Summary
The vertebrate heart develops from mesoderm and requires inductive signals secreted from early endoderm. During embryogenesis, Nkx2.5 acts as a key transcription factor and plays essential roles for heart formation from Drosophila to human. In mice, Nkx2.5 is expressed in the early first heart field, second heart field pharyngeal mesoderm, as well as pharyngeal endodermal cells underlying the second heart field. Currently, the specific requirements for Nkx2.5 in the endoderm versus mesoderm with regard to early heart formation are incompletely understood. Here, we performed tissue-specific deletion in mice to dissect the roles of Nkx2.5 in the pharyngeal endoderm and mesoderm. We found that heart development appeared normal after endodermal deletion of Nkx2.5 whereas mesodermal deletion engendered cardiac defects almost identical to those observed on Nkx2.5 null embryos (Nkx2.5−/−). Furthermore, re-expression of Nkx2.5 in the mesoderm rescued Nkx2.5−/− heart defects. Our findings reveal that Nkx2.5 in the mesoderm is essential while endodermal expression is dispensable for early heart formation in mammals.
Keywords: Nkx2.5, pharyngeal endoderm, pharyngeal mesoderm, second heart field, heart development
Introduction
The induction and development of the early embryonic heart is a dynamic process in vertebrates and require inductive signals secreted from the endoderm. During gastrulation, the cardiac precursors reside in the anterior region of the primitive streak. They migrate anterolaterally and form the lateral plate mesoderm that divides into somatic and splanchnic mesoderm 1–3. As embryo folding occurs, the splanchnic mesoderm cells fuse at the midline to form a cardiac crescent. Subsequently, the first heart field (FHF) precursors in the cardiac crescent further fuse to form a linear heart tube that develops to the left ventricle 1–3. The second heart field (SHF) is a subpopulation of cells located in the pharyngeal mesoderm. It contains precursors that give rise to the outflow tract, right ventricle, and atria 2,4–7.
The lateral plate and cardiac crescent mesoderm lie sub-adjacently to the endoderm. Studies from amphibians, birds and mice suggested that early endoderm elaborates heart-inducing factors (e.g., Bmps, Fgfs, Wnt inhibitors) that induce nascent mesoderm toward cardiac fates 8–16. During SHF development, endoderm-derived signals continue to play essential roles to govern SHF formation and deployment in a non-cell autonomous fashion. In mice, sonic hedgehog (Shh) is expressed in the pharyngeal endoderm (E7.5-10.5). Ablation of Shh in the endoderm or its receptor smoothened (Smo) in the SHF leads to aortic arch and outflow tract malformations 17,18. Shh is required for SHF cell proliferation and survival, and regulates migration of hedgehog-responsive cells from SHF into atrial septum and pulmonary trunk 19,20. It is largely unknown, however, whether and how the other pharyngeal endodermal signals contribute to heart formation during SHF development. This is an important and intriguing question given that several genes critical for SHF development are expressed in both pharyngeal mesoderm and endoderm (e.g., Fgf8 and Tbx1), and their endodermal expression is essential for proper SHF formation 21–25.
Nkx2.5 is a homeobox transcription factor and plays fundamental roles for early heart formation and function from Drosophila to human 26. Mice lacking Nkx2.5 (Nkx2.5−/−) die at E9.5-10.5 with severely underdeveloped heart 27,28. Haploinsufficiency for NKX2.5 in humans also causes congenital heart defects with a variety of malformations including atrial septal defect (ASD), double-outlet right ventricle (DORV) and tetralogy of Fallot 29,30. During early embryogenesis, Nkx2.5 is expressed in the FHF, SHF pharyngeal mesoderm and its adjacent endodermal cells. In this study, we performed genetic analysis and attempted to determine specific requirements of Nkx2.5 in the endoderm and mesoderm with regard to early SHF development.
Material and Methods
Animal models
Foxa2MerCreMer (denoted as Foxa2MerCreMer/+) 31, Mesp1Cre (denoted as Mesp1Cre/+) 32, Nkx2.5flox/flox (denoted as Nkx2.5f/f) 33 and Rosa26LacZ reporter (denoted as R26RLacZ/LacZ ) 34 mouse lines were described previously. To generate a conditional Nkx2.5 expression mouse model, a floxed H2B-GFP-4XpolyA cassette followed by 3XFLAG was targeted into Nkx2.5 start codon in mice. 3XFLAG sequence is fused in frame with Nkx2.5 1st exon (Fig. 7). Tamoxifen (Sigma, T5648) was administrated to the pregnant mice through oral gavage 31. Mouse husbandry was carried out according to an approved IACUC protocol at the Icahn School of Medicine at Mount Sinai.
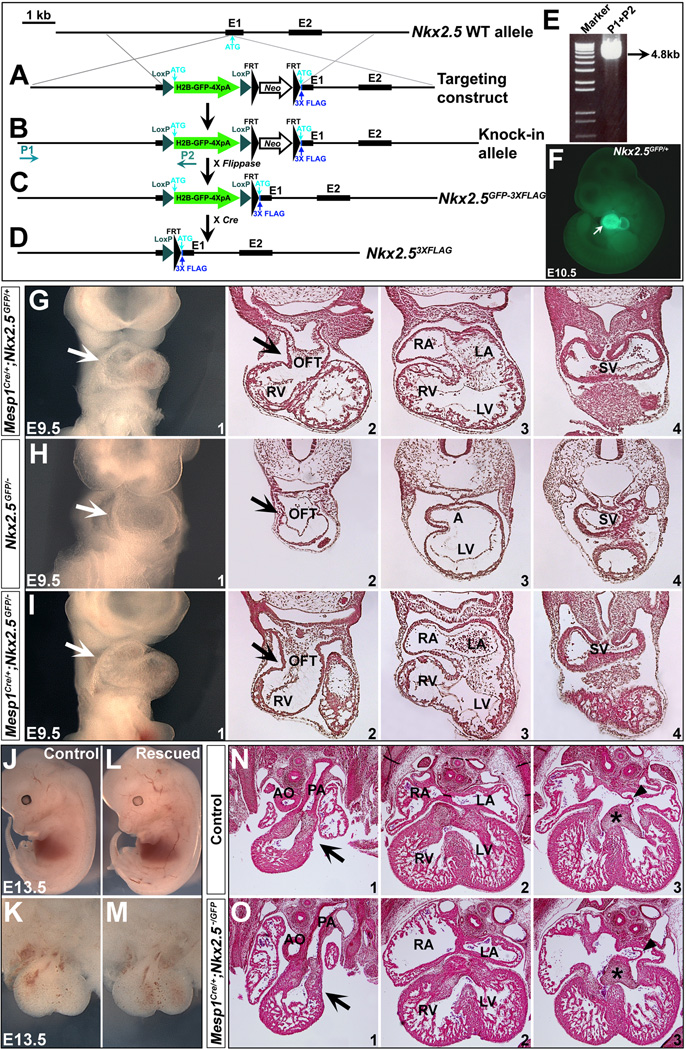
Figure 7. Normal SHF development in the Nkx2.5 null mice after re-expressing of Nkx2.5 in the mesoderm.
(A-F) Generation of Nkx2.5HH2B-GFP-3XFLAG knock-in mice. An H2B-GFP-Neo-3XFLAG cassette (loxP-H2B-GFP-4XpolyA-loxP-FRT-Neo-FRT-3XFLAG) was inserted into Nkx2.5 exon 1 (6 bp upstream of ATG, Neo cassette is flanked by two FRT sites). 3XFLAG has a Kozak sequence at the 5' end. Long-range PCR was performed to screen ES cells with a primer external to 5' targeting construct arm (P1) and a reverse primer within H2B-GFP cassette (P2) (A,B). Targeted ES clones were identified with a recombinant band of 4.8 kb further confirmed by DNA sequencing (E). Nkx2.5H2B-GFP-Neo-3XFLAG/+ mice derived from the positive ES cells were crossed to Flippase65 to remove Neo (C, Nkx2.5H2B-GFP-3XFLAG/+, also denoted as Nkx2.5GFP/+). These mice were further crossed to Protamine-Cre to obtain Nkx2.53XFLAG/+ animals (D). GFP expression in Nkx2.5GFP/+ embryo mirrors Nkx2.5 expression in the heart at E10.5 (arrow in F). (G,J,K,N) At E9.5 and E13.5, control mice (Mesp1Cre/+;Nkx2.5GFP/+) displayed normal heart development. (H) Nkx2.5GFP/− is null for Nkx2.5. (I,L,M,O) Mesp1Cre/+;Nkx2.5GFP/− mouse hearts developed normally at E9.5 and E13.5. G1/H1/I1 are frontal views (E9.5) and G2-4/H2-4/I2-4 are transverse sections of the embryos at comparable locations. J/L are embryos at E13.5 in left lateral view. K/M are front views of hearts from J/L, respectively. N1-3/O1-3 are transverse sections of hearts (K/M) at comparable locations.
Whole mount RNA in situ hybridization, histology and immunohistochemistry
Whole mount RNA in situ hybridization of mouse embryos was carried out according to Wilkinson’s protocol 35. For histology, whole mount mouse embryos were fixed in 4% paraformaldehyde, dehydrated through graded methanol and embedded in paraffin. Paraffin sections were cut at 8-µm thickness with a Leica RM 2255 microtome. Immunostaining of anti- Nkx2.5 antibody (Abcam ab35842, 1:50) was carried out on 8-µm paraffin sections with citratebased antigen retrieval procedure (10 mM sodium citrate, pH 6.0).
X-gal staining
Mouse embryos were fixed in 4% paraformaldehyde for 30 min. After permeabilization (0.02% Na deoxycholate, 0.01% NP-40 in PBS), embryos were stained in X-gal solution (5 mM Kferricyanide, 5 mM K-ferrocyanide, 2 mM MgCl2, 1 mg/ml X-gal in PBS) for 12 h.
Scanning Electron Microscopy (SEM) analysis
A standard SEM sample preparation procedure was applied to process mouse embryos 5. In brief, embryos were primarily fixed with 3% glutaraldehyde with a 0.2 M Na cacodylate buffer followed by fixation in 1% osmium tetroxide for 2 h, dehydratred in graded steps of ethanol and then critical point dried, sputter coated with gold-palladium and observed with a Hitachi S13300 scanning electron microscope at Mount Sinai.
Results and discussion
Nkx2.5 is expressed in the early pharyngeal endoderm during mouse embryogenesis
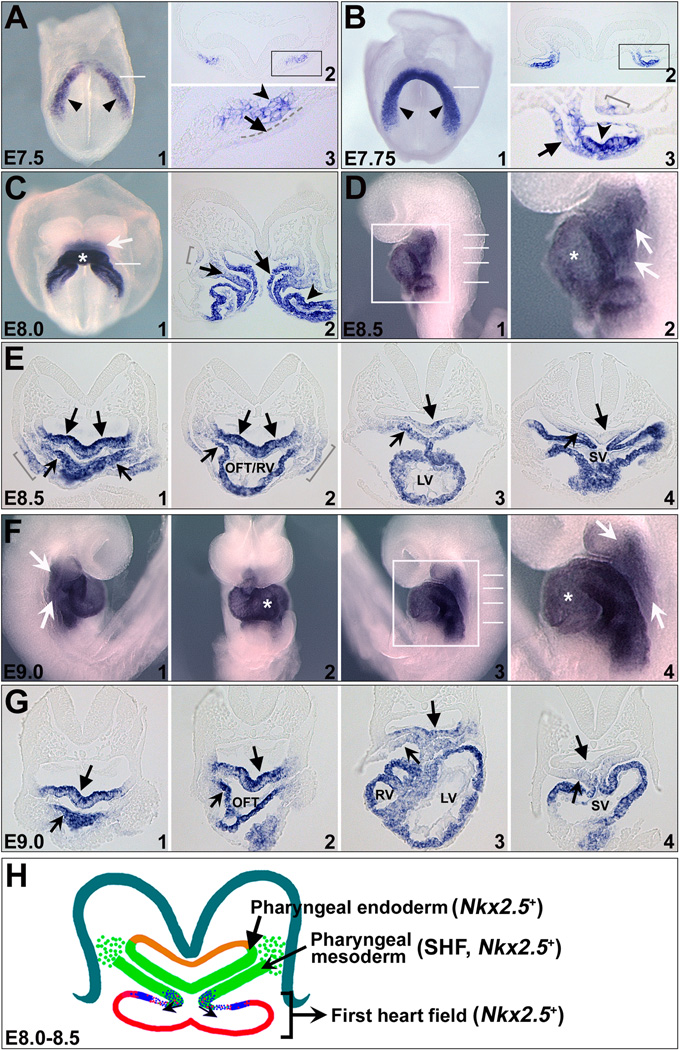
We performed whole mount RNA in situ hybridization to detect Nkx2.5 transcripts on E7.5-9.0 mouse embryos. As previously described 36–39, Nkx2.5 is expressed in the early cardiac crescent at E7.5-7.75 (unnotched arrowheads in Fig. 1 A1,B1). As development proceeds, the cardiac crescent cells fuse at the midline to form a linear heart tube, and then loops with addition of cells from the SHF (Fig. 1 C,D,F). Nkx2.5 expression encompasses all the cardiac regions, including primitive left ventricle (asterisks in Fig. 1 C1,D2,F2/4), SHF-derived outflow tract and right ventricle, as well as atria and sinus venosus (Fig. 1 E,F,G). Nkx2.5 is also detected in the pharyngeal region adjacent to the heart (arrows in Fig. 1 C1,D2,F1/4). Transverse sections of E7.75-9.0 embryos revealed that Nkx2.5+ cells are located in the pharyngeal mesoderm (notched arrows in Fig. 1 C2,E,G) and its adjacent endoderm (unnotched arrows in Fig. 1 C2,E,G) and ectoderm cells (brackets in Fig. 1 B3,C2,E1/2). We examined a series of E7.5-8.0 embryos and found that Nkx2.5 is first detected in the pharyngeal endoderm at ~E7.75 (unnotched arrows in Fig. 1 B2/3,A3). In addition, its pharyngeal expression is detected in the anterior but not in the posterior regions on E8.0-9.0 embryos (arrows in Fig. 1 E,G).
Figure 1. Expression of Nkx2.5 in the FHF, SHF and pharyngeal endoderm during early mouse embryogenesis.
(A-G) Whole-mount RNA in situ hybridization of Nkx2.5 expression in E7.5-E9.0 mouse embryos. A1/B1/C1 are frontal views and D1 is a left lateral view of mouse embryos at corresponding stages. F1/2/3 are right lateral, frontal and left lateral views of E9.0 embryo. A2/B2/C2/E1-5/G1-5 are transverse sections of embryos with approximate positions shown by white lines in A1/B1/C1/D1/F3. A3/B3/D2/F4 are high magnification images of A2/B2/D1/F3 in the square areas, respectively. Unnotched arrowheads in A1/B1 indicate cardiac crescent. Unnotched arrows in A3/B3 indicate splanchnic endoderm. Notched arrowheads in A3/B3 indicate cardiogenic mesoderm. Arrows in C/D/F indicate Nkx2.5 expression in the pharyngeal regions, including pharyngeal mesoderm (notched arrows in C2/E/G) and endoderm (unnotched arrows in C2/E/G) and ectoderm (brackets in B3,C2,E1/2). Asterisks in C/D/F indicate FHF cells. (H) Schematic of Nkx2.5 expression in E8.0-E8.5 mouse embryos with transverse section in the pharyngeal region. Nkx2.5 is expressed in both the FHF-derived linear heart tube (red) and pharyngeal mesoderm SHF (green, notched arrow), as well as pharyngeal endoderm (green, unnotched arrow). OFT, outflow tract; RV, right ventricle; LV, left ventricle.
Morphogenetic defects of Nkx2.5−/− hearts are apparent as early as E8.5 27,28. Given that Nkx2.5 is dynamically expressed in the pharyngeal endoderm and mesoderm (cardiac mesoderm and pharyngeal mesoderm), we investigated whether it has functions in both tissue layers during early cardiac development.
Elimination of Nkx2.5 in the pharyngeal endoderm does not affect heart formation
To determine potential roles for Nkx2.5 in the pharyngeal endodermal during heart development, we crossed Nkx2.5f/f;R26RLacZ/LacZ mice to Foxa2MerCreMer/+;Nkx2.5f/f mice. In this genetic cross, 50% embryos are anticipated to be mutants (Foxa2MerCreMer/+;Nkx2.5f/f;R26RLacZ/+), and the remaining 50% are controls (Nkx2.5f/f;R26RLacZ/+). The Foxa2MerCreMer allele has been shown to confer rapid, robust Cre activity in the pharyngeal endoderm, notochord, and floorplate after tamoxifen administration 31. The R26RLacZ reporter allele was used to document Cre efficiency.
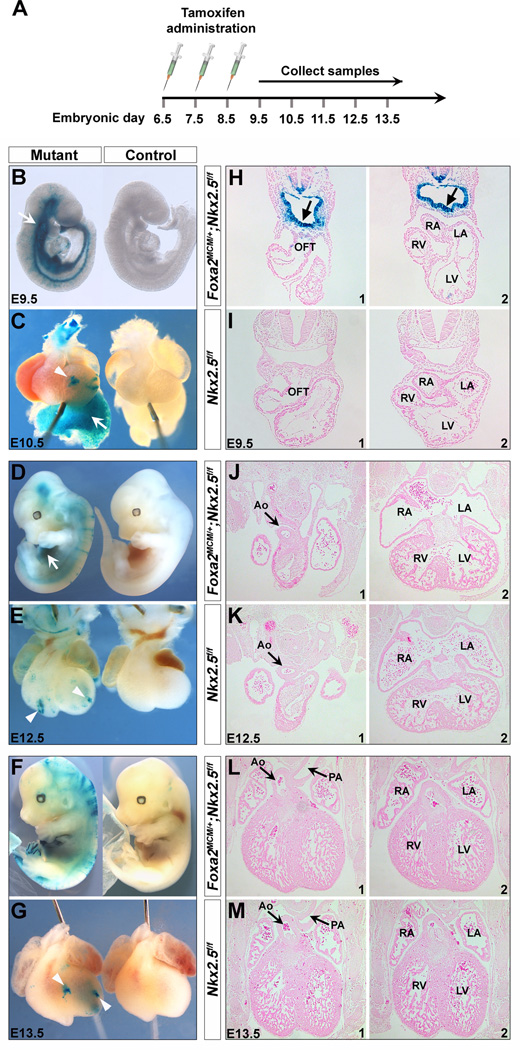
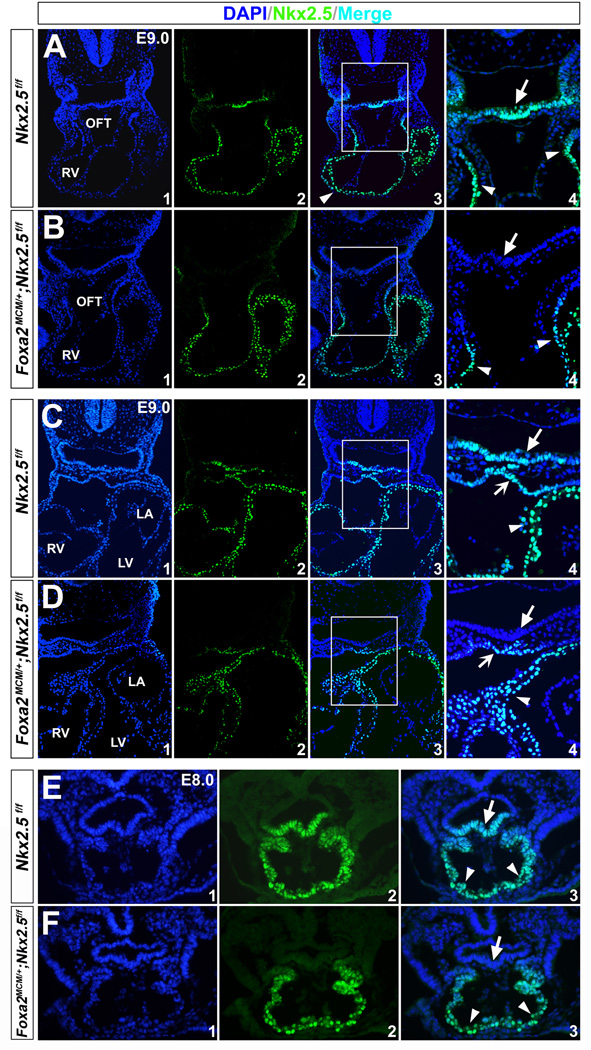
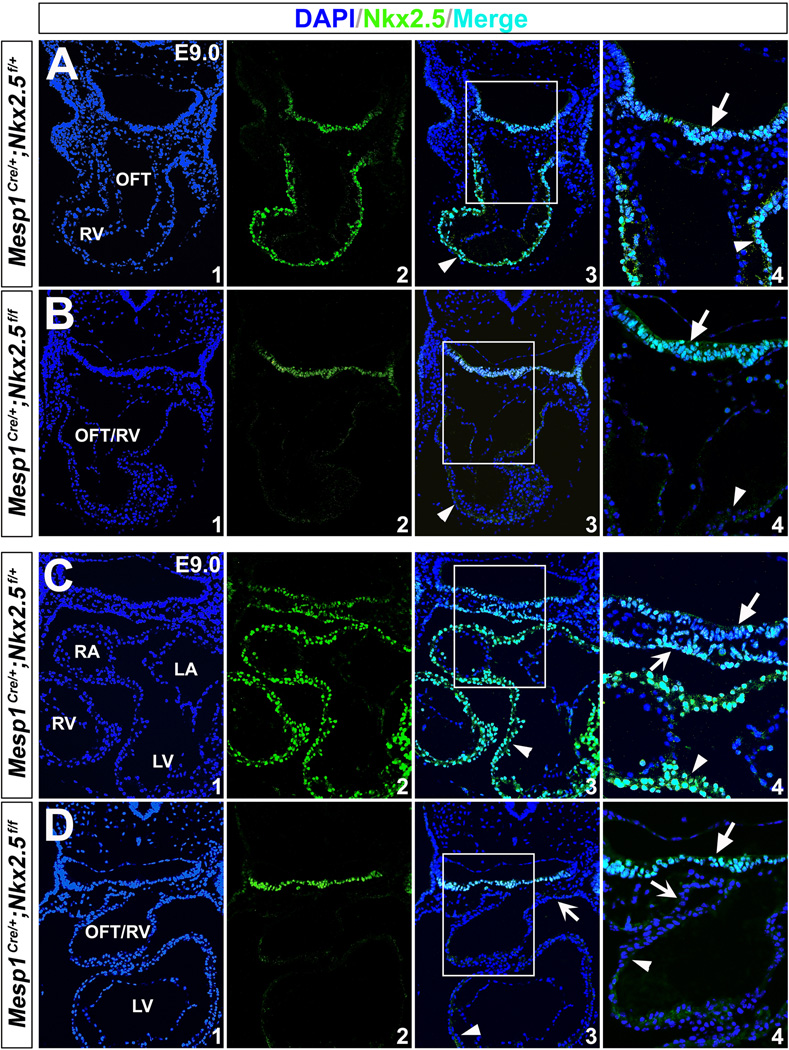
To ensure complete Cre excision, we administrated tamoxifen daily to the pregnant dams for three consecutive days from E6.5 (0.12 mg/g body weight, oral gavage) 31, and harvested the embryos at E9.5-13.5 (Fig. 2A). Robust and uniform X-gal staining was detected in the pharyngeal endoderm and other Foxa2-expressing cells (e.g., notochord and floorplate) in mouse embryos at E9.5 (Fig. 2 B,H and Fig. S1). We further performed Nkx2.5 immunostaining on mutant embryos at E9.0 and E8.0, and found that its expression was specifically removed in the pharyngeal endoderm, but not the heart or pharyngeal mesoderm (Fig. 3). These results indicate that Foxa2MerCreMer indeed mediates effective Cre recombination in the pharyngeal endoderm of Foxa2MerCreMer/+;Nkx2.5f/f;R26RLacZ/+ mice as early as E8.0.
Figure 2. Disruption of Nkx2.5 in the pharyngeal endoderm with Foxa2MerCreMer.
(A) Diagram of the time course of tamoxifen administration to pregnant females at 6.5, E7.5 and E8.5. Embryos were harvested at E9.5 (B), E10.5 (C), E12.5 (D) and E13.5 (F). (B-G) X-gal+ cells were detected in the pharyngeal endoderm (arrows in B and H) and endoderm-derived liver tissues (arrows in C and D) of mutant embryos (Foxa2MerCreMer/+;Nkx2.5f/f;R26RLacZ/+). Control littermates (Nkx2.5f/f;R26RLacZ/+) were negative for X-gal staining (B-G). Scatted X-gal+ cells were detected in the mutant hearts (arrowheads in C, E and G) 31. (H-M) Transverse sections of the embryos. Mutant hearts displayed indistinguishable morphology from their littermates. RA, right atrium; LA, left atrium; Ao, aorta; PA, pulmonary artery.
Figure 3. Nkx2.5 protein is eliminated in the pharyngeal endoderm by Foxa2MerCreMer with tamoxifen induction.
Immunostaining of Nkx2.5 on transverse sections of mutant (Foxa2MerCreMer/+;Nkx2.5f/f; R26RLacZ/+, B/D,F) and control embryos (Nkx2.5f/f;R26RLacZ/+, A/C,E) at E9.0 and E8.0. A/B, C/D and E/F are comparable sections of the control and mutant embryos at corresponding stages. (A,C,E) Consistent with RNA in situ hybridization, Nkx2.5 protein was detected in the myocardial wall (arrowheads), pharyngeal mesoderm (notched arrows) and underlying endodermal cells (unnotched arrows). (B,D,F) In the mutants, Nkx2.5 immunoreactivity was eliminated in the pharyngeal endoderm (unnotched arrows), but persisted normally in the heart tube (arrowheads) and pharyngeal mesoderm (notched arrows). A4/B4/C4/D4 are high magnification images for A3/B3/C3/D3 in the square areas, respectively.
We injected a total of 26 pregnant female mice and collected 73 E9.5 (17 litters), 10 E10.5 (2 litters), 8 E11.5 (2 litters), 13 E12.5 (2 litters) and 13 E13.5 (3 litters) mutant embryos. Interestingly, the mutant mice exhibited normal heart formation at all embryonic stages assayed (Fig. 2 B,D,F), and they can survive to birth (even to adulthood). Cardiac shape of the mutant embryos was indistinguishable from their littermate controls (Fig. 2 C,E,G). Further histological analysis of the mutant hearts also supported these initial observations: no abnormalities were detected in outflow tract or right ventricle formation (Fig. 2 H,I), aorta or pulmonary artery division (Fig. 2 J-M), or chamber septation (Fig. 2 L,M). These cardiac components are derived from, and regulated by, the SHF 5,17–25. As reported previously 31, we detected a few scattered Xgal+ cells in the mutant hearts (arrowheads in Fig. 2 C,E,G). This may be due to transient Foxa2 expression in the anterior mesoderm at the late streak and early bud stage 31,40. These scattered recombination events had no detectable effect on the cardiac development of mutant embryos.
To address Nkx2.5 function at the molecular level in Foxa2MerCreMer/+;Nkx2.5f/f mutants, we examined expression of genes in the pharyngeal endoderm and/or mesoderm with critical roles for SHF development, including Isl1 5, Shh 17–20, Tbx1 23,25,42, Fgf4/8/10 5,21,22,24,43–46, Bmp4/7 5,47,48 and Foxa2/c1/c2/h1 49–51 at E9.5. RNA in situ hybridization revealed expression of these genes was in general unchanged in the mutants (Figs. 4 A-F and S2, data not shown for Foxc1/c2/h1).
Figure 4. Endodermal deletion of Nkx2.5 has little impact on gene expressions in the pharyngeal endoderm and mesoderm.
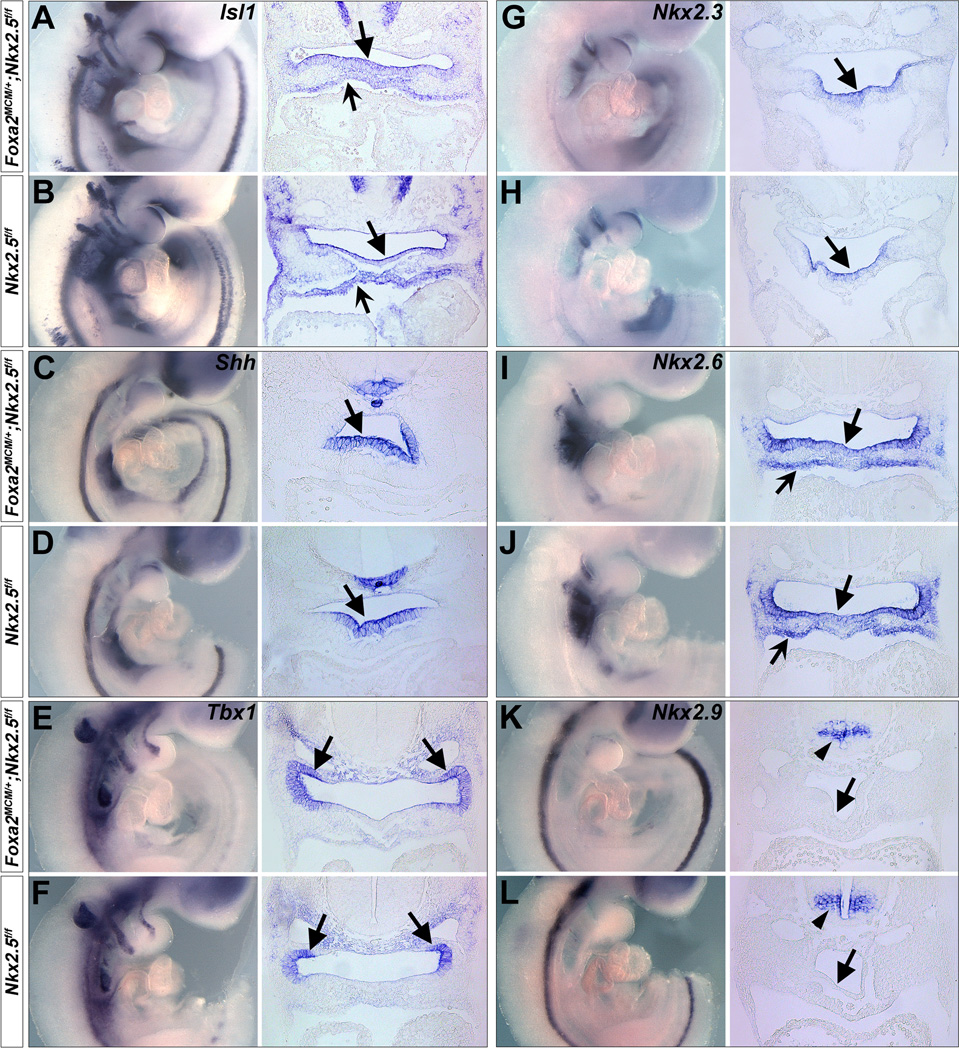
(A-F) Isl1, Shh and Tbx1 are expressed in the pharyngeal endoderm (unnotched arrows) and/or mesoderm (notched arrows) (A,C,E), and their expression is unchanged in Foxa2MerCreMer/+;Nkx2.5f/f embryos (B,D,F). (G-L) Nkx2.3 is expressed in the pharyngeal endoderm (G, unnotched arrows) and Nkx2.6 is expressed in both pharyngeal endoderm (I, unnotched arrows) and mesoderm (notched arrows). Nkx2.9 is expressed in the neural tube but not in the pharyngeal endoderm (K, unnotched arrows). Expression of these Nk-2 genes is normal in Foxa2MerCreMer/+;Nkx2.5f/f embryos. H/J/L are corresponding controls.
Mesodermal deletion of Nkx2.5 engenders similar cardiac defects to Nkx2.5 null mice
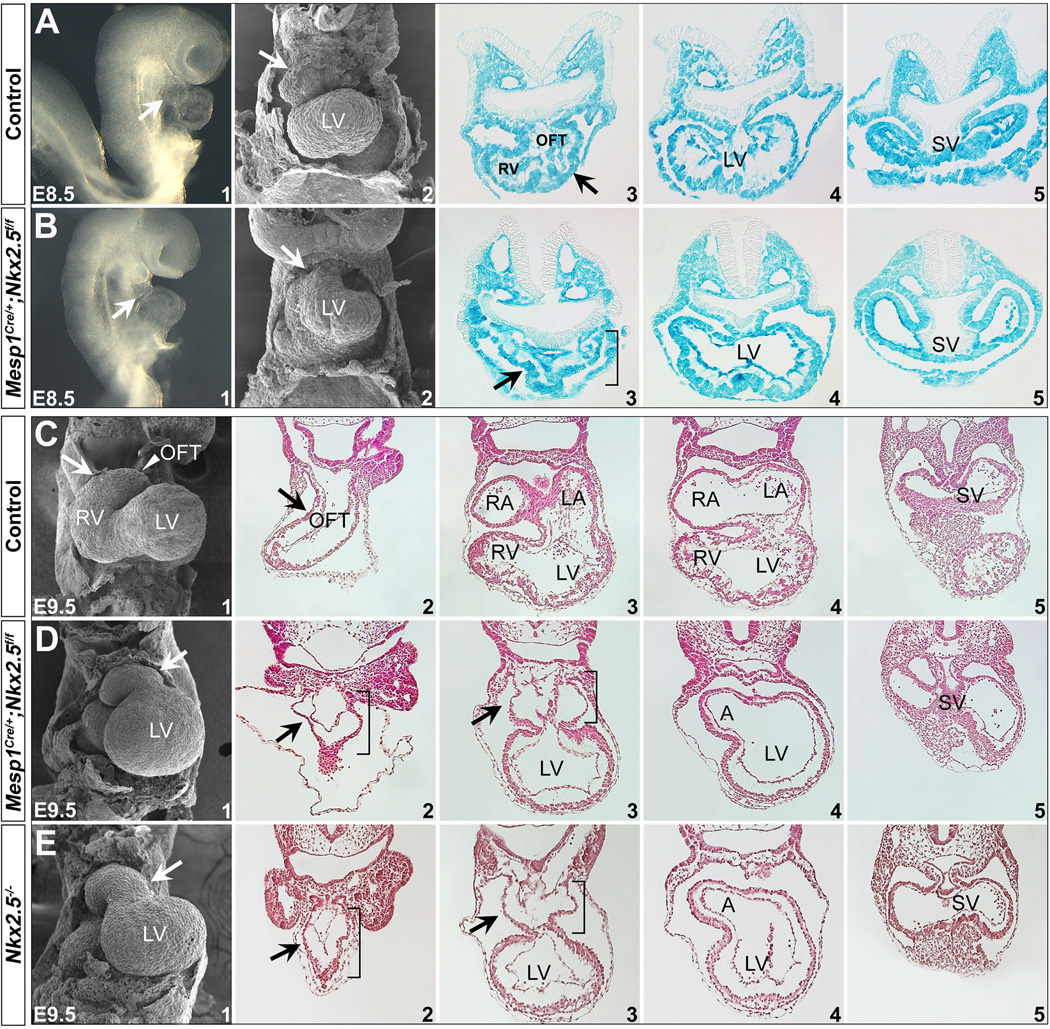
We next deleted Nkx2.5 function using Mesp1Cre which is specifically expressed in nascent mesoderm during gastrulation 32,41. Mesp1Cre progeny include all the cells in the heart and the pharyngeal mesoderm, but no cells in the endoderm or ectoderm (Fig. S3 and Ref. 32). We crossed Mesp1Cre/+;Nkx2.5f/+ mice to Nkx2.5f/f;R26RLacZ/LacZ and found all of the mutant embryos (Mesp1Cre/+;Nkx2.5f/f;R26RLacZ/+) had severely misshaped hearts as early as E8.5 (Fig. 5 A1,B1). We examined the mutant hearts with SEM which revealed perturbation of outflow tract and right ventricle formation at E8.5 (arrows in Fig. 5 A2,B2). Transverse sections of the mutant embryos also showed underdeveloped, shortened outflow tracts and right ventricles (Fig. 5 A3-5,B3-5 and bracket in B3). X-gal staining indicated mesoderm-specific deletion in both controls and mutants (Fig. 5 A3-5,B3-5). We further performed immunostaining and found Nkx2.5 protein was specifically eliminated in the mesoderm (heart, pharyngeal mesoderm), but was normal in the pharyngeal endoderm in the mutants at E9.0 (Fig. 6).
Figure 5. Disruption of Nkx2.5 in the mesoderm with Mesp1Cre.
(A,B) Mouse embryos with mesoderm-specific deletion of Nkx2.5 have cardiac malformations at E8.5. A1/B1 are right lateral views and A2/B2 are frontal views with SEM. Cardiac OFT and RV were severely underdeveloped in the mutants (arrows in B1,2). Transverse sections of control (Mesp1Cre/+;Nkx2.5f/+;R26RLacZ/+) and mutant (Mesp1Cre/+;Nkx2.5f/f;R26RLacZ/+) stained with X-gal indicates Mesp1Cre lineage includes mesoderm tissues (heart and pharyngeal mesoderm), but not ectoderm or endoderm (A3-5 and B3-5, and Fig. S3). OFT and RV defects were shown by transverse sections (arrow and bracket in A3/B3). (C-E) At E9.5, mutant hearts are unlooped and the OFT and RV are hypoplastic (D1). The general morphology of the mutant hearts is similar to that of Nkx2.5−/− hearts at E9.5 (E1). C2-5, D2-5 and E2-5 are transverse sections of the hearts. Brackets in D/E indicate hypoplastic OFT and RV.
Figure 6. Nkx2.5 expression is eliminated in the mesoderm by Mesp1Cre.
Nkx2.5 immunostaining on mutants (Mesp1Cre/+;Nkx2.5f/f;R26RLacZ/+) and littermate controls (Mesp1Cre/+;Nkx2.5f/+;R26RLacZ/+) at E9.0. (A,C) In controls, Nkx2.5 expression was detected in the myocardial wall (arrowheads), pharyngeal mesoderm (notched arrows) and endoderm cells (unnotched arrows). (B,D) In the mutants, Nkx2.5 expression was unaffected in the pharyngeal endoderm (unnotched arrows), but was absent in the heart tube (arrowheads) and pharyngeal mesoderm (notched arrows). A4/B4/C4/D4 are high magnification images for A3/B3/C3/D3 in the square areas, respectively.
We compared the general morphology of mesoderm mutants (Mesp1Cre/+;Nkx2.5f/f) to Nkx2.5−/− embryos at E9.5. Both mutant classes had hearts that did not loop with indistinguishable malformed shape in outflow tract and right ventricle (Fig. 5 D and E). Compared with controls (Fig. 5 C), Nkx2.5−/− and Mesp1Cre/+;Nkx2.5f/f embryos had a hypoplastic outflow tract and right ventricle, indicating perturbed SHF formation. Moreover, Mesp1Cre/+;Nkx2.5f/f mice did not survive beyond E10.5, as seen in Nkx2.5−/− embryos 27,28. These observations suggest a pivotal and decisive role of mesodermal Nkx2.5 for early heart development.
Re-expression of Nkx2.5 in the mesoderm rescues Nkx2.5 null SHF defects
To test whether Nkx2.5 expression in the pharyngeal mesoderm is sufficient for early SHF development, we generated a conditional Nkx2.5-expressing mouse model Nkx2.5H2B-GFP-3XFLAG (denoted as Nkx2.5GFP/+) by inserting a loxP-H2B-GFP-4X polyA-loxP-3XFLAG cassette into the start codon of Nkx2.5 through gene targeting (Fig. 7 A-C). The 3XFLAG codons are in frame with Nkx2.5 cDNA sequences. In mice bearing this allele, GFP expression is under control of endogenous Nkx2.5 regulatory elements (Fig. 7 F). The 3XFLAG-Nkx2.5 fusion protein is produced when the H2B-GFP-4X polyA cassette is removed (by Cre excision, Fig. 7 D). We crossed Nkx2.5GFP/+ to Protamine-Cre mice and Nkx2.53XFLAG/+ animals were obtained. Further intercross of Nkx2.53XFLAG/+ showed that Nkx2.53XFLAG/3XFLAG homozygous mice were viable and normal as their wild-type littermates from embryonic stages to adulthood (data not shown), suggesting 3XFLAG-Nkx2.5 fusion protein retains Nkx2.5 activity.
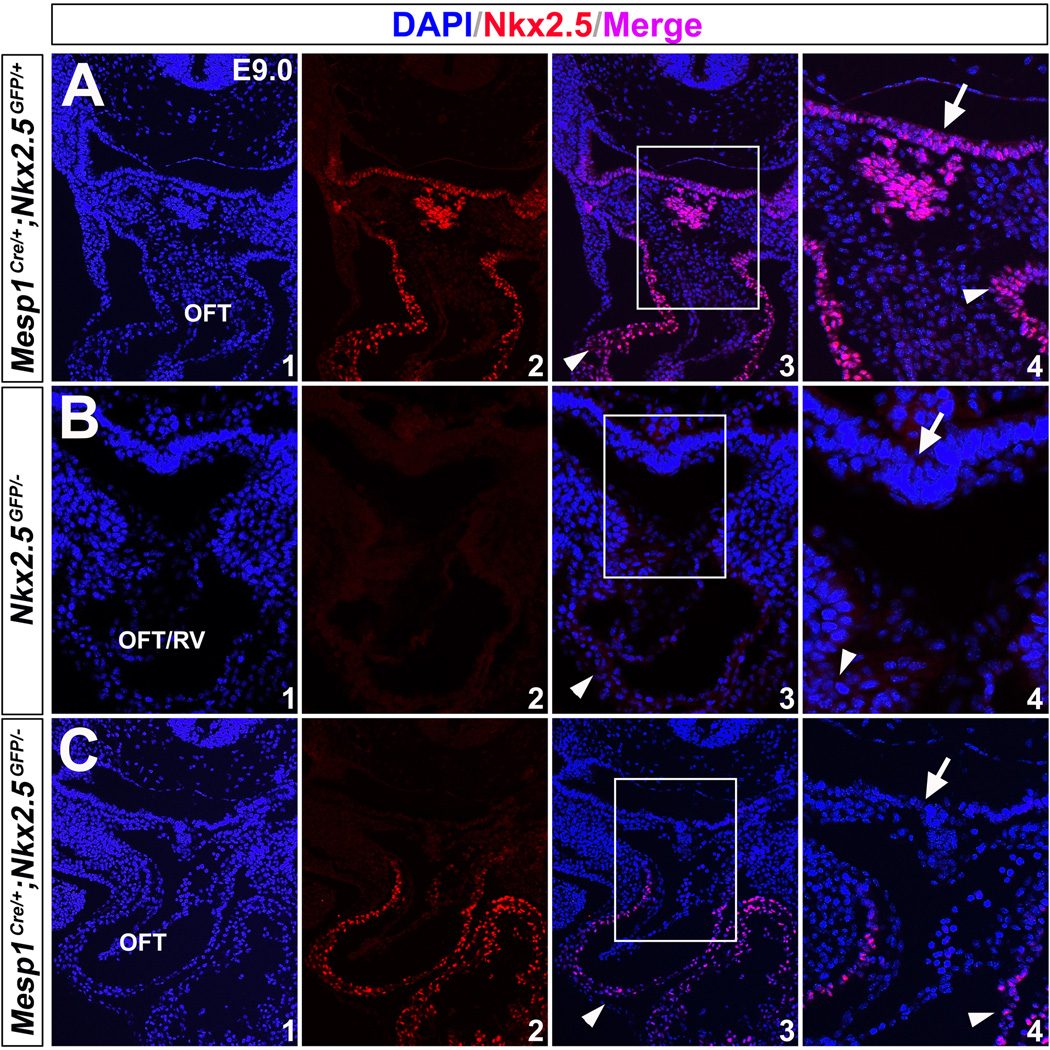
We crossed Mesp1Cre/+;Nkx2.5+/− mice to Nkx2.5GFP/+ mice and Nkx2.5GFP/− embryos were obtained (Fig. 7 H). They displayed malformed hearts as Nkx2.5−/− at E9.5 (Fig. 5 E), indicating Nkx2.5GFP is a functional null allele of Nkx2.5. Mesp1Cre/+;Nkx2.5GFP/+ hearts developed normally. As discussed above, the Nkx2.5GFP allele in Mesp1Cre/+;Nkx2.5GFP/− mice will generate 3XFLAG-Nkx2.5 fusion protein competent to wild-type Nkx2.5 in the mesoderm. Intriguingly, Mesp1Cre/+;Nkx2.5GFP/− mice restored normal development in the heart at E9.5- E13.5 (Fig. 7 I,L,M). Immunostaining confirmed Nkx2.5 was only detected in the mesodermal lineages (Fig. 8 C, arrowhead), but not in the pharyngeal endoderm (Fig. 8 C4, unnotched arrow) of these embryos. Of note, mice with genotype Mesp1Cre/+;Nkx2.5GFP/GFP can survive to birth with normal morphology (data not shown). These results indicate that mesodermal Nkx2.5 expression is sufficient for early SHF development.
Figure 8. Re-expression of Nkx2.5 in the mesoderm of Mesp1Cre/+;Nkx2.5GFP/− mice.
(A) Normal expression of Nkx2.5 in the pharyngeal endoderm (unnotched arrows) and mesoderm/heart (arrowheads) on the control mice (Mesp1Cre/+;Nkx2.5GFP/+) at E9.0. (B) Nkx2.5 was not detected in Nkx2.5GFP/− embryos. (C) Nkx2.5 is produced in the mesoderm/heart (arrowheads), but not endoderm (unnotched arrows), on Mesp1Cre/+;Nkx2.5GFP/− mice. A4/B4/C4 are high magnification images for A3/B3/C3 in the square areas, respectively.
Based on these results, we reason that Nkx2.5-regulated paracrine signals from the pharyngeal endoderm, if any, have minimal or no effect on the development of SHF. Mesodermal deletion of Nkx2.5 caused virtually identical cardiac phenotypes to Nkx2.5−/− hearts, and that re-expression of Nkx2.5 in the mesoderm rescued Nkx2.5GFP/− cardiac defects, conclusively demonstrated that mesodermal, but not endodermal, Nkx2.5 expression provides the requisite signals for regulating early SHF development.
Nkx2.5 is a homologue of Drosophila tinman and belongs to Nk-2 class of homeobox genes 52. In vertebrates, several Nk-2 homologues, including Nkx2.3 53, Nkx2.6/2.8 54–57, Nkx2.7 53, and Nkx2.9 58,59 are expressed in the pharyngeal endoderm and mesoderm, with a pattern that overlaps both temporally and spatially with Nkx2.5 during early cardiogenesis. In Xenopus, Nkx2.5 and Nkx2.3 regulate heart formation in a functionally redundant manner 60. Zebrafish Nkx2.5 and Nkx2.7 also function redundantly to control cardiac morphogenesis 61,62. In mice, Nkx2.5−/−;Nkx2.6−/− double mutant have severely disrupted pharyngeal endoderm formation 63, whereas Nkx2.5−/− or Nkx2.6−/− single mutation displayed normal pharynx development 63,64. Our observation that endodermal deletion of Nkx2.5 permitted normal cardiac formation may indicate a redundant activity of Nkx2.5 with other Nk-2 family members in the pharyngeal endoderm for SHF development. In examining Foxa2MerCreMer/+;Nkx2.5f/f embryos, we detected normal Nkx2.3 and Nkx2.6 pharyngeal endodermal expression (Fig. 4 G-J). Nkx2.7 orthologue was not present in mice, and Nkx2.9 was only expressed in the neural tube (Fig. 4 K,L). We speculate the overlapping endodermal expression of Nkx2.3 and Nkx2.6 may compensate Nkx2.5 loss in the pharyngeal endoderm in Foxa2MerCreMer/+;Nkx2.5f/f embryos. In the future, it will be of interest to investigate the compound mutations of Nkx2.5 with other Nk-2 genes in the pharyngeal endoderm, to understand whether Nk-2 family genes act redundantly to regulate SHF formation through the endoderm.
Supplementary Material
Highlights.
Nkx2.5 is a key cardiac transcription factor expressed in the early FHF, SHF and pharyngeal endoderm.
Mouse heart development appears normal after endodermal deletion of Nkx2.5.
Mesodermal disruption of Nkx2.5 engenders almost identical cardiac defects to Nkx2.5 null mice.
Specific re-expression of Nkx2.5 in the mesoderm rescues Nkx2.5 null cardiac defects.
Nkx2.5 expression in the mesoderm is essential but its endodermal expression is dispensable for early SHF development.
Acknowledgments
The authors thank Drs. Yumiko Saga (National Institute of Genetics, Japan) and Ken Chien (MGH, Boston, USA) for their generosity in providing the Mesp1Cre/+ and Nkx2.5flox/flox mice, respectively. We are also very grateful to Dr. Bruce Gelb for critical reading of this manuscript, and Dr. Kevin Kelly in the Transgenic Core of Mount Sinai for generating mouse models. A.N.K is supported by an NIH T32 training grant. C.L.C. is supported by grants from the NIH/NHLBI (1R01HL095810 and 1K02HL094688), the American Heart Association (0855808D) and the March of Dimes Foundation (5-FY07-642).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima Y, et al. Heart development before beating. Anat Sci Int. 2009;84:67–76. doi: 10.1007/s12565-009-0025-2. [DOI] [PubMed] [Google Scholar]
- 4.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 5.Cai CL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mjaatvedt CH, et al. The outflow tract of the heart is recruited from a novel heartforming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 7.Waldo KL, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 8.Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- 9.Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- 10.Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- 11.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 15.Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Madabhushi M, Lacy E. Anterior visceral endoderm directs ventral morphogenesis and placement of head and heart via BMP2 expression. Dev Cell. 21:907–919. doi: 10.1016/j.devcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol. 2006;295:756–763. doi: 10.1016/j.ydbio.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 19.Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol. 2009;330:305–17. doi: 10.1016/j.ydbio.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macatee TL, et al. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park EJ, et al. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold JS, et al. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- 24.Ilagan R, et al. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey RP, et al. Homeodomain factor Nkx2-5 in heart development and disease. Cold Spring Harb Symp Quant Biol. 2002;67:107–114. doi: 10.1101/sqb.2002.67.107. [DOI] [PubMed] [Google Scholar]
- 27.Lyons I, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 29.Schott JJ, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 30.Prall OW, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park EJ, et al. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev Dyn. 2008;237:447–453. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- 32.Saga Y, et al. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 33.Pashmforoush M, et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 34.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson DG. In Situ Hybridization: A Practical Approach. New York: Oxford University Press; 1992. [Google Scholar]
- 36.Searcy RD, Vincent EB, Liberatore CM, Yutzey KE. A GATA-dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development. 1998;125:4461–4470. doi: 10.1242/dev.125.22.4461. [DOI] [PubMed] [Google Scholar]
- 37.Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 39.Kasahara H, Bartunkova S, Schinke M, Tanaka M, Izumo S. Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ Res. 1998;82:936–946. doi: 10.1161/01.res.82.9.936. [DOI] [PubMed] [Google Scholar]
- 40.Tam PP, Steiner KA. Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development. 1999;126:5171–5179. doi: 10.1242/dev.126.22.5171. [DOI] [PubMed] [Google Scholar]
- 41.Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, et al. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe Y, et al. Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries. Circ Res. 106:495–503. doi: 10.1161/CIRCRESAHA.109.201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly RG, Buckingham ME. The anterior heart-forming field: voyage to the arterial pole of the heart. Trends Genet. 2002;18:210–216. doi: 10.1016/s0168-9525(02)02642-2. [DOI] [PubMed] [Google Scholar]
- 45.Golzio C, et al. ISL1 directly regulates FGF10 transcription during human cardiac outflow formation. PLoS One. 7:e30677. doi: 10.1371/journal.pone.0030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe Y, et al. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc Natl Acad Sci U S A. 109:18273–18280. doi: 10.1073/pnas.1215360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, et al. Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism. Dev Cell. 19:903–912. doi: 10.1016/j.devcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- 49.Harrelson Z, Kaestner KH, Evans SM. Foxa2 mediates critical functions of prechordal plate in patterning and morphogenesis and is cell autonomously required for early ventral endoderm morphogenesis. Biol Open. 1:173–181. doi: 10.1242/bio.2012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo S, Kume T. Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol. 2006;296:421–436. doi: 10.1016/j.ydbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 51.von Both I, et al. Foxh1 is essential for development of the anterior heart field. Dev Cell. 2004;7:331–345. doi: 10.1016/j.devcel.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 52.Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- 53.Lee KH, Xu Q, Breitbart RE. A new tinman-related gene, nkx2.7, anticipates the expression of nkx2.5 and nkx2.3 in zebrafish heart and pharyngeal endoderm. Dev Biol. 1996;180:722–731. doi: 10.1006/dbio.1996.0341. [DOI] [PubMed] [Google Scholar]
- 54.Brand T, Andree B, Schneider A, Buchberger A, Arnold HH. Chicken NKx2-8, a novel homeobox gene expressed during early heart and foregut development. Mech Dev. 1997;64:53–59. doi: 10.1016/s0925-4773(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 55.Boettger T, Stein S, Kessel M. The chicken NKX2.8 homeobox gene: A novel member of the NK-2 gene family. Dev Genes Evol. 1997;207:65–70. doi: 10.1007/s004270050092. [DOI] [PubMed] [Google Scholar]
- 56.Reecy JM, et al. Chicken Nkx-2.8: a novel homeobox gene expressed in early heart progenitor cells and pharyngeal pouch-2 and -3 endoderm. Dev Biol. 1997;188:295–311. doi: 10.1006/dbio.1997.8641. [DOI] [PubMed] [Google Scholar]
- 57.Biben C, Hatzistavrou T, Harvey RP. Expression of NK-2 class homeobox gene Nkx2-6 in foregut endoderm and heart. Mech Dev. 1998;73:125–127. doi: 10.1016/s0925-4773(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 58.Pabst O, Herbrand H, Arnold HH. Nkx2-9 is a novel homeobox transcription factor which demarcates ventral domains in the developing mouse CNS. Mech Dev. 1998;73:85–93. doi: 10.1016/s0925-4773(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 59.Newman CS, Krieg PA. tinman-related genes expressed during heart development in Xenopus. Dev Genet. 1998;22:230–238. doi: 10.1002/(SICI)1520-6408(1998)22:3<230::AID-DVG5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 60.Fu Y, Yan W, Mohun TJ, Evans SM. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development. 1998;125:4439–4449. doi: 10.1242/dev.125.22.4439. [DOI] [PubMed] [Google Scholar]
- 61.Tu CT, Yang TC, Tsai HJ. Nkx2.7 and Nkx2.5 function redundantly and are required for cardiac morphogenesis of zebrafish embryos. PLoS One. 2009;4:e4249. doi: 10.1371/journal.pone.0004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Targoff KL, et al. Nkx genes are essential for maintenance of ventricular identity. Development. 140:4203–4213. doi: 10.1242/dev.095562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka M, Schinke M, Liao HS, Yamasaki N, Izumo S. Nkx2.5 and Nkx2.6, homologs of Drosophila tinman, are required for development of the pharynx. Mol Cell Biol. 2001;21:4391–4398. doi: 10.1128/MCB.21.13.4391-4398.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka M, Yamasaki N, Izumo S. Phenotypic characterization of the murine Nkx2.6 homeobox gene by gene targeting. Mol Cell Biol. 2000;20:2874–2879. doi: 10.1128/mcb.20.8.2874-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.