Abstract
Purpose
Although previous studies have implicated a variety of hormone-related risk factors in the etiology of male breast cancers, no previous studies have examined the effects of endogenous hormones.
Patients and Methods
Within the Male Breast Cancer Pooling Project, an international consortium comprising 21 case-control and cohort investigations, a subset of seven prospective cohort studies were able to contribute prediagnostic serum or plasma samples for hormone quantitation. Using a nested case-control design, multivariable unconditional logistic regression analyses estimated odds ratios and 95% CIs for associations between male breast cancer risk and 11 individual estrogens and androgens, as well as selected ratios of these analytes.
Results
Data from 101 cases and 217 matched controls were analyzed. After adjustment for age and date of blood draw, race, and body mass index, androgens were found to be largely unrelated to risk, but circulating estradiol levels showed a significant association. Men in the highest quartile had an odds ratio of 2.47 (95% CI, 1.10 to 5.58) compared with those in the lowest quartile (trend P = .06). Assessment of estradiol as a ratio to various individual androgens or sum of androgens showed no further enhancement of risk. These relations were not significantly modified by either age or body mass index, although estradiol was slightly more strongly related to breast cancers occurring among younger (age < 67 years) than older men.
Conclusion
Our results support the notion of an important role for estradiol in the etiology of male breast cancers, similar to female breast cancers.
INTRODUCTION
Male breast cancer is a rare condition, comprising only approximately 1% of all breast malignancies.1 Given its rarity, it has been difficult to study, and its etiology remains elusive. Genetic risk factors, including relations with familial history and BRCA gene mutations,2 are well established, but other environmental risk factors are less clear.
Female breast cancer is well recognized as being influenced by hormonal factors.3 It seems the same is true for male breast cancer, given that studies have identified high risks related to obesity,4–10 physical inactivity,4,9,10 exogenous hormone use,11–14 and diabetes.7,15 Investigations have also reported high risks among patients with Klinefelter syndrome (condition characterized by 46-XXY karyotype and relative excesses of estrogens in relation to androgens)16–18 as well as gynecomastia (enlargement of male mammary glands often associated with hormonal perturbations).8 Collectively, these findings emphasize the need for assessing the roles of endogenous hormones in relation to male breast cancers. High levels of both estrogens and androgens have been implicated in female breast cancer,19,20 but studies have not yet been conducted to assess their roles in the etiology of male breast cancer.
We recently reported results regarding hormone-related risk factors from the Male Breast Cancer Pooling Project, a consortium of 21 case-control and cohort investigations.5 From seven of the contributing cohort studies, we were able to access prediagnostic serum or plasma samples, from which hormones could be measured. We report herein the results of this analysis, in which we were able to assess male breast cancer risk in relation to various estrogens and androgens and their ratios.
PATIENTS AND METHODS
Study Population
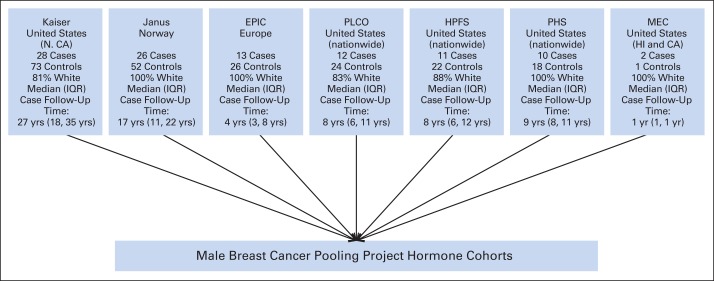
Male breast cancer cases and matched controls were derived from seven cohorts (Fig 1) that had been part of the Male Breast Cancer Pooling Project and could contribute prediagnostic serum or plasma samples.21–27 These studies contributed deidentified data and biologic materials after institutional review board and data-sharing agreement approvals. Breast cancer cases were required to be incident (ie, diagnosed after exposure assessment) but did not have to be the first diagnosed cancer. Risk factor information was available primarily from completed questionnaires, although in one study,23 such data were obtained via linkage with population registries.
Fig 1.
Cohort studies contributing biologic samples for endogenous hormone assays in Male Breast Cancer Pooling Project. EPIC, European Prospective Investigation Into Cancer and Nutrition; HPFS, Health Professionals Follow-Up Study; IQR, interquartile range; MEC, Multiethnic Cohort Study of Diet and Cancer; N. CA, northern California; PHS, Physicians' Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Screening Trial.
We asked each study to provide 40 controls per case matched on sex, race, year of birth (± 1 year), year of cohort entry (± 1 year), and exit date (diagnosis of cancer [excluding nonmelanoma skin cancer], death, loss to follow-up, or end of follow-up ≥ date of diagnosis of index case).5 If the index case had ≥ 0.7 mL serum/plasma available for hormone quantitation, we requested that two of the 40 controls be selected using the following additional criteria: ≥ 0.7 mL serum/plasma available, year of blood draw (± 1 year), and number of freeze/thaw cycles. We were unable to identify a complete set of controls for all matched sets, and one study21 attempted to match three controls per case; thus, in total, there were 101 breast cancer cases and 217 controls.
Laboratory Analysis
In collaboration with the Pharmacogenomics Laboratory of Laval University, Québec City, Québec, Canada, we quantitatively assessed the following unconjugated sex steroid hormones by gas chromatography–mass spectrometry: dehydroepiandrosterone, androstenediol, androstenedione, testosterone, dihydrotestosterone (DHT), androsterone, estrone (E1), and estradiol (E2). Using liquid chromatography–tandem mass spectrometry, we measured glucuronide derivatives of androgens, namely 3-androstanediol-3 glucuronide (3α-diol-3G), 3-androstanediol-17 glucuronide (3α-diol-17G), and androsterone glucuronide (ADT-G). No sulfates are reported. These hormones cover a wide array and key positions of the sex steroid biosynthesis pathway, including both androgens and estrogens (Fig 2). Cases, their matched controls, and blinded quality control (QC) samples from each cohort were randomly assigned throughout the batches with matched sets assayed in the same batch. At the time of random assignment, an additional four blinded QC samples from the same two individuals were added to each batch. Results from these QC samples were used to assess the assays across the entire study. Except for 3α-diol-17G, overall coefficients of variance (CVs) ranged from 2.5% to 12.3%; for 3α-diol-17G, the overall CV was 43.5% because of one outlier observation. With this removed, the CV for 3α-diol-17G was 5.0%.
Fig 2.
Schematic of sex steroid hormone metabolism. Sex steroid hormones that were quantitated are underlined. (*) Note that only nine are underlined, but 11 assays were conducted; this is because 3-androstanediol glucuronide (3α-diol-G) was quantitated as separate metabolites of 3-androstanediol-3 glucuronide and 3-androstanediol-17 glucuronide. ADT, androsterone; ADT-G, androsterone glucuronide; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone.
Statistical Analysis
To assess associations between each hormone and male breast cancer, we used logistic regression models to estimate covariate-adjusted odds ratios (ORs) and 95% CIs. Before these logistic regression analyses, we adjusted all hormones to reduce the influence of study-related variability.28 Using all participants with baseline (prediagnostic) quantitation, we regressed each log-transformed hormone on study and age. Study betas were summed and divided by the number of studies minus one. This value was subtracted from each of the study betas to generate study-specific correction factors, which were subtracted from the log-hormone concentrations to generate individual-level, study-corrected log hormone concentrations.
Each exposure was assessed after being categorized into quartiles using cut points based on the exposure distribution of all participants with baseline hormone quantitation, as well as assessed as a continuous metric with standardization to half the value of the interquartile range, such that continuous estimates of association were approximately per-quartile increase in exposure.29 In addition to assessing individual exposures, we also assessed combinations and ratios of hormones that were metabolically close. These a priori–specified exposures included: E2 to testosterone ratio; testosterone to DHT ratio; E1 to androstenedione ratio; E2 to E1 ratio; parent estrogens (E1 plus E2); E2 to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio; and parent estrogens (E1 plus E2) to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio.
We performed both conditional and unconditional analyses of the data. Conditional models using original or study-adjusted hormone values, and unconditional models using original hormone values with model adjustment for study, did not materially alter the results. Therefore, we present results from the unconditional analyses using the study-adjusted hormone values. In these analyses, we adjusted for race, date of blood draw (continuous calendar years), and age at blood draw (continuous years). Modeling dates or ages as categorical, instead of continuous, variables had minimal effects on risk. We also assessed whether adjustment for study, body mass index (BMI), family history of breast cancer, diabetes, cigarette smoking (ever v never, currency, pack-years, duration, and intensity), and alcohol consumption (currency and grams consumed per day) changed OR estimates by > 10%. None of these covariates consistently altered the estimates obtained, but we included BMI as a continuous variable in the fully adjusted model, given previous evidence that this is associated with both male breast cancer risk and hormone levels and because adjustment resulted in slight modifications of risk. Because we did not have information on Klinefelter syndrome or gynecomastia from any studies, we could not measure potential confounding effects. We assessed whether relations between hormones and male breast cancer were modified by several risk factors by performing likelihood ratio tests of nested models with and without a hormone–risk factor interaction term. Heterogeneity was assessed in the same way using a hormone-study interaction term. All tests were two sided, and P values < .05 were considered statistically significant. Analyses were conducted using STATA software (version 13; STATA, College Station, TX).
RESULTS
Among the 101 male breast cancer cases and 217 controls, the average age at blood draw was 51.6 and 50.9 years, respectively (Table 1). The mean age at diagnosis among cases was 66.9 years.
Table 1.
Distributions of Examined Variables by Case-Control Status of Men Included in Male Breast Cancer Pooling Project (n = 318)
| Variable | Controls (n = 217) |
Cases (n = 101) |
P* | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Demographic† | |||||
| Age at blood draw, years‡ | .62 | ||||
| Mean | 50.93 | 51.59 | |||
| SD | 11.62 | 11.57 | |||
| Age at diagnosis or pseudodiagnosis, years‡ | .56 | ||||
| Mean | 67.72 | 66.91 | |||
| SD | 11.12 | 11.03 | |||
| BMI, kg/m2 | .94 | ||||
| Mean | 25.79 | 25.62 | |||
| SD | 4.21 | 3.37 | |||
| Diabetes, % | 3.81 | 1.02 | .18§ | ||
| Family history of breast cancer, % | 4.00 | 23.08 | .07§ | ||
| Ever smoked, % | 67.36 | 52.31 | .04§ | ||
| Current smokers, % | 26.39 | 18.46 | .11§ | ||
| Pack-years smoked | 22.50 | 7.50 to 37.50 | 12.50 | 3.85 to 22.50 | .03 |
| Cigarette smoking duration, years | 25.00 | 15.00 to 29.00 | 15.00 | 10.00 to 25.00 | .14 |
| Cigarette smoking intensity, cigarettes per day | 20.00 | 10.00 to 30.00 | 12.22 | 10.00 to 25.00 | .03 |
| Current alcohol consumption, % | 81.54 | 89.66 | .16§ | ||
| Alcohol consumption, g/d | 13.54 | 1.83 to 13.54 | 13.54 | 5.51 to 27.74 | .18 |
| Hormonal | |||||
| DHEA, nmol/L | 6.36 | 4.11 to 9.78 | 6.03 | 3.62 to 9.46 | .63 |
| Androstenediol, pmol/L | 2,668.28 | 1,936.95 to 3,867.49 | 2,694.79 | 1,992.94 to 3,871.21 | .96 |
| Androstenedione, nmol/L | 2.60 | 1.94 to 3.41 | 2.77 | 2.11 to 3.23 | .61 |
| Testosterone, nmol/L | 13.07 | 10.02 to 16.40 | 14.09 | 10.39 to 17.05 | .31 |
| DHT, pmol/L | 1,409.71 | 1,089.15 to 1,823.06 | 1,435.25 | 1,147.00 to 1,936.13 | .53 |
| 3α-diol-3G, nmol/L | 2.97 | 2.22 to 4.35 | 2.74 | 2.03 to 4.21 | .36 |
| 3α-diol-17G, nmol/L | 6.64 | 4.54 to 9.24 | 6.45 | 4.65 to 8.15 | .57 |
| ADT, pmol/L | 674.84 | 508.92 to 894.94 | 645.90 | 489.70 to 866.17 | .54 |
| ADT-G, nmol/L | 72.97 | 57.08 to 105.06 | 74.08 | 52.09 to 97.18 | .44 |
| Estrone, pmol/L | 81.02 | 64.54 to 105.20 | 84.63 | 70.70 to 111.27 | .22 |
| Estradiol, pmol/L | 64.09 | 50.69 to 84.82 | 73.08 | 57.28 to 87.46 | .03 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; BMI, body mass index; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; IQR, interquartile range; SD, standard deviation.
Wilcoxon rank sum test, unless otherwise indicated.
Demographic variables generally assessed at time of study entry.
Matching factors in study.
χ2 test for statistical difference between cases and controls. Family history of breast cancer information was available for only 12% of the study subjects.
A family history of breast cancer in a first-degree relative was more common among the cases than the controls, whereas there were no major differences with respect to mean BMI or history of diabetes. Cases were significantly less likely than controls to report a history of cigarette smoking but somewhat more likely to report having consumed alcohol. Quantitation of the primary sex steroid hormones revealed levels that would be expected from a middle-age male population using mass spectrometry technologies.30–32
Among the controls, androgen levels declined significantly with age at blood draw, whereas estrogen levels increased (Appendix Table A1, online only). In addition, BMI affected many of the hormones, with higher BMI associated with lower androgen and somewhat higher E2 levels. Substantial and significant correlations were found between E2 and E1 (r = 0.74), testosterone and DHT (r = 0.74), testosterone and androstenediol (r = 0.54), testosterone and androstenedione (r = 0.53), and androstenedione and androstenediol (r = 0.47). E2 was significantly correlated with testosterone (r = 0.50), but correlations between other androgens and estrogens were weaker, and many were not statistically significant. Hormone concentrations among controls were similar across studies (Appendix Table A2, online only).
Table 2 summarizes risks associated with hormone analytes after adjustment for race, date at blood draw, and age at blood draw—and then in addition for BMI. Although there were not major differences in the two sets of ORs, we chose to focus on the more fully adjusted estimates, which in some instances were based on slightly reduced numbers, given missing information on BMI. In general, androgens were unrelated to risk, although there was a slightly increased risk associated with elevated testosterone levels (ORQ4 v Q1, 1.53; 95% CI, 0.73 to 3.17; trend P = .59). This relation, however, was much less impressive than those seen for estradiol, where those in the highest quartile had an OR of 2.47 (95% CI, 1.10 to 5.58) compared with those in the lowest quartile (trend P = .06). Estrone was not significantly related to risk (ORQ4 v Q1, 1.32; 95% CI, 0.63 to 2.79; trend P = .60).
Table 2.
Unconditional Logistic Regression of Serum and Plasma Hormone Levels and Male Breast Cancer Risk
| Variable | Controls | Cases | OR* | 95% CI | P | Controls | Cases | OR† | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| DHEA, nmol/L | ||||||||||
| < 3.96 | 54 | 31 | 1.00 | Referent | 50 | 30 | 1.00 | Referent | ||
| 3.96 to < 6.07 | 54 | 21 | 0.67 | 0.34 to 1.35 | .27 | 51 | 20 | 0.68 | 0.34 to 1.39 | .29 |
| 6.07 to < 9.44 | 54 | 27 | 0.86 | 0.42 to 1.76 | .67 | 53 | 23 | 0.77 | 0.36 to 1.62 | .49 |
| ≥ 9.44 | 54 | 22 | 0.71 | 0.33 to 1.50 | .37 | 45 | 19 | 0.74 | 0.34 to 1.62 | .45 |
| Continuous | 216 | 101 | 0.90 | 0.60 to 1.33 | .58 | 199 | 92 | 0.90 | 0.58 to 1.40 | .65 |
| Androstenediol, pmol/L | ||||||||||
| < 2,040.95 | 54 | 24 | 1.00 | Referent | 49 | 21 | 1.00 | Referent | ||
| 2,040.95 to < 2,866.19 | 54 | 25 | 1.09 | 0.55 to 2.16 | .82 | 52 | 25 | 1.20 | 0.59 to 2.45 | .61 |
| 2866.19 to > 4,093.17 | 54 | 26 | 1.15 | 0.57 to 2.33 | .69 | 52 | 22 | 1.09 | 0.52 to 2.29 | .82 |
| ≥ 4,093.17 | 54 | 26 | 1.13 | 0.55 to 2.32 | .73 | 46 | 24 | 1.35 | 0.63 to 2.89 | .44 |
| Continuous | 216 | 101 | 1.04 | 0.64 to 1.70 | .88 | 199 | 92 | 1.28 | 0.75 to 2.20 | .37 |
| Androstenedione, nmol/L | ||||||||||
| < 1.95 | 55 | 20 | 1.00 | Referent | 54 | 19 | 1.00 | Referent | ||
| 1.95 to < 2.60 | 53 | 27 | 1.42 | 0.71 to 2.83 | .32 | 47 | 27 | 1.64 | 0.81 to 3.33 | .17 |
| 2.60 to < 3.41 | 54 | 34 | 1.71 | 0.87 to 3.36 | .12 | 48 | 28 | 1.65 | 0.81 to 3.35 | .17 |
| ≥ 3.41 | 54 | 20 | 0.99 | 0.47 to 2.07 | .98 | 50 | 18 | 1.00 | 0.46 to 2.16 | 1.00 |
| Continuous | 216 | 101 | 1.04 | 0.64 to 1.70 | .88 | 199 | 92 | 0.85 | 0.48 to 1.50 | .57 |
| Testosterone, nmol/L | ||||||||||
| < 10.05 | 55 | 21 | 1.00 | Referent | 52 | 19 | 1.00 | Referent | ||
| 10.05 to < 13.17 | 54 | 20 | 0.92 | 0.44 to 1.91 | .82 | 51 | 19 | 0.97 | 0.45 to 2.09 | .94 |
| 13.17 to < 16.41 | 54 | 28 | 1.30 | 0.65 to 2.59 | .46 | 49 | 26 | 1.39 | 0.67 to 2.88 | .38 |
| ≥ 16.41 | 53 | 32 | 1.49 | 0.75 to 2.95 | .25 | 47 | 28 | 1.53 | 0.73 to 3.17 | .26 |
| Continuous | 216 | 101 | 1.17 | 0.68 to 2.02 | .57 | 199 | 92 | 1.18 | 0.64 to 2.17 | .59 |
| DHT, pmol/L | ||||||||||
| < 1,070.34 | 55 | 22 | 1.00 | Referent | 51 | 20 | 1.00 | Referent | ||
| 1,070.34 to < 1,391.97 | 54 | 25 | 1.12 | 0.56 to 2.24 | .74 | 50 | 24 | 1.20 | 0.58 to 2.46 | .62 |
| 1,391.97 to < 1,800.11 | 54 | 25 | 1.13 | 0.57 to 2.26 | .72 | 49 | 21 | 1.04 | 0.50 to 2.18 | .92 |
| ≥ 1,800.11 | 53 | 29 | 1.34 | 0.68 to 2.62 | .40 | 49 | 27 | 1.31 | 0.64 to 2.70 | .46 |
| Continuous | 216 | 101 | 1.17 | 0.75 to 1.83 | .48 | 199 | 92 | 1.20 | 0.73 to 1.94 | .47 |
| 3α-diol-3G, nmol/L | ||||||||||
| < 2.23 | 54 | 32 | 1.00 | Referent | 51 | 30 | 1.00 | Referent | ||
| 2.23 to < 3.01 | 56 | 24 | 0.71 | 0.37 to 1.37 | .30 | 54 | 20 | 0.63 | 0.32 to 1.26 | .19 |
| 3.01 to < 4.40 | 52 | 23 | 0.74 | 0.38 to 1.45 | .38 | 47 | 21 | 0.81 | 0.40 to 1.63 | .55 |
| ≥ 4.40 | 54 | 22 | 0.67 | 0.34 to 1.34 | .26 | 47 | 21 | 0.80 | 0.39 to 1.63 | .53 |
| Continuous | 216 | 101 | 0.83 | 0.57 to 1.19 | .30 | 199 | 92 | 0.94 | 0.66 to 1.35 | .74 |
| 3α-diol-17G, nmol/L | ||||||||||
| < 4.49 | 54 | 23 | 1.00 | Referent | 49 | 20 | 1.00 | Referent | ||
| 4.49 to < 6.56 | 53 | 28 | 1.26 | 0.64 to 2.48 | .50 | 50 | 26 | 1.34 | 0.66 to 2.73 | .42 |
| 6.56 to < 9.24 | 55 | 29 | 1.25 | 0.63 to 2.47 | .52 | 51 | 26 | 1.34 | 0.65 to 2.78 | .43 |
| ≥ 9.24 | 53 | 20 | 0.89 | 0.43 to 1.84 | .76 | 48 | 19 | 1.06 | 0.49 to 2.29 | .89 |
| Continuous | 215 | 100 | 0.98 | 0.65 to 1.46 | .91 | 198 | 91 | 1.06 | 0.69 to 1.62 | .79 |
| ADT, pmol/L | ||||||||||
| < 493.66 | 55 | 29 | 1.00 | Referent | 51 | 27 | 1.00 | Referent | ||
| 493.66 to < 647.62 | 53 | 25 | 0.92 | 0.47 to 1.80 | .81 | 51 | 22 | 0.85 | 0.42 to 1.72 | .66 |
| 647.62 to < 874.96 | 54 | 25 | 0.90 | 0.46 to 1.75 | .75 | 50 | 24 | 0.93 | 0.47 to 1.86 | .84 |
| ≥ 874.96 | 54 | 22 | 0.83 | 0.41 to 1.71 | .62 | 47 | 19 | 0.84 | 0.39 to 1.81 | .65 |
| Continuous | 216 | 101 | 1.10 | 0.71 to 1.69 | .68 | 199 | 92 | 1.04 | 0.61 to 1.75 | .89 |
| ADT-G, nmol/L | ||||||||||
| < 54.96 | 53 | 31 | 1.00 | Referent | 49 | 30 | 1.00 | Referent | ||
| 54.96 to < 70.27 | 54 | 19 | 0.60 | 0.29 to 1.22 | .16 | 53 | 16 | 0.52 | 0.24 to 1.10 | .09 |
| 70.27 to < 101.09 | 54 | 30 | 0.94 | 0.48 to 1.82 | .85 | 49 | 27 | 0.95 | 0.47 to 1.89 | .88 |
| ≥ 101.09 | 54 | 20 | 0.64 | 0.30 to 1.35 | .24 | 47 | 18 | 0.69 | 0.31 to 1.50 | .35 |
| Continuous | 215 | 100 | 0.76 | 0.48 to 1.21 | .25 | 198 | 91 | 0.81 | 0.49 to 1.35 | .42 |
| Estrone, pmol/L | ||||||||||
| < 67.00 | 53 | 22 | 1.00 | Referent | 49 | 20 | 1.00 | Referent | ||
| 67.00 to < 84.45 | 53 | 21 | 0.94 | 0.46 to 1.93 | .87 | 47 | 19 | 0.96 | 0.45 to 2.05 | .92 |
| 84.45 to < 108.18 | 52 | 25 | 1.13 | 0.55 to 2.30 | .74 | 47 | 23 | 1.12 | 0.53 to 2.38 | .76 |
| ≥ 108.18 | 53 | 32 | 1.36 | 0.66 to 2.79 | .40 | 51 | 30 | 1.32 | 0.63 to 2.79 | .47 |
| Continuous | 211 | 100 | 1.22 | 0.69 to 2.17 | .49 | 194 | 92 | 1.17 | 0.65 to 2.10 | .60 |
| Estradiol, pmol/L | ||||||||||
| < 52.23 | 55 | 14 | 1.00 | Referent | 54 | 13 | 1.00 | Referent | ||
| 52.23 to < 65.98 | 54 | 22 | 1.58 | 0.73 to 3.43 | .25 | 47 | 17 | 1.50 | 0.66 to 3.44 | .34 |
| 65.98 to < 86.76 | 54 | 35 | 2.62 | 1.24 to 5.55 | .01 | 48 | 33 | 3.00 | 1.37 to 6.56 | .01 |
| ≥ 86.76 | 53 | 30 | 2.28 | 1.04 to 5.00 | .04 | 50 | 29 | 2.47 | 1.10 to 5.58 | .03 |
| Continuous | 216 | 101 | 1.68 | 0.95 to 2.99 | .08 | 199 | 92 | 1.79 | 0.98 to 3.25 | .06 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; OR, odds ratio.
Adjusted for race, date at blood draw, and age at blood draw. Continuous sex steroid hormone values were standardized to half of difference between 75th and 25th centiles of distribution before correlative analysis.
Adjusted additionally for body mass index as continuous variable.
Further assessment of estrogens as a ratio to various individual androgens or sum of androgens showed no additional discrimination of risk beyond that seen with the estrogens or androgens alone (Table 3). We observed elevated, but not statistically significant, risks for high levels of the ratio of E2 to testosterone (ORQ4 v Q1, 1.95; 95% CI, 0.90 to 4.24; trend P = .14). We also observed a positive relation for the ratio of E2 to the sum of androgens downstream in the metabolic pathway from testosterone (ie, sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT), with the highest quartile providing an OR of 2.27 (95% CI, 0.98 to 5.29; trend P = .34). Although those with high summed E1 plus E2 showed some risk elevation (ORQ4 v Q1, 1.57; 95% CI, 0.70 to 3.53; trend P = .27), there was no further distinction in risk when this measure was examined as a ratio to testosterone levels or sum of androgens.
Table 3.
Unconditional Logistic Regression of Serum and Plasma Hormones and Male Breast Cancer Risk
| Variable | Controls | Cases | OR* | 95% CI | P | Controls | Cases | OR† | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol to testosterone ratio | ||||||||||
| < 0.004 | 55 | 21 | 1.00 | Referent | 50 | 16 | 1.00 | Referent | ||
| 0.004 to < 0.005 | 54 | 24 | 1.15 | 0.57 to 2.32 | .70 | 47 | 23 | 1.57 | 0.73 to 3.38 | .24 |
| 0.005 to < 0.007 | 53 | 24 | 1.19 | 0.58 to 2.45 | .64 | 50 | 23 | 1.45 | 0.66 to 3.16 | .36 |
| ≥ 0.007 | 54 | 32 | 1.57 | 0.78 to 3.13 | .20 | 52 | 30 | 1.95 | 0.90 to 4.24 | .09 |
| Continuous | 216 | 101 | 1.42 | 0.83 to 2.43 | .20 | 199 | 92 | 1.57 | 0.86 to 2.87 | .14 |
| Testosterone to DHT ratio | ||||||||||
| < 0.088 | 55 | 19 | 1.00 | Referent | 51 | 18 | 1.00 | Referent | ||
| 0.088 to < 0.105 | 53 | 34 | 1.82 | 0.92 to 3.61 | .08 | 52 | 32 | 1.73 | 0.85 to 3.50 | .13 |
| 0.105 to < 0.127 | 54 | 19 | 0.97 | 0.46 to 2.06 | .95 | 48 | 16 | 0.92 | 0.42 to 2.03 | .84 |
| ≥ 0.127 | 54 | 29 | 1.49 | 0.74 to 3.01 | .26 | 48 | 26 | 1.54 | 0.74 to 3.21 | .25 |
| Continuous | 216 | 101 | 0.89 | 0.55 to 1.43 | .62 | 199 | 92 | 0.92 | 0.57 to 1.50 | .74 |
| Estrone to androstenedione ratio | ||||||||||
| ≥ 0.025 | 53 | 20 | 1.00 | Referent | 47 | 17 | 1.00 | Referent | ||
| 0.025 to < 0.033 | 53 | 26 | 1.29 | 0.64 to 2.62 | .48 | 48 | 25 | 1.45 | 0.68 to 3.08 | .33 |
| 0.033 to < 0.042 | 53 | 22 | 1.09 | 0.51 to 2.32 | .82 | 48 | 19 | 1.06 | 0.47 to 2.38 | .89 |
| ≥ 0.042 | 52 | 32 | 1.67 | 0.79 to 3.56 | .18 | 51 | 31 | 1.70 | 0.75 to 3.82 | .20 |
| Continuous | 211 | 100 | 1.18 | 0.71 to 1.96 | .51 | 194 | 92 | 1.17 | 0.69 to 1.98 | .56 |
| Estradiol to estrone ratio | ||||||||||
| < 0.679 | 53 | 21 | 1.00 | Referent | 52 | 18 | 1.00 | Referent | ||
| 0.679 to < 0.818 | 53 | 28 | 1.35 | 0.68 to 2.70 | .39 | 46 | 25 | 1.61 | 0.77 to 3.35 | .20 |
| 0.818 to < 0.942 | 52 | 20 | 1.00 | 0.48 to 2.07 | .99 | 50 | 19 | 1.13 | 0.53 to 2.42 | .75 |
| ≥ 0.942 | 53 | 31 | 1.56 | 0.79 to 3.08 | .20 | 46 | 30 | 1.99 | 0.98 to 4.06 | .06 |
| Continuous | 211 | 100 | 1.49 | 0.75 to 2.97 | .26 | 194 | 92 | 1.92 | 0.89 to 4.17 | .10 |
| Sum of estrone plus estradiol | ||||||||||
| < 123.23 | 53 | 18 | 1.00 | Referent | 51 | 16 | 1.00 | Referent | ||
| 123.23 to < 153.58 | 53 | 18 | 0.98 | 0.45 to 2.11 | .96 | 46 | 17 | 1.14 | 0.51 to 2.56 | .74 |
| 153.58 to < 195.12 | 53 | 37 | 2.02 | 1.00 to 4.12 | .05 | 47 | 33 | 2.15 | 1.02 to 4.54 | .04 |
| ≥ 195.12 | 52 | 27 | 1.48 | 0.68 to 3.24 | .32 | 50 | 26 | 1.57 | 0.70 to 3.53 | .27 |
| Continuous | 211 | 100 | 1.40 | 0.78 to 2.51 | .26 | 194 | 92 | 1.38 | 0.76 to 2.51 | .29 |
| Estradiol to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio | ||||||||||
| < 0.0005 | 54 | 18 | 1.00 | Referent | 48 | 15 | 1.00 | Referent | ||
| 0.0005 to < 0.0008 | 54 | 20 | 1.17 | 0.55 to 2.49 | .69 | 49 | 18 | 1.18 | 0.53 to 2.66 | .68 |
| 0.0008 to < 0.0011 | 54 | 25 | 1.49 | 0.70 to 3.19 | .30 | 51 | 23 | 1.50 | 0.66 to 3.37 | .33 |
| ≥ 0.0011 | 52 | 36 | 2.34 | 1.05 to 5.20 | .04 | 49 | 34 | 2.27 | 0.98 to 5.29 | .06 |
| Continuous | 214 | 99 | 1.20 | 0.86 to 1.69 | .29 | 197 | 90 | 1.19 | 0.83 to 1.70 | .34 |
| Sum of estrone plus estradiol to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio | ||||||||||
| < 0.0011 | 53 | 15 | 1.00 | Referent | 47 | 12 | 1.00 | Referent | ||
| 0.0011 to < 0.0016 | 52 | 28 | 1.98 | 0.94 to 4.19 | .07 | 48 | 27 | 2.21 | 0.99 to 4.93 | .05 |
| 0.0016 to < 0.0023 | 53 | 21 | 1.43 | 0.64 to 3.23 | .39 | 48 | 19 | 1.51 | 0.63 to 3.65 | .35 |
| ≥ 0.0023 | 51 | 34 | 2.45 | 1.06 to 5.65 | .04 | 49 | 32 | 2.38 | 0.98 to 5.80 | .06 |
| Continuous | 209 | 98 | 1.09 | 0.78 to 1.51 | .62 | 192 | 90 | 1.05 | 0.75 to 1.47 | .76 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT-G, androsterone glucuronide; DHT, dihydrotestosterone; OR, odds ratio.
Adjusted for race, date at blood draw, and age at blood draw. Continuous sex steroid hormone values were standardized to half of difference between 75th and 25th centiles of distribution before correlative analysis.
Adjusted additionally for body mass index as continuous variable.
We assessed whether there was heterogeneity in hormone relations according to various identified risk factors (Table 4). We saw somewhat stronger associations for most hormones among younger (age < 67 years) compared with older men (eg, highest v lowest quartile for E2: OR, 3.17; 95% CI, 1.06 to 9.42 v OR, 1.70; 95% CI, 0.49 to 5.91), but the difference was not statistically significant (heterogeneity P = .68). No substantial or consistent differences in hormone relations were observed according to other potential risk factors, including BMI (Table 4) or dichotomized exposures of cigarette smoking or alcohol consumption (data not shown).
Table 4.
Unconditional Logistic Regression Analyses of Selected Hormones and Male Breast Cancer According to Age and BMI
| Variable | Age at Diagnosis |
BMI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 67 Years |
≥ 67 Years |
< 25.07 |
≥ 25.07 |
|||||||||
| No. of Exposed Cases | OR* | 95% CI | No. of Exposed Cases | OR* | 95% CI | No. of Exposed Cases | OR† | 95% CI | No. of Exposed Cases | OR† | 95% CI | |
| Testosterone, nmol/L | ||||||||||||
| < 10.02 | 7 | 1.00 | Referent | 12 | 1.00 | Referent | 7 | 1.00 | Referent | 12 | 1.00 | Referent |
| 10.02 to < 13.07 | 12 | 1.45 | 0.48 to 4.39 | 7 | 0.65 | 0.21 to 2.02 | 9 | 1.31 | 0.40 to 4.29 | 10 | 0.83 | 0.30 to 2.32 |
| 13.07 to < 16.40 | 15 | 1.82 | 0.63 to 5.28 | 11 | 1.12 | 0.38 to 3.29 | 13 | 1.64 | 0.54 to 4.98 | 13 | 1.39 | 0.51 to 3.82 |
| ≥ 16.40 | 16 | 2.08 | 0.71 to 6.11 | 12 | 1.10 | 0.38 to 3.21 | 16 | 2.07 | 0.69 to 6.24 | 12 | 1.26 | 0.46 to 3.49 |
| Continuous | 50 | 1.32 | 0.57 to 3.02 | 42 | 0.98 | 0.39 to 2.44 | 45 | 1.41 | 0.64 to 3.13 | 47 | 1.01 | 0.39 to 2.64 |
| Estradiol, pmol/L | ||||||||||||
| < 50.69 | 7 | 1.00 | Referent | 6 | 1.00 | Referent | 9 | 1.00 | Referent | 4 | 1.00 | Referent |
| 50.69 to < 64.09 | 11 | 2.06 | 0.70 to 6.11 | 6 | 0.91 | 0.24 to 3.45 | 10 | 1.65 | 0.57 to 4.77 | 7 | 1.51 | 0.37 to 6.12 |
| 64.09 to < 84.82 | 19 | 3.56 | 1.27 to 9.94 | 14 | 2.43 | 0.72 to 8.16 | 13 | 2.53 | 0.89 to 7.17 | 20 | 3.81 | 1.94 to 13.86 |
| ≥ 84.82 | 13 | 3.17 | 1.06 to 9.42 | 16 | 1.70 | 0.49 to 5.91 | 13 | 2.20 | 0.74 to 6.57 | 16 | 3.47 | 0.92 to 13.11 |
| Continuous | 50 | 3.08 | 1.21 to 7.84 | 42 | 1.21 | 0.53 to 2.75 | 45 | 1.66 | 0.75 to 3.69 | 47 | 2.32 | 0.90 to 5.96 |
| Estradiol to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio | ||||||||||||
| < 0.0005 | 12 | 1.00 | Referent | 3 | 1.00 | Referent | 8 | 1.00 | Referent | 7 | 1.00 | Referent |
| 0.0005 to < 0.0007 | 11 | 0.94 | 0.36 to 2.47 | 7 | 1.46 | 0.29 to 7.36 | 10 | 1.11 | 0.37 to 3.34 | 8 | 1.49 | 0.44 to 5.10 |
| 0.0007 to < 0.0010 | 13 | 1.38 | 0.52 to 3.66 | 10 | 1.35 | 0.29 to 6.32 | 9 | 1.54 | 0.46 to 5.18 | 14 | 1.45 | 0.47 to 4.48 |
| ≥ 0.0010 | 12 | 2.71 | 0.97 to 7.61 | 22 | 1.94 | 0.40 to 9.30 | 16 | 2.06 | 0.63 to 6.74 | 18 | 2.89 | 0.81 to 10.24 |
| Continuous | 48 | 1.65 | 0.88 to 3.09 | 42 | 1.01 | 0.65 to 1.56 | 43 | 1.37 | 0.74 to 2.57 | 47 | 1.10 | 0.71 to 1.69 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT-G, androsterone glucuronide; BMI, body mass index; DHT, dihydrotestosterone; OR, odds ratio.
Adjusted for race, date at blood draw, age at blood draw, and BMI (continuous). Continuous sex steroid hormone values were standardized to half of difference between 75th and 25th centiles of distribution before correlative analysis.
Adjusted for race, date at blood draw, and age at blood draw.
We also assessed whether hormone associations differed according to whether tumors were diagnosed within or after 10 years of blood draw, but these analyses revealed no distinctive differences (Appendix Table A3, online only). Analyses that specifically excluded cases diagnosed within the first 3 years after blood draw also showed no major risk differences (data not shown).
DISCUSSION
In this apparent first-time assessment, we found that male breast cancer risk was influenced by prediagnostic endogenous estradiol. Circulating androgen levels did not seem to be associated with much risk alteration; thus, when we examined estradiol in relation to androgens, there was no additional enhancement of risk beyond that already seen with estradiol levels.
In observing a relation of male breast cancer with high estradiol levels, results are consistent with those for postmenopausal female breast cancer.19,20,33–36 Interestingly, the magnitude of risk associated with high levels of estrogens is similar for female and male breast cancers, being on the order of two- to three-fold for the highest versus lowest quartiles of estradiol levels. Although estrogen-mediated carcinogenesis is not well understood, potential mechanisms include mutagenic action and stimulation of cell proliferation, which may increase risk of neoplastic transformation and/or neoplastic progression.37,38
Although the etiologic role of androgens in male breast cancer is unclear, studies in women support associations independent of estrogens.19,20,36 Androgens may increase breast cancer risk directly by increasing cell growth and proliferation or indirectly by peripheral conversions to estrogens within a number of tissues, including adipose and breast tissues.39 Although androgens are a likely relevant physiologic mechanism with respect to the development of estrogen receptor (ER) –responsive tumors, we did not have complete information on either ERs or androgen receptors for the tumors studied and thus could not differentiate risks according to tumor subtype. On the basis of other studies,40 we can assume that most of the male breast cancers we studied would have been ER positive and probably androgen receptor positive. Although it would have been of interest to examine hormone relations according to hormone receptor status of the tumors, it is noteworthy that some recent studies of female breast cancer have found hormone relationships to prevail for both hormone receptor–positive and –negative tumors,41 suggesting that hormones may act through molecular pathways that do not directly involve the receptors found within the tumor itself.
The incidence of male breast cancer is approximately 100× lower than that of female breast cancer, which likely reflects sex differences in breast cancer pathogenesis, including the numbers and types of cells available for carcinogenic transformation.42 Gynecomastia is a recognized risk factor for male breast cancer, and it is believed to develop mainly because of a disequilibrium between free estrogen and androgen in breast tissue.43 We had no information on the development of gynecomastia and thus could not determine whether the effects of estrogens on male breast cancer were mediated through more tissue at risk. However, data from the case-control studies that contributed data to this pooling project5 and that collected information on gynecomastia indicated that it was a fairly rare event. This would support that high levels of estrogens could be a biomarker of risk even in the absence of diagnosed gynecomastia.
It has been proposed that male breast cancer may arise as a result of a high ratio of estrogens to androgens.44 This speculation derives mainly from findings that patients with Klinefelter syndrome are at an elevated risk of male breast cancer. Such patients, during adolescence, begin to exhibit elevated levels of gonadotropins and decreased levels of testosterone, resulting in their characteristic body proportions and gynecomastia.17 In adults, low testosterone levels are a cardinal feature of Klinefelter syndrome,45 along with high estradiol levels from overexpression of aromatase CYP19.46 However, our results only infer that the ratio of estradiol to testosterone and the sum of various androgens may be associated with male breast cancer; none of these analyses were statistically significant at P < .05.
In postmenopausal female breast cancer, there is a high correlation between BMI and estrogen levels, specifically free estradiol levels.47 Although estradiol seems to remain a significant risk factor after adjustment for BMI, the reverse is apparently not true; the association of BMI with postmenopausal breast cancer in two studies entirely disappeared after adjustment for free estradiol levels.48,49 In addition, there is evidence in female breast cancer that exogenous estrogens50–52 have stronger effects on relative risks in thin women (eg, BMI < 25 kg/m2), supporting that obese women have high estrogen levels that prevent additional effects of other hormones.
These findings in women therefore stimulated our interest in evaluating confounding and effect modifications of hormone levels by BMI in men. However, we did not find that there were large differences in hormone relations after adjustment for BMI, nor did we find that hormone relations varied substantially by BMI level. This may reflect that hormones are not as strongly influenced by BMI in men compared with women, although we did find some evidence of variations of hormone levels by BMI, particularly androgens, which were inversely correlated, as has been noted by others.53–55 However, we could not specifically assess relations with free testosterone, given that we did not measure sex hormone–binding globulin (SHBG). Free testosterone may be more influenced by BMI than total testosterone, because as SHBG levels decrease, the levels of free testosterone increase, requiring less total testosterone to maintain the feedback loop. A similar relation with BMI has been seen among women with respect to free versus total estradiol levels.48
Our results did suggest some possible effect modification of hormones by age at development of breast cancer, with estradiol being more strongly related to younger- than older-onset cancers. Although female breast cancer is recognized as showing distinctive clinical and risk factor differences by age at diagnosis,56 much less is known regarding these parameters for male breast cancer. One large series recently reported that younger patients with male breast cancer had types of tumors that are generally associated with a poor prognosis in women, including ER- and/or progesterone receptor–negative tumors and human epidermal growth receptor 2–positive tumors.40 It is, however, unclear whether these or other tumor characteristics would be influenced by endogenous hormones.
This study had a number of strengths but some limitations as well. Although this is the only prospective study to our knowledge to assess endogenous hormones in relation to male breast cancer risk, the number of cases for analysis was modest, reflecting the general rarity of this disease. Samples were collected before diagnosis, oftentimes many years and at varying times before diagnosis. We had no information on SHBG, and although we had information on various risk factors, we did not have access to some parameters that would have been of interest, including BRCA status, gynecomastia, and Klinefelter syndrome. Finally, we lacked information on clinical parameters, including hormone receptors.
In conclusion, in this investigation to assess the role of endogenous hormones in the etiology of male breast cancer, we found, as in postmenopausal female breast cancer, a strong relation with estradiol levels. Androgens were much less important predictors, and as a result, the ratio of estrogens to androgens was not as important as has been previously speculated. Future studies may benefit from a focus on the mediating effects of estrogens on breast cancer among men with gynecomastia and/or Klinefelter syndrome.
Glossary Terms
- logistic regression analysis:
a multivariable regression model in which the log of the odds of a time-fixed outcome event (eg, 30-day mortality) or other binary outcome is related to a linear equation.
- logistic regression model:
a multivariable prediction model in which the log of the odds of a time-fixed outcome event or other binary outcome is related to a linear equation.
Appendix
The principal investigators from each of the European Prospective Investigation Into Cancer and Nutrition centers that contributed cases were: Heiner Boeing, Rudolph Kaaks (Germany); Göran Hallmans, Jonas Manjer (Sweden); Timothy J. Key, Nick Wareham (United Kingdom); Kim Overvad, Anne Tjønneland (Denmark); Domenico Palli, Paolo Vineis, Rosario Tumino (Italy); Maria José Sánchez (Spain); and Antonia Trichopoulou (Greece).
Table A1.
Spearman Correlation Coefficients Among Sex Steroid Hormone Measures in MBCPP Controls (n = 217)
| Hormone | DHEA | Androstenediol | Androstenedione | Testosterone | DHT | 3α-diol-3G | 3α-diol-17G | ADT | ADT-G | E1 | E2 | Age at Blood Draw | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHEA | 1.00 | ||||||||||||
| Androstenediol | 0.61 | 1.00 | |||||||||||
| Androstenedione | 0.55 | 0.47 | 1.00 | ||||||||||
| Testosterone | 0.20 | 0.54 | 0.53 | 1.00 | |||||||||
| DHT | 0.15 | 0.37 | 0.34 | 0.74 | 1.00 | ||||||||
| 3α-diol-3G | 0.33 | 0.30 | 0.26 | 0.11 | −0.02 | 1.00 | |||||||
| 3α-diol-17G | 0.16 | 0.24 | 0.08 | 0.15 | 0.09 | 0.53 | 1.00 | ||||||
| ADT | 0.71 | 0.48 | 0.56 | 0.31 | 0.31 | 0.28 | 0.06 | 1.00 | |||||
| ADT-G | 0.57 | 0.43 | 0.27 | 0.16 | 0.08 | 0.58 | 0.46 | 0.50 | 1.00 | ||||
| E1 | 0.11 | 0.12 | 0.42 | 0.33 | 0.22 | 0.00 | 0.07 | 0.27 | 0.03 | 1.00 | |||
| E2 | −0.03 | 0.21 | 0.27 | 0.50 | 0.34 | −0.06 | 0.11 | 0.14 | −0.02 | 0.74 | 1.00 | ||
| Age at blood draw | −0.49 | −0.34 | −0.05 | 0.10 | 0.14 | −0.02 | −0.19 | −0.29 | −0.42 | 0.44 | 0.40 | 1.00 | |
| BMI, kg/m2 | −0.10 | −0.14 | −0.16 | −0.19 | −0.34 | 0.10 | −0.19 | −0.23 | −0.02 | 0.09 | 0.15 | 0.14 | 1.00 |
NOTE. Bold font indicates significance at .05 level.
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; BMI, body mass index; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; MBCPP, Male Breast Cancer Pooling Project.
Table A2.
Hormone Medians and IQRs for Controls by Study
| Hormone Variable | EPIC (n = 26) |
HPFS (n = 22) |
Janus (n = 52) |
Kaiser (n = 73) |
||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| DHEA, nmol/L | 5.79 | 4.13 to 7.43 | 3.30 | 2.34 to 6.36 | 9.98 | 6.54 to 13.70 | 6.06 | 4.41 to 8.31 |
| Androstenediol, pmol/L | 2,440.17 | 1,865.78 to 2,960.50 | 1,876.82 | 1,188.21 to 2,668.28 | 3,781.97 | 2,780.06 to 5,192.30 | 2,544.48 | 1,934.24 to 3,635.27 |
| Androstenedione, nmol/L | 2.63 | 2.08 to 3.14 | 2.21 | 1.65 to 3.27 | 2.71 | 2.05 to 3.38 | 2.43 | 1.76 to 2.93 |
| Testosterone, nmol/L | 12.70 | 11.25 to 15.94 | 14.00 | 10.71 to 16.86 | 14.51 | 11.39 to 17.92 | 12.04 | 8.35 to 14.59 |
| DHT, pmol/L | 1,602.91 | 1,300.63 to 2,107.15 | 1,281.16 | 1,015.90 to 1,846.84 | 1,494.93 | 1,169.02 to 1,878.91 | 1,292.41 | 1,027.23 to 1,727.27 |
| 3α-diol-3G, nmol/L | 2.95 | 2.24 to 3.82 | 2.50 | 1.72 to 3.54 | 3.87 | 2.74 to 5.55 | 2.70 | 2.09 to 3.88 |
| 3α-diol-17G, nmol/L | 6.24 | 4.42 to 8.89 | 5.24 | 4.11 to 11.16 | 6.89 | 4.44 to 9.44 | 6.88 | 4.96 to 9.24 |
| ADT, pmol/L | 641.06 | 508.92 to 757.44 | 493.78 | 428.11 to 674.84 | 757.82 | 521.93 to 1,077.61 | 695.56 | 532.82 to 951.34 |
| ADT-G, nmol/L | 63.97 | 53.53 to 78.85 | 55.75 | 44.42 to 75.08 | 99.62 | 74.08 to 131.94 | 72.41 | 57.31 to 108.61 |
| Estrone, pmol/L | 80.15 | 69.56 to 118.07 | 91.56 | 85.31 to 98.95 | 67.52 | 56.19 to 86.56 | 75.59 | 59.55 to 94.89 |
| Estradiol, pmol/L | 62.92 | 54.74 to 78.03 | 79.31 | 64.09 to 85.00 | 53.62 | 42.57 to 68.93 | 63.92 | 47.95 to 80.31 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; BMI, body mass index; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; EPIC, European Prospective Investigation Into Cancer and Nutrition; HPFS, Health Professionals Follow-Up Study; IQR, interquartile range; MEC, Multiethnic Cohort Study of Diet and Cancer; PHS, Physicians' Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Screening Trial.
Table A3.
Logistic Regression of Hormones and Male Breast Cancer Stratified by Interval Between Blood Draw and Diagnosis
| Variable | Interval < 10 Years |
Interval ≥ 10 Years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | OR* | 95% CI | P | Controls | Cases | OR* | 95% CI | P | |
| DHEA, nmol/L | ||||||||||
| < 3.96 | 25 | 18 | 1.00 | Referent | 29 | 13 | 1.00 | Referent | ||
| 3.96 to < 6.07 | 20 | 8 | 0.50 | 0.17 to 1.46 | .20 | 34 | 12 | 0.83 | 0.32 to 2.15 | .69 |
| 6.07 to < 9.44 | 21 | 9 | 0.50 | 0.16 to 1.52 | .22 | 33 | 18 | 1.32 | 0.49 to 3.52 | .59 |
| ≥ 9.44 | 9 | 4 | 0.54 | 0.13 to 2.17 | .38 | 45 | 17 | 0.91 | 0.34 to 2.43 | .86 |
| Continuous | 75 | 39 | 0.64 | 0.24 to 1.69 | .37 | 141 | 60 | 0.96 | 0.62 to 1.48 | .84 |
| Androstenediol, pmol/L | ||||||||||
| < 2,040.95 | 21 | 12 | 1.00 | Referent | 33 | 12 | 1.00 | Referent | ||
| 2,040.95 to < 2,866.19 | 21 | 10 | 0.83 | 0.29 to 2.37 | .73 | 33 | 15 | 1.31 | 0.52 to 3.30 | .57 |
| 2,866.19 to < 4,093.17 | 18 | 9 | 0.88 | 0.29 to 2.65 | .81 | 36 | 16 | 1.34 | 0.52 to 3.45 | .54 |
| ≥ 4,093.17 | 15 | 8 | 0.94 | 0.30 to 3.00 | .92 | 39 | 17 | 1.26 | 0.49 to 3.26 | .64 |
| Continuous | 75 | 39 | 0.84 | 0.33 to 2.14 | .71 | 141 | 60 | 1.09 | 0.60 to 1.98 | .77 |
| Androstenedione, nmol/L | ||||||||||
| < 1.95 | 23 | 9 | 1.00 | Referent | 32 | 11 | 1.00 | Referent | ||
| 1.95 to < 2.60 | 16 | 12 | 1.96 | 0.66 to 5.79 | .22 | 37 | 14 | 1.07 | 0.42 to 2.69 | .89 |
| 2.60 to < 3.41 | 16 | 12 | 1.83 | 0.61 to 5.49 | .28 | 38 | 21 | 1.55 | 0.64 to 3.72 | .33 |
| ≥ 3.41 | 20 | 6 | 0.74 | 0.22 to 2.48 | .62 | 34 | 14 | 1.15 | 0.45 to 2.97 | .77 |
| Continuous | 75 | 39 | 0.59 | 0.23 to 1.54 | .29 | 141 | 60 | 1.32 | 0.74 to 2.38 | .35 |
| Testosterone, nmol/L | ||||||||||
| < 10.05 | 14 | 6 | 1.00 | Referent | 41 | 15 | 1.00 | Referent | ||
| 10.05 to < 13.17 | 23 | 8 | 0.80 | 0.23 to 2.82 | .73 | 31 | 10 | 0.88 | 0.34 to 2.26 | .79 |
| 13.17 to < 16.41 | 18 | 12 | 1.54 | 0.46 to 5.17 | .48 | 36 | 16 | 1.16 | 0.49 to 2.73 | .73 |
| ≥ 16.41 | 20 | 13 | 1.49 | 0.45 to 4.97 | .52 | 33 | 19 | 1.56 | 0.67 to 3.61 | .30 |
| Continuous | 75 | 39 | 0.82 | 0.31 to 2.15 | .68 | 141 | 60 | 1.46 | 0.75 to 2.87 | .27 |
| DHT, pmol/L | ||||||||||
| < 1,070.34 | 14 | 8 | 1.00 | Referent | 41 | 14 | 1.00 | Referent | ||
| 1,070.34 to < 1,391.97 | 19 | 12 | 1.10 | 0.35 to 3.46 | .87 | 35 | 12 | 1.00 | 0.41 to 2.47 | .99 |
| 1,391.97 to < 1,800.11 | 18 | 8 | 0.78 | 0.23 to 2.61 | .69 | 36 | 16 | 1.28 | 0.55 to 2.99 | .57 |
| ≥ 1,800.11 | 24 | 11 | 0.80 | 0.26 to 2.49 | .70 | 29 | 18 | 1.84 | 0.79 to 4.32 | .16 |
| Continuous | 75 | 39 | 0.84 | 0.42 to 1.68 | .63 | 141 | 60 | 1.59 | 0.87 to 2.89 | .13 |
| 3α-diol-3G, nmol/L | ||||||||||
| < 2.23 | 22 | 16 | 1.00 | Referent | 32 | 16 | 1.00 | Referent | ||
| 2.23 to < 3.01 | 22 | 7 | 0.43 | 0.15 to 1.27 | .13 | 34 | 17 | 0.96 | 0.41 to 2.23 | .92 |
| 3.01 to < 4.40 | 18 | 8 | 0.61 | 0.20 to 1.84 | .38 | 34 | 14 | 0.74 | 0.30 to 1.80 | .51 |
| ≥ 4.40 | 13 | 8 | 0.83 | 0.26 to 2.62 | .74 | 41 | 13 | 0.58 | 0.24 to 1.42 | .23 |
| Continuous | 75 | 39 | 0.92 | 0.56 to 1.51 | .74 | 141 | 60 | 0.70 | 0.41 to 1.17 | .17 |
| 3α-diol-17G, nmol/L | ||||||||||
| < 4.49 | 20 | 8 | 1.00 | Referent | 34 | 15 | 1.00 | Referent | ||
| 4.49 to < 6.56 | 22 | 12 | 1.48 | 0.47 to 4.70 | .51 | 31 | 16 | 1.15 | 0.49 to 2.73 | .75 |
| 6.56 to < 9.24 | 15 | 10 | 1.77 | 0.54 to 5.76 | .34 | 40 | 18 | 1.00 | 0.43 to 2.33 | 1.00 |
| ≥ 9.24 | 17 | 8 | 1.18 | 0.34 to 4.06 | .80 | 36 | 11 | 0.68 | 0.27 to 1.72 | .42 |
| Continuous | 74 | 38 | 1.04 | 0.48 to 2.22 | .92 | 141 | 60 | 0.89 | 0.54 to 1.48 | .66 |
| ADT, pmol/L | ||||||||||
| < 493.66 | 20 | 17 | 1.00 | Referent | 35 | 11 | 1.00 | Referent | ||
| 493.66 to < 647.62 | 25 | 8 | 0.36 | 0.12 to 1.03 | .06 | 28 | 17 | 2.10 | 0.83 to 5.34 | .12 |
| 647.62 to < 874.96 | 18 | 9 | 0.57 | 0.20 to 1.62 | .29 | 36 | 15 | 1.44 | 0.57 to 3.68 | .44 |
| ≥ 874.96 | 12 | 5 | 0.45 | 0.13 to 1.62 | .22 | 42 | 17 | 1.46 | 0.56 to 3.77 | .44 |
| Continuous | 75 | 39 | 0.45 | 0.14 to 1.45 | .18 | 141 | 60 | 1.32 | 0.82 to 2.13 | .26 |
| ADT-G, nmol/L | ||||||||||
| < 54.96 | 23 | 18 | 1.00 | Referent | 30 | 13 | 1.00 | Referent | ||
| 54.96 to < 70.27 | 22 | 8 | 0.43 | 0.15 to 1.25 | .12 | 32 | 11 | 0.77 | 0.28 to 2.10 | .61 |
| 70.27 to < 101.09 | 20 | 9 | 0.52 | 0.18 to 1.52 | .23 | 34 | 21 | 1.32 | 0.53 to 3.26 | .55 |
| ≥ 101.09 | 9 | 4 | 0.51 | 0.13 to 2.09 | .35 | 45 | 14 | 0.69 | 0.26 to 1.83 | .46 |
| Continuous | 74 | 39 | 0.60 | 0.24 to 1.50 | .27 | 141 | 59 | 0.77 | 0.44 to 1.35 | .37 |
| Estrone, pmol/L | ||||||||||
| < 67.00 | 12 | 7 | 1.00 | Referent | 41 | 15 | 1.00 | Referent | ||
| 67.00 to < 84.45 | 16 | 5 | 0.55 | 0.14 to 2.22 | .40 | 37 | 16 | 1.17 | 0.50 to 2.71 | .72 |
| 84.45 to < 108.18 | 19 | 9 | 0.84 | 0.23 to 3.07 | .80 | 33 | 16 | 1.39 | 0.58 to 3.31 | .46 |
| ≥ 108.18 | 27 | 18 | 1.20 | 0.35 to 4.08 | .77 | 26 | 12 | 1.21 | 0.47 to 3.11 | .69 |
| Continuous | 74 | 39 | 1.14 | 0.47 to 2.76 | .77 | 137 | 59 | 1.24 | 0.58 to 2.68 | .58 |
| Estradiol, pmol/L | ||||||||||
| < 52.23 | 12 | 3 | 1.00 | Referent | 43 | 11 | 1.00 | Referent | ||
| 52.23 to < 65.98 | 19 | 7 | 1.56 | 0.33 to 7.44 | .58 | 35 | 15 | 1.67 | 0.68 to 4.12 | .26 |
| 65.98 to < 86.76 | 20 | 13 | 3.14 | 0.68 to 14.39 | .14 | 34 | 21 | 2.55 | 1.06 to 6.16 | .04 |
| ≥ 86.76 | 24 | 16 | 3.34 | 0.71 to 15.80 | .13 | 29 | 13 | 1.86 | 0.71 to 4.87 | .21 |
| Continuous | 75 | 39 | 1.48 | 0.59 to 3.71 | .40 | 141 | 60 | 1.80 | 0.85 to 3.79 | .12 |
| Estradiol to testosterone ratio | ||||||||||
| < 0.004 | 17 | 4 | 1.00 | Referent | 38 | 17 | 1.00 | Referent | ||
| 0.004 to < 0.005 | 18 | 11 | 2.66 | 0.70 to 10.09 | .15 | 36 | 13 | 0.80 | 0.34 to 1.88 | .61 |
| 0.005 to < 0.007 | 20 | 10 | 2.27 | 0.56 to 9.21 | .25 | 33 | 14 | 0.95 | 0.40 to 2.28 | .91 |
| ≥ 0.007 | 20 | 14 | 3.22 | 0.85 to 12.27 | .09 | 34 | 16 | 1.10 | 0.47 to 2.58 | .83 |
| Continuous | 75 | 39 | 1.48 | 0.63 to 3.46 | .36 | 141 | 60 | 1.31 | 0.64 to 2.67 | .45 |
| Testosterone to DHT ratio | ||||||||||
| < 0.088 | 18 | 6 | 1.00 | Referent | 37 | 13 | 1.00 | Referent | ||
| 0.088 to < 0.105 | 20 | 14 | 2.13 | 0.63 to 7.20 | .22 | 33 | 18 | 1.56 | 0.66 to 3.69 | .31 |
| 0.105 to < 0.127 | 21 | 8 | 1.14 | 0.31 to 4.16 | .85 | 33 | 11 | 0.95 | 0.37 to 2.42 | .92 |
| ≥ 0.127 | 16 | 11 | 2.11 | 0.61 to 7.34 | .24 | 38 | 18 | 1.28 | 0.54 to 3.02 | .58 |
| Continuous | 75 | 39 | 0.91 | 0.51 to 1.61 | .74 | 141 | 60 | 0.81 | 0.36 to 1.84 | .62 |
| Estrone to androstenedione ratio | ||||||||||
| < 0.025 | 15 | 11 | 1.00 | Referent | 40 | 20 | 1.00 | Referent | ||
| 0.025 to < 0.033 | 19 | 13 | — | — | 38 | 15 | 0.77 | 0.34 to 1.76 | .54 | |
| 0.033 to < 0.042 | 27 | 15 | — | — | 34 | 8 | 0.44 | 0.16 to 1.18 | .10 | |
| ≥ 0.042 | 27 | 15 | — | — | 25 | 16 | 1.42 | 0.57 to 3.55 | .45 | |
| Continuous | 74 | 39 | 1.14 | 0.54 to 2.42 | .73 | 137 | 59 | 1.21 | 0.59 to 2.47 | .60 |
| Estradiol to estrone ratio | ||||||||||
| < 0.679 | 20 | 7 | 1.00 | Referent | 33 | 13 | 1.00 | Referent | ||
| 0.679 to < 0.818 | 17 | 12 | 2.06 | 0.65 to 6.56 | .22 | 36 | 16 | 1.23 | 0.50 to 3.00 | .65 |
| 0.818 to < 0.942 | 18 | 10 | 1.64 | 0.51 to 5.32 | .41 | 34 | 9 | 0.72 | 0.27 to 1.93 | .51 |
| ≥ 0.942 | 19 | 10 | 1.55 | 0.48 to 5.05 | .47 | 34 | 21 | 1.66 | 0.71 to 3.87 | .24 |
| Continuous | 74 | 39 | 1.81 | 0.49 to 6.59 | .37 | 137 | 59 | 1.42 | 0.62 to 3.26 | .40 |
| Sum of estrone plus estradiol | ||||||||||
| < 123.23 | 12 | 5 | 1.00 | Referent | 41 | 13 | 1.00 | Referent | ||
| 123.23 to < 153.58 | 15 | 7 | 1.19 | 0.29 to 4.90 | .81 | 38 | 11 | 0.89 | 0.35 to 2.26 | .81 |
| 153.58 to < 195.12 | 22 | 11 | 1.29 | 0.34 to 4.96 | .71 | 31 | 26 | 2.81 | 1.20 to 6.58 | .02 |
| ≥ 195.12 | 25 | 16 | 1.72 | 0.43 to 6.84 | .44 | 27 | 9 | 1.04 | 0.37 to 2.94 | .93 |
| Continuous | 74 | 39 | 1.28 | 0.52 to 3.18 | .59 | 137 | 59 | 1.46 | 0.67 to 3.16 | .34 |
| Estradiol to sum of ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio | ||||||||||
| < 0.0005 | 11 | 4 | 1.00 | Referent | 43 | 14 | 1.00 | Referent | ||
| 0.0005 to < 0.0008 | 14 | 2 | 0.43 | 0.07 to 2.88 | .39 | 40 | 16 | 1.29 | 0.55 to 3.04 | .56 |
| 0.0008 to < 0.0011 | 21 | 12 | 1.99 | 0.48 to 8.31 | .35 | 33 | 13 | 1.34 | 0.52 to 3.42 | .55 |
| ≥ 0.0011 | 27 | 20 | 3.23 | 0.69 to 15.08 | .14 | 25 | 16 | 2.25 | 0.84 to 5.97 | .10 |
| Continuous | 73 | 38 | 1.17 | 0.77 to 1.78 | .47 | 141 | 59 | 1.41 | 0.77 to 2.57 | .26 |
| Sum of estrone plus stradiol to sum ADT-G, 3α-diol-3G, 3α-diol-17G, and DHT ratio | ||||||||||
| < 0.0011 | 9 | 4 | 1.00 | Referent | 44 | 11 | 1.00 | Referent | ||
| 0.0011 to < 0.0016 | 17 | 4 | 0.56 | 0.11 to 2.81 | .48 | 35 | 22 | 2.72 | 1.13 to 6.56 | .03 |
| 0.0016 to < 0.0023 | 18 | 10 | 1.45 | 0.33 to 6.37 | .62 | 35 | 11 | 1.36 | 0.49 to 3.76 | .55 |
| ≥ 0.0023 | 28 | 20 | 2.19 | 0.49 to 9.74 | .30 | 23 | 14 | 2.71 | 0.94 to 7.85 | .07 |
| Continuous | 72 | 38 | 1.09 | 0.74 to 1.60 | .66 | 137 | 58 | 1.22 | 0.63 to 2.38 | .56 |
Abbreviations: 3α-diol-3G, 3-androstanediol-3 glucuronide; 3α-diol-17G, 3-androstanediol-17 glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; OR, odds ratio.
Adjusted for race, date at blood draw, and age at blood draw. Continuous sex steroid hormone values were standardized to half of difference between 75th and 25th centiles of distribution before correlative analysis.
Footnotes
Supported in part by intramural funds from the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI); by NCI Research Grant No. CA167552 (for the Health Professionals Follow-Up Study); and by NCI Grants No. CA097193, CA34944, CA40360, HL26490, and HL34595 (for the Physicians' Health Study). The EPIC (European Prospective Investigation Into Cancer and Nutrition) study was supported in part by the Deutsche Krebshilfe, Deutsches Krebsforschungszentrum, and Federal Ministry of Education and Research (Germany); the Swedish Cancer Society, Swedish Scientific Council, and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research United Kingdom and UK Medical Research Council (United Kingdom); Danish Cancer Society (Denmark); Italian Association for Research on Cancer, National Research Council Italy, and HuGeF Foundation (Italy); Instituto de Salud Carlos III Red Temática de Investigación Cooperativa en Cáncer (Spain); and the Hellenic Health Foundation, Stavros Niarchos Foundation, and Hellenic Ministry of Health and Social Solidarity (Greece).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Louise A. Brinton, Michael B. Cook
Financial support: Louise A. Brinton
Administrative support: Louise A. Brinton, Michael B. Cook
Provision of study materials or patients: Louise A. Brinton, Karin B. Michels, Howard Sesso, Stephen K. Van Den Eeden, Elio Riboli, Elisabete Weiderpass
Collection and assembly of data: Louise A. Brinton, Tim J. Key, Laurence N. Kolonel, Karin B. Michels, Howard D. Sesso, Giske Ursin, Stephen K. Van Den Eeden, Dominick Parisi, Laurel Habel, Claudine J. Isaacs, Elio Riboli, Elisabete Weiderpass, Michael B. Cook
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prediagnostic Sex Steroid Hormones in Relation to Male Breast Cancer Risk
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Louise A. Brinton
No relationship to disclose
Tim J. Key
No relationship to disclose
Laurence N. Kolonel
No relationship to disclose
Karin B. Michels
No relationship to disclose
Howard D. Sesso
Research Funding: Pfizer (Inst)
Giske Ursin
No relationship to disclose
Stephen K. Van Den Eeden
Research Funding: GlaxoSmithKline, Takeda Pharmaceuticals, Takeda Pharmaceuticals (I), sanofi-aventis (I), Abbott Molecular
Shannon N. Wood
No relationship to disclose
Roni T. Falk
No relationship to disclose
Dominick Parisi
No relationship to disclose
Chantal Guillemette
No relationship to disclose
Patrick Caron
No relationship to disclose
Véronique Turcotte
No relationship to disclose
Laurel Habel
Research Funding: Takeda Pharmaceuticals (Inst), Genentech (Inst)
Claudine J. Isaacs
No relationship to disclose
Elio Riboli
No relationship to disclose
Elisabete Weiderpass
No relationship to disclose
Michael B. Cook
No relationship to disclose
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710–711. doi: 10.1136/jmg.2009.075176. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 4.Brinton LA, Richesson DA, Gierach GL, et al. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst. 2008;100:1477–1481. doi: 10.1093/jnci/djn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton LA, Cook MB, McCormack V, et al. Anthropometric and hormonal risk factors for male breast cancer: Male breast cancer pooling project results. J Natl Cancer Inst. 2014;106:djt465. doi: 10.1093/jnci/djt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Avanzo B, La Vecchia C. Risk factors for male breast cancer. Br J Cancer. 1995;71:1359–1362. doi: 10.1038/bjc.1995.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewertz M, Holmberg L, Tretli S, et al. Risk factors for male breast cancer: A case-control study from Scandinavia. Acta Oncol. 2001;40:467–471. doi: 10.1080/028418601750288181. [DOI] [PubMed] [Google Scholar]
- 8.Guénel P, Cyr D, Sabroe S, et al. Alcohol drinking may increase risk of breast cancer in men: A European population-based case-control study. Cancer Causes Control. 2004;15:571–580. doi: 10.1023/B:CACO.0000036154.18162.43. [DOI] [PubMed] [Google Scholar]
- 9.Hsing AW, McLaughlin JK, Cocco P, et al. Risk factors for male breast cancer (United States) Cancer Causes Control. 1998;9:269–275. doi: 10.1023/a:1008869003012. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KC, Pan S, Mao Y. Risk factors for male breast cancer in Canada, 1994-1998. Eur J Cancer Prev. 2002;11:253–263. doi: 10.1097/00008469-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kanhai RC, Hage JJ, van Diest PJ, et al. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24:74–80. doi: 10.1097/00000478-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Medras M, Filus A, Jozkow P, et al. Breast cancer and long-term hormonal treatment of male hypogonadism. Breast Cancer Res Treat. 2006;96:263–265. doi: 10.1007/s10549-005-9074-y. [DOI] [PubMed] [Google Scholar]
- 13.Symmers WS. Carcinoma of breast in trans-sexual individuals after surgical and hormonal interference with the primary and secondary sex characteristics. BMJ. 1968;2:83–85. doi: 10.1136/bmj.2.5597.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SR, Evans PJ, Holland PA, et al. Invasive breast cancer after initiation of testosterone replacement therapy in a man: A warning to endocrinologists. Endocr Pract. 2008;14:201–203. doi: 10.4158/EP.14.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DB, Jimenez LM, McTiernan A, et al. Breast cancer in men: Risk factors with hormonal implications. Am J Epidemiol. 1992;135:734–748. doi: 10.1093/oxfordjournals.aje.a116360. [DOI] [PubMed] [Google Scholar]
- 16.Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100:814–818. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paduch DA, Fine RG, Bolyakov A, et al. New concepts in Klinefelter syndrome. Curr Opin Urol. 2008;18:621–627. doi: 10.1097/MOU.0b013e32831367c7. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Schoemaker MJ, Higgins CD, et al. Cancer incidence and mortality in men with Klinefelter syndrome: A cohort study. J Natl Cancer Inst. 2005;97:1204–1210. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 19.Key TJ. Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids. 2011;76:812–815. doi: 10.1016/j.steroids.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Key TJ, Appleby PN, Reeves GK, et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–1019. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutler JL, Ramcharan S, Feldman R, et al. Multiphasic checkup evaluation study: 1. Methods and population. Prev Med. 1973;2:197–206. doi: 10.1016/0091-7435(73)90064-9. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 23.Jellum E, Andersen A, Lund-Larsen P, et al. The JANUS serum bank. Sci Total Environ. 1993;139-140:527–535. doi: 10.1016/0048-9697(93)90049-c. [DOI] [PubMed] [Google Scholar]
- 24.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: Baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 26.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation Into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 27.Sesso HD, Gaziano JM, VanDenburgh M, et al. Comparison of baseline characteristics and mortality experience of participants and nonparticipants in a randomized clinical trial: The Physicians' Health Study. Control Clin Trials. 2002;23:686–702. doi: 10.1016/s0197-2456(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 28.Birmann BM, Neuhouser ML, Rosner B, et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood. 2012;120:4929–4937. doi: 10.1182/blood-2012-03-417253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson AL, Lorentzon M, Vandenput L, et al. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J Clin Endocrinol Metab. 2009;94:1033–1041. doi: 10.1210/jc.2008-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huhtaniemi IT, Tajar A, Lee DM, et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry: Relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- 32.Lee DM, Ulubaev A, Tajar A, et al. Endogenous hormones, androgen receptor CAG repeat length and fluid cognition in middle-aged and older men: Results from the European Male Ageing Study. Eur J Endocrinol. 2010;162:1155–1164. doi: 10.1530/EJE-09-0970. [DOI] [PubMed] [Google Scholar]
- 33.Dallal CM, Tice JA, Buist DS, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: A case-cohort study within B∼FIT. Carcinogenesis. 2014;35:346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falk RT, Brinton LA, Dorgan JF, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: A nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuhrman BJ, Schairer C, Gail MH, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaaks R, Tikk K, Sookthai D, et al. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status: Results from the EPIC cohort. Int J Cancer. 2014;134:1947–1957. doi: 10.1002/ijc.28528. [DOI] [PubMed] [Google Scholar]
- 37.Yager JD. Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention—A review. Steroids. doi: 10.1016/j.steroids.2014.08.006. [epub ahead of print on August 24, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue W, Yager JD, Wang JP, et al. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Simpson ER, Clyne C, Rubin G, et al. Aromatase: A brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 40.Chavez-Macgregor M, Clarke CA, Lichtensztajn D, et al. Male breast cancer according to tumor subtype and race: A population-based study. Cancer. 2013;119:1611–1617. doi: 10.1002/cncr.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James RE, Lukanova A, Dossus L, et al. Postmenopausal serum sex steroids and risk of hormone receptor-positive and -negative breast cancer: A nested case-control study. Cancer Prev Res (Phila) 2011;4:1626–1635. doi: 10.1158/1940-6207.CAPR-11-0090. [DOI] [PubMed] [Google Scholar]
- 42.Popli MB, Popli V, Bahl P, et al. Pictorial essay: Mammography of the male breast. Indian J Radiol Imaging. 2009;19:278–281. doi: 10.4103/0971-3026.57207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braunstein GD. Clinical practice: Gynecomastia. N Engl J Med. 2007;357:1229–1237. doi: 10.1056/NEJMcp070677. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:20–26. [PubMed] [Google Scholar]
- 45.Wikström AM, Dunkel L. Testicular function in Klinefelter syndrome. Horm Res. 2008;69:317–326. doi: 10.1159/000117387. [DOI] [PubMed] [Google Scholar]
- 46.Wosnitzer MS, Paduch DA. Endocrinological issues and hormonal manipulation in children and men with Klinefelter syndrome. Am J Med Genet C Semin Med Genet. 2013;163C:16–26. doi: 10.1002/ajmg.c.31350. [DOI] [PubMed] [Google Scholar]
- 47.Key TJ, Appleby PN, Reeves GK, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 49.Rinaldi S, Key TJ, Peeters PH, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: A study within the EPIC cohort. Int J Cancer. 2006;118:2832–2839. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- 50.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 51.Brinton LA, Richesson D, Leitzmann MF, et al. Menopausal hormone therapy and breast cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:3150–3160. doi: 10.1158/1055-9965.EPI-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breast cancer and hormone replacement therapy. Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer—Collaborative group on hormonal factors in breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 53.Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jasuja GK, Travison TG, Davda M, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2013;68:733–740. doi: 10.1093/gerona/gls216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trabert B, Graubard BI, Nyante SJ, et al. Relationship of sex steroid hormones with body size and with body composition measured by dual-energy X-ray absorptiometry in US men. Cancer Causes Control. 2012;23:1881–1891. doi: 10.1007/s10552-012-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jatoi I, Anderson WF. Qualitative age interactions in breast cancer studies: A mini-review. Future Oncol. 2010;6:1781–1788. doi: 10.2217/fon.10.139. [DOI] [PubMed] [Google Scholar]