evaluating patients with prostate cancer over a 12-month period after ADT initiation, concurrently evaluating patients with prostate cancer not exposed to ADT and men with no history of cancer of similar age and education level. To address the issue of clinical relevance, analyses were not limited to comparisons of mean-level performance but also focused on the extent to which cognitive performance was impaired based on standard criteria.18,19 It was hypothesized that ADT recipients would show worsening mean-level cognitive performance and higher rates of impaired cognitive performance over time than control participants. In addition, exploratory analyses examined genetic variants, patient age, cognitive reserve, and symptomatology as moderators of the impact of ADT on cognitive functioning. Specifically, we examined whether genetic inheritance, age, cognitive reserve, depressive symptoms, fatigue, or hot flash interference in combination with ADT would confer a so-called double hit, in which ADT recipients with at-risk alleles, older age, less cognitive reserve, or greater symptomatology would demonstrate the greatest decrements in cognitive functioning.
PATIENTS AND METHODS
Participants
Eligibility criteria for all participants were as follows: age ≥ 18 years, ability to speak and read English, educational level ≥ sixth grade, no history of stroke, no demonstrated impaired mental status (Short Portable Mental Status Examination score < 3), and no visual, auditory, or psychiatric conditions that would preclude participation. Additional eligibility criteria for patients with prostate cancer receiving ADT were as follows: diagnosed with nonmetastatic or asymptomatic metastatic prostate cancer, scheduled to start or started ADT in past month and to receive it for ≥ 6 months, no treatment for other cancers in previous 12 months, no history of brain cancer or cranial irradiation, and no ADT treatment in previous 12 months or antiandrogen in previous 6 months. Additional eligibility criteria for patients with prostate cancer not treated with ADT were as follows: diagnosed with nonmetastatic prostate cancer, no history of other cancers except nonmelanoma skin cancer, undergone prostatectomy but no other prostate cancer treatment, no history of recurrence, and not receiving testosterone supplementation. Additional eligibility criteria for men with no cancer were as follows: no history of cancer except nonmelanoma skin cancer and not receiving testosterone supplementation.
Procedure
Data were collected between September 2008 and October 2013. Written informed consent was obtained before initiation of study procedures. Participants were paid $80 at each evaluation. This study was approved by the University of South Florida Institutional Review Board.
Patients were recruited from the Moffitt Cancer Center (patients with prostate cancer receiving and not receiving ADT) and the James A. Haley Veterans' Hospital (patients with prostate cancer not receiving ADT). Control participants without prostate cancer were recruited through use of information obtained from Marketing Systems Group (Fort Washington, PA). Patients with prostate cancer not receiving ADT and participants without prostate cancer were recruited to be matched to patients with prostate cancer receiving ADT on age (within 5 years) and educational level (≤ 12, 13 to 16, or ≥ 17 years); patients with prostate cancer not receiving ADT were also matched to those with prostate cancer receiving ADT on time since prostate cancer diagnosis (within 6 months). Baseline assessments were completed by patients with prostate cancer receiving ADT before or within 21 days of starting ADT and 6 and 12 months later. Those with prostate cancer not receiving ADT and participants without prostate cancer were assessed at similar time intervals. Appendix Figures A1 to A3 (online only) provide information about participant flow. The larger numbers of patients with prostate cancer not receiving ADT and participants without prostate cancer reflect that, for participants receiving ADT whose control participant not receiving ADT or control participant without prostate cancer withdrew, an additional matched control was recruited.
Measures
Demographic and clinical information.
Self-reported demographics and medical comorbidities20 were assessed at baseline. Time since diagnosis and Gleason scores were assessed via medical record review.
Cognitive performance.
Neuropsychological testing was conducted by clinical psychology graduate students trained and supervised by experienced clinical psychologists (H.S.L.J., M.B.-J., P.B.J.). Tests with established reliability and validity in older individuals were used (Table 1), including those recommended by the International Cognition and Cancer Task Force (ICCTF).18 All tests have previously been used in studies with patients with prostate cancer receiving ADT.10 Estimated full-scale intelligence quotient was assessed at baseline as a measure of cognitive reserve.
Table 1.
Summary of Neuropsychological Test Battery
| Domain | Test |
|---|---|
| Verbal memory | HVLT-R Total Recall21 |
| HVLT-R Delayed Recall21 | |
| WMS-III Logical Memory II22 | |
| Visual memory | BVMT-R Total Recall23 |
| BVMT-R Delayed Recall23 | |
| Attention | Color Trails 124 |
| WMS-III Digit Span22 | |
| WMS-III Spatial Span22 | |
| SDMT Items Completed25 | |
| Executive function | Color Trails 224 |
| COWA26 | |
| TIADL27 | |
| Cognitive reserve | NART Full-Scale IQ28 |
Abbreviations: BVMT-R, Brief Visuospatial Memory Test–Revised; COWA, Controlled Oral Word Association Test; HVLT-R, Hopkins Verbal Learning Test–Revised; IQ, intelligence quotient; NART, National Adult Reading Test; SDMT, Symbol Digit Modalities Test; TIADL, Timed Instrumental Activities of Daily Living Test; WMS-III, Wechsler Memory Scale–III.
Self-reported measures.
At baseline, all participants completed the Center for Epidemiologic Studies Depression Scale,29 the Fatigue Symptom Inventory,30 and the Hot Flash–Related Daily Interference Scale.31 These measures have been used previously in prostate cancer research.32–36
Single-Nucleotide Polymorphism Selection
Single-nucleotide polymorphisms (SNPs) were selected based on evidence of association with cognitive impairment, depression, fatigue, or circadian rhythm in clinical or nonclinical populations, with preference given to: location in coding regions or known transcription factor binding sites, nonsynonymous polymorphisms, and minor allele frequency (MAF) of ≥ .20 in the HapMap population of Utah residents of northern and western European ancestry.37 A total of 494 SNPs were initially identified and 384 retained after an iterative custom panel design process.
Genotyping
Genomic DNA was extracted from blood obtained using Gentra Puregene tissue kits (Qiagen, Valencia, CA). DNA samples were genotyped using the Illumina GoldenGate assay (Illumina, San Diego, CA) and genotyped using the BeadStudio algorithm by the Moffitt Molecular Genomics Core.
Statistical Analyses
Analyses were restricted to the 58 patients with prostate cancer receiving ADT, 84 patients with prostate cancer not receiving ADT, and 88 participants without prostate cancer who completed the baseline assessment and at least one follow-up assessment. Those ineligible based on this rule (n = 45) did not differ from eligible participants on demographic or clinical variables within any group (P for all comparisons ≥ .10). Fisher's exact and t tests were conducted to identify group differences on demographic and clinical factors. Those significant at P < .10 were included as covariates in all multivariable analyses. To reduce the number of analyses performed and type I error rate, omnibus tests were performed on mean-level and impaired cognitive performance before proceeding to additional analyses.
Mean-level cognitive performance comprised the average of each participant's t scores (derived using published norms) on all cognitive tests. Changes in scores over time and their interaction with group membership were examined using mixed models with SAS PROC MIXED software (version 9.4; SAS Institute, Cary, NC), allowing for use of all available data at each assessment without imputing missing data.38
In accordance with ICCTF guidelines,18 impaired cognitive performance was defined as scoring ≥ 1.5 standard deviations (SDs) below published norms on ≥ two tests or scoring ≥ 2.0 SDs below published norms on ≥ one test. In addition, a second, more stringent39 criterion that is commonly used40 in the cancer and cognition literature was applied: scoring ≥ 2.0 SDs below published norms on ≥ one test. Change in impaired performance over time was evaluated with generalized estimating equation analyses using SAS PROC GENMOD (version 9.4; SAS Institute), which also allowed for use of all available data at each assessment without imputing missing data.41 Fully adjusted odds ratios (ORs) compared odds of impairment between groups at each assessment. Consistent with previous research on ADT recipients,9 logistic regression analyses were used to compare rates of impaired cognitive performance between groups on each test at 12 months. Per ICCTF guidelines,18 the percentage of participants who demonstrated impaired cognitive performance on zero, one, or ≥ two tests was calculated. The number of tests with impaired performance was compared between groups using logistic regression analyses. Because the ICCTF-recommended criterion for cognitive impairment is for overall impairment, logistic regression analyses used the test impairment criterion of scoring ≥ 2.0 SDs below norms. A two-sided α level of 0.05 was set for statistical significance.
Logistic regression analyses using JMP Genomics (SAS Institute) evaluated genetic predictors of change in impaired cognitive performance using additive and dominant models. Impaired cognitive performance at 12 months was regressed on baseline impaired performance, comorbidities, education, group status, genotype, and group-by-genotype interaction. Analyses were restricted to SNPs in Hardy-Weinberg equilibrium, demonstrating a MAF of ≥ 1% and missing genotype data in < 20% in the current sample. A positive false discovery rate was used to control for multiple comparisons; SNPs with q < .05 were considered statistically significant.42 Because ancestry informative markers were not measured, and few participants self-identified as nonwhite, genetic analyses were limited to the 214 participants who self-identified as white to reduce extraneous variance resulting from race.
RESULTS
Preliminary Analyses
Table 2 lists demographic and clinical characteristics. The group of patients with prostate cancer receiving ADT reported more comorbidities and had higher Gleason scores than the group with prostate cancer not receiving ADT and was less likely to be white or better educated than the groups of patients with prostate cancer not receiving ADT and participants without prostate cancer (P for all comparisons ≤ .04). Therefore, comorbidities, race, and years of education were included as covariates in subsequent analyses; Gleason scores were not, because ADT treatment is often prescribed for more advanced disease. At the 12-month assessment, 76% of the ADT group was still receiving ADT.
Table 2.
Demographic and Clinical Characteristics of Sample (N = 231)
| Characteristic | ADT+ (n = 58) |
ADT− (n = 84) |
CA− (n = 88) |
P* |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ADT+ Versus ADT− | ADT+ Versus CA− | ADT+ Versus All Controls | |
| Age, years | .76 | .21 | .36 | ||||||
| Mean | 67.31 | 67.72 | 69.10 | ||||||
| SD | 8.87 | 7.37 | 8.00 | ||||||
| Time since diagnosis, years | .34 | — | — | ||||||
| Mean | 3.68 | 4.45 | — | ||||||
| SD | 5.03 | 4.68 | — | ||||||
| Comorbidity index score | .003 | .32 | .03 | ||||||
| Mean | 2.90 | 2.41 | 2.72 | ||||||
| SD | 1.05 | 0.85 | 1.09 | ||||||
| Education, years | .03 | .03 | .01 | ||||||
| ≤ 12 | 23 | 40 | 17 | 20 | 18 | 21 | |||
| 13 to 16 | 29 | 50 | 50 | 60 | 54 | 61 | |||
| ≥ 17 | 6 | 10 | 17 | 20 | 16 | 18 | |||
| Race | .02 | .04 | .004 | ||||||
| White | 49 | 84 | 81 | 96 | 84 | 95 | |||
| Nonwhite | 9 | 16 | 3 | 4 | 4 | 5 | |||
| Ethnicity | .65 | .65 | .38 | ||||||
| Hispanic | 1 | 2 | 3 | 4 | 4 | 5 | |||
| Non-Hispanic | 57 | 98 | 81 | 96 | 84 | 95 | |||
| Gleason score | < .001 | — | — | ||||||
| 4 to 6 | 9 | 16 | 38 | 45 | — | ||||
| 7 | 22 | 38 | 39 | 46 | — | ||||
| 8 | 18 | 31 | 2 | 2 | — | ||||
| 9 to 10 | 6 | 10 | 0 | 0 | — | ||||
| Missing | 3 | 5 | 5 | 6 | — | ||||
Abbreviations: ADT+, patients with prostate cancer receiving androgen-deprivation therapy; ADT−, patients with prostate cancer not receiving androgen-deprivation therapy; CA−, participants without prostate cancer; SD, standard deviation.
P values calculated using Fisher's exact tests for categorical variables and t tests for continuous variables. Missing levels were excluded from calculation of P values.
Because neither mean-level cognitive performance nor impaired cognitive performance differed between the group of patients with prostate cancer not receiving ADT and the group of participants without prostate cancer at any assessment (P for all comparisons ≥ .41; Appendix Table A1, online only), these groups were combined into a single control group, thus improving statistical power and reducing the number of analyses performed.
Differences in Cognitive Performance
Change over time in mean-level cognitive performance did not differ between the ADT group and combined control group (P = .71). Group differences were observed in change in impaired cognitive performance over time using both criteria for cognitive impairment (P for both comparisons ≤ .05; Fig 1). Using the ICCTF-recommended criterion, rates of impaired performance did not differ between groups at baseline (OR, 1.21; 95% CI, 0.66 to 2.22); however, the ADT group was more likely to demonstrate impaired performance than the control group within 6 (OR, 1.71; 95% CI, 1.01 to 2.89) and 12 months (OR, 2.42; 95% CI, 1.27 to 4.61). Similarly, with the more stringent impairment criterion, rates of impaired performance did not differ between groups at baseline (OR, 1.00; 95% CI, 0.53 to 1.89); however, the ADT group was more likely to demonstrate impaired performance than the control group within 6 (OR, 1.72; 95% CI, 1.03 to 2.87) and 12 months (OR, 2.97; 95% CI, 1.54 to 5.72). Using both impairment criteria, impaired performance decreased over time in the 12-month period in the control group (P for both analyses ≤ .05) but did not change in the ADT group (P for both analyses ≥ .22). This pattern of results remained when the four participants receiving ADT who completed the baseline assessment shortly after ADT initiation and the 13 participants initially receiving ADT but no longer receiving ADT at 12 months were excluded.
Fig 1.
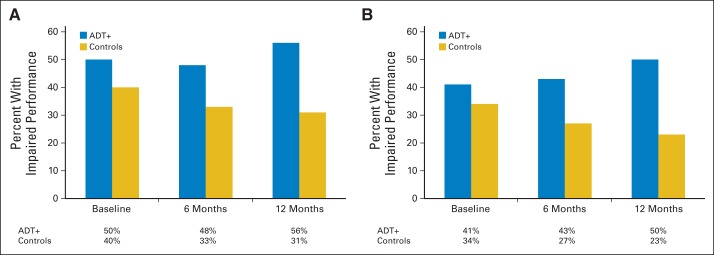
Observed rates of cognitive impairment in group of patients with prostate cancer receiving androgen-deprivation therapy (ADT+) and control group. Criteria for impaired cognitive performance: (A) scoring ≥ 1.5 standard deviations (SDs) below published norms on ≥ two tests or 2.0 SDs below published norms on ≥ one test (group differences in change over time P = .05); (B) scoring ≥ 2.0 SDs below published norms on ≥ one test (group differences in change over time P = .01).
Analyses examining the number of impaired tests indicated no group differences at baseline or within 6 months (P for both comparisons ≥ .15); however, the ADT group was more likely to be impaired on ≥ two tests within 12 months (P = .001; Fig 2). Rates of impaired performance by test are listed in Table 3. The ADT group was more likely to demonstrate impaired performance on Color Trails 2 (P = .05).
Fig 2.
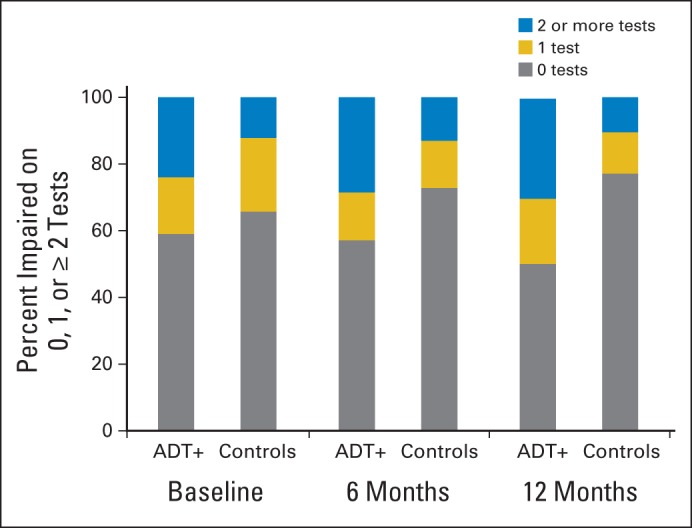
No. of tests on which participants demonstrated performance ≥ 2.0 standard deviations below published norms at each assessment. ADT+, patients with prostate cancer receiving androgen-deprivation therapy.
Table 3.
Unadjusted Rates of Impaired Cognitive Performance on Each Test at 12 Months
| Cognitive Test | Percentage With Impaired Performance* |
P† | |
|---|---|---|---|
| ADT+ | Controls | ||
| Verbal memory | |||
| HVLT-R Total Recall | 14 | 5 | .33 |
| HVLT-R Delayed Recall | 26 | 11 | .10 |
| WMS-III Logical Memory II | 4 | 6 | .27 |
| Visual memory | |||
| BVMT-R Total Recall | 14 | 5 | .25 |
| BVMT-R Delayed Recall | 10 | 6 | .92 |
| Attention | |||
| Color Trails 1 | 16 | 5 | .08 |
| WMS-III Digit Span | 2 | 1 | .85 |
| WMS-III Spatial Span | 2 | 1 | .93 |
| SDMT | 0 | 0 | — |
| Executive function | |||
| Color Trails 2 | 16 | 4 | .05 |
| COWA | 14 | 5 | .17 |
| TIADL | 2 | 1 | .83 |
Abbreviations: ADT+, patients with prostate cancer receiving androgen-deprivation therapy; BVMT-R, Brief Visuospatial Memory Test–Revised; COWA, Controlled Oral Word Association Test; HVLT-R, Hopkins Verbal Learning Test–Revised; SDMT, Symbol Digit Modalities Test; TIADL, Timed Instrumental Activities of Daily Living Test; WMS-III, Wechsler Memory Scale–III.
≥ 2.0 standard deivations below norms on ≥ one test.
P values calculated using logistic regression analyses controlling for medical comorbidities, race, and years of education.
Predictors of Change in Impaired Cognitive Performance
Of 384 SNPs measured, 31 were excluded based on the quality-control parameters described. Using the ICCTF-recommended criterion, 15 SNPs were associated with change in impaired cognitive performance using the additive model, and 12 were associated using the dominant model (P for both comparisons < .05; Data Supplement). Using the more stringent criterion, 25 SNPs were associated with change in impaired cognitive performance using the additive model, and 33 were associated using the dominant model (P for both comparisons < .05; Data Supplement). One variant (rs1047776 in GNB3) also met the cutoff of q < .05 using this criterion. Whereas the rate of impaired cognitive performance decreased over time in the control group and in patients with prostate cancer receiving ADT with ≥ one A allele (AA or AG), it more than doubled over time in the 44% of patients with prostate cancer receiving ADT with wild type (GG; Fig 3). Patients receiving ADT with wild type demonstrated higher odds of impaired cognitive performance within 12 months than patients receiving ADT with ≥ one A allele (OR, 14.00; 95% CI, 2.97 to 66.09). Logistic regression analyses indicated that age, baseline cognitive reserve, depression, fatigue, and hot flash interference did not moderate the impact of ADT on change in impaired cognitive performance using either impairment criterion (P for all comparisons ≥ .09).
Fig 3.
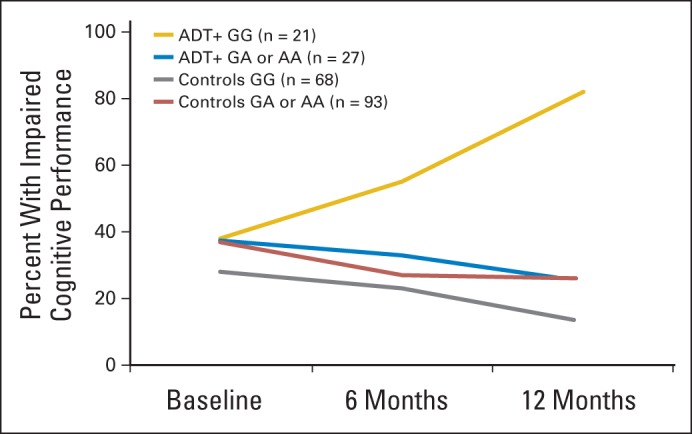
Observed rates of scoring ≥ 2.0 standard deviations below norms on ≥ one test in group of patients with prostate cancer receiving androgen-deprivation therapy (ADT+) and control group by rs1047776 genotype.
DISCUSSION
Although there were no differences between groups in changes in mean-level performance, ADT-treated patients were more likely to exhibit impaired cognitive performance than a control group of prostatectomy-treated patients with prostate cancer and men with no history of prostate cancer. This was true using either criterion for impaired cognitive performance. The odds of impaired performance in ADT recipients were approximately 70% higher than in controls within 6 months and > twice that of controls within 12 months. ADT recipients were also more likely to show impaired performance on multiple tests within 12 months and perform at an impaired level on a test of executive function.
These findings stand in contrast to those from a previous study, in which cognitive functioning was also assessed over a 12-month period in ADT recipients and in prostate cancer and healthy control groups.9 In that study, the authors found no evidence of higher rates of impaired performance among ADT-treated patients and no differences in rates of impairment on specific tests.9 Differences in criteria used to define impairment may explain the divergent findings. The previous study defined impairment as a decline of ≥ one SD below baseline level on ≥ one test, whereas in our study, it was defined using two sets of criteria, more in line with existing guidelines,18 allowing for direct comparison with other studies in the cancer literature.18,40,43–45 Interestingly, both studies found evidence suggesting that practice effects (ie, improvement over time as function of repeated exposure to same tests) were limited primarily to control groups. These practice effects underscore the importance of including control groups exposed to the same neuropsychological tests and suggest that a key feature of ADT-related cognitive changes may be inability to learn based on prior experience rather than sudden and obvious decline in current ability. Both studies generally failed to find differences in rates of impairment on specific tests within 12 months. In our study, ADT recipients demonstrated impaired performance on one test of executive function but not on two others. Taken together, the findings suggest there is no domain-specific pattern of cognitive impairment associated with ADT. Accordingly, clinicians may find that ADT-treated patients complaining of cognitive change report a variety of problems.
This is the first study to our knowledge to examine whether genetic polymorphisms moderate the impact of ADT on cognitive function. Previous research in patients with cancer has suggested that polymorphisms in certain genes (eg, APOE and COMT) confer increased risk for cancer-related cognitive changes.11,12,46,47 Although variants in these genes were included in our study, they were not associated with cognitive function. In contrast, rs1047776 in GNB3 was found to be associated with cognitive decline among patients but not controls. GNB3, involved in modulating transmembrane signaling pathways, was examined based on research suggesting associations with cognitive function and several patient-reported outcomes in noncancer populations.48,49 Most previous research has focused on rs5443 (C825T), which is in high linkage disequilibrium with rs1047776 (D′ = .92),50 reporting that it is associated with cognitive function, sleep, depression, hypertension, and obesity.51–54 To our knowledge, only one other study has reported on rs1047776, finding that the A (v G) allele was associated with worse sleep in older adults.53
Our study has several limitations. It was observational in design and recruited a relatively small sample size. Larger observational studies and randomized trials need to be conducted to more definitively evaluate the impact of ADT on cognitive function. Several patients completed the baseline assessment shortly after ADT initiation, and some were no longer receiving ADT at 12 months. However, excluding these patients did not change the pattern of significant findings. Some cognitive domains assessed in previous studies, such as working memory, were not assessed in our study. No corrections were made for multiple statistical comparisons in the primary analyses; however, an omnibus approach to evaluating cognitive changes was used to conserve the number of comparisons performed. The sample possessed limited racial and ethnic diversity and was composed primarily of college-educated individuals. Controls with history of prostate cancer were limited to patients treated with prostatectomy; whether the same pattern of differences would be evident using a control group of patients treated with radiotherapy instead is unknown. Genetic analyses were limited to those who self-identified as white, because ancestry-informative markers were not measured, thereby limiting the generalizability of the genetic findings. Future studies should aim to validate and replicate the genetic findings. Lastly, because assessments were only conducted at 6 and 12 months after starting ADT, this study could not determine whether group differences emerged before the 6-month assessment. Nevertheless, this study is among the largest controlled prospective, longitudinal investigations of cognitive functioning in ADT-treated patients with prostate cancer and the first to our knowledge to report on the potential moderating role of genetic polymorphisms on cognitive functioning in this patient population.
In conclusion, these findings serve to raise awareness that some patients with prostate cancer receiving ADT may experience changes in cognitive function within the first 6 months after starting ADT that are likely to persist through 12 months. These findings may have implications for discussions of risks and benefits of ADT. Clinicians may also consider inquiring about changes in cognitive functioning that may have occurred after starting ADT and refer patients for assessment and treatment as needed.
Supplementary Material
Glossary Terms
- allele:
an alternative form of a gene (in diploids, one member of a pair) that is located at a specific position on a specific chromosome.
- androgen deprivation therapy (ADT):
treatment that suppresses or blocks the production or action of male hormones.
- genotype:
the specific genetic makeup of a given individual. Although genotypes give rise to the phenotype of an individual, genotypes and phenotypes are not always correlative. For example, some genotypes are expressed only under specific environmental conditions.
- single nucleotide polymorphism (SNP):
natural variations in the genomic DNA sequence present in greater than 1% of the population, with single nucleotide polymorphisms representing DNA variations in a single nucleotide. Single nucleotide polymorphisms are being widely used to better understand disease processes, thereby paving the way for genetic-based diagnostics and therapeutics.
Appendix
Table A1.
Summary of Neuropsychological Test Battery
| Group | Impairment Criterion (%) |
|
|---|---|---|
| ≥ 1.5 SDs on ≥ Two Tests or ≥ 2.0 SDs on ≥ One Test | ≥ 2.0 SDs on ≥ One Test | |
| ADT+ | ||
| Baseline | 50 | 41 |
| 6 months | 48 | 43 |
| 12 months | 56 | 50 |
| ADT− | ||
| Baseline | 38 | 31 |
| 6 months | 37 | 30 |
| 12 months | 32 | 23 |
| CA− | ||
| Baseline | 41 | 37 |
| 6 months | 28 | 25 |
| 12 months | 31 | 23 |
Abbreviations: ADT+, patients with prostate cancer receiving androgen-deprivation therapy; ADT−, patients with prostate cancer not receiving androgen-deprivation therapy; CA−, participants without prostate cancer; SD, standard deviation.
Fig A1.
Participant flow for patients with prostate cancer receiving androgen-deprivation therapy (ADT+).
Fig A2.
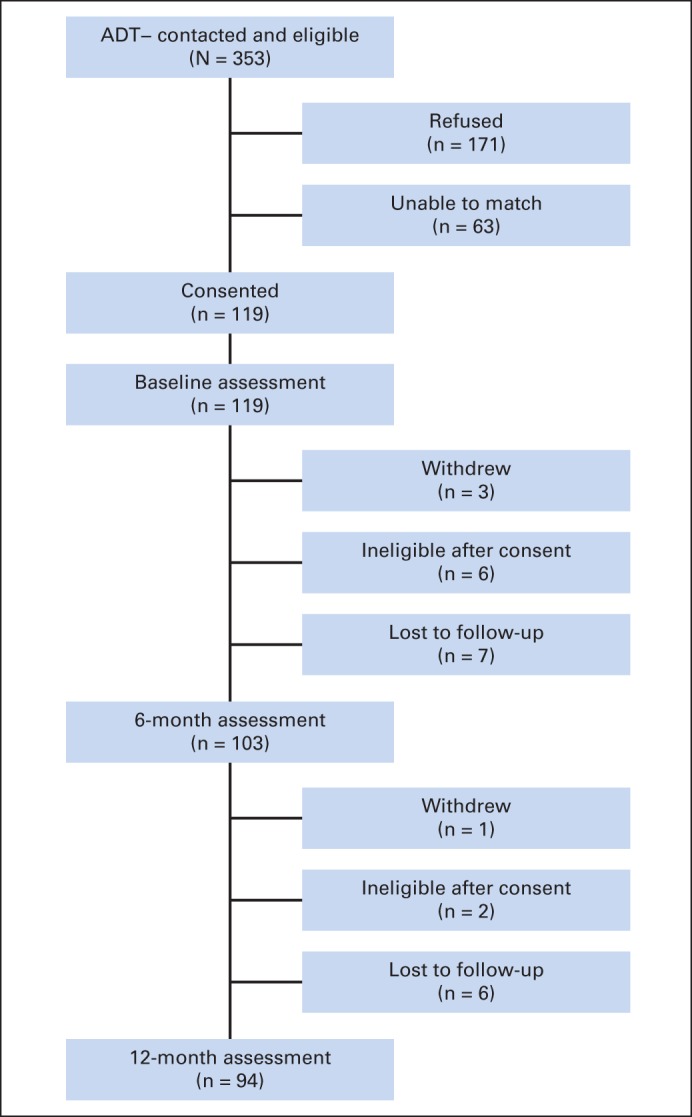
Participant flow for patients with prostate cancer not receiving androgen-deprivation therapy (ADT−).
Fig A3.
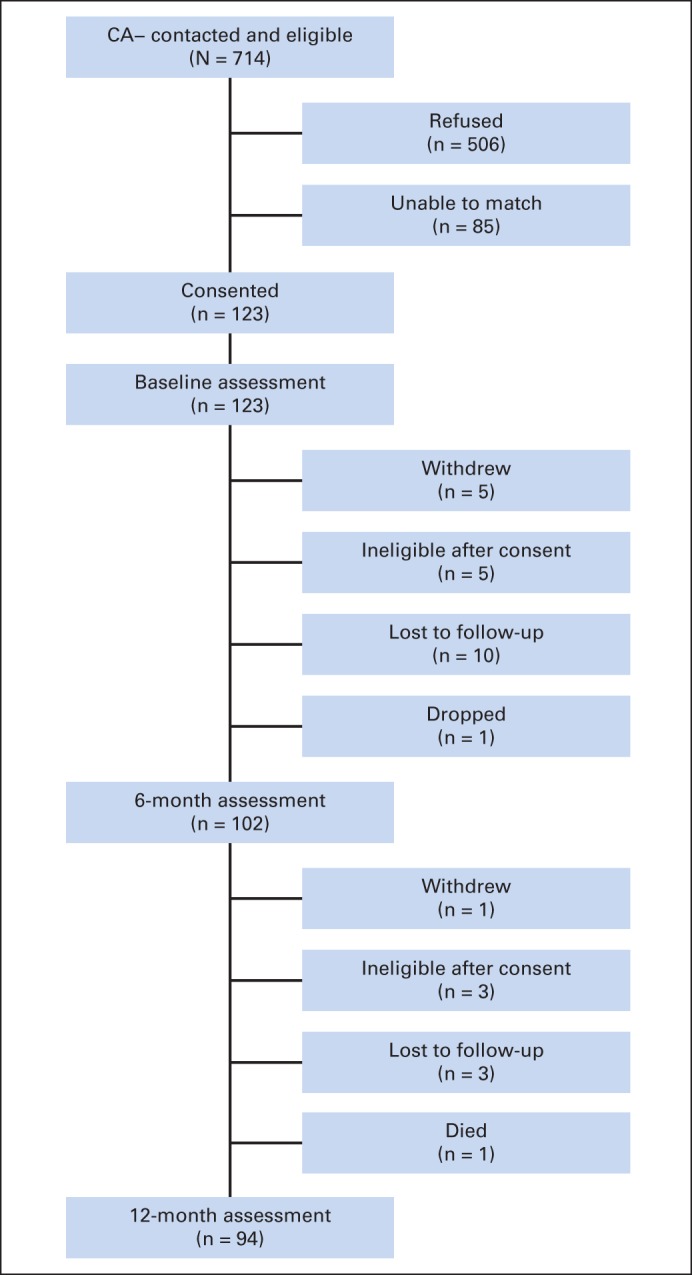
Participant flow for men without prostate cancer (CA−).
Footnotes
Listen to the podcast by Dr Slovin at www.jco.org/podcasts
Supported by Grants No. R01-CA132803 and R25-CA090314 from the National Cancer Institute (P.B.J.) and in part by the Biostatistics Core and Molecular Genomics Core at the Moffitt Cancer Center (Grant No. P30-CA076292 from the National Cancer Institute).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Brian D. Gonzalez, Heather S.L. Jim, Margaret Booth-Jones, Brent J. Small, Jong Y. Park, Paul B. Jacobsen
Provision of study materials or patients: Philippe E. Spiess, Mayer N. Fishman
Collection and assembly of data: Brian D. Gonzalez, Heather S.L. Jim, Margaret Booth-Jones, Jong Y. Park, Paul B. Jacobsen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Course and Predictors of Cognitive Function in Patients With Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Brian D. Gonzalez
No relationship to disclose
Heather S.L. Jim
No relationship to disclose
Margaret Booth-Jones
No relationship to disclose
Brent J. Small
No relationship to disclose
Steven K. Sutton
No relationship to disclose
Hui-Yi Lin
No relationship to disclose
Jong Y. Park
No relationship to disclose
Philippe E. Spiess
Consulting or Advisory Role: Medivation
Mayer N. Fishman
No relationship to disclose
Paul B. Jacobsen
Consulting or Advisory Role: Onyx Pharmaceuticals, Philips Healthcare
Research Funding: Pfizer, On Q Health
Travel, Accommodations, Expenses: Onyx Pharmaceuticals
REFERENCES
- 1.Ahles TA, Root JC, Ryan EL. Cancer-and cancer treatment–associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2011;6:647–653. doi: 10.1016/j.urolonc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Psychooncology. doi: 10.1002/pon.3608. [epub ahead of print on June 13, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23:1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 6.Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: A review. Maturitas. 2011;69:322–337. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Jim HS, Small BJ, Patterson S, et al. Cognitive impairment in men treated with luteinizing hormone-releasing hormone agonists for prostate cancer: A controlled comparison. Support Care Cancer. 2010;18:21–27. doi: 10.1007/s00520-009-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joly F, Alibhai S, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176:2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 9.Alibhai SMH, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5030–5037. doi: 10.1200/JCO.2010.30.8742. [DOI] [PubMed] [Google Scholar]
- 10.McGinty HL, Phillips KM, Jim HS, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: A systematic review and meta-analysis. Support Care Cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 12.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–856. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012;2:231–238. doi: 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 19.Lezak M. Neuropsychological Assessment (ed 3) New York, NY: Oxford University Press; 1995. [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Benedict RH, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale (WMS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 23.Benedict RH. Brief Visuospatial Memory Test–Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 24.D'Elia L. Color Trails Test: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 25.Smith A. Symbol Digit Modalities Test: Manual. Torrance, CA: Western Psychological Corporation; 2002. [Google Scholar]
- 26.Spreen O, Straus E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (ed 2) New York, NY: Oxford University Press; 1998. [Google Scholar]
- 27.Owsley C, Sloane M, McGwin G, Jr, et al. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- 28.Nelson HE, Willison J. National Adult Reading Test (NART) Windsor, United Kingdom: Nfer-Nelson; 1991. [Google Scholar]
- 29.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter JS. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 32.Jim HS, Park JY, Permuth-Wey J, et al. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: Preliminary findings. Brain Behav Immun. 2012;26:1030–1036. doi: 10.1016/j.bbi.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korfage I, Essink-Bot M, Janssens A, et al. Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow-up. Br J Cancer. 2006;94:1093–1098. doi: 10.1038/sj.bjc.6603057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beer TM, Benavides M, Emmons SL, et al. Acupuncture for hot flashes in patients with prostate cancer. Urology. 2010;76:1182–1188. doi: 10.1016/j.urology.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulloa EW, Salup R, Patterson SG, et al. Relationship between hot flashes and distress in men receiving androgen deprivation therapy for prostate cancer. Psychooncology. 2009;18:598–605. doi: 10.1002/pon.1427. [DOI] [PubMed] [Google Scholar]
- 37.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 38.Newell A, Rosenbloom PS. Anderson JR. Cognitive Skills and Their Acquisition. Hillsdale, NJ: Lawrence Earlbaum Associates; 1981. Mechanisms of skill acquisition and the law of practice; pp. 1–55. [Google Scholar]
- 39.Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog–methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006;95:125–129. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- 40.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 41.Diggle P, Heagerty P, Liang KY, et al. Analysis of Longitudinal Data (ed 2) New York, NY: Oxford University Press; 2002. [Google Scholar]
- 42.Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol. 2002;64:479–498. [Google Scholar]
- 43.Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 44.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 45.Wefel JS, Vidrine DJ, Veramonti TL, et al. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2011;117:190–196. doi: 10.1002/cncr.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correa DD, Satagopan J, Baser RE, et al. APOE polymorphisms and cognitive functions in patients with brain tumors. Neurology. 2014;83:320–327. doi: 10.1212/WNL.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koleck TA, Bender CM, Sereika SM, et al. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum. 2014;41:E313–E325. doi: 10.1188/14.ONF.E313-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruano D, Abecasis GR, Glaser B, et al. Functional gene group analysis reveals a role of synaptic heterotrimeric G proteins in cognitive ability. Am J Hum Genet. 2010;86:113–125. doi: 10.1016/j.ajhg.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bullido MJ, Ramos MC, Ruiz-Gómez A, et al. Polymorphism in genes involved in adrenergic signaling associated with Alzheimer's. Neurobiol Aging. 2004;25:853–859. doi: 10.1016/j.neurobiolaging.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Johnson AD, Handsaker RE, Pulit SL, et al. SNAP: A Web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casiglia E, Giordano N, Tikhonoff V, et al. Cognitive functions across the GNB3 C825T polymorphism in an elderly Italian population. Neurol Res Int. 2013;2013:597034. doi: 10.1155/2013/597034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen PS, Yeh TL, Lee IH, et al. Effects of C825T polymorphism of the GNB3 gene on availability of dopamine transporter in healthy volunteers: A SPECT study. Neuroimage. 2011;56:1526–1530. doi: 10.1016/j.neuroimage.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 53.Evans DS, Parimi N, Nievergelt CM, et al. Common genetic variants in ARNTL and NPAS2 and at chromosome 12p13 are associated with objectively measured sleep traits in the elderly. Sleep. 2013;36:431–446. doi: 10.5665/sleep.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-León S, Janssens A, González-Zuloeta Ladd A, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2007;13:772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


