Abstract
Purpose
Treatment and prognosis of pediatric non-Hodgkin lymphoma (NHL) have improved dramatically in the last 30 years. However, the St Jude NHL staging classification for pediatric NHL was developed more than 35 years ago. The most recent Lugano lymphoma staging classification focused on adult lymphoma. Furthermore, major limitations of the current pediatric NHL staging classification include lack of consideration of new distinct pediatric NHL histologic entities; absence of recognition of frequent skin, bone, kidney, ovarian, and other organ involvement; and lack of newer precise methods to detect bone marrow and CNS involvement, minimal disease quantification, and highly sensitive imaging technologies.
Methods
An international multidisciplinary expert panel convened in Frankfurt, Germany, in 2009 at the Third International Childhood, Adolescent and Young Adult NHL Symposium to develop a revised international pediatric NHL staging system (IPNHLSS), addressing limitations of the current pediatric NHL staging system and creating a revised classification. Evidence-based disease distribution and behavior were reviewed from multiple pediatric cooperative group NHL studies.
Results
A revised IPNHLSS was developed incorporating new histologic entities, extranodal dissemination, improved diagnostic methods, and advanced imaging technology.
Conclusion
This revised IPNHLSS will facilitate more precise staging for children and adolescents with NHL and facilitate comparisons of efficacy across different treatment strategies, various institutions, multicenter trials, and cooperative groups by allowing for reproducible pediatric-based staging at diagnosis and relapse.
INTRODUCTION
Dramatic improvements have occurred over the past 35 years in childhood and adolescent non-Hodgkin lymphoma (NHL) prognosis.1–14 Currently, localized or limited stage NHL (stage I to II) has an approximate 95% to 100% 5-year event-free survival (EFS) rate. Furthermore, the prognosis for children with advanced-stage disease (stage III to IV) has doubled from a 5-year EFS of approximately 40% 30 years ago to more than 80%.1–7,10–14
The original St Jude childhood and adolescent NHL staging system from 1980 is still used today.15 However, over the last 35 years, there has been a significant increase in identification of new pathologic entities; improvements in cytogenetic, molecular, and immunophenotypic characterizations of disease; new diagnostic methods for the detection of minimal disseminated (MDD) or residual disease (MRD); and major advances in imaging applicable to childhood and adolescent NHL. Furthermore, different pediatric cancer cooperative groups and academic institutions have developed and used different risk stratifications incorporating clinical staging.1–4,7,11,13–16
Limitations of Current Pediatric NHL Staging System
The St Jude staging system is primarily based on clinicopathologic features of childhood Burkitt's lymphoma (BL) and lymphoblastic lymphoma (LL).15 Stage is determined by the number and anatomic pattern of disease sites, their resectability, and involvement of marrow and the CNS.15 Since the introduction of the St Jude staging system, the pathologic classification of NHL has changed significantly, and new subtypes of pediatric NHL have been identified, some of which display unique patterns of organ involvement, including mucosal sites, skin, bone, ovary, and kidney.
Limitations of Ann Arbor and More Recent Lugano Classification
The original Ann Arbor staging system reported by Lister et al17 was designed without input from the pediatric oncology community and did not reference specific pediatric NHL disease entities or clinical patterns. Similarly, the most recent update, the Lugano classification, recently reported by Cheson et al,18 was developed without input from the pediatric oncology community and does not reference specific pediatric NHL disease entities.
METHODS
An international (North America, Europe, and Australia) subcommittee of multidisciplinary experts (pediatric oncology, hematopathology, imaging, and biology) in childhood and adolescent NHL was convened to develop a revised staging classification. Disease distribution and behavior of specific pediatric NHL histologic subtypes from multiple pediatric NHL trials from five pediatric cooperative groups over the last 30 years were reviewed. New pathologic entities, methods of minimal disease detection, and advances in imaging and disease extent in pediatric NHL were also reviewed. At the Third International Symposium on Childhood, Adolescent and Young Adult NHL held in Frankfurt, Germany, in 2009, a revised St Jude childhood and adolescent staging classification was presented to the international community of investigators of childhood and adolescent NHL that incorporated evidence-based disease spread and behavior derived from multiple studies of pediatric cooperative groups.1–14 The final version was approved at the Fourth International Childhood, Adolescent and Young Adult NHL Symposium in New York, New York, in 2012. Our report presents this proposed revised staging classification of childhood and adolescent NHL, representing a multidisciplinary international collaboration of experts in childhood and adolescent NHL.
RESULTS
Pathologic Classification
A pathologic diagnosis of NHL is dependent on identification of specific morphologic features in combination with immunophenotype that can be determined by immunohistochemical staining of paraffin-embedded fixed tissue or flow cytometric analysis of fresh tumor cells,19–25 allowing for assignment of specific lymphoid lineage (ie, T cell, B cell, or natural killer cell) and identifying patterns associated with cellular differentiation state or cell of origin.19–25
MDD Detection at Diagnosis
Identification of new disease-specific markers and recent technical advances have increased our ability to detect MDD with high sensitivity and specificity.22–29 Molecular assessment of disease extent may contribute to a renewed definition of disease stage in which macroscopic, microscopic, and molecular features are integrated. This integrated approach may also have consequences on risk stratification of patients within the same subtype of NHL.26–28,30
BL.
Since the early use of specific MYC-IGH gene rearrangements as markers in BL, it has become evident that molecular techniques, such as long-distance polymerase chain reaction (PCR), could be used to complement diagnosis and determine marrow or blood involvement.22,29,31 Recently, the analysis of idiotypic immunoglobulin H rearrangements has be used to detect MDD in the great majority of patients with B-cell NHL (BL and diffuse large B-cell lymphoma), with a sensitivity in the range of 0.01% to 0.001%.25 Moreover, patients with high-risk BL with minimal disease involvement of the marrow at diagnosis had a significantly worse prognosis than patients with MDD-negative BL (Fig 1).30
Fig 1.
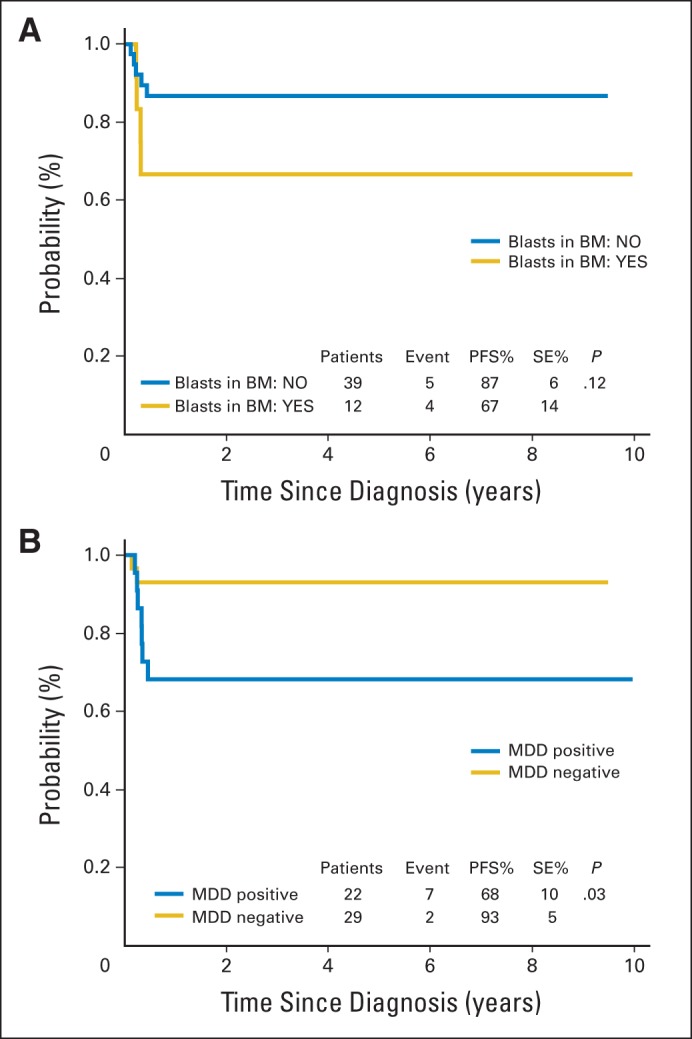
Survival analysis. Progression-free survival (PFS) analysis (A) in patients with Burkitt's lymphoma at high risk (Berlin-Frankfurt-Munster high-risk group R4) according to morphologic bone marrow (BM) status at diagnosis and (B) according to minimal disseminated disease (MDD) status in BM at diagnosis. Data adapted.30
LL.
The immunophenotype and genetic abnormalities of LL cells are similar to those of acute lymphoblastic leukemia cells. The sensitive and specific methodologies used for MRD monitoring in acute lymphoblastic leukemia—including PCR amplification of specific genetic abnormalities and clonal immunoglobulin or T-cell receptor gene rearrangements and flow cytometric analysis with a combination of LL blast specific markers—can be used to detect submicroscopic disseminated disease in either blood or marrow in LL, with a sensitivity of 0.01% and high specificity (Fig 2).26,32–34
Fig 2.
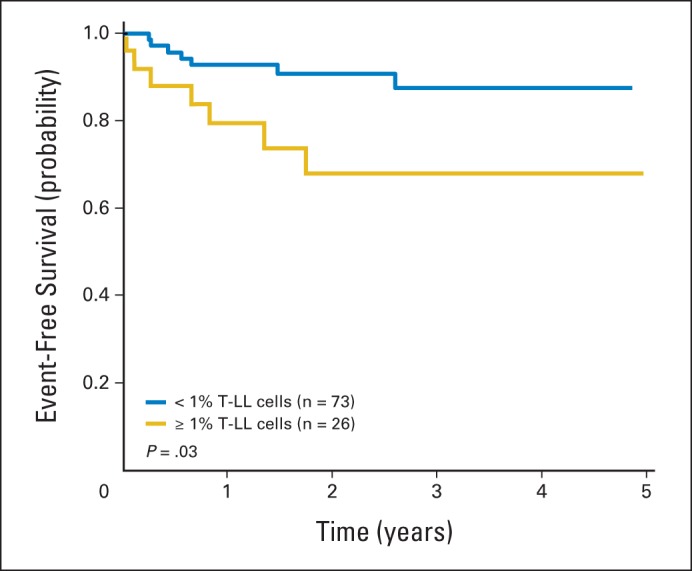
Event-free survival according to levels of T-cell lymphoblastic lymphoma (T-LL) cells in bone marrow at diagnosis measured by flow cytometry. Data adapted.26
Anaplastic large-cell lymphoma.
The nucleophosmin anaplastic lymphoma kinase (ALK) fusion gene (NPM-ALK) from the t(2;5)(p23;q35) chromosomal translocation can be found in more than 90% of pediatric cases of anaplastic large-cell lymphoma (ALCL) and can be used as a molecular disease marker, detectable by PCR or immunohistochemistry for ALK protein overexpression.27,35,36 Availability of anti-ALK antibody has also significantly increased the ability to detect low levels of ALCL tumor cells infiltrating the marrow that could not be identified by morphologic examination of marrow smears or trephines. PCR-based assays have allowed assessment of the negative prognostic role of MDD at diagnosis in ALCL (Fig 3),24,27,37 as has flow cytometric analysis of marrow and blood using anti-ALK–specific antibodies.37
Fig 3.
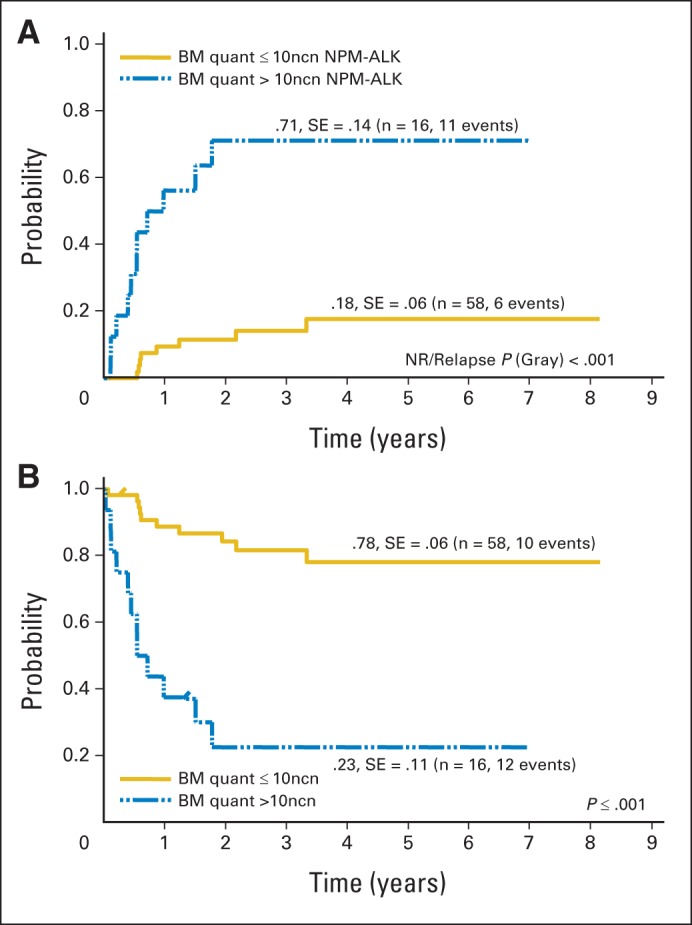
Outcome of patients with anaplastic large-cell lymphoma according to quantitative polymerase chain reaction (PCR) results for NPM-ALK in bone marrow. (A) Cumulative incidence of relapse and (B) Kaplan-Meier estimates of event-free survival of 74 patients with either negative qualitative bone marrow (BM) PCR or positive qualitative BM PCR using quantitative (quant) PCR results and NPM-ALK cutoff copy number of 10 of 104 copies of ABL (normalized copy number). NR, nonrelapse. Data adapted.27
Advances in Diagnostic Imaging
Contrast-enhanced computed tomography (CT) has served as the main imaging modality for defining the burden and extent of pediatric NHL since the St Jude staging system was introduced.15 Magnetic resonance imaging (MRI) has been used in staging of pediatric NHL as an alternative to CT for patients with contraindications to iodinated contrast and as a preferred method to evaluate primary skeletal or CNS involvement. A reappraisal of the role of diagnostic imaging in staging of pediatric NHL is warranted in view of advances in CT and MRI technology, availability of [18F]fluorodeoxyglucose (FDG) –positron emission tomography (PET), development of combined PET-CT and PET-MRI scanners, and characterization of the relative diagnostic efficacy of these imaging modalities. Newer applications, such as perfusion CT and spectral CT, permit characterization of tumor properties beyond just size and enable the transition of CT from a purely morphologic to a functional imaging technique.38 Also, new MRI techniques, such as perfusion MRI, diffusion-weighted MRI, magnetic resonance elastography, and magnetic resonance spectroscopy, permit investigation of tumor metabolism and function rather than just morphology.39 More recently, Klenk et al40 compared whole-body diffusion MRI with ferumoxytol-T1–weighted images with FDG-PET–CT in children and young adults with cancer. Unfortunately in this study, only five patients with NHL were studied, and none were age younger than 8 years. Ferumoxytol, an iron-containing compound, is not currently approved in children, and the serious adverse events related to this iron compound in children are currently unknown.
The coregistration of FDG-PET and CT or MRI images provided by combined PET-CT and PET-MRI scanners allows more precise correlation of radiopharmaceutical uptake with anatomic sites, permitting better differentiation between physiologic and tumor uptake and more accurate tumor localization. Tumor cells that hyperconcentrate the glucose analog FDG because of increased glucose transporter activity and glycolysis are detectable by FDG-PET imaging. Increased FDG uptake is exhibited by more than 97% of sites of the aggressive high-grade forms of NHL typically encountered in the pediatric population and in 80% to 90% of sites of more rare, indolent forms of NHL, such as follicular lymphoma and extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue.41,42 The more recent report by Barrington et al43 revising the staging and response criteria by PET-CT and the proposed 5-point scale to grade response was entirely focused on adult diffuse large B-cell lymphoma and follicular lymphoma histologies, with no reference to pediatric NHL disease entities.
New Proposed International Pediatric NHL Staging System
The St Jude staging system has been an invaluable tool to define disease extent in pediatric NHL (Table 1).15 Our proposed revised international staging system includes modifications in stage definitions and the inclusion of new information, such as additional staging information, to incorporate recent medical progress (Tables 2 and 3).
Table 1.
Murphy Staging System
| Murphy Staging System |
|---|
| Stage I |
| Single tumor (extranodal) or single anatomical area (nodal), with exclusion of mediastinum or abdomen |
| Stage II |
| Single tumor (extranodal) with regional node involvement |
| ≥ Two nodal areas on same side of diaphragm |
| Two single (extranodal) tumors ± regional node involvement on same side of diaphragm |
| Primary GI tract tumor, usually in ileocecal area, ± involvement of associated mesenteric nodes only* |
| Stage III |
| Two single tumors (extranodal) on opposite sides of diaphragm |
| ≥ Two nodal areas above and below diaphragm |
| All primary intrathoracic tumor (mediastinal, pleural, thymic) |
| All extensive primary intra-abdominal disease* |
| All paraspinal or epidural tumors, regardless of other tumor sites |
| Stage IV |
| Any of above with initial involvement of CNS and/or bone marrow involvement† |
NOTE. Data adapted.15
Distinction is made between apparently localized GI tract lymphoma versus more extensive intra-abdominal disease because of their quite different patterns of survival after appropriate therapy. Stage II disease is typically limited to segment of gut ± associated mesenteric nodes only, and primary tumor can be completely removed grossly by segmental excision. Stage III disease typically exhibits spread via lymphatics to para-aortic and retroperitoneal nodes via intra-peritoneal dissemination to form implants and plaques along mesentery or peritoneum or by direct infiltration of structures adjacent to primary tumor. Ascites may be present, and complete resection of all gross tumor is not possible.
If marrow involvement is present initially, No. of abnormal cells must be ≤ 25% in otherwise normal marrow aspirate with normal peripheral blood picture.
Table 2.
International Pediatric Non-Hodgkin Lymphoma Staging System*
| International Pediatric Non-Hodgkin Lymphoma Staging System |
|---|
| Stage I |
| Single tumor with exclusion of mediastinum and abdomen (N; EN; B or S: EN-B, EN-S) |
| Stage II |
| Single EN tumor with regional node involvement |
| ≥ Two N areas on same side of diaphragm |
| Primary GI tract tumor (usually in ileocecal area), ± involvement of associated mesenteric nodes, that is completely resectable (if malignant ascites or extension of tumor to adjacent organs, it should be regarded as stage III) |
| Stage III |
| ≥ Two EN tumors (including EN-B or EN-S) above and/or below diaphragm |
| ≥ Two N areas above and below diaphragm |
| Any intrathoracic tumor (mediastinal, hilar, pulmonary, pleural, or thymic) |
| Intra-abdominal and retroperitoneal disease, including liver, spleen, kidney, and/or ovary localizations, regardless of degree of resection (except primary GI tract tumor [usually in ileocecal region] ± involvement of associated mesenteric nodes that is completely resectable) |
| Any paraspinal or epidural tumor, regardless of whether other sites are involved |
| Single B lesion with concomitant involvement of EN and/or nonregional N sites |
| Stage IV |
| Any of the above findings with initial involvement of CNS (stage IV CNS), BM (stage IV BM), or both (stage IV combined) based on conventional methods |
NOTE. For each stage, type of examination and degree of BM and CNS involvement should be specified. Based on classification proposed by Murphy.15
Abbreviations: B, bone; BM, bone marrow; EN, extranodal; N, nodal; S, skin.
Table 3.
Additional Staging Information
| Additional Staging Information |
|---|
| BM involvement |
| Stage IV disease, resulting from BM involvement, is currently defined by morphologic evidence of ≥ 5% blasts or lymphoma cells by BM aspiration; this applies to any histologic subtype and will be maintained in IPNHLSS |
| For each stage, type and degree of BM involvement (by BM aspiration) should be specified, using abbreviations below to identify involvement: |
| BMm: BM positivity by morphology (specify % lymphoma cells) |
| BMi: BM positivity by immunophenotypic methods (immunohistochemical or flow-cytometric analysis; specify % lymphoma cells) |
| BMc: BM positivity by cytogenetic or FISH analysis (specify % lymphoma cells) |
| BMmol: BM positivity by molecular techniques (PCR based; specify level of involvement) |
| Same approach should be used for PB involvement (ie, PBm, PBi, PBc, PBmol) |
| Definition of BM involvement should be obtained from analysis of bilateral BM aspirates and BM biopsy |
| CNS involvement |
| CNS is considered involved in case of: |
| Any CNS tumor mass (identified by imaging techniques [ie, CT, MRI]) |
| Cranial nerve palsy that cannot be explained by extradural lesions |
| Blasts morphologically identified in CSF |
| Condition that defines CNS positivity should be specified: CNS positive/mass, CNS positive/palsy, CNS positive/blasts |
| CSF status: CSF positivity is based on morphologic evidence of lymphoma cells |
| CSF should be considered positive when any No. of blasts is detected |
| CSF unknown (not performed, technical difficulties) |
| Similarly to BM, type of CSF involvement should be described whenever possible |
| CSFm: CSF positivity by morphology (specify No. of blasts/μL) |
| CSFi: CSF positivity by immunophenotype methods (immunohistochemical or flow cytometric analysis; specify % lymphoma cells) |
| CSFc: CSF positive by cytogenetic or FISH analysis (specify % lymphoma cells) |
| CSFmol: CSF positivity by molecular techniques (PCR based; specify level of involvement) |
NOTE. Until sufficient data are available, PET should be used with caution for staging, and PET results should be compared and discussed in light of other more consolidated imaging approaches.
Abbreviations: BM, bone marrow; CT, computed tomography; FISH, fluorescent in situ hybridization; IPNHLSS, International Pediatric Non-Hodgkin Lymphoma Staging System; MRI, magnetic resonance imaging; PB, peripheral blood; PBc, PB positivity by cytogenetic or FISH analysis; PBi, PB positivity by immunophenotype methods; PBm, PB positivity by morphology; PBmol, PB positivity by molecular techniques; PCR, polymerase chain reaction; PET, positron emission tomography.
Stage I.
Because different pathologic types of NHL, including those most recently defined by the WHO,20 present with different localization patterns in terms of both region and tissue involved, we have defined in detail specific sites of involvement, particularly with reference to nodal versus extranodal sites, specifying for the latter skin or bone involvement (Table 2). This is of special importance for selected NHL subtypes, including ALCL, which more frequently may arise with isolated localization to skin or with skin involvement together with dissemination of disease to lymph nodes and extranodal sites. Knowledge of whether extranodal localizations relate to bone lesions may also be relevant, because localized bone disease may have a better prognosis than other disease sites, at least in some NHL subtypes.44
Stage II.
In the proposed revised international pediatric NHL staging system (IPNHLSS), multiple organ involvement is considered stage III, irrespective of localization in relation to the diaphragm (Table 2). In addition, selected limited abdominal involvement, such as ileocecal involvement with or without adjacent mesenteric node involvement (that is completely resectable), is considered stage II. When there is concomitant malignant ascites or the tumor extends to different adjacent organs, the international and multidisciplinary expert panel was concordant in considering those conditions as nonlocalized disease. Thus, they should be regarded as stage III to differentiate them from the true localized abdominal NHL. Some of the specifications originally included as footnotes to the St Jude staging system15 were incorporated into the new IPNHLSS, with the aim of keeping the definition of stage II as clear as possible. Some specifications on stage II previously included in the St Jude staging definition were therefore eliminated (Tables 1 and 2).
Stage III.
The considerations related to definition of stage II disease also affect the definition of stage III NHL. In particular, two or more extranodal tumor localizations should be considered stage III, independent of their localization in relation to the diaphragm. In addition, tumor localization, including lymph nodes, skin, bone, ovary, and kidney, should be explicitly specified (Table 2). Definition of intrathoracic tumor was clarified to include hilar and pulmonary localizations of disease in addition to mediastinal, pleural, and thymic sites. For abdominal and retroperitoneal localizations, except for ileocecal disease (with or without local lymph node involvement) that is completely resected, any other disease in the abdomen should be considered stage III. Explicit mention of liver, spleen, kidney, or ovarian localization of NHL has also been introduced into this new system for more specific clarification. Also, a single bone lesion together with nonregional nodal or extranodal disease should be considered stage III disease. Paraspinal or epidural tumors are also defined as stage III disease, independent of whether other sites are involved. This was addressed in the original version of the St Jude staging system; however, in the new IPNHLSS, it has been more clearly specified (Table 2).
Stage IV.
Stage IV pediatric NHL has historically been defined as marrow and/or CNS involvement; this definition has been refined in the revised IPNHLSS. However, the past definition did not specify which site conferred the feature of stage IV or whether there was concomitant involvement of both marrow and CNS. From published data, it is evident that marrow and, even more importantly, CNS disease have different prevalence in different selected NHL subtypes; for example, CNS involvement is rare in ALCL.7,24,45 Moreover, CNS involvement usually carries a more negative prognostic impact than marrow involvement, and CNS positivity represents a significant risk factor even in pediatric NHL subtypes.4,46 Directly linked to definition of stage IV are the methods used to detect marrow or CNS involvement, given that by using different technical approaches, there may be remarkably different degrees of sensitivity. This may influence the definition of stage IV and consequently the risk classification of patients, with obvious therapeutic and prognostic implications. To this aim, additional staging information (Table 3) should be collected that specifies features of marrow and CNS involvement, particularly related to sensitivity and methods of analysis used for their detection, but we recommend defining as stage IV those with bone marrow (BM) positivity by morphology or CSF positivity by morphology and leaving patients with BM positivity by cytogenic or fluorescent in situ hybridization (FISH) analysis, by immunophenotypic methods, or by molecular techniques in the stages otherwise assigned (Table 3); the same criteria apply to CSF. Thus, there might be a patient with stage II (eg, extranodal Waldeyer ring) BM positivity and CSF negativity by molecular techniques or another patient with abdominal stage III BM negativity by cytogenic or FISH analysis and BM positivity by molecular techniques.
Additional Staging Information
Although morphologic examination of marrow smears and morphologic examination of CSF are still routinely performed, use of specific monoclonal antibodies and new techniques, including flow cytometry and PCR, have added much in terms of specificity and sensitivity to our ability to detect marrow and CNS involvement.25,26,28,29,47 As discussed, this has clear implications on the definition of stage IV and, consequently, on therapeutic decisions. Therefore, it would be necessary to gain information on the levels of sensitivity and specificity by which the presence of malignant cells have been detected. To this aim, four categories corresponding to different technical levels of sensitivity and/or specificity were identified. They include BM morphology, immunohistochemical or flow cytometry methods, cytogenetic or FISH analysis, and molecular methods (PCR based; Table 3). However, to maintain continuity with the previous staging system and avoid upstaging patients on the sole basis of more sensitive techniques, we propose maintaining the definition of stage IV based on standard morphologic identification of blasts or tumor cells. These supplementary categories would be applied to the analysis of marrow and CNS. For the latter, based on emerging evidence that CNS involvement may have different implications on the overall management and prognosis of patients with NHL, depending on whether CNS positivity is the result of the presence of CNS tumor mass, neurologic deficits (ie, cranial nerve palsy), or presence of blasts in the CSF, type of CNS involvement should be reported.
DISCUSSION
For full clinical use of a staging system, we need to identify the objectives of the classification and the methodology and techniques used for staging. A staging system is most valuable when it guarantees reproducibility over extended periods of time, is applicable to different subtypes of the disease of interest, and has relevance for prognosis and treatment stratification.
Similar to the recent Lugano classification report updating the adult lymphoma Ann Arbor staging classification, revision is currently needed for the pediatric NHL classification.18 Several pediatric cancer staging systems have been revised over the last 20 years.48–50 However, other classifications, distinct from staging classification, can address more accurately issues related to prognosis and therapy. This is the case in risk groups developed for pediatric NHL, where variables other than disease localization and distribution are accounted for, including histologic subtype, lactate dehydrogenase level, and so on.
After careful evaluation and discussion of several aspects involved in the definition of stage in pediatric and adolescent NHL, agreement was reached in the international expert panel to maintain the general structure of the St Jude staging system, while introducing some modifications and more explicit indications on peculiar sites of disease, keeping in mind common clinical practice in particular entities.15 Moreover, it was decided to keep the staging system clearly distinct from risk categories, where staging is usually only one of the determinants.15 Different subtypes of childhood and adolescent NHL have diverse risk factors, and some risk factors exist only in the context of specific treatment strategies. Keeping staging as independent as possible from risk categories will eventually help compare diseases, prognoses, and treatment outcomes in different settings.
From the beginning of this project, we recognized that important progress in understanding the biology of NHL would affect prognosis and clinical management. However, technology to detect specific biologic features is often limited in accessibility, and the impact may still be influenced by therapy. For this reason and because there is evidence that specific disease characteristics, including MDD, may have a relevant influence on outcome, we introduced an additional staging information section, with the aim of encouraging clinicians and researchers to collect information on selected items related to marrow and CNS involvement. Thus, technical advances would be incorporated into a more comprehensive definition of stage and serve as a background for future studies based on available evidence. In specific conditions, only standard parameters will be collected, and less updated technologies may be used to define disease extent. This decision maintains continuity with a 30-year-old staging classification but also incorporates the most significant elements of the recent biologic and technologic progress. However, we were cautious not to overestimate the potential of new technology, such as PET scanning, that is yet to be fully validated for staging of childhood and adolescent NHL.
This revised staging system should achieve the main goals of any staging system, but it will also guarantee sufficient flexibility for further modification and future improvements. The primary aim of this revision was to provide a template that allows for a clear definition of disease extent for pediatric NHL, which can then be used in risk stratification processes, in which prognostic groups will be defined as extent of disease, but includes other biologic and pathologic features, depending on the specific type of pediatric NHL. With future collaborative studies now focusing on each pediatric histologic subtype, the precise validation of the new IPNHLSS will become more evident in the near future and will allow us to incorporate into the staging system new advances to improve the management and outcome of children and adolescents with NHL on a global scale.
Acknowledgment
This article is dedicated to Angelo Rosolen, MD, for all of his expert clinical care and brilliant research in the field of childhood and adolescent non-Hodgkin lyphoma. We thank Lauren Harrison, RN, MSN, and Erin Morris, RN, BSN, for their organizational skills and invaluable assistance in the preparation of this article.
Footnotes
See accompanying article on page 2106
Supported in part by grants from the Pediatric Cancer Research Foundation and Grant No. 1R13CA171748-01 from the National Cancer Institute (M.S.C.) and by Grant No. CA 21765 from the National Cancer and grants from the American Lebanese Syrian Associated Charities (J.T.S.).
Presented in part at the Third and Fourth International Childhood, Adolescent and Young Adult NHL Symposia, Frankfurt, Germany, June 11-13, 2009, and New York, NY, November 1-3, 2012, respectively.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Revised International Pediatric Non-Hodgkin Lymphoma Staging System
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Angelo Rosolen
No relationship to disclose
Sherrie L. Perkins
No relationship to disclose
C. Ross Pinkerton
No relationship to disclose
R. Paul Guillerman
Consulting or Advisory Role: PTC Therapeutics, Vertex Pharmaceuticals
John T. Sandlund
No relationship to disclose
Catherine Patte
No relationship to disclose
Alfred Reiter
No relationship to disclose
Mitchell S. Cairo
Consulting or Advisory Role: sanofi-aventis
Speakers' Bureau: sanofi-aventis
REFERENCES
- 1.Abromowitch M, Sposto R, Perkins S, et al. Shortened intensified multi-agent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: Report from the Children's Oncology Group. Br J Haematol. 2008;143:261–267. doi: 10.1111/j.1365-2141.2008.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugières L, Le Deley MC, Rosolen A, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: Results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27:897–903. doi: 10.1200/JCO.2008.18.1487. [DOI] [PubMed] [Google Scholar]
- 3.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairo MS, Sposto R, Hoover-Regan M, et al. Childhood and adolescent large-cell lymphoma (LCL): A review of the Children's Cancer Group experience. Am J Hematol. 2003;72:53–63. doi: 10.1002/ajh.10262. [DOI] [PubMed] [Google Scholar]
- 5.Cairo MS, Sposto R, Perkins SL, et al. Burkitt's and Burkitt-like lymphoma in children and adolescents: A review of the Children's Cancer Group experience. Br J Haematol. 2003;120:660–670. doi: 10.1046/j.1365-2141.2003.04134.x. [DOI] [PubMed] [Google Scholar]
- 6.Gentet JC, Patte C, Quintana E, et al. Phase II study of cytarabine and etoposide in children with refractory or relapsed non-Hodgkin's lymphoma: A study of the French Society of Pediatric Oncology. J Clin Oncol. 1990;8:661–665. doi: 10.1200/JCO.1990.8.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: Results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV B-cell non-Hodgkin lymphoma: A Children's Oncology Group report. Leukemia. 2013;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman S, Smith L, Galardy P, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: A Children's Oncology Group Report. Br J Haematol. 2014;167:394–401. doi: 10.1111/bjh.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: State of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 11.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patte C, Philip T, Rodary C, et al. High survival rate in advanced-stage B-cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: Results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol. 1991;9:123–132. doi: 10.1200/JCO.1991.9.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol. 2008;142:329–347. doi: 10.1111/j.1365-2141.2008.06988.x. [DOI] [PubMed] [Google Scholar]
- 14.Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: A report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–958. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: Dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- 16.Pinkerton R. Continuing challenges in childhood non-Hodgkin's lymphoma. Br J Haematol. 2005;130:480–488. doi: 10.1111/j.1365-2141.2005.05598.x. [DOI] [PubMed] [Google Scholar]
- 17.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe E, Harris N, Stein H, et al. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 21.Perkins SL. Work-up and diagnosis of pediatric non-Hodgkin's lymphomas. Pediatr Dev Pathol. 2000;3:374–390. doi: 10.1007/s100249910052. [DOI] [PubMed] [Google Scholar]
- 22.Busch K, Borkhardt A, Wössmann W, et al. Combined polymerase chain reaction methods to detect c-myc/IgH rearrangement in childhood Burkitt's lymphoma for minimal residual disease analysis. Haematologica. 2004;89:818–825. [PubMed] [Google Scholar]
- 23.Mussolin L, Pillon M, Conter V, et al. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J Clin Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. [DOI] [PubMed] [Google Scholar]
- 24.Mussolin L, Pillon M, d'Amore ES, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005;19:1643–1647. doi: 10.1038/sj.leu.2403888. [DOI] [PubMed] [Google Scholar]
- 25.Shiramizu B, Goldman S, Kusao I, et al. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (stage III/IV) B-cell non-Hodgkin lymphoma: A Children's Oncology Group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coustan-Smith E, Sandlund JT, Perkins SL, et al. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: A report from the children's oncology group. J Clin Oncol. 2009;27:3533–3539. doi: 10.1200/JCO.2008.21.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damm-Welk C, Busch K, Burkhardt B, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2007;110:670–677. doi: 10.1182/blood-2007-02-066852. [DOI] [PubMed] [Google Scholar]
- 28.Damm-Welk C, Schieferstein J, Schwalm S, et al. Flow cytometric detection of circulating tumour cells in nucleophosmin/anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: Comparison with quantitative polymerase chain reaction. Br J Haematol. 2007;138:459–466. doi: 10.1111/j.1365-2141.2007.06672.x. [DOI] [PubMed] [Google Scholar]
- 29.Mussolin L, Basso K, Pillon M, et al. Prospective analysis of minimal bone marrow infiltration in pediatric Burkitt's lymphomas by long-distance polymerase chain reaction for t(8;14)(q24;q32) Leukemia. 2003;17:585–589. doi: 10.1038/sj.leu.2402828. [DOI] [PubMed] [Google Scholar]
- 30.Mussolin L, Pillon M, d'Amore ES, et al. Minimal disseminated disease in high-risk Burkitt's lymphoma identifies patients with different prognosis. J Clin Oncol. 2011;29:1779–1784. doi: 10.1200/JCO.2010.32.8161. [DOI] [PubMed] [Google Scholar]
- 31.Akasaka T, Muramatsu M, Ohno H, et al. Application of long-distance polymerase chain reaction to detection of junctional sequences created by chromosomal translocation in mature B-cell neoplasms. Blood. 1996;88:985–994. [PubMed] [Google Scholar]
- 32.Campana D. Monitoring minimal residual disease in pediatric hematologic malignancies. Clin Adv Hematol Oncol. 2007;5:876–877, 915. [PubMed] [Google Scholar]
- 33.Stark B, Avigad S, Luria D, et al. Bone marrow minimal disseminated disease (MDD) and minimal residual disease (MRD) in childhood T-cell lymphoblastic lymphoma stage III, detected by flow cytometry (FC) and real-time quantitative polymerase chain reaction (RQ-PCR) Pediatr Blood Cancer. 2009;52:20–25. doi: 10.1002/pbc.21823. [DOI] [PubMed] [Google Scholar]
- 34.van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–611. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- 35.Lamant L, Meggetto F, al Saati T, et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease: Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996;87:284–291. [PubMed] [Google Scholar]
- 36.Stein H, Foss HD, Dürkop H, et al. CD30(+) anaplastic large cell lymphoma: A review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- 37.Mussolin L, Bonvini P, Ait-Tahar K, et al. Kinetics of humoral response to ALK and its relationship with minimal residual disease in pediatric ALCL. Leukemia. 2009;23:400–402. doi: 10.1038/leu.2008.184. [DOI] [PubMed] [Google Scholar]
- 38.Guillerman RP. Newer CT applications and their alternatives: What is appropriate in children? Pediatr Radiol. 2011;41(suppl 2):534–548. doi: 10.1007/s00247-011-2163-7. [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie JD, Vasanawala SS. State-of-the-art in pediatric body and musculoskeletal magnetic resonance imaging. Semin Ultrasound CT MR. 2010;31:86–99. doi: 10.1053/j.sult.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Klenk C, Gawande R, Uslu L, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: A prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15:275–285. doi: 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 41.Guillerman RP, Voss SD, Parker BR. Leukemia and lymphoma. Radiol Clin North Am. 2011;49:767–797. vii. doi: 10.1016/j.rcl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Shankar A, Fiumara F, Pinkerton R. Role of FDG PET in the management of childhood lymphomas: Case proven or is the jury still out? Eur J Cancer. 2008;44:663–673. doi: 10.1016/j.ejca.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lones MA, Perkins SL, Sposto R, et al. Non-Hodgkin's lymphoma arising in bone in children and adolescents is associated with an excellent outcome: A Children's Cancer Group report. J Clin Oncol. 2002;20:2293–2301. doi: 10.1200/JCO.2002.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Salzburg J, Burkhardt B, Zimmermann M, et al. Prevalence, clinical pattern, and outcome of CNS involvement in childhood and adolescent non-Hodgkin's lymphoma differ by non-Hodgkin's lymphoma subtype: A Berlin-Frankfurt-Munster Group Report. J Clin Oncol. 2007;25:3915–3922. doi: 10.1200/JCO.2007.11.0700. [DOI] [PubMed] [Google Scholar]
- 46.Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin's lymphoma: Results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coustan-Smith E, Sancho J, Hancock ML, et al. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002;100:2399–2402. doi: 10.1182/blood-2002-04-1130. [DOI] [PubMed] [Google Scholar]
- 48.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 49.Chantada G, Doz F, Antoneli CB, et al. A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer. 2006;47:801–805. doi: 10.1002/pbc.20606. [DOI] [PubMed] [Google Scholar]
- 50.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]


