Abstract
Equine protozoal myeloencephalitis (EPM) is a serious disease of horses, and its management continues to be a challenge for veterinarians. The protozoan Sarcocystis neurona is most commonly associated with EPM. S. neurona has emerged as a common cause of mortality in marine mammals, especially sea otters (Enhydra lutris). EPM-like illness has also been recorded in several other mammals, including domestic dogs and cats. This paper updates S. neurona and EPM information from the last 15 years on the advances regarding life cycle, molecular biology, epidemiology, clinical signs, diagnosis, treatment and control.
Keywords: Sarcocystis neurona, Equine protozoal myeloencephalitis, EPM, Horse, Marine mammals, Genetics, Epidemiology, Life cycle, Structure, Lesions, Clinical signs, Diagnosis, Treatment, Prevention
1. Introduction
Equine protozoal myeloencephalitis (EPM) due to the protozoan parasite, Sarcocystis neurona continues to be a serious neurological disease of horses in the Americas. Since the publication of a comprehensive review on this subject in 2001(Dubey et al., 2001a), many advances have occurred in the biology of this parasite, including the discovery of its full life cycle. S. neurona has emerged as an important cause of mortality in marine mammals. Since the parasite is easy to cultivate in many cell lines and can be genetically manipulated, S. neurona has been used as model to study several aspects of cell cycle including synthesis of the apicomplexan plastid called the apicoplast (Vaishnava et al., 2005). Recently the S. neurona genome was sequenced and annotated, the first for the genus Sarcocystis, potentially leading to discovery of better diagnostic methods and therapies (Blazejewski et al., 2015). Here we have reviewed information on its life cycle, biology, clinical disease in many species of animals, diagnosis, treatment and prevention.
2. Methods and literature citation
An attempt has been made to cite all peer-reviewed papers published after 2000, excluding meeting abstracts and conference proceedings. Although the related protozoa Neospora spp. can occasionally cause EPM, this review is restricted to S. neurona.
3. Biology
3.1. Hosts
S. neurona has a wide host range relative to other species in the genus Sarcocystis (Fig. 1, Table 1). The North American opossum (Didelphis virginiana) and the South American opossum (D. albiventris) are its known definitive hosts; whether S. neurona can occur in other species of South American opossums continues to be investigated. Several other animal species are its intermediate or aberrant hosts (Table 1). In some hosts, only schizonts have been identified and these are considered aberrant hosts. Mature sarcocysts are essential for the completion of the life cycle. Hosts in which mature sarcocysts have been demonstrated are the intermediate hosts. Laboratory-raised opossums excreted sporocysts after feeding naturally infected skunk, raccoon, sea otter or armadillo muscle (Cheadle et al., 2001c; Cheadle et al., 2001d; Tanhauser et al., 2001; Dubey et al., Dubey et al., 2001b, Dubey et al., 2001c), indicating that they are proven intermediate hosts of S. neurona. Mansfield et al (2008) reported brown-headed cowbirds as intermediate host for S. neurona but this finding needs confirmation.
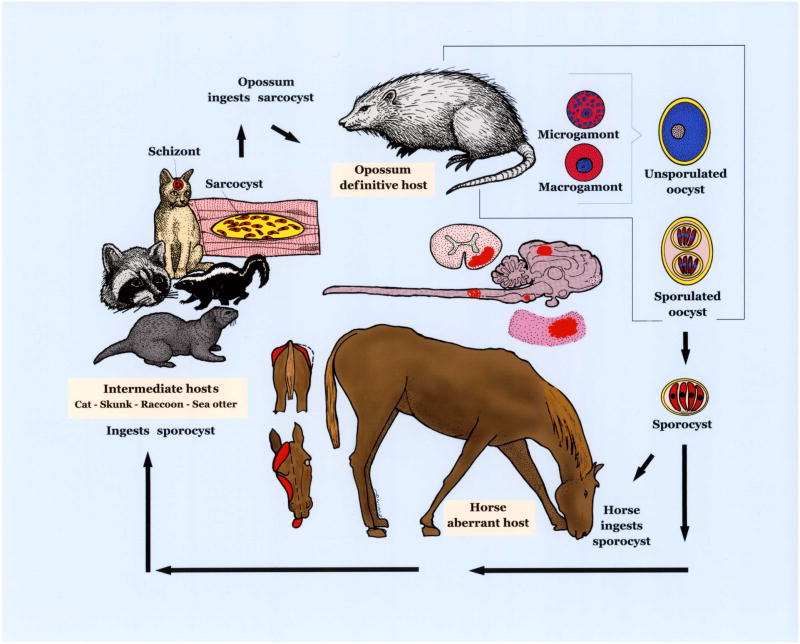
Figure 1.
Life cycle of S. neurona. Opossums are the definitive host and other animals are aberrant/intermediate hosts. S. neurona parasitizes and causes lesions (in red) in the brain and spinal cord of horses. Affected horses can have neurological signs, including abnormal gait, dysphagia, and muscle atrophy depicted.
Table 1.
Intermediate /aberrant hosts of S. neurona.
| Proven intermediate hostsa (see Table 4) | S. neurona isolated from host tissue by bioassay in mice or cell culture (see Table 4) | S. neurona – like sarcocysts in sections | Clinical disease reported (see Tables 7–9) | Only antibody found (see Table 6) |
|---|---|---|---|---|
| Cat Skunk Raccoon Armadillo Sea otter |
Horse Sea otter Pacific harbor seal Harbor porpoise Raccoon |
Dog Horse Mink Bobcatb |
Horse Pony Zebra Raccoon Red pandab Cat Ferret Dog, Skunk Lynx Sea otter Pacific harbor seal Sea lion |
Ring tailed lemur Blue eyed black lemur Black and white ruffed lemur Beaver |
Laboratory raised opossums shed sporocysts after consuming infected muscle; sporocysts were infective to KO mice.
Unpublished.
Based on high prevalence of S. neurona-like sarcocysts in raccoons and the success in completing the raccoon and opossum cycle in the laboratory, the raccoon appears to be an important intermediate host in North America (JPD own observations).
3.2. Structure and life cycle
3.2.1. Structure
Schizonts and sarcocysts are tissues stages in intermediate hosts. In naturally infected horses with neurological signs, S. neurona schizonts have been found only in the central nervous system (CNS). Both neural and inflammatory cells in the CNS may be parasitized. As many as 13 schizonts and several hundred merozoites were present in one neuron (Dubey et al., 2001a).
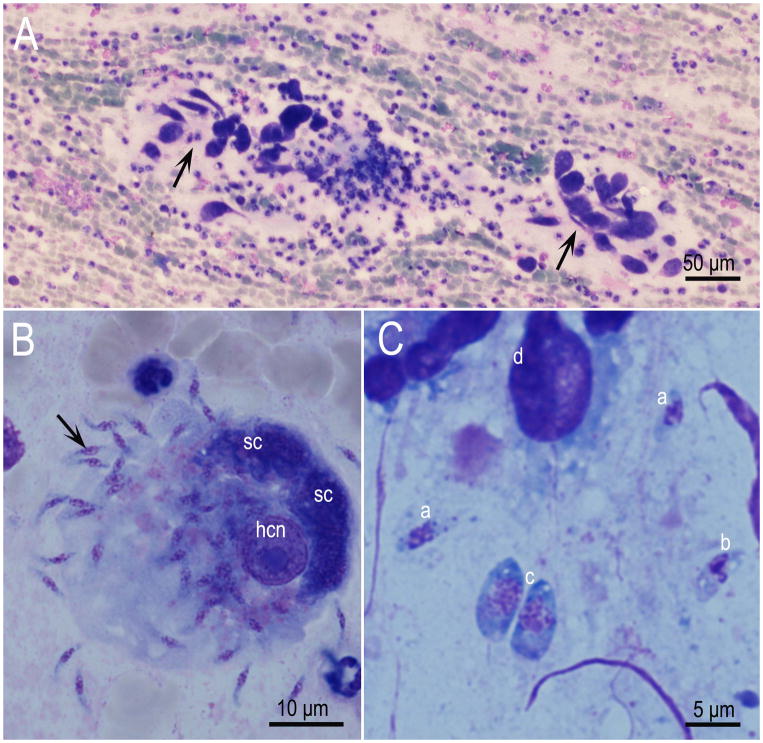
Schizonts divide by endopolygeny where the nucleus becomes multilobed before merozoites are formed (Figs. 2–4). The schizogonic cycle may be asynchronous; schizonts of different maturity can be found in a single cell (Figs. 5, 6, 7). Only one morphologic type of schizonts have been found. Mature schizonts in the CNS are up to 30 μm long and they may be oval, round, elongated or irregular in shape. Merozoites are approximately 7–8 μm ×1–2 μm (Fig. 2). Ultrastructurally, longitudinally cut merozoites in sections were 7.3 × 1.7 μm and they contained same organelles as described in merozoites of other Sarcocystis species, including absence of rhoptries (Speer and Dubey, 2001).
Figure 2.
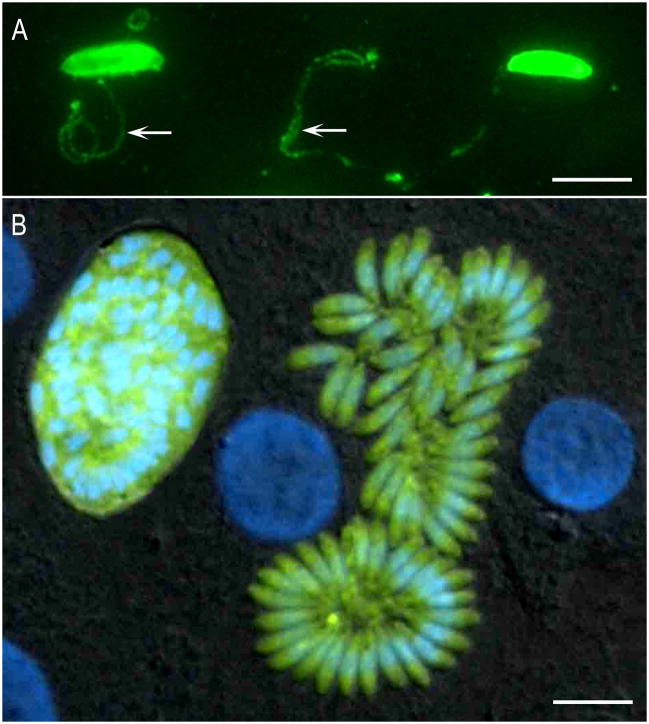
Fluorescence images of S. neurona. (A) Images of gliding S. neurona merozoites stained with a monoclonal antibody (2A7-18) to the surface protein of Sn-SAG1. The formation of the trails is similar to those reported for Toxoplasma gondii. The trails are readily visualized by staining with antibodies to the major surface antigens. Gliding occurs on a variety of substrates, including coated chamber slides (50% PBS and fetal bovine serum). (B) Transgenic clone of Sarcocystis neurona expressing yellow fluorescent protein. Differential interference contrast image with epifluorescence image overlay showing a bovine turbinate cell monolayer containing a late-stage schizont and a mature schizont of a clone of S. neurona that stably expresses YFP. Host cell and parasite nuclei were stained with DAPI (blue). Bar= 10μm.
Figure 4.
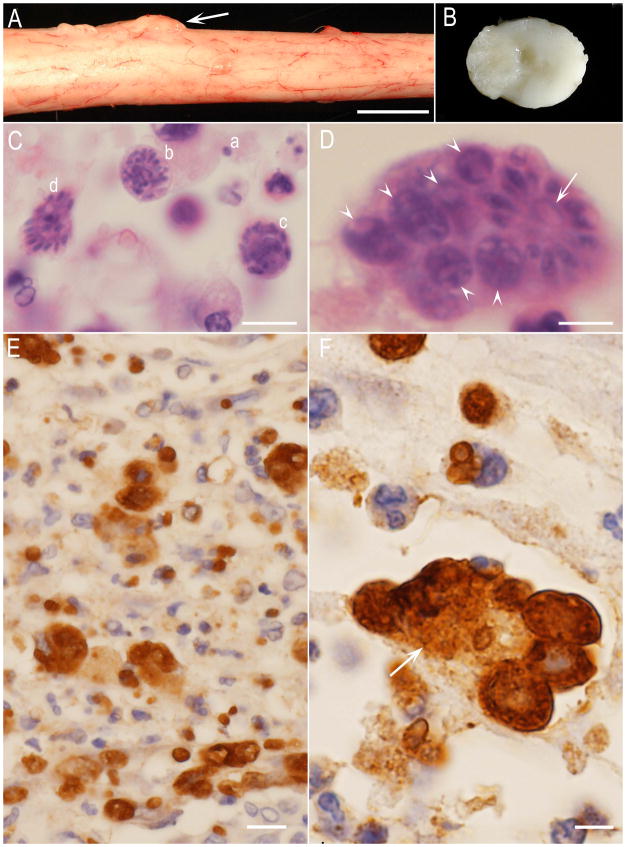
S. neurona -associated lesions in the spinal cord of a dog reported by Gerhold et al. (2014). (A) Nodular growth (arrow) between spinal nerve roots. Unstained. Bar =1cm. (B) Cut section of the spinal cord in Fig. 11A. The protruding lesion is extended in to the central canal. Unstained. C to F histological sections. C and D, hematoxylin and eosin stain, E and F immunohistochemical staining with S. neurona antibody. Bar in C, D, and F=5 μm, and in E=20 μm. (C) Note different developing schizogonic stages in one field-merozoite (a), an immature schizont with multilobed nucleus (b), schizonts with developing merozoites (c,d). (D) At least 7 schizonts within a phagocytic cell. Arrow points to a mature schizont with residual body. Arrowheads point to immature schizonts. (E) Numerous free merozoites and schizonts. The organisms appear larger in size after immunostaining. (F) A phagocytic host cell (arrow) similar to that in Fig. 11D showing several schizonts (specimens courtesy of Shelley J Newman, and Amanda Crews).
Figure 5.
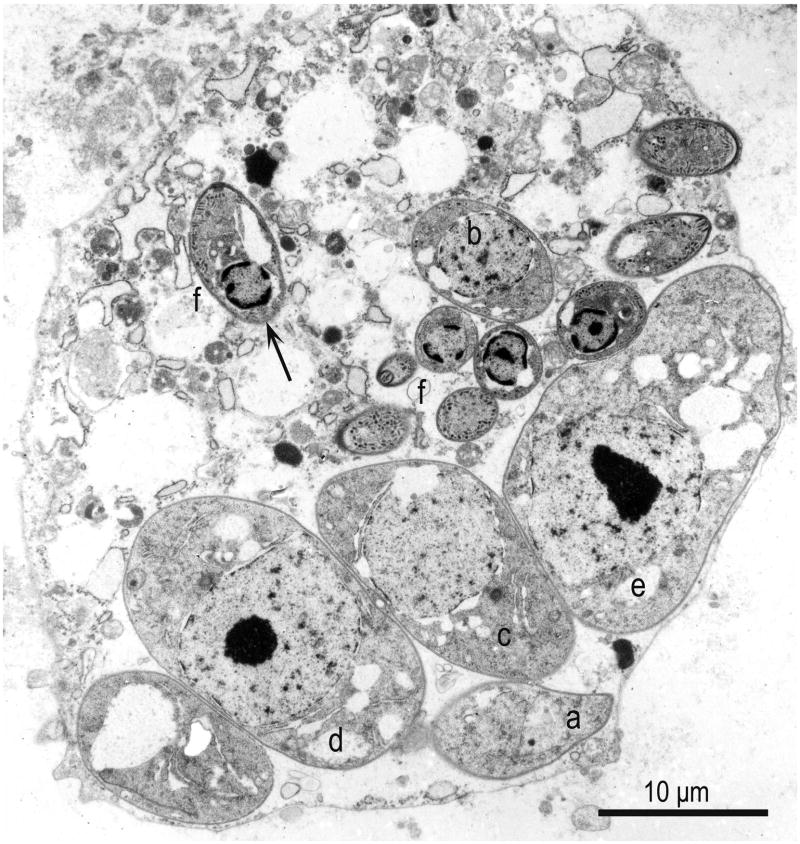
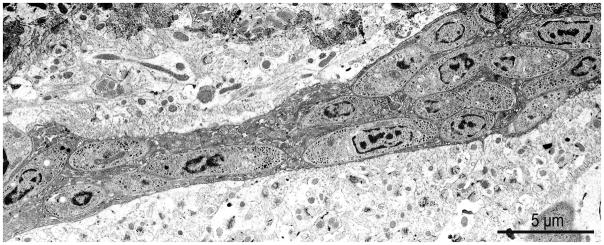
TEM of an infected neural cell in the brain of a raccoon naturally infected with S. neurona. Note asynchronous schizogony with six developing schizonts, in presumed order of development (a-e), and nine merozoites (f). Arrow points to a longitudinally merozoite with a conoid at one end and a subterminal nucleus. The host cell is degerated but parasite structures are fairly well preserved (From Dubey et al., 1991c).
Figure 6.
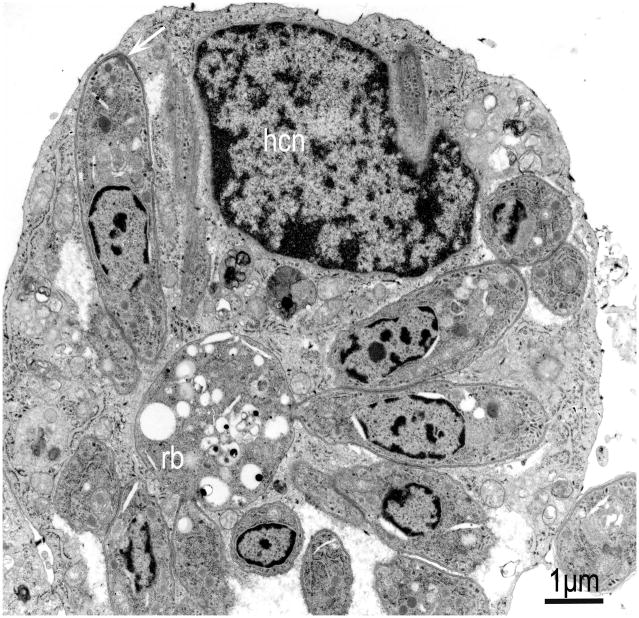
TEM of a bovine turbinate cell culture infected with S. neurona. Mature schizont with non conoidal end of merozoites still attached to a residual body (rb). Note one longitudinally cut merozoite (arrow) that has separated from the schizont, and the host cell nucleus (hcn). (From Dubey, 2004).
Figure 7.
S. neurona merozoites free in cytoplasm of an unmyelinated axon in the cerebellum of an experimentally infected KO mouse. (From Fritz and Dubey, 2002).
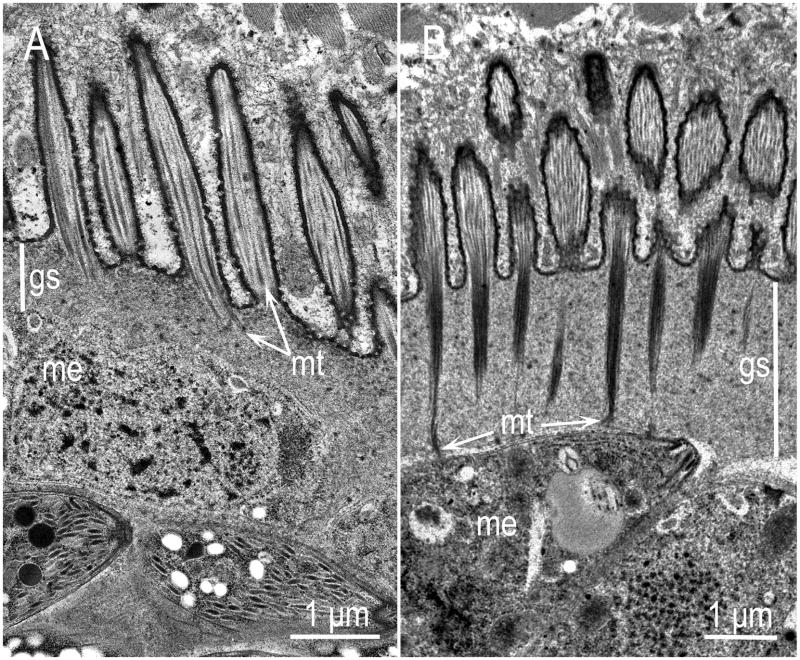
Sarcocysts have been demonstrated in skeletal and cardiac muscles and brain. Sarcocysts in brain are often round, and smaller in size than in skeletal muscle (Thomas et al., 2007; Miller et al., 2009). Additionally, sarcocysts in myocardium are smaller than those in skeletal muscle. Mature S. neurona sarcocysts are microscopic and the sarcocyst wall appears striated under the light microscope (Fig. 8). In 1-μm sections stained with Toluidine blue, villar protrusions on the cyst wall are tapered and the tips are denser than at the base (Fig. 8C). Ultrastructurally, the mature S. neurona sarcocyst wall has villar protrusions that are up to 2.8 μm long and 0.4 μm wide (Dubey et al., 2001d). The villar protrusions have microtubules that extend from tip to the base, but have no granules (Fig. 9). Few of these microtubules extend deeper in the ground substance but they are electron lucent and not prominent compared with the other Sarcocystis species of the horse in North America, S. fayeri (Fig. 9). Bradyzoites in sections are 4.8–6.5 × 1.0–1.3 μm in size, and contain only two rhoptries (Fig. 10; Dubey et al., 2001d).
Figure 8.
S. neurona sarcocysts in histological sections of skeletal muscle. Arrowheads point to striated cyst wall. Arrows point to thickening of the villar tips. (A) Cat, 144 DPI. From Dubey et al., 2002).Toluidine blue stain. (B) Raccoon, 77 DPI. Hematoxylin and eosin. (C) Toluidine blue stain. (B and C from Stanek et al., 2002).
Figure 9.
Comparison of the cyst walls of S. neurona (A) and S. fayeri (B) sarcocysts by TEM. The cyst walls, including the ground substance layer (gs) of S. fayeri are thick, the microtubules (mt) are more electrondense and extend up to the pellicle of the zoites whereas the cyst walls of S. neurona are comparatively thin, the microtubules are few, and never extend deep in the gs. (From Stanek et al., 2002, and Saville et al., 2004b).
Figure 10.
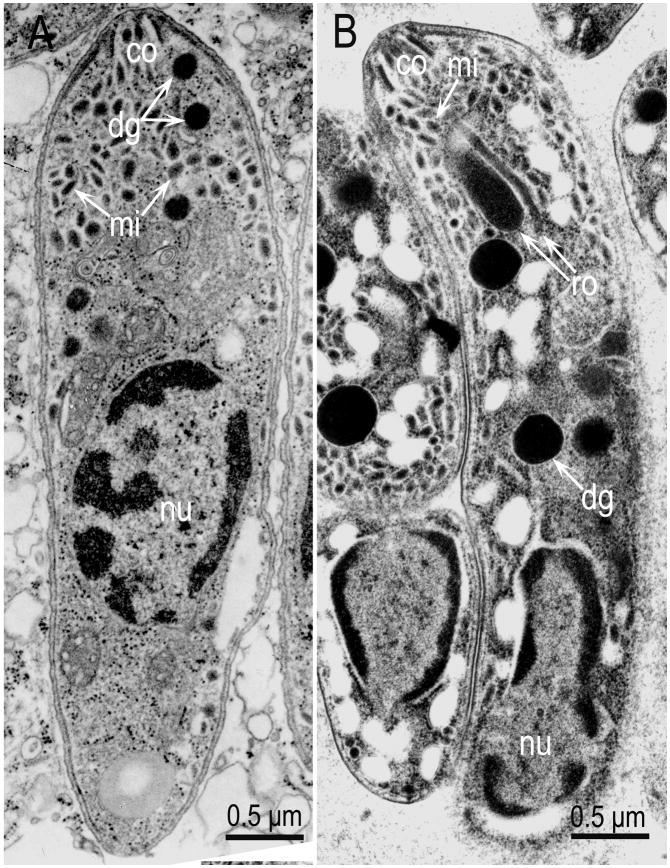
Comparison of a merozoite and a bradyzoite of S. neurona. (A) Merozoite in the brain of a naturally infected horse (From Dubey et al., 1998). (B) Bradyzoite in sarcocyst in an experimentally infected cat (From Dubey et al., 2001d). Note the location of nucleus (nu), central in merozoite, and terminal in bradyzoite, and the absence of rhoptries in merozoite. Also note conoid (co), micronemes (mn) and dense granules (dg). Dense granules are often mistaken for rhoptries unless their elongated portions are visible.
S. neurona sporocysts are 11.3 x 8.2 μm in size. Sporozoites are slender, and have 2–4 rhoptries but no crystalloid body (Lindsay et al., 2004). The absence of a crystalloid body in sporozoites is notable because the crystalloid body was present in S. cruzi and other species of Sarcocystis sporozoites examined ultrastructurally (Dubey et al., 1989).
3.2.2. Migration and development
Migration and development of S. neurona has been studied in an experimental aberrant host (interferon gamma gene knock-out [KO] mouse), experimentally in a natural host (raccoon), and natural aberrant host (ponies) inoculated with S. neurona. The number of asexual generations (schizogony) has not been determined.
3.2.2.1. Mouse modeling schizogony
Only schizonts are produced in the KO mouse. After oral inoculation of S. neurona sporocysts, sporozoites excyst in the small intestine and parasitemia has been detected 1–8 days post inoculation (DPI) (Dubey, 2001a; Dubey, 2001b). By 1 DPI, sporozoites can be detected histologically in mesenteric lymph nodes. Mature schizonts with merozoites were first detected histologically starting 8 DPI. Beginning 13 DPI, schizonts were seen consistently in the brain. The other organs parasitized were lung, heart, liver, intestine, retina, and kidney. Although all regions of brain and spinal cords were parasitized, over 90% of organisms were seen in the cerebellum of KO mice (Fritz and Dubey, 2002). The infectivity of sporocysts in KO mice by the oral route paralleled the subcutaneous route, irrespective of the strain of S. neurona (Dubey et al., 2001e). All infected KO mice died and the dose did not affect clinical signs (Cheadle et al., 2001a; Cheadle et al., 2002). The strain of the KO mouse (C57BL/6-Black or BALB/c White–derived) did not affect outcome of disease. Of the 40 mice inoculated with graded doses, 26 mice became infected and died of sarcocystosis; the day of deaths were 25–36 (21 mice), and 49 (2 mice), 60 (1 mouse) and 69 (1 mouse) (Dubey et al., 2013). Thus all affected mice died by 70 DPI; clinical signs were more severe in C57Bl/6-genetic background as compared to the BALB/c line (Dubey et al., 2013). Severe combined immunodeficiency (SCID) mice were not susceptible to S. neurona infection (Marsh et al. 1997a). Thus the genetic background and specific cellular immune components of mice play a role in their susceptibility and the parasite’s ability to replicate and cause disease. Cell cultured merozoites were infective to KO mice but bradyzoites (from muscles of experimentally infected raccoon) were not (Dubey et al., 2013).
3.2.2. 2. Raccoon modeling of sarcocyst and bradyzoite development
Development of S. neurona was studied in ten raccoons euthanized 1–77 days after oral inoculation with SN37R strain S. neurona sporocysts derived from experimentally infected opossums (Stanek et al., 2002); the SN37R strain had been passaged 10 cycles through laboratory raised raccoons and opossums, ensuring the absence of extraneous species. Parasitemia was detected three and five DPI. Individual zoites, interpreted as sporozoites, were seen in histological sections of intestines at one and three DPI. Schizonts with merozoites were consistently detected in five raccoons euthanized seven-22 DPI. Schizonts were not detected in two raccoons euthanized 37 and 77 DPI. At 22 and 37 DPI, sarcocysts were immature (containing only metrocytes); they were up to 125 μm long at 37 DPI, and up to 270 μm long at 37 DPI. Sarcocysts at 77 DPI were mature. The sarcocyst wall was up to 2.5 μm thick and the slender villar protrusions were 2.5 μm long (Stanek et al., 2002). None of these raccoons had clinical signs. In another study, two raccoons were each fed five million sporocysts, one raccoon became lame and was euthanized 14 DPI (Dubey et al., 2001b). The second raccoon developed neurological signs and was euthanized 22 DPI. Histologically, both raccoons had encephalomyelitis associated with schizonts. Additionally, sarcocysts were seen in muscle of the raccoon euthanized 22 DPI (Dubey et al., 2001b). Thus, the raccoon-opossum cycle has been repeated under laboratory conditions.
3.2.3. Feline modeling of sarcocysts and bradyzoite development
Sarcocysts also developed in cats after oral inoculation with S. neurona sporocysts (Dubey et al., 2000). At 36, 45, and 57 DPI, sarcocysts were immature. At 144 DPI, sarcocysts were up to 700 μm long and opossums fed these sarcocysts shed S. neurona sporocysts. Butcher et al. (2002) found sarcocysts in muscle of one cat 43 days after the cat was inoculated parenterally with 10 million S. neurona merozoites; an opossum fed the sarcocysts from cat muscle did not shed sporocysts. Sarcocysts found in another cat euthanized day 50 after inoculation with 10 million merozoites were infective to an opossum as evidenced by sporocyst excretion. Thus, bona fide S. neurona sarcocysts have been produced in cats following inoculation with culture-derived merozoites.
3.2.3. Equine modeling of early parasite infection
Attempts were made to study migration and development of S. neurona in six ponies after oral inoculation with 250,000,000 sporocysts (Elitsur et al., 2007); the ponies were serologically negative to S. neurona before inoculation and euthanized 1, 3, 5, 7, and 9 DPI. Viable S. neurona were isolated by bioassay in KO mice and in cell culture inoculated with tissue homogenates of pony mesenteric lymph nodes at 1, 2, and 7 DPI, liver at 2, 5, and 7 DPI, and from lungs at 5, 7, and 9 DPI. The parasite was not detected histologically in tissues of any pony. However, encephalitic lesions were detected in sections of brain and spinal cords of two ponies euthanized 7 and 9 DPI. One pony euthanized 9 DPI had IgM antibodies to S. neurona (Elitsur et al., 2007).
3.3. Related Sarcocystis spp., their detection, and excretion of sporocysts by definitive hosts
Large numbers of sporocysts can be excreted in feces of opossums (Dubey, 2000); Table 2). Opossums are the definitive hosts for S. neurona and 3 other named species, S. speeri, S. falcatula, and S. lindsayi (Box et al., 1984; Dubey and Lindsay, 1999; Tanhauser et al., 1999a; Tanhauser et al., 1999b; Dubey et al., 2001j). Cheadle et al. (2001b) provided morphologic measurements of possibly five species of Sarcocystis-like sporocysts in feces of 17 naturally-infected opossums. Sporocysts of S. neurona were 10.7 x 7.0 μm, S. speeri were 12.2 x 8.8 μm, strain-1085 of Sarcocystis sp. were 10.9 x 6.8 μm, and S. falcatula were 11.0 x 7.1 μm; thus the differences were within 2-μm range. Additionally, the identity of sporocysts of each species was from naturally-infected opossums and the species identification was not definitive. Cheadle et al. (2001b) reported sporocysts of another organism in feces of 10 opossums; sporocysts were 19.4 x 10.5 μm in size and had Stieda body-like structures at both poles. In our opinion, these are unlikely to be Sarcocystis sporocysts. Bioassay has been used to distinguish sporocysts of different species in opossum feces. The KO mice and budgerigars have been used to differentiate viable S. neurona and S. falcatula in opossum feces; S. neurona and S. speeri are not infective to budgerigars and S. falcatula is not infective to KO mice (Dubey and Lindsay, 1998). S. speeri and S. neurona in KO mice can be distinguished immunohistochemically (Dubey and Lindsay, 1999; Dubey, 2000). Molecular methods have been developed to distinguish S. falcatula and S. neurona, (Tanhauser et al., 1999a,b). Very few S. neurona sporocysts are lethal for KO mice. (Dubey et al., 2013) and 1000 S. speeri sporocysts are lethal for KO mice (Dubey and Lindsay, 1999).
Table 2.
Prevalence of Sarcocystis sporocysts in feces/intestines of opossum (Didelphis virginiana) in the USA.
| Location | Year | No. tested | With sporocysts (%) | S. neurona | Other species | S. neurona Isolates | Reference |
|---|---|---|---|---|---|---|---|
| LA, MD, VA, GA, FL, PA | 1998–99 | 44 | 24 (54.4) | 14 (31.8%)-bioassay in KOM | 19 (43.1%) S. falcatula-bioassay in budgerigars, 8 (18.8%) S. speeri-bioassay in KO mice | SN8-15OP | Dubey (2000) |
| MS | 1999–2000 | 72 | 24 (33.3) | 19 (26.3%) | Not investigated, 1 opossum infected with S. speeri | SN16 to 34OP | Dubey et al. (2001i) |
| MI | 1996–2002 | 206 | 37 (18) | 23 (11.1%) PCR-RFLP | 4 (1.94) S. falcatula based on PCR-RFLP, | Not stated | Elsheikha et al. (2004d) |
| CA | 2005–2008 | 288 | 53 (18.4) | 17-PCR-ITS-1 | Not stated | OP-1-13 | Rejmanek et al. (2009) |
Ca=California, Fl=Florida, GA=Georgia, LA=Louisiana, MD=Maryland, MI=Michigan,, MS =Mississippi, PA=Pennsylvania, VA= Virginia.
Molecular PCR-RFLP methods have been developed to unambiguously distinguish S. neurona, from S. falcatula (Tanhauser et al., 1999a,b), and PCR-DNA sequencing using pan-genus primers anchored within the 18S and 5.8S ssuRNA gene array, that amplify across the ITS-1 locus (Gibson et al., 2011), distinguish among the opossum infective Sarcocystis species. For example, across the ~1100 nucleotide ITS1 locus, S. lindsayi and S. falcatula differ from S. neurona at 54 and 26 nucleotide positions and possess 14 and 3 INDELs respectively by DNA sequencing (GenBank Accession Nos: AF387164; AF098244; AY009113, respectively). No ITS1 DNA sequences have been deposited in GenBank for S. speeri. Primers developed within the ITS1 (called ITS1500) are now routinely used to identify infection by opossum derived Sarcocystis spp. and DNA sequencing of the PCR amplicons distinguish between S. speeri, S. lindsayi, S. falcatula, and S. neurona (Miller et al, 2009).
S. neurona sporocysts have been found in 6–31% of opossums examined in the USA (Table 2). Three of the surveys listed in Table 2 examined factors associated with S. neurona positivity (Table 3). Season, body condition, and presence of young in pouch were associated with the presence of sporocysts. How these factors affect the presence of sporocysts in opossums is uncertain because Sarcocystis does not multiply in definitive hosts, and little is known of immunity to re-infection. The availability of infected intermediate hosts during spring might account for the results shown in Table 3.
Table 3.
Factors associated with Sarcocystis infections in opossums in the USA.
| Risk factor | Percentage of positive (n) | |||
|---|---|---|---|---|
| Mississippi (Rickard et al., 2001) | Michigan (Elsheikha et al., 2004b,d) | California (Rejmanek et al., 2010) | ||
| Age | Adult | 28 (14/50) | 14.5 (30/206) | 6.7 (14/206) |
| Juvenile | 22.7 (5/22) | 3.3 (7/206) | 3.7 (3/81) | |
| Gender | Female | 29.4 (10/34) | 9.7 (20/206) | 4.4 (6/136) |
| Male | 23.6 (9/38) | 8.25 (17/206) | 7.5 (11/146) | |
| Female with pouch young | Present | 33.3 (6/18) | no data | 8.3 (2/24) |
| Absent | 25 (4/16) | no data | 5.2 (4/76) | |
| Season | Spring | 34.2 (13/38) | 3.3 (7/206) | 9.1 (14/154) |
| Summer | no data | 9.2 (19/206) | ||
| Autumn | no data | 3.3 (7/206) | 2.2 (3/133) | |
| Winter | 17.6 (6/34) | 1.9 (4/206) | ||
| Total number tested | 72 | 206 | 288 | |
| Total positive (%) | 26.3 (19) | 17.9 (37) | 5.9 (17) | |
The white-eared opossum, D. albiventris, is found in Argentina, Brazil, Bolivia, Paraguay, and Uruguay but S. neurona sporocysts have been isolated only from D. albiventris from Brazil (Dubey et al., 2001g). S. neurona sporocysts have not yet been identified in Didelphis marsupialis and Didelphis aurita (Casagrande et al., 2009).
4. In vitro cultivation, cell and molecular biology
4.1. In vitro cultivation
Viable S. neurona has been isolated from many hosts (Table 4). Numerous cell lines can support the growth of S. neurona (Table 4). In most EPM horses, the number of S. neurona in CNS tissue is low. Therefore, culture flasks seeded with CNS homogenates should be incubated for at least two months because some strains are slow to adapt in cell cultures. Once established, S. neurona can complete schizogonic development in three days. S. neurona has also been isolated from sporocysts purified from the intestines of opossums either directly in cell cultures (Murphy and Mansfield, 1999; Elsheikha et al., 2003), or by feeding sporocysts to KO mice and then recovering S. neurona in cell cultures from mouse brains. The isolation of S. neurona from sporocysts via KO mice is advantageous because it removes, S. falcatula, a common parasite in opossum feces; S. falcatula is not infective to KO mice (Marsh et al., 1997b; Dubey and Lindsay, 1998). Cell cultures infected with S. neurona are useful for in vitro screening of anti-S. neurona compounds and other aspects of biology (Lindasy and Dubey, 2000; Bowman et al., 2001; Ellison et al., 2001; Lindsay and Dubey, 2001a, b; Marsh et al., 2001a; Gargala et al., 2005; Gargala et al., 2009).
Table 4.
In vitro cultivation of S. neurona.
| Host | year | Tissuea,b | Cell type initial culturec | Strain designation | Reference |
|---|---|---|---|---|---|
| Horse | 1990 | Spinal cord | M617 | SN1 | Dubey et al. (1991a) |
| 1990 | Spinal cord | M617 | SN2 | Davis et al. (1991) | |
| 1991 | Spinal cord | M617 | SN3 | Granstrom et al. (1992) | |
| 1991 | Spinal cord | M617 | SN4 | Davis et al. (1991) | |
| 1992 | Spinal cord | M617 | SN5 | Granstrom et al. 1994) | |
| 1998 | Spinal cord | M617,Esp | SN6 | Dubey et al. (1999a) | |
| 1997 | Spinal cord | M617,EK,Esp | SN7 | Dubey et al. (2001f) | |
| 1999 | Spinal cord | ED (not BT, DT) | SN-MU1,2 | Marsh et al. (2001b) | |
| 1994–5 | Spinal cord | M617 | UCD1,2,3 | Marsh et al. (1996, 1997a) | |
| 2009 | Brain | Not stated | H1756, H1801 | Rejmanek et al. (2010) | |
| 1997–98 | Spinal cord or brain | ED | MIH 1-8 | Mansfield et al. (2001) | |
| Sea otter | Brain | CV-1,BT | SN-OT1 | Lindsay et al.(2000b) | |
| 1999 | Brain | MA104 | SO SN1 | Miller et al. (2001a) | |
| 2009 | Brain | MA104 | SO1-3 | Miller et al. (2009) | |
| Brain | MA104 | Miller et al. (2010) | |||
| 2005–2008 | Brain | MA104 | 23 isolatese | Rejmanek et al. (2010) | |
| Pacific harbor seal | Brain, CSF | MA104 | HS1 | Miller et al. (2001b) | |
| Harbor porpoise (Phocoena phocoena) | 2006 | Brain | Not stated | HP060325 | Rejmanek et al. (2010) |
| Raccoon (Procyon lotor) | 2001 | Muscleb | Not applicable | SN37R | Dubey et al. (2001b); Sofaly et al. (2002) |
| Cat (Felis catus) | 2000 | Muscleb | Not applicable | Sn-Mucat-2 | Turay et al. (2002b); Butcher et al. (2002) |
| Armadillo (Dasypus novemcinctus) | 2000 | Muscleb | Not applicable | Not stated | Cheadle et al. (2001c) |
| Sea otter (Enhydra lutris) | 2001 | Muscleb | Not applicable | SN-OT-2f | Dubey et al.(2001c) |
| Brown-headed cowbird (Molothrus ater) | 1999 | Muscleb | Not applicable | MICB1 | Mansfield et al. (2008) |
| South American opossum (Didelphis albiventris) | 1999 | Intestinea | EK,BT | SN35-OP, SN36-OP | Dubey et al. (2001g) |
| North American opossum (Didelphis virginiana) | 1999 | Intestinea | ED,M617 | SN8 to15OP | Dubey (2000) |
| Intestinea | ED,BT,EK,M617 | SN16 to34OP | Dubey et al. (2001i) | ||
| 2005–2008 | Intestinec | MA104 | OP 26,48,68,134, 166,187,201,2 12,226,L49,GA 3,GA7,OPBF2 | Rejmanek et al. (2010) d |
By feeding sporocysts to KO mice, and invitro cultivation from infected tissues of KO mice.
By feeding naturally infected muscle to laboratory-raised opossums, and the bioassay of sporocysts shed into KO mice.
BT=bovine turbinate, CV1=African green monkey; DT=deer testes, ED=equine dermal, EK=equine kidney, ESp= equine spleen, M617= bovine monocytes. MA104=monkey kidney.
Invitro cultivation directly from sporozoites released from sporocysts.
SO 4387, 4413, 4530, 4653, 4697, 4711, 4725, 4755, 4786, 4834, 4928, 4970, 4972, 5002, 5073, 5110, 5226, 5259, 5263, 5274, 5278, 5283, 5296.
designated here.
4.2. Cell biology
Because the parasite is easy to cultivate in many cell lines and can be genetically manipulated, S. neurona has been used as model to study several aspects of cell biology. Biologically, S. neurona has many characteristics in common with Toxoplasma gondii, and multiple reagents and techniques developed for T. gondii have been applied to studying the biology of S. neurona. In turn, the S. neurona culture system has contributed to the understanding of organelle development that is difficult to resolve with the T. gondii cultures. One example is the information on plastid replication obtained by using the S. neurona system. Compared with T. gondii (<6 h), the nuclear division in S. neurona merozoites is prolonged (three days), and thus events during parasite development can be easily followed. The nuclear and plastid replication were followed by Vaishnava et al. (2005) in bovine turbinate cells infected with the SN3 isolate of S. neurona. In this culture system, 64 merozoites were formed in three days. During schizogonic development, the nucleus continued to grow, became lobulated; five cycles of chromosome replication occurred without nuclear division (Vaishnava et al., 2005). After the sixth and the final division, the nuclear lobes bifurcated into two giving rise to 64 merozoites. The apicoplast in S. neurona was a four-layered, tubular structure without microcristae and was closely associated with the nucleus at all stages of division. During nuclear division, the apicoplast stayed associated with the nuclear lobes like a flexible hose. When the nucleus divided, the apicoplast fragmented and one lobe was incorporated into the budding merozoite.
4.3. Gene discovery and characterization
Compared to other prominent members of the Apicomplexa, there is limited information concerning the molecular composition of S. neurona. The initial efforts to generate nucleic acid sequence data for S. neurona were conducted in the context of phylogenetic analyses, development of diagnostic probes, and included the use of RAPD assays and sequence analysis of the 18S rRNA locus (Fenger et al., 1994; Granstrom et al., 1994; Dame et al., 1995; Marsh et al., 1999; Tanhauser et al., 1999a, b; Elsheikha et al., 2005a; Elsheikha et al., 2005b; Elsheikha et al., 2006a; Elsheikha et al., 2006b; Elsheikha and Mansfield, 2007; Elsheikha, 2009) to identify species-specific genetic markers. Some of these investigations led to PCR-based tests that detect S. neurona and/or distinguish it from other closely-related species (Fenger et al., 1995; Tanhauser et al., 1999a, b).
Early studies to identify and characterize protein-encoding genes of S. neurona utilized traditional molecular biology approaches. Sequence analysis of random clones from a cDNA library produced from the UCD-1 strain and standard immunoscreening of a cDNA library produced from the SN3 strain independently identified the S. neurona major surface antigen SnSAG1 (Ellison et al., 2002; Howe et al., 2005) which is homologous to the gene family of SAG/SRS surface proteins that have been investigated extensively in T. gondii (Jung et al., 2004; Lekutis et al., 2001; Wasmuth et al., 2012). Polyclonal antibodies against T. gondii enolase 2 (ENO2) followed by mass spectrometry were used successfully to identify the S. neurona ENO2 homolog (Bolten et al., 2008; Wilson et al., 2004). Biochemical characterization of S. neurona merozoites demonstrated the presence of serine protease activity at defined molecular weights, but the encoding gene(s) was not identified (Barr and Warner, 2003).
To better facilitate gene discovery in S. neurona, an expressed sequence tag (EST) sequencing project was performed using cDNA libraries constructed from the merozoite stage of the EPM isolates SN3 and SN4 (Howe, 2001; Li et al., 2003; Li et al., 2004). This project generated 15388 partial gene sequences from S. neurona, with another 6332 ESTs produced from the closely-related S. falcatula for comparative purposes. The resulting database of sequences represented only a portion of the genes present in S. neurona, but it permitted more efficient approaches to identify and select parasite sequences for further investigation. While a majority of S. neurona genes were chosen for study based on their homology to T. gondii molecules, predicted structural features of the encoded protein and cDNA abundance (i.e., number of ESTs) were also useful criteria for selection of interesting genes.
Along with the major surface antigen SnSAG1 that was identified by traditional approaches, three additional SnSAG paralogues designated SnSAG2, SnSAG3, and SnSAG4 were revealed in the ESTs generated from the SN3 strain of S. neurona (Howe et al., 2005). The SnSAGs are located on the surface of the extracellular merozoite stage of S. neurona and are present throughout intracellular development of the schizont as well. Additionally, analyses of the bradyzoites and sporozoites demonstrated that expression of the SnSAGs is differentially regulated during the life cycle (Gautam et al., 2011), similar to the SAG/SRS surface antigens in T. gondii and N. caninum. The specific function (s) of the SnSAGs has not been defined. However, evidence for T. gondii has implicated several of the TgSAGs as cell adhesins and modulators of host immunity (Dzierszinski et al., 2000; Rachinel et al., 2004; Kim and Boothroyd, 2005;), so it seem reasonable to suggest a similar role for the SnSAGs. Since the SnSAGs elicit strong humoral responses in infected animals (Ellison et al., 2002; Howe et al., 2005; Liang et al., 1998; Ellison and Witonsky, 2009), they have been used as target molecules to develop serologic tests for detection of antibodies against S. neurona (Ellison et al., 2003a; Hoane et al., 2005; Yeargan and Howe, 2011). Interestingly, it is apparent that all strains of S. neurona do not possess the same repertoire of SnSAG genes. This antigenic diversity was initially revealed in an S. neurona isolate from a horse in Missouri (Marsh et al., 2001b). Further examination of this strain, designated Sn-MU1 revealed that it lacked the SnSAG1 gene that is transcribed abundantly in the UCD-1 and SN3 strains (Hyun et al., 2003). Subsequent immunologic analysis of a collection of 14 S. neurona strains using antiserum against SnSAG1 demonstrated that this surface antigen is not expressed by multiple strains, and the lack of expression was shown to be due to the absence of the SnSAG1 gene (Howe et al., 2008). Those parasite strains that lack SnSAG1 were found to express one of two alternative major surface antigens that were call SnSAG5 and SnSAG6 (Crowdus et al., 2008; Wendte et al., 2010b). While it is conceivable that additional alternative major SnSAG paralogues exist, analysis of much more extensive collections of parasite strains suggest that SnSAG1, SnSAG5, or SnSAG6 will be predominant in the S. neurona strains circulating in nature (Rejmanek et al., 2010,; Wendte et al., 2010a). The genes for these three SnSAG paralogues seem to be mutually exclusive to one another, since all strains of S. neurona that have been analyzed possess sequence for only SnSAG1 or SnSAG5 or SnSAG6; no strain has been found to possess more than one of these genes. Analysis of synonymous versus non-synonymous mutations in the SnSAG1 and SnSAG5 gene sequences suggests there may be evolutionary advantages to altering some regions of these surface proteins (Elsheikha and Mansfield, 2004b).
The S. neurona surface protein 1 (SnSPR1) gene is a seemingly novel sequence that is not paralogous to the SnSAG surface antigens but does encode a merozoite surface protein (Zhang and Howe, 2008). Instead of relying on homology to select the sequence for investigation, this gene was chosen for study based on its abundance in the S. neurona EST collection and the prediction that the encoded protein had an amino-terminal signal peptide and a carboxyl-terminal glycolipid anchor addition, consistent with a cell surface protein. In addition to localizing to the merozoite surface, SnSPR1 was shown to be a low molecular weight protein (approximately 14 kDa) that is present throughout intracellular development of the S. neurona schizont. Despite being an abundant protein based on the number of ESTs that match the SnSPR1 sequence, this protein does not appear to elicit the robust immune response seen for the SnSAG surface antigens.
Genes encoding secretory proteins of S. neurona have been identified based on homology. A putative SnMIC10 sequence exhibited approximately 30% identical to the TgMIC10 and NcMIC10 orthologues of T. gondii and N. caninum, respectively, and examination of the native protein in merozoites using antiserum raised against recombinant SnMIC10 revealed characteristics consistent with it being a microneme protein of S. neurona (Hoane et al., 2003). A dithiol-dependent nucleoside triphosphate hydrolase (SnNTP1) was similarly identified based on sequence similarity to the two TgNTPase isoforms of T. gondii (Zhang et al., 2006). Although localization to the apical end of the merozoite was unexpected for SnNTP1, it was found to be part of the secreted fraction of S. neurona, consistent with it being a dense granule protein. Interestingly, both SnMIC10 and SnNTP1 are not expressed for much of S. neurona intracellular development, with these two proteins seen only in late schizonts containing newly-forming daughter merozoites (Hoane et al., 2003; Zhang et al., 2006). While this was expected for SnMIC10 since microneme proteins participate in host cell invasion and should not be needed during endopolygeny, it was unanticipated for SnNTP1 since the TgNTPases are important for intracellular growth of T. gondii (Asai et al., 2002; Nakaar et al., 1999).
4.4. Molecular genetic tools
To enhance the study of S. neurona, basic methods have been established for DNA transfection, transient expression of transgenes, and selection of stably transformed clones of this parasite. Luciferase, beta-galactosidase (beta-gal), yellow fluorescent protein (YFP), and red fluorescent protein (RFP) have been used successfully as reporter molecules in S. neurona (Gaji and Howe, 2009; Gaji et al., 2006; Vaishnava et al., 2005). Mutant dihydrofolate reductase-thymidylate synthase (DHFR-TS) that confers resistance to pyrimethamine can be used to achieve stable transformation of S. neurona. This selection system was used to produce transgenic S. neurona clones that stably express either beta-gal or YFP, which are useful for monitoring parasite growth or invasion rates in-vitro (Gaji et al., 2006). Additionally, fluorescence-activated cell sorting (FACS) of YFP expression can be used to select stable clones of S. neurona that do not contain a drug resistance gene (Figure 2). Recently, the hypoxanthine-xanthine-guanine phosphoribosyltransferase gene of S. neurona (SnHXGPRT) was successfully disrupted by double homologous recombination using a gene-targeting plasmid (Dangoudoubiyam et al., 2014). The SnHXGPRT-deficient mutant clones (SndeltaHXG) were selected by their resistance 6-thioxanthine (6-TX), a toxic analog of xanthine, and could be complemented with the T. gondii HXGPRT gene, rendering the SndeltaHXG parasites resistant to mycophenolic acid (MPA). Thus, the SndeltaHXG clone provides an efficient system for both positive and negative selection of stable transgenic S. neurona. Collectively, the molecular genetic capabilities that have been developed for S. neurona make possible a variety of new experimental approaches, including gene knockouts, complementation studies, and gene regulation assays. Indeed, the value of these molecular tools has been demonstrated in studies examining the development and segregation of the S. neurona apicoplast during endopolygeny (Vaishnava et al., 2005) and identification of sequence elements involved in promoter function (Gaji and Howe, 2009).
4.5. Population genetics
Compared to other prominent parasites within the Apicomplexa, there have been only limited analyses performed to examine the population genetic structure of S. neurona strains circulating throughout the Americas. Initial efforts focused on developing markers capable of distinguishing between the different Sarcocystis species that infect the opossum definitive host, including S. neurona, S. falcatula, S. lindsayi, and S. speeri. The first molecular characterization was performed using the small subunit ribosomal RNA (ssuRNA; 18S) marker on Sarcocystis strains isolated from horses and established that S. neurona resolved phylogenetically within the family Sarcocystidae, suggesting a close relationship to S. muris (Fenger et al., 1994). Follow-up work at the 18S locus indicated S. neurona was synonymous with S. falcatula, the parasite that cycles between opossums and birds (Dame et al., 1995). Phylogenetic resolution between S. neurona and S. falcatula was finally achieved upon DNA sequencing the internal transcribed spacer 1 (ITS-1) locus within the ssuRNA gene array, which identified 12 nucleotide differences between the two parasites (Marsh et al., 1999). In the absence of gene-specific molecular markers, panels of random amplified polymorphic DNA (RAPD) markers were next developed to differentiate S. neurona isolates from other related coccidia (Granstrom et al., 1994) and within the S. neurona species (Tanhauser et al., 1999a; Elsheikha and Mansfield, 2007). These investigations led to the development of PCR-based tests used to detect S. neurona and/or distinguish it from other closely-related coccidia (Fenger et al., 1995; Tanhauser et al., 1999a, b).
During the last 15 years, a large collection of S. neurona strains have been isolated from a variety of geographic regions and host species. To ascertain the true genetic diversity among circulating strains and to address whether specific S. neurona strains are associated with increased disease risk, a serious effort has been pursued to develop a panel of genetic markers capable of resolving the parasite’s population genetic structure, its evolutionary biology, and the extent to which it expands in nature either sexually (by uniparental mating or outcrossing) or, akin to its close cousin T. gondii, whether it is capable of asexual transmission by carnivory among its intermediate host range. Currently, all evidence indicates that, unlike T. gondii, Sarcocystis bradyzoites are not infectious to intermediate hosts (Dubey et al., 2013). To answer these important questions, a series of genetic markers of varying resolution have been developed. The first set of widely applied sequence-specific markers for population genetic analyses were derived from two RAPD markers originally described by Tanhauser et al. (1999a). A restriction fragment length polymorphism (RFLP) at the 33/54 locus was used to distinguish S. neurona from S. falcatula, and nucleotide sequence polymorphisms at the 25/396 locus identified two major alleles that resolved North American from South American S. neurona strains (Rosenthal et al., 2001). To estimate whether the S. neurona population genetic structure was genetically diverse or clonal, Asmundsson and Rosenthal (2006) and Asmundsson et al. (2006) developed and applied 12 highly polymorphic microsatellite markers against 34 predominantly North American Sarcocystis samples collected from both definitive and intermediate hosts isolated from diverse geographical origins (Sundar et al., 2008). This important study established that substantial allelic and genotypic diversity exists among circulating S. neurona strains and showed that one genotype is more prevalent than expected for a strictly outbred population, indicating that some degree of clonal expansion has occurred within the species (Asmundsson et al., 2006).
The identification of widespread S. neurona infections in marine pinnipeds and the discovery of a large epizootic that resulted in the death of approximately 2% of the federally-listed threatened southern sea otter population, during a 3 week period in 2004, fostered the necessity to develop a more comprehensive genotyping scheme to determine the transmission dynamics of the parasite and whether specific genotypes of S. neurona circulating in the marine ecosystem were more pathogenic (Rosonke et al., 1999; Lindsay et al., 2000b; Lindsay et al., 2001a; Miller et al., 2001a; Miller et al, 2001b; Dubey et al., 2001c; Miller et al., 2010). In 2010, a panel of gene-specific molecular markers of varying phylogenetic resolution was developed to increase the discriminatory power of the molecular markers used for Sarcocystis population genetic analyses. This work established a comprehensive multi-locus sequence typing (MLST) approach capable of resolving strains at the genus, species and intra-species level (Wendte et al., 2010b; Rejmanek et al., 2010). The markers consisted of a plastid-encoded RNA polymerase b gene (RPOb) and a cytochrome c oxidase 1 (CO1) gene encoded within the apicoplast and mitochondrial organellar genomes respectively, both of which are maternally inherited and exist as useful markers to detect genetic exchange (or hybridization) between strains based on incongruity between nuclear and organellar genome phylogenies. Hence, when two nearly identical MLST genotypes possess different organellar genomes, outcrossing is supported. In addition, the discovery of a family of polymorphic SnSAG surface antigen genes orthologous to the highly informative SRS genotyping markers encoded by T. gondii (Howe et al., 2005; Howe et al., 2008) identified a series of intra-specific genetic markers (annotated SnSAG1, 3, 4, 5, and 6) that possessed sufficient allelic diversity to chart the parasite’s population genetic structure and produce the first genetic history model for S. neurona (Wendte et al., 2010b). MLST sequence level analysis identified 12 genetic types, two of which were predominant (56/87; 64%) and accounted for the majority of infections in the USA, based on the inheritance of different allele combinations encoded by the SnSAG genes among the 87 S. neurona-infected samples collected from varying hosts and geographic locations (Wendte et al., 2010a). These data supported a population structure that is both clonal and punctuated by a series of genetic types that evolved by sexual recombination through the definitive opossum host. Such an intermediate population structure is similar to that described for T. gondii (Grigg and Sundar, 2009). To explain the existence of dominant clonotypes within the population structure for S. neurona, Wendte et al. (2010a, b) showed using a series of high resolution microsatellite markers that S. neurona, like T. gondii, is capable of uniparental mating, and established selfing (or sexual amplification of a single clone) as a genetic mechanism for the clonal propagation of the species. S. neurona is not known to be infectious between intermediate hosts, because it is thought that the tissue-encysted sarcocyst form is obligatory committed to its sexual life cycle within its definitive opossum host (Wendte et al., 2010a). The extent to which uniparental mating versus outcrossing is impacting the population genetic structure of S. neurona, or its capacity to generate and expand specific strains capable of causing disease epidemics, is not currently known. This lack of knowledge certainly underscores the necessity to expand sample collection among intermediate and definitive hosts, to increase the number of informative polymorphic phylogenetic markers to improve the resolution of the current MLST typing scheme, and to pursue whole genome comparative studies in order to produce an accurate genetic history model for the species and assess the extent to which genetic recombination is impacting the parasite’s population genetics in this genome-sequencing era.
4.6. S. neurona genome
As a genus, Sarcocystis arguably exists as the most successful protozoan parasite in nature, largely because all vertebrates, including birds, reptiles, fish and mammals can be infected by at least one Sarcocystis species. Despite its widespread prevalence and the relative ease of genetically manipulating mutants in S. neurona and propagating them in vitro, the lack of a physical or genetic map has hampered the development of this parasite as a model system for genetic analyses. This is largely because S. neurona chromosomes do not condense, nor can they be resolved by pulse-field gel electrophoresis. The recent whole genome shot-gun sequencing of the S. neurona SO SN1 genome has rectified this knowledge gap and produced the first molecular karyotype for the genus, which should greatly facilitate future genetic and comparative genomic studies on this important pathogen (Blazejewski et al., 2015).
Combining Roche 454 and Illumina Hi-Seq reads at ~200X coverage, the S. neurona genome assembled into a molecular karyotype of 116 genomic scaffolds with a combined size of 127 Mbp, which was over twice the size of the N. caninum, T. gondii, and H. hammondi genomes (Reid et al., 2012; Walzer et al., 2013). The existence of a high proportion of repetitive Type II transposons, DNA- and LINE-element sequences totaling 31 Mbp accounted for nearly half of the increased genome size, with the remaining due to increased average intron length and intergenic region sizes that were > 3X that of Toxoplasma. The largest scaffold was 9.2 Mbp in length, and an additional 3.1 Mbp of sequence was encoded within 2950 unscaffolded contigs (that were each >500 bp in size). When RNA-Seq data was combined with the genomic data into an annotation pipeline, a complement of 7093 genes were identified, 5853 of which were expressed during merozoite growth. The predicted gene complement was similar to that found in N. caninum, T. gondii, and E. tenella, however, only limited chromosome-wide synteny was observed for homologous genes between S. neurona and T. gondii, with the largest block comprised of only 43 genes. This established that significant genome rearrangement has occurred between Sarcocystis and that of Toxoplasma and Neospora, which are largely syntenic across all chromosomes. In addition to its nuclear genome, the S. neurona apicoplast genome was largely conserved across the coccidians, with a few key differences. S. neurona has lost its rpl36 gene, it had only one copy of the tRNA-Met ORF, and has two distinct RNA polymerase C2 genes. In common with Toxoplasma, it was missing ORF A. Furthermore, genome comparisons showed that S. neurona shared more orthologs with Toxoplasma (3169) than with Eimeria (1759), consistent with Sarcocystis being more closely related to Toxoplasma than to Eimeria. Additionally, S. neurona encoded 1285 (18%) genes that showed no detectable homology with any other species, underscoring the opportunity for investigators to now identify new gene families within the Sarcocystis genus that promote their success in nature.
The tissue-encysting coccidia have evolved many families of dense granule (GRA), rhoptry kinase (ROPK), microneme (MIC), and surface protein adhesins known collectively as the SRS to promote their ability to disseminate and establish long-term, chronic infections in their intermediate hosts. Comparative genomic, transcriptomic and metabolic data analyses between S. neurona and other coccidian genomes has established that the S. neurona invasion machinery is largely conserved, but that it only has a limited set of ROPK and GRA proteins. Fifteen ROPK orthologs have been identified, which is significantly smaller than E. tenella (n=27), N. caninum (n=44) and T. gondii (n=55). Moreover, none of the ROPK proteins previously identified as important murine virulence genes (ROP5, ROP18) or those that have the ability to alter host immune effector function by altering STAT3/6 (ROP16) or MAP kinase signaling (ROP38) are encoded within the S. neurona genome, indicating that S. neurona pathogenesis and infectivity is not dependent on the inactivation of these pathways; this may also explain why this parasite is not infectious in immunocompetent rodents. In addition, S. neurona did not encode orthologs of the majority of GRA proteins expressed by T. gondii and N. caninum, including GRA6, GRA15, GRA24 and GRA25 which are known to regulate NFAT4, NF-kB, p38a MAP kinase and CXCL1/CCL2 levels, respectively, during acute infection. In fact, only two GRA protein orthologs were identified in S. neurona, GRA10, and GRA12. These data underscore the differences between S. neurona and closely-related coccidia (e.g., T. gondii and N. caninum), and may indicate that Sarcocystis does not require an expanded repertoire of GRA and ROPK genes to promote recrudescence of infection; specifically, Sarcocystis bradyzoites appear to be terminally committed to the gamont stage, thus requiring ingestion by its definitive host to complete its life cycle.
Of particular note, S. neurona encoded a reduced set of only 23 SRS surface protein adhesins, significantly less than the 109 and 246 SRS proteins encoded by T. gondii and N. caninum respectively (Reid et al., 2012; Wasmuth et al., 2012). Twenty of the SnSRS proteins were distributed across 11 of the major scaffolds, and the majority existed as single genes. Only one genomic locus (SnSRS7 on scaffold 4) possessed an array of paralogs that presumably evolved by tandem gene duplication. Structural evidence supports the resolution of the SRS protein domain into eight different families, and the 23 SRS genes were predominantly comprised of family 2 (fam2) SRS domains, which are known to play an important and integral role in preserving the bradyzoite cyst wall structure in T. gondii (Tomita et al., 2013). These findings suggest that fam2 SnSRS domain-containing proteins emerged in the common ancestor of S. neurona and T. gondii to provide these parasites with the ability to extend their host range and penetrance within an ecosystem by forming long-lived, infectious tissue cysts in a vast array of intermediate prey hosts for their respective opossum and felid definitive hosts.
With the publication of the first S. neurona genome, it should now be possible to produce the necessary phylogenomic datasets that focus on important questions pertaining to the extent to which the genus utilizes its sexual cycle to generate outbreak strains that alter its host range and specificity. Equally, it provides the template to scaffold gene and protein expression datasets that will facilitate the identification of critical proteins for development of novel control measures that are urgently required to control the pathogenesis of this important protozoan pathogen.
5. Serologic prevalence
5.1. Equids
Prevalence of antibodies is dependent on the distribution of opossums in the area, type, and age of horses sampled, geographical location, and the serological test used (Table 5). Sera in several studies were tested in one laboratory by using Western blot (WB) against low molecular proteins (marked WBb) in Table 5. In these studies, prevalence was around 50% of horses tested. Seroprevalence increased with age (Blythe et al., 1997; Saville et al., 1997; Dubey et al., 1999b; Tillotson et al., 1999; Bentz et al., 2003; Rossano et al., 2001; Yeargan et al., 2013). In these surveys, the horses tested were mostly older than 1 year of age. It is likely that some of the foals tested had maternal-acquired antibodies. In one study, foals born to 33 seropositive mares were bled before suckling, 1 day after colostrum ingestion, and again at monthly intervals (Cook et al., 2001). All foals were seronegative before suckling, all became seropositive one day after suckling, and 31 of 33 became seronegative by nine months of age. The decay of antibody was probably related to the concentration of IgG in the colostrums. In another study, the median time of decay of maternal-acquired S. neurona antibodies in foals was 96 days, and these antibodies disappeared by 230 days of birth (Durate et al., 2004a). These results are in contrast to the study by Pivoto et al. (2014) who reported antibodies in 61 (37.3%) of 181 mares at parturition and in pre-suckle blood samples of 6.6% foals born to these mares. These observations need confirmation because current evidence indicates that transplacental or lactogenic transmission of S. neuronais very uncommon or absent. In an epidemiologic investigation of S. neurona seropositivity of horses in California tested by IFAT, there was no evidence for the transplacental transfer of S. neurona antibodies or parasite; all 366 pre-suckling foal sera were seronegative (Durate et al., 2004b). In one study of 174 foals born to mares on a farm with very high seroprevalence to S. neurona (90%) found only a single foal with a pre-suckle antibody titer (Pusterla et al., 2014).
Table 5.
Seroprevalence of S. neurona antibodies in equids.
| Location | Year sampled | Type, source | No. tested | Test, cut-off | Positive | Reference | |
|---|---|---|---|---|---|---|---|
| No | % | ||||||
|
USA PA |
1993–94 | Thoroughbred farmsa | 117 | WBb | 53 | 45.3 | Bentz et al. (1997) |
| OR | Not stated | Horses from 4 regions, >3 month old | 334 | WBb | 149 | 45.0 | Blythe et al. (1997) |
| OH | 1993–94 | 37 breeds, state widec | 1056 | WBb | 560 | 53 | Saville et al. (1997) |
| CO | 1995–96 | 593 horses and 15 ponies | 608 | WBb | 204 | 33.6 | Tilloston et al. (1999) |
| OK | 2001 | Banked sera | 798 | WBb | 712 | 89.2 | Bentz et al. (2003) |
| WY | Not stated | Wild horses | 276 | WBb | 18 | 6.5 | Dubey et al. (2003b) |
| SAT, 1:50 | 39 | 14.1 | |||||
| CA, FL, MO, MT | Not stated | Sera, diagnostic labs | 208 | IFAT, 1:100 | 49 | 26.0 | Vardeleon et al. (2001) |
| MI | 1997 | 1056 horses, 63 ponies, 3 donkeys, 1 mule from 98 herds | 1121 | WBd | 627 | 56.0 | Rossano et al. (2001, 2003) |
| 40 states | 2010–11 | 40 states, sera submitted to diagnostis lab | 3123 | IFAT, 1:80 | 865 | 27.6 | Pusterla et al. (2014) |
| Canada | 2001 | Western Canada | 239 | WBb | 0 | 0 | Dubey et al. (2003b) |
| Argentina | 1996 | Chaco Province | 76 | WBb | 27 | 35.5 | Dubey et al. (1999c) |
| Argentina | 2006–2010 | 9 Provinces | 640 | WBb | 167 | 26.1 | Moré et al. (2014) |
| Brazil | 1998 | Thoroughbreds | 101 | WBb | 36 | 36.0 | Dubey et al. (1999b) |
| Not stated | 10 states | 961 | ELISA SAG4e | 669 | 69.6 | Hoane et al. (2006) | |
| Not stated | Rio Grande do Sul | 181 | ELISA-crude extract | 61 | 33.7 | Pivoto et al. (2014) | |
| Costa Rica | Not stated | 7 Provinces | 315 | SnSAG2 ELISAe | 133 | 42.2 | Dangoudoubiyam et al.(2011) |
| India | 123 | WBb | 1 | 0.8 | Brown et al. (2006) | ||
| Spain | Galicia | 384 | SnSAG2 ELISAf | 9 | 2.3 | Arias et al. (2012) | |
| France | 2 farms in Normandy | 50 | SAT | 18 | 36.0 | Pitel et al. (2003) | |
| Korea | Jeju island | Thoroughbred Jeju island | 191 | WB | 0 | 0 | Gupta et al. (2002) |
| Mexico | Durango state | Durango state | 495 | rSnSAG2/4/3ELISA | 240 | 48.5 | Yeargan et al. (2013) |
20% of horses from each farm with a population of 580 horses; 4-week old to 26 year old, 1 4-week old might have colostrally-acquired antibodies.
Western-blot, Granstrom et al. (1993)
Every 36th sample submitted to diagnostic lab for equine infectious anemia.
WB, blo treated with bovine antibodies against S. cruzi antigen Rossano et al., 2000).
Reactive to 30–35 kDa protein. Of these 26.1% reactive to 16–17 kDA protein, 5 (3.62%) reactive to rSnSAG2 ELISA, 4 (3%) reactive in SnSAG4/3 ELISA
CA=California, CO=Colorado, MI=Michigan, MO=Missouri, MS=Mississippi, OH=Ohio, OK=Oklahoma OR=Oregon, PA=Pennsylvania, WY=Wyoming.
Opossums are the only known definitive hosts for S. neurona, and they are found only in Americas. Theoretically, therefore, horses in other countries should have no exposure to S. neurona. Consistent with this, S. neurona antibodies were not detected by immunoblotting in any of 191 horses from South Korea (Gupta et al., 2002) and in only one of 123 horses born in India (Brown et al., 2006). However, seropositivity in horses from France (Pitel et al., 2002; Pitel et al., 2003) and Spain (Arias et al., 2012) is puzzling. Pitel et al. (2002) found S. neurona antibodies by WB in 28 horses with EPM like clinical signs. In a follow up study they found seropositivity in 18 (36%) of 50 healthy horses tested by the SAT. Even more intriguing are results from horses tested from Spain. Of 138 horses tested by immunoblotting, 26.1% to 82.6% of horses were seropositive, based on the antibodies directed against immunodominant molecules at low (16–17 kDa) or high (30–35 kDa) molecular weights (MW); notably, Granstrom et al. (1993) used antibodies directed against proteins lower than 16 kDA for interpretation of the WB. Retesting of these 138 samples with the rSnSAG2 ELISA and rSnSAG4/3 ELISAs revealed that only five sera (3.6%) were reactive in rSnSAG2 ELISA, and only four (3%) were reactive in rSnSAG4/3 ELISAs; only one serum was strongly reactive in both types of ELISA tests (Arias et al., 2012). A further set of sera from 246 horses were tested by rSnSAG2 ELISA; 9 (2.34%) of 384 were seropositive. None of these 384 horses travelled out of Spain. Consequently, it was suggested that the immune-reactivity observed in the immunoblots was due to cross-reactivity with another species of Sarcocystis that infects horses in Spain. Currently, S. neurona has not been isolated nor detected by PCR in European horses.
It has been suggested that there may be another definitive host for S. neurona in Europe, or there is cross reactivity among Sarcocystis species in horses to account for the seropositivity in European horses (Pitel et al., 2003). In this respect, another species of Sarcocystis, S. bertrami occurs in horses in Europe (Dubey et al., 1989). Whether there is cross reactivity between S. bertrami and S. neurona, and if the type of antibody-testing or antigen preparations account for this reactivity has not been completely investigated. In the USA, only one non-S. neurona species of Sarcocystis, S. fayeri, has been found in horses. To resolve the question of cross-reactivity between S. fayeri and S. neurona, Saville et al. (2004b) orally inoculated three seronegative ponies, 105, 106, or 107 S. fayeri sporocysts collected from dogs fed infected muscle obtained from horses in Texas. The fourth pony was an uninoculated control. Sera from ponies were tested by employing three tests (WB, indirect fluorescent antibody test [IFAT], and S. neurona direct agglutination test (SAT) before dosing sporocysts and then weekly thereafter. With the WB using the strict interpretation criteria that were previously established (Granstrom et al., 1993), antibodies specific to S. neurona were not found in any pony at 0, 2, 37, and 79 DPI. This negative reactivity was further confirmed using a recombinant SnSAG1 protein. (Gupta et al., 2002) By SAT, only one sample collected day 37 from the pony dosed with 107 S. fayeri sporocysts was positive at 1:50 dilution. By contrast, all three inoculated ponies were seropositive by IFAT up to 1:400 dilution whereas the control pony was seronegative. (Saville et al., 2004b) This experiment provides conclusive evidence that S. neurona infection can be distinguished from S. fayeri infection. It is important that the WB results are interpreted correctly since it was apparent that these S. fayeri-infected ponies had antibodies that recognized numerous S. neurona antigens (Hoane et al., 2005a).
5.2. Cats and other animals
There is limited knowledge on the specificity of the serological tests in non-equid species but available serological S. neurona surveys in other hosts are summarized here. Antibodies were found in 1–40% of cats in USA (Table 6). As a contrast, S. neurona antibodies were not found by SAT in any of the 502 domestic cats from Brazil that were tested (Dubey et al., 2002c).
Table 6.
Serological prevalence of S. neurona in non equid, non marine mammals in the USA.
| Host | Locationa | Year | Type | No. | Test, cut off | No. positive (%) | Reference |
|---|---|---|---|---|---|---|---|
| Cat (Felis cat us) | VA | 232 | IFAT, 40 | 22 (9.0) | Hsu et al. (2010) | ||
| PA | 209 | 10 (5.0) | |||||
| MS | NS | Stray | 9 | WB | 1 (11.1) | Turay et al. (2002b) | |
| MI | 1999–2001 | Pets | 196 | IFAT, 20 | 10 (27) | Rossano et al., (2002) | |
| OH | 2001 | Horse farms | 35 | SAT, 25 | 14 (40.0) | Stanek et al.(2003) | |
| 2001 | Spay/neuter | 275 | SAT, 25 | 27 (10.0) | |||
| FL | NS | humane shelter, feral | 100 | WB | 5 (5.0) | Gillis et al. (2003) | |
| Skunk (Mephitis mephitis) | CT | NS | 24 | SAT, 50 | 11(46) | Mitchell et al. (2002) | |
| Raccoon (Procyon lotor) | CT | 12 | 100 | Mitchell et al. (2002) | |||
| PA,MA, FL, NJ | 99 | SAT, 50 | 58 (59.6) | Lindsay et al. (2001b) | |||
| VA | 2001–2 | Feral | 469 | SAT, 50 | 433 (92.3) | Hancock et al.(2004) | |
| Opossum (Didelphis virginiana) | CT | 7 | 0 | Mitchell et al.(2002) | |||
| FL | Not stated | Feral | 20 | SAT, filter paper | 0 | Cheadle et al. (2006) | |
| Beaver (Castor Canadensis) | MA | 62 | SAT, 25 | 4(6.0) | Jordan et al. (2005) | ||
| Lemur Ring-tailed (Lemur catta) | 52 | SAT, 50 | 2 (1.9) | Yabsley et al. (2007) | |||
| Black-and-white ruffed lemur(Varecia variegata uffed) | 4 | 1 (25.0) | |||||
| Black–eyed lemur (Eulemur macaco flavfrons) | 6 | 0 | |||||
| Armadillo (Dasypus novemcinctus) | FL | Not stated | 2 | SAT, 1:50 | 2 (100) | Cheadle et al. (2006) |
CT=Connecticut, FL=Florida, NJ=New Jersey, MA=Massachusetts, MS=Mississippi, OH=Ohio, PA=Pennsylvania, VA=Virginia.
SAT=S. neurona agglutination test. IFAT=indirect fluorescent antibody test, Wb=western blot.
Antibodies (IFAT, 1:25) were found in two of 63 capybaras (Hydrochoerus hydrochaeris) from Brazil (Valadas et al., 2010).
Antibodies to S. neurona were not found in experimentally (Cheadle et al., 2006) or naturally exposed (Houk et al., 2010) opossums.
Antibodies were detected in skunks following oral inoculation of sporocysts (Cheadle et al. 2001d).
6. Clinical infections
61. Horses, pony, zebra
EPM is often a progressively debilitating disease affecting the CNS of horses. The clinical signs may vary from acute to insidious onset of focal or multifocal signs of neurologic disease involving the brain, brainstem, spinal cord or any combination of the areas of the CNS (Figs. 11, 12). Some horses affected with EPM have abnormal upper airway function, unusual or atypical lameness or even seizures. In severe cases, the horse may have difficulty with standing, walking, or swallowing, and the disease may progress very rapidly. In some horses, the disease appears to stabilize or remain static for a time period.
Figure 11.
Two horses with clinical EPM. Top, ataxic and bottom a mare with urinary inconsitance.
Figure 12.
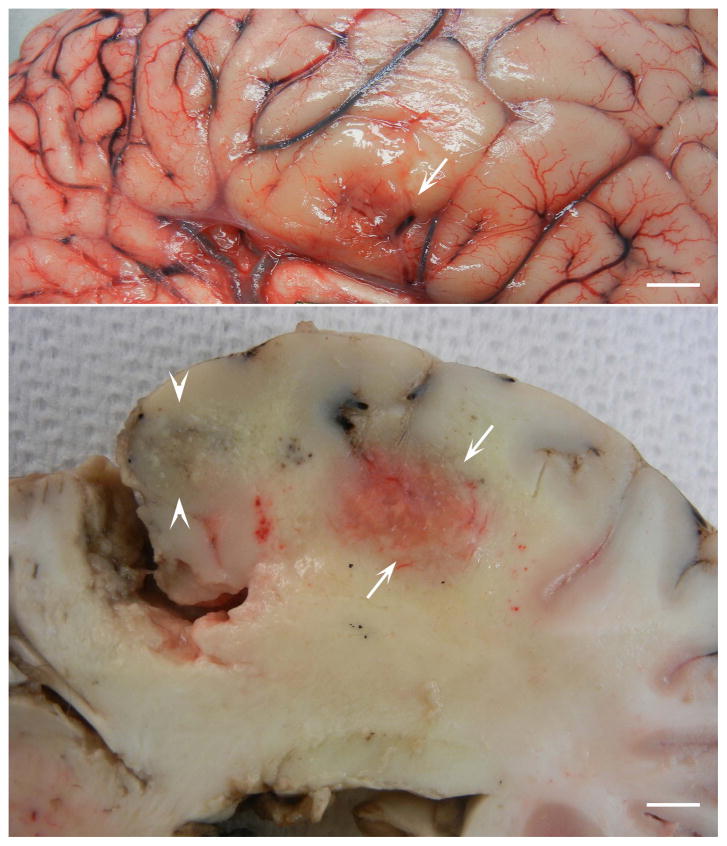
Surface (top) and cut (bottom) views of cerebrum of a 20 year old Paint horse with histologically and PCR confirmed EPM. The horse had a six day history of muscle fasciculations, bruxism, difficulty eating and drinking, and circling to the left with head pressing. Note hemorrhagic and yellow discolored areas indicative of necrosis. Bar = 5 mm. (Courtesy of Uneeda Bryant).
The early clinical signs of stumbling and frequent interference are often easily confused with a lameness of either the thoracic and/or the pelvic limbs. In many horses the disease tends to have a gradual progression of clinical signs including ataxia, but in some horses mild clinical signs followed by a rapidly progressive course have been observed. In these horses if blood monitoring, using either western blot, IFAT or ELISA testing, changes in antibody concentration, sometimes dramatic changes, are often observed. On physical examination, the vital signs are usually normal, although variations in body condition from very thin to obese along with clinical depression may also be observed. Neurological examination often reveals an asymmetric weakness, ataxia and spasticity involving all four limbs. Frequently, areas of hypoalgesia or complete sensory loss may be noted. The most frequent brain or cranial nerve deficits observed in horses appear to be head tilt, depression, facial nerve paralysis, and difficulty swallowing, upper airway dysfunction such as dorsal displacement of the soft palate and laryngeal hemiplegia have been noted although signs are not limited to these areas (Fig. 9). Gait abnormalities are often a result of damage to the brainstem or spinal cord and may be quite variable depending on the location and severity of the lesion.
Most horses affected with EPM are bright and alert at presentation; however, any horse with signs of neurologic disease is a candidate to have EPM. At the time of initial examination, most horses have normal blood values. One of the most helpful clinical signs is that horses with EPM often have asymmetric gait deficits with focal muscle atrophy. This can be a useful differentiating feature and may help direct one towards a clinical diagnosis of EPM rather than another neurological diseases.
The pathogenesis of EPM is not clear. Clinical signs of EPM are dependent on the area of the CNS parasitized. For example, involvement of the cerebrum may cause depression, behavior changes or seizures. Lesions in the brainstem and spinal cord often cause gait abnormalities, incoordination caused by involvement of ascending and descending tracts, and any of a variety of signs attributable to damaged cranial nerve nuclei. Severe damage in the gray matter that innervates muscles of the limbs can produce weakness followed by atrophy of innervated muscles. The quadriceps and gluteal as well as the temporalis muscles are often atrophied; S. neurona has not been found in affected muscles.
Factors governing severity of EPM are unknown. Clinical EPM is often reported in well cared race horses three to six years of age. Clinical EPM does not seem to be associated with poor nutrition or known concurrent infections. There are no confirmed reports of clinical EPM in horses younger than six months of age. Gray et al. (2001) reported EPM-like disease in a two-month old Appaloosa colt with facial paralysis since two days after birth; S. neurona antibodies were found in its CSF by immunoblot, and it responded favorably to pyrimethamine and trimethoprim-sulfadiazine medication.
Until now, EPM has been confirmed histologically only in horses born and raised in the Americas, coincident with the geographical range of opossums. However, EPM has been diagnosed in horses exported from the Americas. In Japan, Katayama et al. (2003) confirmed S. neurona immunohistochemically in a Thoroughbred, 15 months after importation from Kentucky. From the published reports, it appears EPM is rare in equids other than horses. One case was diagnosed in a ten year old pony from Maryland (Dubey and Miller, 1986; Dubey and Hamir, 2000). Marsh et al. (2000) reported EPM in an eight year old Grant’s zebra (Equus burchelli bohmi) in California.
Prevalence of EPM in horses was estimated at 0.5 to 1% of the horse population (National Animal Health Monitoring System. EPM: Equine Protozoal Myeloencephalitis in the US. Ft. Collins, CO: US Department of Agriculture: Animal and Plant Health Inspection Service: Veterinary Services; 2001; #N312.0501. Available at: http// nahms.aphis.usda.gov/Equine/eq98EPM.pdf. Accessed August 28, 2007.). Clinical disease is sporadic and more than one case is seldom seen at a particular farm. However, clusters of cases have occurred in a few instances, which would suggest all of the risk factors necessary for disease were at those facilities. EPM has been reported in siblings.
The pathogenesis of S. neurona in horses is unclear because it has been difficult to reliably induce disease experimentally in horses. Attempts at inducing EPM in horses are summarized in Table 7. Although horses developed clinical disease, attempts to demonstrate S. neurona in histological sections were essentially unsuccessful. Several factors including the stage of the parasite inoculated, dose, age of the horse, immunological status, endogenous and exogenous stress during experiment were examined by different investigators. Clinical outcome was not affected whether sporocysts were derived from naturally-infected versus experimentally-infected opossums. The dose did not significantly affected outcome, as feeding as few as 100 sporocysts induced clinical disease (Sofaly et al., 2002). Administration of corticosteroids to horses also did not affect the outcome of the experiments (Cutler et al., 2001; Saville et al., 2001). A transport stress model proved valuable in helping to induce clinical EPM. In a series of experiments young horses selected from northwest Canada where opossums are not present were transported by road for 55 h and then dosed with S. neurona sporocysts as the horses were unloaded from trucks. In the transport-stressed horses, seroconversion to S. neurona was sooner and clinical signs were more severe than in horses not stressed. A second transport stress after a two week rest did not affect the severity of EPM (Saville et al., 2001; Saville et al., 2004a). Arabian SCID foals lacking T and B cells were successfully infected with S. neurona with demonstrable live parasites in blood and tissues; however, these foals did not develop clinical signs of EPM (Long et al., 2002; Sellon et al., 2004). Parasitemia was demonstrable in an immunocompetent horse dosed daily with S. neurona for 112 days (Rossano et al., 2005b). All five horses inoculated with cultured S. neurona merozoites directly in the subarachnoid space seroconverted but only one developed clinical signs (Lindsay et al., 2000). S. neurona can circulate in equine tissues within lymphocytes (Lindsay et al., 2006). To facilitate dissemination of S. neurona merozoites and their entry into the CNS, Ellison et al. (2003b, 2004) first infected equine peripheral blood mononuclear cells (PBMCs) with merozoites in vitro, and after five h incubation, the infected cells were inoculated intravenously back into horses. Five of the six inoculated horses developed signs of clinical EPM starting the first week post inoculation. This experiment was repeated with eight additional horses with similar results (Witonsky et al., 2008). However, not all horses inoculated with merozoites in PBMCs develop EPM (Heskett and MacKay, 2008).
Table 7.
Attempts to induce clinical disease in horses after inoculation with S. neurona.
| No. | Type | S. neurona | Clinical disease | Durati on (days) | Diagnosis | Reference | |
|---|---|---|---|---|---|---|---|
| Bioassay | Histology | ||||||
| 5 | 3–6 month foals | Naturally infected opossums (NIO) | Clinical signs in all, 28–42 DPI, neurological, lesions in spinal cord of 4 | 40–110 | Negative | Negative | Fenger et al. (1997) |
| 8a | 3–4 years | NIO | lesions spinal cord in 7 of 8 horses | 90 | Negative | Negative | Cutler et al. (2001) |
| 9a | Foals | NIO | Clinical signs more severe in stressedb horses | 44–63 | Negative | Negative | Saville et al. (2001) |
| 20a | 4–5 month old | Experimentally infected opossums (EIO)d | Dose response 100, 1000, 10000,100000, 1000000, 4 horses per group. Clinical signs in all, histologic lesions inconsistent | 30 | Negative | Negative | Sofaly et al. (2002) |
| 24a | 4–5 month old | EIOd | Stressed horsesb, second timec | 40 | Negative | Negative | Saville et al. (2004a) |
| 6 | EIOd | 100–1000 sporocysts daily for 112 days | Positive, blood of 1 horse | No data | Rossano et al. (2005b) | ||
| 1 | Arabian 5-month old SCID foal | NIO | Knuckling 39 DPI, euthanized 53 DPI. S. neurona isolated from brain and blood | 53 | Positive | Long et al. (2002) | |
| 2 | Arabian | NIO | Neurological signs in 1 | 90 | Negative | Negative | Sellon et al. (2004b) |
| 4 | Arabian | 500 million merozoites from culture, inoculated intravenously | 3 horses neurological | 41, 73, 80, 471 | Positive in 1 horse brain | Negative | |
| 1 | Arabian SCID foals | 500 million merozoites from culture, inoculated intravenously in to 1 foal | NO clinical signs | 21,32 | Li, Sp,Sk of 1 foal, H and Sk of second foal | Negative | |
| 2 | Arabian SCID foals | NIO | NO clinical signs | 96 | Li, Lu, Sp, H, Sk | Negative | |
| 5 | 500000 to 5000000 merozoites inoculated intrathecally | NO clinical signs | 132 | Not done | Negative | Lindsay et al. (2000c) | |
| 6 | 6000 merozoites in lymphocytes were inoculated intravenously daily for 14 days | All horses developed clinical signs | 90 | Not done | Positive | Ellison et al. (2004) | |
| 8 | 6000 merozoites from culture inoculated intravenously, daily for 14 days. | All horses developed clinical signs | 55 | Not done | No data | Witonsky et al. (2008) | |
| 5a | 625,000 sporocysts NIO | Mild ataxia in 3 | 84 | Negative | MacKay et al. (2008) | ||
horses obtained from Northwest Canada outside the range of opossums.
Transport stress model. Horses transported for 55 hours and dosed soon after arrival.
Horses transported after rest period.
Isolate SN-37R—obtained by serial passage between laboratory raised opossums and raccoons.
SCID= severe combined immunodeficiency disease.
H=heart, Li=liver,Lu=lung, Sk=skeletal muscle, Sp=spleen.
Immunity is considered to play an important role in clinical outcome of EPM but data from naturally and experimentally infected horses are not conclusive (Furr and Pontzer, 2001; Furr et al., 2001a; Tornquist at al., 2001; Njoku et al., 2002; Marsh et al., 2004; Spencer et al., 2004; Scott et al., 2005; Spencer et al., 2005; Pusterla et al., 2006; Yang et al., 2006; Witonsky et al., 2008). Interferon gamma ((IFN-gamma) is essential for controlling the development of EPM, as evidenced by studies in mice. Immunocompetent out-bred and inbred BALB/c or C57Bl/6 mice are not susceptible to S. neurona infection whereas the parasite is fatal to either BALB/c or C57Bl/6-derived interferon gamma gene KO mice (Rosypal et al., 2002; Dubey and Lindsay, 1998; Witonsky et al., 2003a; Witonsky et al., 2003b, Witonsky et al., 2005a; Witonsky et al., 2005b; Dubey et al., 2013). Both IFN-gamma KO and nude mice are susceptible to infection and disease development whereas, SCID mice did not show signs of parasite survival or disease development (Marsh et al., 1997; Sellon et al., 2004a). The humoral response is less critical in protection, as suggested by the fact that B cell deficient mice are not susceptible to infection and mice that die of severe disease have antibodies to S. neurona (Witonsky et al., 2005a,b).
6.2. Marine mammals
6.2. 1. Sea otter (Enhydra lutris)
Reports of S. neurona infection in sea otters are summarized in Table 8. Among marine mammals, causes of mortality are best known for sea otters because during long periods of time all dead sea otters recovered by wildlife services were examined at necropsy. Sea otters are resident on the coasts of California, Washington, Alaska, British Columbia, and Russia, but S. neurona has been definitively identified only in sea otters from California and Washington, which is consistent with the distribution of opossums. Data in Table 8 are based on dead or sick animals. Goldstein et al. (2011) found S. neurona antibodies at a titer of 1:320 in 2 of 74 otters from Kodiak, USA, but not in 89 otters from Bering Island, Russia.
Table 8.
Reports of clinical S. neurona infections in sea otters in USA.
| Location | Year | No. of sea otters examined | No. positive | Observations, tissues parasitized | Bioassay | Reference |
|---|---|---|---|---|---|---|
| Zoo, OR | 1997 | 1 | 1 | Antemortem diagnosis, biopsy muscle, CSF–WB positive, medicated with pyrimethamine, euthanized. Schizonts in brain, sarcocysts in muscle | Not done | Rosonke et al. (1999) |
| Monterey Bay, CA | 2000 | 1 | 1 | Meningoencephalitis, schizonts in brain, sarcocysts in muscle and heart. | Positive | Lindsay et al. (2000b) |
| Grays Harbor County, WA | 2000 | 1 | 1 | Convulsions, died. Meningoencephalitis, schizonts in brain. Co-infected with T. gondii. | Positive | Lindsay et al. (2001a) |
| Monterey Bay, CA | 1999 | 1 | 1 | Pup-estimated 16 week old. Meningoencephalitis, schizonts in brain. | Positive | Miller et al. (2001a) |
| CA | 1998–2001 | 105 | 7 | Only conventional histology. Encephalitis, schizonts in brain. Causes of mortality detailed. T. gondii in 17 otters. | Not done | Kreuder et al. (2003) |
| CA, WA | 1985–2004 | 344 | 39 | Encephalitis in 17 otters from CA and in 5 from WA. S. neurona alone in 22 otters, dual S. neurona and T. gondii in 12, T. gondii in 7. Sarcocysts in muscles of 18 of 18 otters examined. | Not done | Thomas et al. (2007) |
| CA | 2004 | 3 | 3 | Lymphadenopathy, enlarged heart. Encephalitis, schizonts in brain. Sarcocysts in brain and muscle, proven by TEM. | Positive | Miller et al. (2009) |
| Morro Bay, CA | 2004 | 63 otters dead in an epidemic | 8 | 16 necropsied and tested for S. neurona. S. neurona antibodies in 15, 1 pup seronegative. S. neurona isolated from 8, T. gondii isolated from 6. Encephalitis and myositis in 15 | Positive | Miller et al. (2010) |
| Copalis Beach, WA | 2010 | 1 | 1 | Retinitis, and encephalitis with schizonts. | Not done | Dubey and Thomas (2011) |
CA=California, OR=Oregon, WA=Washington.
Most diagnosed cases were from Southern sea otters (Enhydra lutris neresis) from California, compared with Northern sea otters (Enhydra lutris kenyoni) from Washington (Table 8). Since 1992, more than 1000 sea otters were examined at necropsy and protozoal encephalitis was identified as an important cause of their mortality (Kreuder et al., 2003; Thomas et al., 2007; Miller et al., 2010). Among various causes of mortality in 105 sea otters, S. neurona encephalitis was diagnosed in 7 otters (Kreuder et al., 2003), which was an underestimate because diagnosis was made only when protozoa were identified in lesions based on conventional histological examination; immunohistochemistry was not performed. In a detailed study of encephalitic lesions, S. neurona was identified alone in 22 of 39 cases of encephalitis, and dual infection with T. gondii in another 12 otters among 344 sea otters (Thomas et al., 2007). Most of these deaths were sporadic. An epizootic of protozoal encephalitis in sea otters was recorded by Miller et al. (2009, 2010). Within one month, 63 sick and dead otters were recovered near Morro Bay, California. This outbreak provided the opportunity for epidemiologic and etiological investigations. Seizures, muscle tremors, paresis, were noted in live stranded otters. A few animals had tachycardia, and respiratory distress (Miller et al., 2010). Of these otters, 15 were studied in detail. Antibodies (IFAT>1:2,560–1:81,920) to S. neurona were present in all 14 adult otters; one pup was seronegative (<1:40). Viable S. neurona were isolated from brains of eight adults. Predominant gross findings were enlarged heart with mottling, lymphadenopathy, and adipose petechiation. Microscopically, meningoencephalitis in association with schizonts and myositis associated with sarcocysts predominated. Immature and mature sarcocysts were seen in muscles, heart, and brain (Miller et al., 2009; Miller et al., 2010).
In an epidemiological investigation, Shapiro et al. (2012) found that the cause of mortality in sea otters was 12 times more likely S. neurona once an increased run-off due to rain had occurred in the prior 1–2 months. S. neurona was identified in 33 of 205 sea otters; however, it is not certain if these were new cases or previously included in the reports listed in Table 7.
Encephalitis is the predominant lesion in sea otters, and both T. gondii and S. neurona can be found together, sometimes in the same histological section. In general, the S. neurona-associated lesions are more severe than associated with T. gondii (Thomas et al., 2007).
6.2. 2. Pacific harbor seals (Phoca vitulina richardsi)
There are three reports of S. neurona mortality in seals. Lapointe et al. (1998) found encephalitis in seven seals; six of them were stranded on the California coast, and the seventh was captive. Among these seals was a juvenile, one week of age at capture that had tremors that lasted 17 days. Schizont-associated encephalitis was found in all seven animals, and the diagnosis was confirmed immunohistochemically. S. neurona antibodies were found by immunoblot in four of five seals tested. Four seals had myocarditis, and sarcocysts were found in the heart of one seal.
Subsequently, Miller et al. (2001b) isolated viable S. neurona from an adult seal that was stranded near Monterey, California. The seal had encephalitis in association with S. neurona and T. gondii infection.
Mylniczenko et al. (2008) reported antemortem diagnosis and treatment in a seal. A 20-year old captive seal developed tremors and dysphagia. Findings of S. neurona antibodies in serum and CSF, and negative tests for other infections suggested the diagnosis of S. neurona. The animal improved clinically after treatment with ponazuril at 10 mg/kg/oral for three months (Mylniczenko et al., 2008).
6. 2. 3. California sea lion (Zalophus californianus)
S. neurona infection was diagnosed ante-mortem in a California sea lion stranded ashore in Marin County, California (Carlson-Bremer et al., 2012). The animal was lethargic. Acute myositis was diagnosed based on histological examination of biopsy, serum biochemical tests, and finding of elevated antibodies to S. neurona (IFAT>1:5120–1:20480). The alanine aminotransferase and creatinine kinase values were elevated. Treatment with ponazuril (10 mg/kg/oral for 5 days) resulted in clinical improvement. The animal was returned to its habitat three months after it was found stranded.
The finding of S. neurona in nine of ten asymptomatic sea lions is most interesting. Of the ten adult healthy California sea lions euthanized to protect fish stocks, nine were infected with S. neurona, and one had concurrent T. gondii infection (Gibson et al., 2011). These results were based on the detection of the parasite DNA in samples of heart, brain, muscle, and lymph nodes.
6.3. Miscellaneous animals
Results are summarized in Table 9. Most of these cases were from cats, dogs, and raccoons. Solitary reports were from ferret, fisher, lynx. and skunk. A major outbreak occurred in mink. Unusual findings are commented upon.
Table 9.
Reports of clinical S. neurona infections in non-equid and non marine animals in USA and Canada.
| Host | Location | No., age | Clinical signs | Diagnostic methods | Organs parasitized | Reference |
|---|---|---|---|---|---|---|
| Cat (Felis catus) | California | 1,13-week old | lame after fall, euthanized | IHC | B, Sc | Dubey et al. (1994); Dubey and Hamir (2000) |
| Illinois | 1, 4-month old | Neurologic, post surgery | IHC | B, Sc | Dubey et al.(2003a) | |
| Indiana | 1, 5-month | Neurologic, antemortem dignosis | PCR | CSF | Bisby et al. (2010) | |
| Canada lynx (Felis canadensis) | New York Zoo | 1, 13-year | Encephalitis | IHC | B | Forest et al. (2001) |
| Dog (Canis familiaris) | Illinois | 1, 6 year old | Myositis | IHC, PCR | Sk | Vashisht et al. (2005) |
| Maryland, Illinois, Arkansas, Oklahoma | 7, retrospective | Neurologic, dermal, pneumonia, | IHC, PCR | B, K, Lu, skin,Sc | Dubey et al. (2006):Dubey et al. (1991b); Trasti et al. (1999) | |
| Mississippi | 1, 1.5 year old | Ataxia, neurological | IHC, PCR | B | Cooley et al., (2007) | |
| Mississippi | 1, 2 month old | Neurologic | IHC | B, E, L, H, I, Nt, T, | Dubey et al. (2014) | |
| Tennessee | 1, 2-yearold | Acute onset of parapresis | IHC, PCR | Sc | Gerhold et al. (2014) | |
| Raccoon (Procyon lotor) | Ohio | 1 | Neurological, euthanized | IHC | B | Dubey et al. (1990); Dubey et al. (1991c); Dubey and Hamir (2000) |
| Oregon | 2 | Encephalitis and myocarditis, no evidence of MVI | IHC | B, H | Hamir and Dubey, (2001) | |
| New York | 1 | Neurologic, MVI | IHC | B | Stoffregen and Dubey (1991); Dubey and Hamir (2000) | |
| Illinois, | 1 | Neurologic, MVI | Hist. | B | Thullin et al. (1992) | |
| Mink (Mustela vison) | Oregon, | 2 | Neurologic | IHC | B | Dubey and Hedstrom (1993) |
| Ferret (Mustela putorius furo) | British Columbia, Canada | 1 | Neurologic, respiratory, MVI | IHC,PCR | A, B, H, K, Li, Lu, Nt, Ln, Mo, Sk, Sp, | Britton et al. (2010) |
| Fisher (Martes pennanti) | Maryland | 1 | Neurologic | IHC,PCR | B | Gerhold et al.(2005) |
| Canada lynx (Lynx canadensis) | New York zoo | 1 | Neurologic | IHC | B | Forest et al. (2001) |
| Striped skunk (Mephitis mephitis) | Indiana | 1 | Neurologic | IHC,PCR | B, Li, Lu, Nt, | Burcham et al. (2010) |
| Bald eagle (Haliaeetus leucocephalus) | Missouri | 1 | Neurologic signs | IHC | B | Olson et al. (2007) |
A=adrenal B=brain, H=heart, I=intestine, Li=liver, Lu=lung, Mo=blood monocytes, Nt=nasal turbinates, Sk= skeletal muscle, Sc= spinal cord, Sp=spleen, T=tongue
MVI=concurrent Morvilli Virus Infection.
6.3.1. Raccoons (Procyon lotor)
There are several reports of S. neurona encephalitis in raccoons (Table 9). These raccoons were examined at necropsy because of suspicion of rabies. The concurrent morbillivirus infection (MVI) is considered to aggravate severity of disease. However, in two raccoons evidence for MVI was not found; in these animals the encephalitis was granulomatous rather than pyonecrotic or pyogranulomatous seen in animals with concurrent MBI (Hamir and Dubey, 2001). The most severe disease diagnosed was in a juvenile raccoon (Dubey et al., 1990, Dubey et al., 1991c). The raccoon had neurological signs, was unsteady and could not maintain its balance. Within a week of onset of clinical signs, its condition deteriorated, was euthanized, and a complete necropsy was performed. Protozoa-associated lesions were confined to cerebrum. Numerous S. neurona schizonts and free merozoites were observed in lesions.
6.3.2. Mink (Mustela vison)
On a farm in Oregon, USA, 50 of 15500 minks had died within two weeks (Dubey and Hedstrom, 1993). The affected mink were weak, ataxic, and anorectic. Complete necropsy was performed on two, one-month old female mink. Grossly, a discolored area was found in cerebrum of one of the two minks. Microscopically, both mink had severe meningoencephalitis associated with numerous schizonts of S. neurona. Mink with progressive neurologic disease can also have variable number of sarcocysts present (Ramos Vara et al., 1997).
6.3.3. Domestic cat (Felis catus)
Three reports of S. neurona-like infection in cats need explanation because of unusual presentations. The first cat diagnosed was a three month old that became ill two weeks after a fall and developed hemiparesis (Dubey et al., 1994). The second cat developed neurological signs three days after routine surgery for castration (Dubey et al., 2003a). In both of these cats, lesions and S. neurona schizonts were confined to CNS. Additionally, S. neurona-like sarcocysts were seen in the brain of the second cat. In the third cat, the diagnosis was made antemortem, and thus a detailed clinicopathologic could be documented (Bisby et al., 2010).
Efforts to induce clinical disease in cats were unsuccessful. Four inoculated with 10 million culture derived S. neurona merozoites (Butcher et al., 2002) and five cats orally inoculated with >1000 S.neurona sporocysts (Dubey et al., 2000) became infected but remained subclinical; three of these cats had been administered rather high doses of methyl prednisone acetate (Dubey et al., 2000).
6.3.4. Dog (Canis familiaris)
Of the 10 dogs diagnosed with S. neurona-like infections (Table 9), three reports need an explanation because of unusual presentations. The first case was a dog from Illinois (Dubey et al., 1991). Protozoa were seen in the skin of this dog. The dog was misdiagnosed as Sarcocystis canis infection (Dubey and Speer, 1991). Subsequently, it was diagnosed as S. neurona based on immunohistochemistry (Dubey et al., 2006). This dog had pustular dermatitis with many merozoites and schizonts found in a smear made from the oozing fluid (Fig. 3).
Figure 3.
S. neurona schizonts and merozoites in tissue smears. Giemsa stain. (A) Smear from a pustular dermal ulcer from a Rottweiler dog reported by Dubey et al. (1991b). Note several intensely stained schizonts (arrows) among leukocytes and RBC. (B) Infected dermal cell with immature schizonts (sc) and free elongated merozoites compared with size or RBC. Note host cell nucleus (hcn). (C) Smear of brain of a red panda. Note two different sized merozoites (a), a merozoite-shaped schizont with lobed nucleus (b), and two schizonts with large nucleus (c), and an intensely stained schizont (d). (Smear courtesy of Timothy Walsh, unpublished).
A two-month old dog was thought to be congenitally infected with S. neurona-like organisms; it had focal severe encephalitis, retinitis, and myositis-associated with schizonts and merozoites (Dubey et al., 2014). Mature sarcocysts were found in its skeletal muscle. Schizonts were also present in sections of nasal turbinates but without inflammation.
Recently, a two-year old dog had acute onset of paraparesis (Gerhold et al., 2014). At necropsy large nodular masses were found between nerve roots of thoracic spinal cord (Fig. 4). Microscopically, the nodular growth had mixed cellular infiltrates and necrosis associated with protozoa, thought to be T. gondii. However, in the opinion of one of us (JPD) these structures are S. neurona schizonts (not T. gondii) in different stages of maturation; they reacted strongly to S. neurona antibody and not with T. gondii antibodies (JPD, own observations).
Attempts to induce clinical disease in dogs were unsuccessful; two dogs orally inoculated with 1,000,000 sporocysts remained subclinical despite administration of corticosteroids (JPD own observations).
6.3.5. Ferret (Mustela putorius furo)
The case of an infected ferret is unusual for two reasons. Firstly, the animal had massive infection of S. neurona affecting the nasal turbinates (Britton et al., 2010). Secondly, the protozoal infection might have been triggered by vaccination with live modified MVI vaccination. The ferret had been administered one dose of live canine distemper vaccine (CDV) one week before onset of rhinitis. In dogs, vaccination with CDV can trigger T. gondii infection (Dubey, 2010).
6.3.6. Canada lynx (Felis lynx canadensis)
A 13 year old lynx from a zoo had granulomatous encephalitis both in grey and white matter throughout the cerebral cortex (Forest et al., 2001). Schizonts were seen in neuropil as well as in vascular endothelium.
6.3.7. Fisher (Martes pennati)
A wild–caught, ataxic fisher was found to have no fear of humans and was attacked by a dog (Gerhold et al., 2005). Euthanasia and complete necropsy were performed to exclude rabies. Numerous S. neurona schizonts were identified in encephalitic lesions and the diagnosis was confirmed by PCR. Additionally, mature S. neurona-like sarcocysts were identified in skeletal muscle.
6.3.8. Striped skunk (Mephitis mephitis)
Disseminated S. neurona infection was diagnosed in a juvenile skunk with concurrent MVI (Burcham et al., 2010). Schizonts were seen in sections of brain, lung, liver, and nasal epithelium.
6.3.9. Bald eagle (Haliaeetus leucocephalus)
Sarcocystis-associated encephalitis was diagnosed in a captive bald eagle (Olson et al., 2007). Confirmation of S. neurona infection is needed because this is a unique report of this parasite in an avian species. Recently, another Sarcocystis species, S. calchasi was diagnosed to cause encephalitis in birds (Wunschmann et al., 2011; Olias et al., 2010).
7. Diagnosis
7.1. Horses
7.1.1. General observations
Although many neurologic disorders affect the horse, EPM remains the most commonly diagnosed infectious equine neurologic disease in the Americas (Dubey et al., 2001a; Morley et al., 2001; Furr et al., 2002; Morley et al., 2008). A complete neurologic examination and implementation of a thorough diagnostic plan to rule out differential diagnoses are essential prerequisites to laboratory testing and appropriate interpretation of clinical signs and death. Many ancillary diagnostic procedures may be required to differentiate primary musculoskeletal disorders and other neurologic diseases from EPM.
7.1.2. Blood chemistry
EPM does not produce consistent detectable changes in complete and differential cell counts or serum chemistry values. CSF in horses with EPM is generally normal S. neurona merozoites have not been observed in CSF.
7.1.3. Polymerase chain reaction
PCR testing of equine CSF may provide information regarding the presence of S. neurona DNA in the CNS. However, the sensitivity of the PCR test appears to be much lower than initially estimated, possibly because intact merozoites rarely enter CSF. Nonetheless, The PCR test may be a useful adjunct for the post-mortem diagnosis of EPM in selected cases when it is used on tissues collected at necropsy.
7.1.2. Serological tests
Several serological tests have been used to detect antibodies to S. neurona in animals. The Western blot (WB) test, also called the immunoblot test, was the first assay developed for detection of antibodies against S. neurona and diagnosis of EPM (Granstrom et al., 1993). This assay has been available for over two decades and continues to be offered by several diagnostic testing laboratories. Methods and data used to validate WB were reviewed by Dubey et al. (2001a). Subsequent to its development, the S. neurona WB test has been modified to try to improve the diagnostic accuracy and/or to make the assay semiquantitative. Use of bovine serum against Sarcocystis cruzi to “block” cross-reacting antigens was reported to yield sensitivity and specificity approaching 100% (Rossano et al., 2000). However, evaluation of this modified WB protocol by other investigators found a lower sensitivity/specificity of 89% and 69%, respectively, when applied to a more extensive sample set (Daft et al., 2002; Duarte et al., 2003). A WB method used by a commercial testing laboratory (Neogen, Inc., Lansing, Michigan, USA) involved measuring the intensity of the antibody/antigen response to yield a unitless number referred to as the “relative quotient” (RQ). This test is no longer offered by the company.
Development of the WB test provided a tremendous boost to EPM diagnosis, but the Western blot technique is primarily a research tool that is fairly laborious and requires significant expertise to interpret accurately. Consequently, multiple “second generation” serologic assays have been developed that provide greater throughput and are more informative. Two assays developed using whole S. neurona merozoites as antigen were the S. neurona SAT (Lindsay and Dubey, 2001a) and the IFAT (Duarte et al., 2003; Duarte et al., 2004c). Usefulness of SAT was further evaluated in experimentally-infected cats, raccoons, and ponies (Dubey et al., 2002a; Saville et al., 2004b). Both the SAT and the IFAT can be used to obtain an end-point antibody titer, which is a large improvement over the non-quantitative result provided by the WB test. The SAT is advantageous since it can be used for detection of antibodies in multiple different animal species, and the assay has been employed in a variety of research studies (Table 5). However, the SAT has not been routinely employed for EPM diagnosis and is not presently offered as a commercial test. The IFAT was optimized and validated at the University of California-Davis (Duarte et al., 2003; Duarte et al., 2004), and testing is currently available from the University of California Veterinary Diagnostic Laboratory. Although specialized instrumentation is needed (i.e., a fluorescence microscope), interpretation of parasite fluorescence requires expertise, and concerns have been raised about cross-reactivity with the non-pathogenic species S. fayeri (Saville et al., 2004b), serologic results from the IFAT will be generally accurate when the assay is performed by individuals with proper training.
Enzyme-linked immunosorbent assays (ELISAs) are easy to perform, allow for relatively high-throughput testing, and provide a more objective interpretation of results relative to the other existing assays, particularly the WB. Several ELISAs have been developed using S. neurona antigens expressed as recombinant proteins in E. coli (Ellison et al., 2003a; Hoane et al., 2005b; Yeargan and Howe, 2011) or in Baculovirus (Gupta et al., 2004). Thus far, these ELISAs are all based on the family of related S. neurona merozoite surface antigens (SnSAGs) (Ellison et al., 2002; Howe et al., 2005), which are good serologic targets since they are abundant and immunogenic. Importantly, antigenic diversity has been found among different strains of S. neurona (Crowdus et al., 2008; Howe et al., 2008; Hyun et al., 2003; Marsh et al., 2001b; Wendte et al., 2010b), so all of the SnSAGs are not equally valid for use in serologic assays.
ELISAs based on the SnSAG2, SnSAG3, and SnSAG4 surface antigens have been shown to be accurate for detecting and quantifying antibodies against S. neurona in equine serum and CSF samples (Hoane et al., 2005a; Yeargan and Howe, 2011). To help overcome the negative impact of antigenic diversity in the S. neurona population and the varied immune responses that occur in different horses, fusion of SnSAG3 and SnSAG4 into a single chimeric protein (rSnSAG4/3) and concurrent analysis with two ELISAs (rSnSAG2 ELISA and rSnSAG4/3 ELISA) have been employed for commercial testing of equine samples (Equine Diagnostic Solutions, LLC, Lexington, KY, USA). Although homologues of the SnSAG antigens are present in the related parasite S. falcatula (Wendte et al., 2010a), this does not hinder the specificity of the SnSAG2 and SnSAG4/3 ELISA results since S. falcatula, is incapable of infecting and causing seroconversion in horses (Cutler et al., 1999). Furthermore, extensive validation studies have shown that the SnSAG ELISAs are specific and do not cross-react with serum from horses infected with other species of Sarcocystis (Arias et al., 2012; Hoane et al., 2005). Hence, these surface proteins are valuable immunologic markers that accurately detect infection with S. neurona.
An ELISA based on the SnSAG1 surface antigen was described (Ellison et al., 2003a) and has been offered for EPM diagnosis by Antech, Inc. This test provides an end-point titer, and values greater than 1:100 in serum are reported to indicate active infection. This test and cut-off point has not been rigorously evalued, however, the cut-off values used for a positive diagnosis appear to be arbitrary. More critically, the SnSAG1 protein has been shown to be absent from multiple S. neurona isolates (Howe et al., 2008; Hyun et al., 2003; Marsh et al., 2001b), which explains the relatively low accuracy observed with this antigen in independent studies (Hoane et al., 2005; Johnson et al., 2010). An assay that combines SnSAG1 with the two alternative major SnSAGs, SnSAG5 (Crowdus et al., 2008) and SnSAG6 (Wendte et al., 2010b), is presently offered as a stall-side test (Prota, LLC), but no published reports have validated this assay so remains unclear whether the test accurately detects antibodies to S. neurona.
Since EPM occurs only in a small proportion of horses infected with S. neurona, the simple detection of serum antibodies against this parasite has minimal diagnostic value. Detection of antibodies in cerebrospinal fluid (CSF) is more informative, but this analysis will be confounded by blood contamination of the sample during collection as well as normal passive transfer of antibody from the plasma across an intact and healthy blood-CNS barrier (Furr et al., 2011a; Furr et al., 2002). The blood-CNS barrier can also be compromised in neonatal foals. Of 13 foals born to seropositive mares, antibodies were detected in CSF of 12 of 13 tested in S. neurona WB test two to seven days after birth (Cook et al., 2002). The CSF antibodies declined over time but were detectable in three foals between 62 and 90 days; the foals were asymptomatic (Cook et al., 2002). These obstacles to accurate immunodiagnosis of EPM have been reduced in recent years through the development of semi-quantitative assays (e.g., IFAT and ELISA) and use of diagnostic methods that reveal intrathecal antibody production, which indicates active S. neurona infection in CNS. The blood contamination of CSF had no effect on the diagnosis of EPM using the IFAT (Finno et al., 2007).
The Goldman-Witmer coefficient (C-value) and the antigen-specific antibody index (AI) are algorithms that use antibody end-point titers in paired serum and CSF samples to assess whether the amount of pathogen-specific antibody in the CSF is greater than would be present from normal passive transfer across the BBB. Difficulties in interpretation and value of these tests to diagnose EPM were reviewed previously (Dubey et al., 2001a). Use of the SnSAG2 ELISA and the C-value and AI algorithms to examine a sample set of 29 clinical cases demonstrated that these methods provide accurate diagnosis of EPM (Furr et al., 2011). This study also confirmed prior findings that modest blood contamination of the CSF, up to 10,000 red blood cells/μl, will have minimal effect on test results (Finno et al., 2007).
Many horses with EPM have antibody titers in their CSF that are profoundly higher than what should be present due to normal passive transfer across the BBB. Studies using the SnSAG2 and SnSAG4/3 ELISAs to examine a large collection of horses with neurologic disease showed that a simple serum: CSF ratio calculated from the end-point titers can serve as a proxy for the more laborious and expensive C-value or AI (Johnson et al., 2010; Reed et al., 2013). These studies found that the optimal serum: CSF titer ratio was 100:1, which approximates the normal partitioning of proteins (e.g., albumin or total IgG) between the blood and the CSF (Rossano et al., 2003a; Furr et al., 2011). Serum: CSF ratios equal to or lower than 100:1 were indicative of intrathecal antibody production against S. neurona and yielded EPM diagnostic accuracy of approximately 93% sensitivity and 83% specificity for a collection of 128 cases examined (Reed et al., 2013). Of 44 EPM cases, 33 had serum: CSF ratios of 25:1 or less, with nine horses had CSF antibody titers that approached or exceeded their serum titers (i.e., ratio of 1.6:1 or lower). Although IgM antibodies can be detected in CSF or blood of horses experimentally infected with S. neurona (Murphy et al., 2006) this test has not been used in diagnosis of EPM in horses.
Based on statistical modeling of IFAT results from a sample set of naturally and experimentally infected horses, it has been proposed that EPM diagnosis can be achieved from serum titers alone, with minimal additional information provided by CSF testing (Duarte et al., 2004a; Duarte et al., 2006). This idea was not supported by the previously described studies using ELISAs (Johnson et al., 2010; Reed et al., 2013), which showed that serum antibody titers alone are not a good indicator of EPM. This discrepancy is likely not due to differences in the serologic assays, but rather can be attributed to differences in the authenticity of the sample sets used for the studies. Specifically, the sample set used by Duarte et al. (Duarte et al., 2004a; Duarte et al., 2006) had only 12 seropositive horses in the non-EPM population (n = 97), which does not represent the seroprevalence of S. neurona that has been documented in many parts of the United States and Central and South America (Table 4). In most regions where S. neurona is endemic, there will be numerous seropositive horses that exhibit signs of neurologic disease not due to EPM, and some of these horses will have serum antibody titers that are quite high. When sample sets that better represented the normal equine population are tested, it becomes apparent that serum antibody titers against S. neurona do not provide accurate EPM diagnosis (Johnson et al., 2010; Reed et al., 2013).
7.3. Necropsy examination
7.3.1. Gross lesions
When present, the gross lesions of EPM in the horse are confined to the CNS. Acute lesions consist of multifocal, randomly-distributed foci of hemorrhages, whereas subacute and chronic lesions show areas of discoloration ranging from pale to dark tan areas and foci of malacia, respectively (Fig. 12). Although the brainstem is more often involved than other areas of the brain, the lesions are more frequently seen in the spinal cord. In rare cases, lesions may be present in both the brain and the spinal cord of a horse.
7.3.2. Microscopic lesions
Microscopically, the predominant lesion is multifocal to coalescing areas of hemorrhage, nonsuppurative inflammation, and small foci of necrosis. Perivascular cuffing by mononuclear cells is evident in some of the affected areas, particularly in the meninges. The numbers of S. neurona stages present are often few and difficult to locate in routine histological sections stained with hematoxylin and eosin. Developmental stages of S. neurona are more easily seen if organisms are present in neurons rather than in inflammatory cells. The types of host cells which are infected are not definitively known except that schizonts and merozoites are found in neurons and in macrophages.
7.3.3. Immunohistochemical staining
S. neurona schizonts and merozoites are stained specifically with polyclonal rabbit antibodies (Fig. 4) against cultured-derived S. neurona merozoites (Dubey et al., 1999a; Dubey and Hamir, 2000). The reactivity of S. neurona sarcocysts to S. neurona antibodies is highly variable; immature sarcocysts reacted brilliantly whereas mature sarcocysts were stained irregularly or not at all within the same histological section (Stanek et al., 2002; Thomas et al., 2007; Dubey et al., 2014). Monoclonal antibodies against S. neurona can also be used for immunostaining (Marsh et al., 2002). In most EPM cases organisms are sparse and organisms may not be demonstrable even in cases where diagnosis is confirmed by bioassay in cell culture and demonstration of S. neurona DNA. Not all S. neurona organisms in EPM cases will stain by immunohistochemistry, and it is likely due to the antibody recognition of specific parasite antigens or lack of antigens in the tissue sections. For example the Sn-Mu1 isolate (SnSAG1 minus) did not stain using polyclonal antibodies prepared against the Sn-UCD1 isolate (SnSAG1 positive; Marsh et al., 2001b). However, multiple isolates and histopathology sections from a variety of hosts containing S. neurona merozoites or schizonts are recognized using Mab 2G5-2 which targets a S. neurona antigen conserved (Miller et al., 2009)
8. Treatment
Treatment of horses suspected to have EPM should begin as quickly as possible after clinical signs of the disease are recognized. Treatment appears to result in successful recovery in 70 to 75 % of the affected horses, although these estimates are somewhat suspect without post-mortem confirmation.
Anti-protozoal drugs such as sulfonamides and pyrimethamine have been used to treat EPM since 1974 when the protozoal etiology of the disease was recognized. After the first cultivation of S. neurona in 1991, it became possible to test anti-protozoal drugs in vitro. Subsequently, when the KO mouse model was established in 2000, it became possible to test drugs against S. neurona in vivo (reviewed in Dubey et al., 2001a). Since then, various compounds have been developed and are licensed for treatment of EPM in the United States. In addition to ponazuril (Marquis®, Bayer), which was the first FDA-approved EPM medication, diclazuril (Protazi®l, Merck), and a sulfadiazine/pyrimethamine combination drug (ReBalance®, PRN Pharmacal) are currently approved for treatment of EPM.
8.1. Ponazuril
Ponazuril (Marquis®, Bayer Animal Health), is widely used to treat EPM. The dosage commonly used is 5 mg/kg per day for a minimum of 28 days. Ponazuril is a benzeneacetonitrile compound that is related to the herbicide atrazine and may act by inhibiting apicoplast and/or mitochondrial function in the parasite (Harder and Haberkorn, 1989; Mitchell et al., 2005). Ponazuril is technically considered to be a coccidiostat (Pusterla et al., 2013; MacKay et al., 2008). The significance of this mode of action in vivo, however, is totally unknown and is probably unimportant since efficacy studies between coccidiocidal and coccidiostatic drugs provide similar outcomes.
Ponazuril is well absorbed orally, and achieves a steady state concentration of 0.16 ± 0.06 mg/L in the cerebrospinal fluid of horses treated with 5 mg/kg body weight (Furr and Kennedy, 2001). In vitro studies have documented anti S. neurona activity of ponazuril (Lindsay et al., 2000a; Mitchell et al., 2005). In vivo studies in KO mice inoculated with S. neurona sporocysts indicated reduction in severity of clinical signs if the drug was administered 4 DPI (Franklin et al., 2003). Prophylactic administration of ponazuril at 5 mg/kg also reduced severity of clinical signs in experimentally infected horses (Furr et al., 2006). The time to reach steady state concentrations of ponazuril in the CSF of horses is approximately one week. For this reason an initial loading dose is recommended in an effort to achieve therapeutic concentrations more quickly. While the clinical value of this approach has not yet been demonstrated, maximum CSF concentrations of ponazuril were achieved within 28 h when horses were given a loading dose of 15 mg/kg body weight (3X the normal dosage) (Reed et al own observations). A field efficacy study of 101 horses demonstrated approximately 60% efficacy, with 8% of cases relapsing within 90 days if treatment is stopped after 28 days (Furr et al., 2001b). It is expected, although not proven, that the relapse rate (already low) will be even less if treatment is continued for longer than 28 days. Animals typically responded within ten days of beginning treatment although some horses may take up to three weeks following the initiation of treatment before tangible evidence of response is noted. Therefore, a refractory patient often continued to improve even after treatment stopped at 28 days. The baseline neurologic score did not influence outcome in that study. However, success was defined as improvement by one clinical grade, which may be considered unacceptable in severe cases (Furr et al., 2001b). In clinical cases, the animal should be re-evaluated at the end of the treatment period, and then a determination made whether further treatment is needed. In general, if there has been a clinical response yet the horse remains abnormal, a second month of ponazuril is recommended. If finances are limited, treatment can be stopped after 28 days, but the horse should be reexamined after one month to ensure that there is nodeterioration.
Toxicity studies have found ponazuril to be very safe, with no systemic toxicity, even at high doses (30 mg/kg body weight) for up to 56 days (Kennedy et al., 2001). Uterine edema was noted in mares given 30 mg/kg body weight each day for 30 days, however (Kennedy et al., 2001). Treatment of breeding stallions with 10 mg/kg body weight did not affect androgenic hormone production nor spermatogenesis (Welsh et al., 2002). Ponazuril has been used without obvious problems in pregnant mares, but the use of ponazuril in pregnant animals is off-label and owners should be made aware of this fact. As stated earlier, ponazuril was used to treat a sea lion with S. neurona-associated clinical disease (Mylniczenko et al., 2008).
8.2. Diclazuril
Diclazuril (Protazil®, Merck Animal Health) is also a benzeneacitonitrile compound that is chemically similar to ponazuril. Diclazuril is administered at 1 mg/kg body weight as alfalfa-based pellets that are top dressed in the daily grain ration. In a study of 49 horses, diclazuril had a success rate of 58%, when success was defined as improvement by a minimum of one clinical grade (MacKay, 2008). Recent investigation of diclazuril pharmacokinetics has shown that a dose of 0.5 mg/kg body weight (50% of the recommended dose) is sufficient to attain concentrations in plasma and CSF that will inhibit S. neurona growth in culture (Hunyadi et al., 2014). Further study is needed, but these findings suggest that effective treatment of EPM may be possible with lower doses of diclazuril.
Diclazuril is absorbed quickly, especially the sodium salt, and has been found in serum one h after feeding to horses (Dirikolu et al., 2006) Diclazuril has anti-S. neurona activity in vitro (Lindsay and Dubey, 2000) and in vivo (Dubey et al., 2001h). Mice fed diclazuril in pelleted feed (50 parts per million) starting six days before or seven days after feeding S. neurona sporocysts and continuing therapy for a month resulted in the absence of S. neurona stages in the mice. Therapy was less effective when diclazuril was given 12 days or more after feeding sporocysts. These results indicate that diclazuril can inhibit the early stages of S. neurona and may be useful as a prophylactic against S. neurona infections in horses (Dubey et al., 2001h). A dosage of 0.5 mg/kg was suggested as a prophylactic for reducing S. neurona infection in horses. Feeding diclazuril medicated pellets daily from 1–12 months to foals considerably reduced seroconversion to S. neurona in naturally exposed foals compared with foals not medicated with diclazuril (Pusterla et al., 2015).
8.3. Nitazoxanide
The broad-spectrum antimicrobial nitazoxanide (NTZ) and its other derivatives have anti-S. neurona activity in vitro (Garglia et al., 2009). NTZ was approved for the treatment of EPM and marketed as Navigator® (Idexx Pharmaceuticals, Inc.). Although treatment with NTZ was effective against EPM, there were prominent concerns about adverse side-effects (e.g., colic). Subsequently, NTZ was removed from the market and is no longer available.
8.4. Decoquinate
Decoquinate has been used to treat coccidiosis in poultry, cattle, sheep, and goats. In vitro testing has determined that it is effective against S. neurona at low concentrations (Lindsay et al., 2013). Decoquinate in combination with the immunomodulator levamisole has been investigated for treatment of EPM in one published study, with a very high rate of clinical improvement reported after only tendays of treatment (Ellison and Lindsay, 2012). There are substantial concerns with regard to these findings, however, including case selection and assessment, and the diagnostic standard applied. Substantial additional research using confirmed cases needs to be performed before the use of this drug combination can be considered.
8.5. Sulfonamide and pyrimethamine
The sulfonamide and pyrimethamine (S/P) combination has been used widely for the last four decades, but a pre-mixed version of this combination has recently achieved FDA approval and can be purchased commercially (ReBalance®, PRN Pharmacal). The sulfonamide component of this compound competes with para-aminobenzoic acid to inhibit dihydropteroate synthetase activity, while pyrimethamine targets dihydrofolate reductase, which collectively inhibit folate metabolism. The synergistic effects of these compounds block synthesis of nucleic acids and amino acids, ultimately leading to parasite death. Weaknesses of S/P suspension in the treatment of EPM include the prolonged duration of treatment required to affect a positive response, and the toxicity of the compound. Toxicity includes anemia, leukopenia, fetal loss, and fetal abnormalities. A benefit of S/P combinations is the lower cost, yet this may be offset by the extended dosing interval required.
Pyrimethamine has historically been given in combination with sulfa drugs in the treatment of EPM. There is, however, some evidence to suggest a synergistic effect of pyrimethamine when used with the benzeneacitonitrile-group antiprotozoals (ponazuril, diclazuril). While not empirically evaluated in horses with EPM so its effectiveness is not known at this time, it may be beneficial to add this drug to the treatment regimen in refractory cases..
8.6. Pyrantel tartrate
Based on in vitro activity of pyrantel tartrate against S. neurona merozoites (Kruttlin et al., 2001), administration of pyrantel tartrate was considered for prophylactic use against EPM. However, this compound had no anti-S. neurona activity in experimentally infected KO mice (Lindsay and Dubey, 2001b). Similarly, daily administration of pyrantel tartrate for 134 days had no anti-S. neurona activity in experimentally-infected horses (Rossano et al., 2005a).
8.7. Supportive/ancillary therapy
A variety of ancillary and supportive therapies may be indicated for the treatment of horses with EPM (Sellon and Dubey, 2007). Ancillary treatments may include various anti-inflammatory drugs such as phenylbutazone, flunixen meglumine, dimethylsulfoxide (DMSO) or steroids. Corticosteroids can be used to help stabilize horses with serious neurologic abnormalities during the early period of treatment. Long term steroid treatment should be avoided due to their unknown effects upon immune clearance of S. neurona. However, it has been observed that some horses initially worsen slightly and transiently with treatment; hence, prophylactic use of nonsteroidal anti-inflammatory drugs to ameliorate this “treatment crisis” is sometimes advised. In the experience of one author (MF), treatment crisis is rarely a problem, particularly in minimally affected horses. In seriously affected horses, prophylactic anti-inflammatory treatment is probably advisable for the first week. Additional ancillary treatments, such as Vitamin E, homeopathic medications, etc., have not been demonstrated to have any value, and use of these compounds or approaches is not recommended.
Immuno-stimulants have been recommended by some on the presumption that immunosuppression is a component of the pathophysiology of EPM. Levamisole (1 mg/kg PO daily), EqStim (5 ml IM on day 1, 3 and 7, then monthly), or Equimune IV, (1.5 ml IV weekly for three weeks) have all been advocated, however there is no specific information to suggest that these have any positive effect. Another potential beneficial immune modulator is Parapox Ovis Virus Immunomodulator (Zylexis, Pfizer Animal Health). While licensed as an aid in the treatment of horses with EHV-1 and EHV-4, parapox ovis has been demonstrated to increase interferon gamma secretion in treated horses. As interferon gamma is considered a key cytokine in protection against S. neurona infection, this compound might be beneficial in selected animals.
8.7. Duration of treatment
The duration of treatment for EPM is difficult to determine, and when to terminate treatment in a particular horse remains problematic. Duration of treatment appears to be more important than peak concentrations (as long as they exceed minimum inhibitory concentrations). Therefore, horses should be evaluated after 1 month of treatment with ponazuril or diclazuril. If improvement has been noted, but clinical signs remain, then a further month of treatment is recommended. If finances are limiting, or the horse appears clinically normal, then treatment can be discontinued. However, the horse should be examined one month after treatment is completed to ensure that there has been no relapse. Alternatively, a one- to two-month course of S/P can be given to help minimize the chance of relapse; the effectiveness of this approach has not been evaluated.
Treatment until the CSF WB becomes negative has been advocated by some clinicians in the past. Experience has dictated that this is an unachievable goal for most cases. Antibodies can have a lengthy half-life, so horses will exhibit positive titers for long periods. The effects of treatment upon CSF or serum antibody concentrations is unclear, and at the present time repeating these tests appears to have little value in determining treatment success or duration.
9. Epidemiology of EPM in horses
Only two epidemiologic studies have assessed the risk factors associated with clinical EPM in horses in the USA (Saville et al., 2000; Morley et al., 2008). One was done at a tertiary care facility (Saville et al., 2000), which was not an ideal population of horses to reflect risk factors on a national scale. In 1998, a National Animal Health Monitoring System collaborative study with National Agriculture Statistics Services (NASS, USDA) Center for Epidemiology and Animal Health examined data collected from a large number of US equine industry operators (most of them with with three or more horses). More than 900 operators completed survey questions regarding a Control Horse from premises where there is no history of EPM. The analysis was performed and the results were reported (Morley et al., 2008). The following conclusions were drawn:
EPM risk is closely tied to environmental and management factors that impact exposures to opossums, which contaminate the environment. This suggests opossums, or the contamination from these animals may be more commonly found on premises where EPM has occurred. This is coupled with, climate and terrain factors that affect opossum habitat and therefore, S. neurona sporocysts will be present and survive in the environment.
The type of housing for horses (inside or outside), stocking density, the choice of bedding material, safe storage of concentrate feeds all could impact the likelihood of horses being exposed to S. neurona. Operations that stored concentrate feeds in rodent-proof containers had lower odds of detecting EPM than those that did not. Operations that used wood products (eg, shavings, chips, or sawdust) as the predominant bedding material had about 1.4 times lower odds of detecting EPM than did those that used other bedding materials (eg, straw, corn stalks). Operations that housed horses installs or paddocks during the day had small increases in the odds of EPM occurrence when compared with operations that housed horses in pastures, again suggesting stocking density.
Horses that were used primarily for racing had a greater risk compared with horses used primarily for pleasure. Thoroughbreds, Standardbreds, and Warmblood horses had markedly greater odds of disease compared with Quarter Horses, however, there was no detectable difference when comparing other breeds. This phenomenon of primary use and breed may have been indicated due to the intensity of competition and transportation on a regular basis. Age was a risk factor for EPM particularly in young horses had a higher risk of developing EPM than older horses. The youngest horses (6 months to 18 months) had greater than three times the the odds of developing EPM and horses 18 months to five years old had twice the odds of developing EPM when compared with horses >5 years old. Finding age-related differences in the likelihood of disease is suggestive of repeated exposure to S. neurona over time. In addition to age, there was about 2.6 times greater odds of disease if owners reported transporting horses one to six months previously. Several variables were associated with EPM occurrence in individual horses (e.g, age, sex, breed, and primary use), but only one of the variables marking hypothetical stressful events (eg participating in competition) was considered the most important.
Other variables most strongly associated with EPM occurrence were number of resident horses, finding evidence of wildlife on premises, and located near a marsh. It was expected that EPM Cases were more likely to be identified on premises with more horses because the likelihood of identifying a case increased with horse population size. Therefore, operations with >20 horses were two to three times more likely to detect an EPM Case when compared with smaller operations. Operations where there are raccoons or skunks or opossums seen on the premises, the odds of disease increased. Operations where there were modest mean temperatures had the lowest odds of disease and those with moderate temperatures had the highest odds of disease, followed by those with the lowest mean annual temperatures.
Regional ecology was was defined as participating states were allocated to regional groups based upon distributions and density of opossum populations. Region A included the states of Kentucky, Michigan, Missouri, New Jersey, New York, Ohio, Pennsylvania, and Tenneessee. Regional ecology was associated with the likelihood of EPM occurrence in this study; horses were more than twice as likely to have EPM if they resided in states from Region A than if they lived in the other three regions (Morley et al., 2008).
10. Prevention and control
Preventing contamination of feed and water with opossum feces is essential to prevent EPM in animals. Opossums can produce millions of sporocysts that can be excreted in feces for months. Sporocysts are resistant to environmental influences, and most commonly used disinfectants do not kill S. neurona sporocysts (Table 10). Heating to 60°C for 1 minute will kill sporocysts but exposure to 55°C for 5 minutes will not. Although survival of sporocysts in different environmental conditions outdoors has not been tested, sporocysts remained viable at 4 °C for months (Table 10).
Table 10.
Resistance of S. neurona sporocysts to chemical and environmental treatments.
| Reagent/condition | Duration | Viabilitya | Reference |
|---|---|---|---|
| 5.25% NAOCl (bleach) | 1 hr | Killed | Dubey et al. (2002b) |
| 12.56% phenol (wex-cide) | 6 hr | Live | |
| 2% chlorhexide (Novalsn) | 6 h | Live | |
| 1% iodine (Betadine) | 6 hr | Live | |
| 5% 0-benzyk-p-Chlorphenol (TB-plus) | 6 hr | Live | |
| Formalin 10% | 6 hr | Live | |
| 6% benzyl ammonium Chloride (NPD) | 6 hr | ||
| Amonium hydroxide | |||
| 100% | 1 hr | killed | |
| 10% | 6 hr | Live | |
| Thermal exposure | |||
| 60°C | 1 min | killed | |
| 55°C | 5 min | live | |
| 55°C | 15 min | killed | |
| 50°C | 1 hr | killed | |
| 4°C | 34 months | Live | Elsheika et al.(2004c) |
| 4°C | 44 months | killed |
Determined by bioassay in interferon gamma gene knockout (KO) mice.
Currently there is no vaccine for EPM. A killed whole S. neurona merozoite vaccine was conditionally marketed by Fort Dodge in the USA but the product is now withdrawn because of the difficulty of obtaining efficacy data. Several vaccine trials were done with this vaccine and there was no difference between the vaccinates and the controls (Saville et al., unpublished findings).
Acknowledgments
This research was partially funded by the NIAID/Division of Intramural Research to MEG. MEG is a scholar of the Integrated Microbial Biodiversity program of the Canadian Institute for Advanced Research (CIFAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias M, Yeargan M, Francisco I, Dangoudoubiyam S, Becerra P, Francisco R, Sánchez-Andrade R, Paz-Silva A, Howe DK. Exposure to Sarcocystis spp. in horses from Spain determined by Western blot analysis using Sarcocystis neurona merozoites as heterologous antigen. Vet Parasitol. 2012;185:301–304. doi: 10.1016/j.vetpar.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Asai T, Takeuchi T, Diffenderfer J, Sibley LD. Identification of small-molecule inhibitors of nucleoside triphosphate hydrolase in Toxoplasma gondii. Antimicrob Agents Chemother. 2002;46:2393–2399. doi: 10.1128/AAC.46.8.2393-2399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundsson IM, Dubey JP, Rosenthal BM. A genetically diverse but distinct North American population of Sarcocystis neurona includes an overrepresented clone described by 12 microsatellite alleles. Infect Genet Evol. 2006;6:352–360. doi: 10.1016/j.meegid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Asmundsson IM, Rosenthal BM. Isolation and characterization of microsatellite markers from Sarcocystis neurona, a causative agent of equine protozoal myeloencephalitis. Molecular Ecology Notes. 2006;6:8–10. [Google Scholar]
- Barr SC, Warner K. Characterization of a serine protease activity in Sarcocystis neurona merozoites. J Parasitol. 2003;89:385–388. doi: 10.1645/0022-3395(2003)089[0385:COASPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bentz BG, Granstrom DE, Stamper S. Seroprevalence of antibodies to Sarcocystis neurona in horses residing in a county of southeastern Pennsylvania. J Am Vet Med Assoc. 1997;210:517–518. [PubMed] [Google Scholar]
- Bentz BG, Ealey KA, Morrow J, Claypool PL, Saliki JT. Seroprevalence of antibodies to Sarcocystis neurona in equids residing in Oklahoma. J Vet Diagn Invest. 2003;15:597–600. doi: 10.1177/104063870301500617. [DOI] [PubMed] [Google Scholar]
- Bisby TM, Holman PJ, Pitoc GA, Packer RA, Thompson CA, Raskin RE. Sarcocystis sp encephalomyelitis in a cat. Vet Clin Pathol. 2010;39:105–112. doi: 10.1111/j.1939-165X.2009.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewski T, Nursimulu N, Pszenny V, Dangoudoubiyam S, Namasivayam S, Chiasson MA, Chessman K, Tonkin M, Seshadri S, Hung SS, Bridgers J, Ricklefs SM, Boulanger MJ, Dubey JP, Porcella SF, Kissinger JC, Howe DK, Grigg ME, Parkinson J. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a hetroxenous life cycle. mBio. 2015;6:e02445–14. doi: 10.1128/mBio.02445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe LL, Granstrom DE, Hansen DE, Walker LL, Bartlett J, Stamper S. Seroprevalence of antibodies to Sarcocystis neurona in horses residing in Oregon. J Am Vet Med Assoc. 1997;210:525–527. [PubMed] [Google Scholar]
- Bolten KE, Marsh AE, Reed SM, Dubey JP, Toribio RE, Saville WJA. Sarcocystis neurona: molecular characterization of enolase domain I region and a comparison to other protozoa. Exp Parasitol. 2008;120:108–112. doi: 10.1016/j.exppara.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Bowman DD, Iordanescu MA, Chou HH, Horton KH. Sarcocystis neurona and Sarcocystis falcatula: monitoring of schizogony in cell culture using fluorescent nuclear labeling. Vet Parasitol. 2001;95:353–356. doi: 10.1016/s0304-4017(00)00402-7. [DOI] [PubMed] [Google Scholar]
- Box ED, Meier JL, Smith JH. Description of Sarcocystis falcatula Stiles, 1893, a parasite of birds and opossums. J Protozool. 1984;31:521–524. doi: 10.1111/j.1550-7408.1984.tb05495.x. [DOI] [PubMed] [Google Scholar]
- Britton AP, Dubey JP, Rosenthal BM. Rhinitis and disseminated disease in a ferret (Mustela putorius furo) naturally infected with Sarcocystis neurona. Vet Parasitol. 2010;169:226–231. doi: 10.1016/j.vetpar.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Brown CM, Morrow JK, Carleton CL, Ramanathan B, Reddy R, Vaidya V, Karthikeyan SM, Zulfikar AA, Kannadkar VS. Persistence of serum antibodies to Sarcocystis neurona in horses moved from Noarth America to India. J Vet Intern Med. 2006;20:994–997. doi: 10.1892/0891-6640(2006)20[994:posats]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burcham GN, Ramos-Vara JA, Vemulapalli R. Systemic sarcocystosis in a striped skunk (Mephitis mephitis) Vet Pathol. 2010;47:560–564. doi: 10.1177/0300985810363720. [DOI] [PubMed] [Google Scholar]
- Butcher M, Lakritz J, Halaney A, Branson K, Gupta GD, Kreeger J, Marsh AE. Experimental inoculation of domestic cats (Felis domesticus) with Sarcocystis neurona or S. neurona-like merozoites. Vet Parasitol. 2002;107:1–14. doi: 10.1016/s0304-4017(02)00107-3. [DOI] [PubMed] [Google Scholar]
- Carlson-Bremer DP, Gulland FMD, Johnson CK, Colegrove KM, Van Bonn WG. Diagnosis and treatment of Sarcocystis neurona-induced myositis in a free-ranging Califronia sea lion. J Am Vet Med Assoc. 2012;240:324–328. doi: 10.2460/javma.240.3.324. [DOI] [PubMed] [Google Scholar]
- Casagrande RA, Cesar MO, Pena HFJ, Zwarg T, Teixeira RHF, Nunes ALV, Neves DVDA, Gomes M, Quagglia Neto F, Milanello L, Fontenelle JH, Matushima ER. Occurrence of Sarcocystis spp. in opossums (Didelphis aurita and Didelphis albiventris) in regions of the state of São Paulo, Brazil Braz. J Vet Res Anim Sci. 2009;46:101–106. [Google Scholar]
- Cheadle MA, Tanhauser SM, Scase TJ, Dame JB, MacKay RJ, Ginn PE, Greiner EC. Viability of Sarcocystis neurona sporocysts and dose titration in gamma-interferon knockout mice. Vet Parasitol. 2001a;95:223–231. doi: 10.1016/s0304-4017(00)00419-2. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Dame JB, Greiner EC. Sporocyst size of isolates of Sarcocystis shed by the Virginia opossum (Didelphis virginiana) Vet Parasitol. 2001b;95:305–311. doi: 10.1016/s0304-4017(00)00396-4. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Tanhauser SM, Dame JB, Sellon DC, Hines M, Ginn PE, MacKay RJ, Greiner EC. The nine-banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona. Int J Parasitol. 2001c;31:330–335. doi: 10.1016/s0020-7519(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Yowell CA, Sellon DC, Hines M, Ginn PE, Marsh AE, Dame JB, Greiner EC. The striped skunk (Mephitis mephitis) is an intermediate host for Sarcocystis neurona. Int J Parasitol. 2001d;31:843–849. doi: 10.1016/s0020-7519(01)00231-4. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Ginn PE, Lindsay DS, Greiner EC. Neurologic disease in gamma-interferon gene knockout mice caused by Sarcocystis neurona sporocysts collected from opossums fed armadillo muscle. Vet Parasitol. 2002;103:65–69. doi: 10.1016/s0304-4017(01)00550-7. [DOI] [PubMed] [Google Scholar]
- Cheadle MA, Lindsay DS, Greiner EC. Lack of Sarcocystis neurona antibody response in Virginia opossums (Didelphis virginiana) fed Sarcocystis neurona-infected muscle tissue. J Parasitol. 2006;92:652–654. doi: 10.1645/GE-788R.1. [DOI] [PubMed] [Google Scholar]
- Cook AG, Buechner-Maxwell V, Morrow JK, Ward DL, Parker NA, Dascanio JJ, Ley WB, Cooper W. Interpretation of the detection of Sarcocystis neurona antibodies in the serum of young horses. Vet Parasitol. 2001;95:187–195. doi: 10.1016/s0304-4017(00)00390-3. [DOI] [PubMed] [Google Scholar]
- Cook AG, Maxwell VB, Donaldson LL, Parker NA, Ward DL, Morrow JK. Detection of antibodies against Sarcocystis neurona in cerebrospinal fluid from clinically normal neonatal foals. J Am Vet Med Assoc. 2002;220:208–211. doi: 10.2460/javma.2002.220.208. [DOI] [PubMed] [Google Scholar]
- Cooley AJ, Barr B, Rejmanek D. Sarcocystis neurona encephalitis in a dog. Vet Pathol. 2007;44:956–961. doi: 10.1354/vp.44-6-956. [DOI] [PubMed] [Google Scholar]
- Crowdus CA, Marsh AE, Saville WJ, Lindsay DS, Dubey JP, Granstrom DE, Howe DK. SnSAG5 is an alternative surface antigen of Sarcocystis neurona strains that is mutually exclusive to SnSAG1. Vet Parasitol. 2008;158:36–43. doi: 10.1016/j.vetpar.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Cutler TJ, MacKay RJ, Ginn PE, Greiner EC, Porter R, Yowell CA, Dame JB. Are Sarcocystis neurona and Sarcocystis falcatula synonymous? A horse infection challenge. J Parasitol. 1999;85:301–305. [PubMed] [Google Scholar]
- Cutler TJ, MacKay RJ, Ginn PE, Gillis K, Tanhauser SM, LeRay EV, Dame JB, Greiner EC. Immunoconversion against Sarcocystis neurona in normal and dexamethasone-treated horses challenged with S. neurona sporocysts. Vet Parasitol. 2001;95:197–210. doi: 10.1016/s0304-4017(00)00420-9. [DOI] [PubMed] [Google Scholar]
- Daft BM, Barr BC, Gardner IA, Read D, Bell W, Peyser KG, Ardans A, Kinde H, Morrow JK. Sensitivity and specificity of western blot testing of cerebrospinal fluid and serum for diagnosis of equine protozoal myeloencephalitis in horses with and without neurologic abnormalities. J Am Vet Med Assoc. 2002;221:1007–1013. doi: 10.2460/javma.2002.221.1007. [DOI] [PubMed] [Google Scholar]
- Dame JB, MacKay RJ, Yowell CA, Cutler TJ, Marsh A, Greiner EC. Sarcocystis falcatula from passerine and psittacine birds: synonymy with Sarcocystis neurona, agent of equine protozoal myeloencephalitis. J Parasitol. 1995;81:930–935. [PubMed] [Google Scholar]
- Dangoudoubiyam S, Oliveira JB, Víquez C, Gómez-García A, González O, Romero JJ, Kwok OCH, Dubey JP, Howe DK. Detection of antibodies against Sarcocystis neurona, Neospora spp. and Toxoplasma gondii in horses from Costa Rica. J Parasitol. 2011;97:522–524. doi: 10.1645/GE-2722.1. [DOI] [PubMed] [Google Scholar]
- Dangoudoubiyam S, Zhang Z, Howe DK. Purine salvage in the apicomplexan Sarcocystis neurona, and generation of hypoxanthine-xanthine-guanine phosporibosyltransferase-deficient clones for positive-negative selection of transgenic parasites. Parasitology. 2014;141:1399–1405. doi: 10.1017/S0031182014000687. [DOI] [PubMed] [Google Scholar]
- Davis SW, Daft BM, Dubey JP. Sarcocystis neurona cultured in vitro from a horse with equine protozoal myelitis. Equine Vet J. 1991;23:315–317. doi: 10.1111/j.2042-3306.1991.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Dirikolu L, Karpiesiuk W, Lehner AF, Hughes C, Woods WE, Harkins JD, Boyles J, Atkinson A, Granstrom DE, Tobin T. New therapeutic approaches for equine protozoal myeloencephalitis: pharmacokinetics of diclazuril sodium salts in horses. Veterinary Therapeutics. 2006;7:52–63. [PubMed] [Google Scholar]
- Duarte PC, Daft BM, Conrad PA, Packham AE, Gardner IA. Comparison of a serum indirect fluorescent antibody test with two Western blot tests for the diagnosis of equine protozoal myeloencephalitis. J Vet Diagn Invest. 2003;15:8–13. doi: 10.1177/104063870301500103. [DOI] [PubMed] [Google Scholar]
- Duarte PC, Conrad PA, Wilson WD, Ferraro GL, Packham AE, Bowers-Lepore J, Carpenter TE, Gardner IA. Risk of postnatal exposure to Sarcocystis neurona and Neospora hughesi in horses. Am J Vet Res. 2004a;65:1047–1052. doi: 10.2460/ajvr.2004.65.1047. [DOI] [PubMed] [Google Scholar]
- Duarte PC, Conrad PA, Barr BC, Wilson WD, Ferraro GL, Packham AE, Carpenter TE, Gardner IA. Risk of transplacental transmission of Sarcocystis neurona and Neospora hughesi in California horses. J Parasitol. 2004b;90:1345–1351. doi: 10.1645/GE-3372. [DOI] [PubMed] [Google Scholar]
- Duarte PC, Daft BM, Conrad PA, Packham AE, Saville WJ, MacKay RJ, Barr BC, Wilson WD, Ng T, Reed SM, Gardner IA. Evaluation and comparison of an indirect fluorescent antibody test for detection of antibodies to Sarcocystis neurona, using serum and cerebrospinal fluid of naturally and experimentally infected, and vaccinated horses. J Parasitol. 2004c;90:379–386. doi: 10.1645/GE-3263. [DOI] [PubMed] [Google Scholar]
- Duarte PC, Ebel ED, Traub-Dargatz J, Wilson WD, Conrad PA, Gardner IA. Indirect fluorescent antibody testing of cerebrospinal fluid for diagnosis of equine protozoal myeloencephalitis. Am J Vet Res. 2006;67:869–876. doi: 10.2460/ajvr.67.5.869. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Miller S. Equine protozoal myeloencephalitis in a pony. J Am Vet Med Assoc. 1986;188:1311–1312. [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Fayer R. Sarcocystosis of Animals and Man. CRC Press; Boca Raton, FL: 1989. pp. 1–215. [Google Scholar]
- Dubey JP, Hamir AN, Hanlon CA, Topper MJ, Rupprecht CE. Fatal necrotizing encephalitis in a raccoon associated with a Sarcocystis-like protozoon. J Vet Diagn Invest. 1990;2:345–347. doi: 10.1177/104063879000200419. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA. Sarcocystis canis n. sp (Apicomplexa: Sarcocystidae), the etiologic agent of generalized coccidiosis in dogs. J Parasitol. 1991;77:522–527. [PubMed] [Google Scholar]
- Dubey JP, Davis SW, Speer CA, Bowman DD, de Lahunta A, Granstrom DE, Topper MJ, Hamir AN, Cummings JF, Suter MM. Sarcocystis neurona n. sp (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J Parasitol. 1991a;77:212–218. [PubMed] [Google Scholar]
- Dubey JP, Slife LN, Speer CA, Lipscomb TP, Topper MJ. Fatal cutaneous and visceral infection in a Rottweiler dog associated with a Sarcocystis-like protozoon. J Vet Diagn Invest. 1991b;3:72–75. doi: 10.1177/104063879100300115. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Hamir AN, Topper MJ, Brown C, Rupprecht CE. Development of a Sarcocystis-like apicomplexan protozoan in the brain of a raccoon (Procyon lotor) J Helminthol Soc Wash. 1991c;58:250–255. [Google Scholar]
- Dubey JP, Hedstrom OR. Meningoencephalitis in mink associated with a Sarcocystis neurona-like organism. J Vet Diagn Invest. 1993;5:467–471. doi: 10.1177/104063879300500333. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Higgins RJ, Barr BC, Spangler WL, Kollin B, Jorgensen LS. Sarcocystis-associated meningoencephalomyelitis in a cat. J Vet Diagn Invest. 1994;6:118–120. doi: 10.1177/104063879400600126. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS. Isolation in immunodeficient mice of Sarcocystis neurona from opossum (Didelphis virginiana) faeces, and its differentiation from Sarcocystis falcatula. Int J Parasitol. 1998;28:1823–1828. doi: 10.1016/s0020-7519(98)00166-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Lindsay DS. Isolation of a third species of Sarcocystis in immunodeficient mice fed feces from opossums (Didelphis virginiana) and its differentiation from Sarcocystis falcatula and Sarcocystis neurona. J Parasitol. 1998;84:1158–1164. [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS. Sarcocystis speeri n. sp (Protozoa: Sarcocystidae) from the opossum (Didelphis virginiana) J Parasitol. 1999;85:903–909. [PubMed] [Google Scholar]
- Dubey JP, Mattson DE, Speer CA, Baker RJ, Mulrooney DM, Tornquist SJ, Hamir AN, Gerros TC. Characterization of Sarcocystis neurona isolate (SN6) from a naturally infected horse from Oregon. J Eukaryot Microbiol. 1999a;46:500–506. doi: 10.1111/j.1550-7408.1999.tb06067.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Kerber CE, Granstrom DE. Serologic prevalence of Sarcocystis neurona, Toxoplasma gondii, and Neospora caninum in horses in Brazil. J Am Vet Med Assoc. 1999b;215:970–972. [PubMed] [Google Scholar]
- Dubey JP, Venturini MC, Venturini L, McKinney J, Pecoraro M. Prevalence of antibodies to Sarcocystis neurona, Toxoplasma gondii, and Neospora caninum in horses from Argentina. Vet Parasitol. 1999c;86:59–62. doi: 10.1016/s0304-4017(99)00127-2. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Prevalence of Sarcocystis species sporocysts in wild caught opossums (Didelphis virginiana) J Parasitol. 2000;86:705–710. doi: 10.1645/0022-3395(2000)086[0705:POSSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Hamir AN. Immunohistochemical confirmation of Sarcocystis neurona infections in raccoons, mink, cat, skunk and pony. J Parasitol. 2000;86:1150–1152. doi: 10.1645/0022-3395(2000)086[1150:ICOSNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Saville WJA, Lindsay DS, Stich RW, Stanek JF, Speer CA, Rosenthal BM, Njoku CJ, Kwok OCH, Shen SK, Reed SM. Completion of the life cycle of Sarcocystis neurona. J Parasitol. 2000;86:1276–1280. doi: 10.1645/0022-3395(2000)086[1276:COTLCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Migration and development of Sarcocystis neurona in tissues of interferon gamma knockout mice fed sporocysts from a naturally infected opossums. Vet Parasitol. 2001a;95:341–351. doi: 10.1016/s0304-4017(00)00401-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Parasitemia and early tissue localization of Sarcocystis neurona in interferon gamma gene knockout mice fed sporocysts. J Parasitol. 2001b;87:1476–1479. doi: 10.1645/0022-3395(2001)087[1476:PAETLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJA, Reed SM, Granstrom DE, Speer CA. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM) Vet Parasitol. 2001a;95:89–131. doi: 10.1016/s0304-4017(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Saville WJA, Stanek JF, Lindsay DS, Rosenthal BM, Oglesbee MJ, Rosypal AC, Njoku CJ, Stich RW, Kwok OCH, Shen SK, Hamir AN, Reed SM. Sarcocystis neurona infections in raccoons (Procyon lotor): evidence for natural infection with sarcocysts, transmission of infection to opossums (Didelphis virginiana), and experimental induction of neurologic disease in raccoons. Vet Parasitol. 2001b;100:117–129. doi: 10.1016/s0304-4017(01)00500-3. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Rosypal AC, Rosenthal BM, Thomas NJ, Lindsay DS, Stanek JF, Reed SM, Saville WJA. Sarcocystis neurona infections in sea otter (Enhydra lutris): evidence for natural infections with sarcocysts and transmission of infection to opossums (Didelphis virginiana) J Parasitol. 2001c;87:1387–1393. doi: 10.1645/0022-3395(2001)087[1387:SNIISO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Fritz D, Speer CA. Structure of Sarcocystis neurona sarcocysts. J Parasitol. 2001d;87:1323–1327. doi: 10.1645/0022-3395(2001)087[1323:SOSNS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Kwok OCH, Shen SK. The gamma interferon knockout mouse model for Sarcocystis neurona: comparison of infectivity of sporocysts and merozoites and routes of inoculation. J Parasitol. 2001e;87:1171–1173. doi: 10.1645/0022-3395(2001)087[1171:TGIKMM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Mattson DE, Speer CA, Hamir AN, Lindsay DS, Rosenthal BM, Kwok OCH, Baker RJ, Mulrooney DM, Tornquist SJ, Gerros TC. Characteristics of a recent isolate of Sarcocystis neurona (SN7) from a horse and loss of pathogenicity of isolates SN6 and SN7 by passages in cell culture. Vet Parasitol. 2001f;95:155–166. doi: 10.1016/s0304-4017(00)00387-3. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Kerber CE, Kasai N, Pena HFJ, Gennari SM, Kwok OCH, Shen SK, Rosenthal BM. First isolation of Sarcocystis neurona from the South American opossum, Didelphis albiventris, from Brazil. Vet Parasitol. 2001g;95:295–304. doi: 10.1016/s0304-4017(00)00395-2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Fritz D, Lindsay DS, Shen SK, Kwok OCH, Thompson KC. Diclazuril preventive therapy of gamma interferon knockout mice fed Sarcocystis neurona sporocysts. Vet Parasitol. 2001h;94:257–263. doi: 10.1016/s0304-4017(00)00376-9. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Black SS, Rickard LG, Rosenthal BM, Lindsay DS, Shen SK, Kwok OCH, Hurst G, Rashmir-Raven A. Prevalence of Sarcocystis neurona sporocysts in opossums (Didelphis virginiana) from rural Mississippi. Vet Parasitol. 2001i;95:283–293. doi: 10.1016/s0304-4017(00)00394-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Rosenthal BM, Speer CA. Sarcocystis lindsayi n. sp (Protozoa: Sarcocystidae) from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol. 2001j;48:595–603. doi: 10.1111/j.1550-7408.2001.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJA. Serologic responses of cats against experimental Sarcocystis neurona infections. Vet Parasitol. 2002a;107:265–269. doi: 10.1016/s0304-4017(02)00157-7. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay DS, Pena HF, Vianna MC, Gennari SM, Reed SM. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. J Parasitol. 2002b;88:1252–1254. doi: 10.1645/0022-3395(2002)088[1252:EOHTAD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Hill D, Romand S, Thulliez P, Kwok OCH, Silva JCR, Oliveira-Camargo MC, Gennari SM. Prevalence of antibodies to Neospora caninum and Sarcocystis neurona in sera of domestic cats from Brazil. J Parasitol. 2002c;88:1251–1252. doi: 10.1645/0022-3395(2002)088[1251:POATNC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Benson J, Larson MA. Clinical Sarcocystis neurona encephalomyelitis in a domestic cat following routine surgery. Vet Parasitol. 2003a;112:261–267. doi: 10.1016/s0304-4017(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Mitchell SM, Morrow JK, Rhyan JC, Stewart LM, Granstrom DE, Romand S, Thulliez P, Saville WJ, Lindsay DS. Prevalence of antibodies to Neospora caninum, Sarcocystis neurona, and Toxoplasma gondii in wild horses from central Wyoming. J Parasitol. 2003b;89:716–720. doi: 10.1645/GE-66R. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Equine protozoal myeloencephalitis and Sarcocystis neurona. In: Coetzer JAW, Thompson GR, Tustin RC, Kriek NPJ, editors. Infectious Diseases of Livestock with special Reference to southern Africa. Oxford University Press; Ni City, South Africa: 2004. pp. 394–403. [Google Scholar]
- Dubey JP, Chapman JL, Rosenthal BM, Mense M, Schueler RL. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet Parasitol. 2006;137:36–49. doi: 10.1016/j.vetpar.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of Animals and Humans. 2. CRC Press; Boca Raton, FL: 2010. pp. 1–313. [Google Scholar]
- Dubey JP, Thomas NJ. Sarcocystis neurona retinochoroiditis in a sea otter (Enhydra lutris kenyoni) Vet Parasitol. 2011;183:156–159. doi: 10.1016/j.vetpar.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Kwok OCH, Saville WJA. Sarcocystis neurona infection in gamma interferon gene knockout (KO) mice: Comparative infectivity of sporocysts in two strains of KO mice, effect of trypsin digestion on merozoite viability, and infectivity of bradyzoites to KO mice and cell culture. Vet Parasitol. 2013;196:212–215. doi: 10.1016/j.vetpar.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Black SS, Verma SK, Calero-Bernal R, Morris E, Hanson MA, Cooley AJ. Sarcocystis neurona schizonts-associated encephalitis, chorioretinitis, and myositis in a two-month-old dog simulating toxoplasmosis, and presence of mature sarcocysts in muscles. Vet Parasitol. 2014;202:194–200. doi: 10.1016/j.vetpar.2014.02.055. [DOI] [PubMed] [Google Scholar]
- Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000;37:574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- Elitsur E, Marsh AE, Reed SM, Dubey JP, Oglesbee MJ, Murphy JE, Saville WJA. Early migration of Sarcocystis neurona in ponies fed sporocysts. J Parasitol. 2007;93:1222–1225. doi: 10.1645/GE-497R.1. [DOI] [PubMed] [Google Scholar]
- Ellison SP, Greiner E, Dame JB. In vitro culture and synchronous release of Sarcocystis neurona merozoites from host cells. Vet Parasitol. 2001;95:251–261. doi: 10.1016/s0304-4017(00)00391-5. [DOI] [PubMed] [Google Scholar]
- Ellison SP, Omara-Opyene AL, Yowell CA, Marsh AE, Dame JB. Molecular characterization of a major 29 kDa surface antigen of Sarcocystis neurona. Int J Parasitol. 2002;32:217–225. doi: 10.1016/s0020-7519(01)00324-1. [DOI] [PubMed] [Google Scholar]
- Ellison SP, Kennedy T, Brown KK. Development of an ELISA to detect antibodies to rSAG1 in the horse. Int J Appl Res Vet Med. 2003a;1:318–327. [Google Scholar]
- Ellison SP, Kennedy T, Brown KK. Early signs of equine protozoal myeloencephalitis. Int J Appl Res Vet Med. 2003b;1:272–278. [Google Scholar]
- Ellison SP, Greiner E, Brown KK, Kennedy T. Experimental infection of horses with culture-derived Sarcocystis neurona merozoites as a model for equine protozoal myeloencephalitis. Int J Appl Res Vet Med. 2004;2:79–89. [Google Scholar]
- Ellison S, Witonsky S. Evidence that antibodies against recombinant SnSAG1 of Sarcocystis neurona merozoites are involved in infection and immunity in equine protozoal myeloencephalitis. Can J Vet Res. 2009;73:176–183. [PMC free article] [PubMed] [Google Scholar]
- Ellison SP, Lindsay DS. Decoquinate combined with levamisole reduce the clinical signs and serum SAG 1, 5, 6 antibodies in horses with suspected equine protozoal myeloencephalitis. Intern J Appl Res Vet Med. 2012;10:1–7. [Google Scholar]
- Elsheikha HM, Murphy AJ, Fitzgerald SD, Mansfield LS, Massey JP, Saeed MA. Purification of Sarcocystis neurona sporocysts from opossum (Didelphis virginiana) using potassium bromide discontinuous density gradient centrifugation. Parasitol Res. 2003;90:104–109. doi: 10.1007/s00436-002-0789-y. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Mansfield LS. Assessment of Sarcocystis neurona sporocyst viability and differentiation between viable and nonviable sporocysts using propidium iodide stain. J Parasitol. 2004a;90:872–875. doi: 10.1645/GE-262R. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Mansfield LS. Sarcocystis neurona major surface antigen gene 1 (SAG1) shows evidence of having evolved under positive selection pressure. Parasitol Res. 2004b;94:452–459. doi: 10.1007/s00436-004-1237-y. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Fitzgerald SD, Rosenthal BM, Mansfield LS. Concurrent presence of Sarcocystis neurona sporocysts, Besnoitia darlingi tissue cysts, and Sarcocystis inghami sarcocysts in naturally infected opossums (Didelphis virginiana) J Vet Diagn Invest. 2004a;16:352–356. doi: 10.1177/104063870401600419. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Mansfield LS. Prevalence of and risk factors associated with the presence of Sarcocystis neurona sporocysts in opossum (Didelphis virginiana) from Michigan: a retrospective study. Vet Parasitol. 2004b;125:277–286. doi: 10.1016/j.vetpar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murthy AJ, Mansfield LS. Viability of Sarcocystis neurona sporocysts after long-term storage. Vet Parasitol. 2004c;123:257–264. doi: 10.1016/j.vetpar.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Mansfield LS. Prevalence of Sarcocystis species sporocysts in Northern Virginia opossums (Didelphis virginiana) Parasitol Res. 2004d;93:427–431. doi: 10.1007/s00436-004-1150-4. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Mansfield LS. Phylogenetic congruence of Sarcocystis neurona Dubey et al. 1991 (Apicomplexa: Sarcocystidae) in the United States based on sequence analysis and restriction fragment length polymorphism (RFLP) Syst Parasitol. 2005a;61:191–202. doi: 10.1007/s11230-005-3163-5. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Lacher DW, Mansfield LS. Phylogenetic relationships of Sarcocystis neurona of horses and opossums to other cyst-forming coccidia deduced from SSU rRNA gene sequence. Parasitol Res. 2005b;97:345–357. doi: 10.1007/s00436-005-1396-5. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Rosenthal BM, Murphy AJ, Dunams DB, Neelis DA, Mansfield LS. Generally applicable methods to purify intracellular coccidia from cell cultures and to quantify purification efficacy using quantitative PCR. Vet Parasitol. 2006a;135:223–234. doi: 10.1016/j.vetpar.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Schott HC, Mansfield LS. Genetic variation among isolates of Sarcocystis neurona, the agent of protozoal myeloencephalitis, as revealed by amplified fragment length polymorphism markers. Infect Immun. 2006b;74:3448–3454. doi: 10.1128/IAI.01215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikha HM, Mansfield LS. Molecular typing of Sarcocystis neurona: current status and future trends. Vet Parasitol. 2007;149:43–55. doi: 10.1016/j.vetpar.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM. Has Sarcocystis neurona Dubey et al. 1991 (Sporozoa: Apicomplexa: Sarcocystidae) cospeciated with its intermediate hosts? Vet Parasitol. 2009;163:307–314. doi: 10.1016/j.vetpar.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Fenger CK, Granstrom DE, Langemeier JL, Gajadhar A, Cothran G, Tramontin RR, Stamper S, Dubey JP. Phylogenetic relationship of Sarcocystis neurona to other members of the family Sarcocystidae based on the sequence of the small ribosomal subunit gene. J Parasitol. 1994;79:966–975. [PubMed] [Google Scholar]
- Fenger CK, Granstrom DE, Langemeier JL, Stamper S, Donahue JM, Patterson JS, Gajadhar AA, Marteniuk JV, Xiaomin Z, Dubey JP. Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J Parasitol. 1995;81:916–919. [PubMed] [Google Scholar]
- Fenger CK, Granstrom DE, Gajadhar AA, Williams NM, McCrillis SA, Stamper S, Langemeier JL, Dubey JP. Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp sporocysts from the opossum (Didelphis virginiana) Vet Parasitol. 1997;68:199–213. doi: 10.1016/s0304-4017(96)01112-0. [DOI] [PubMed] [Google Scholar]
- Finno CJ, Packham AE, Wilson WD, Gardner IA, Conrad PA, Pusterla N. Effects of blood contamination of cerebrospinal fluid on results of indirect fluorescent antibody tests for detection of antibodies against Sarcocystis neurona and Neospora hughesi. J Vet Diagn Invest. 2007;19:286–289. doi: 10.1177/104063870701900310. [DOI] [PubMed] [Google Scholar]
- Forest TW, Abou-Madi N, Summers BA, Tornquist SJ, Cooper BJ. Sarcocystis neurona-like encephalitis in a Canada lynx (Felis lynx canadensis) J Zoo Wildlife Med. 2001;31:383–387. doi: 10.1638/1042-7260(2000)031[0383:SNLEIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Franklin RP, MacKay RJ, Gillis KD, Tanhauser SM, Ginn PE, Kennedy TJ. Effect of a single dose of ponazuril on neural infection and clinical disease in Sarcocystis neurona-challenged interferon-gamma knockout mice. Vet Parasitol. 2003;114:123–130. doi: 10.1016/s0304-4017(03)00127-4. [DOI] [PubMed] [Google Scholar]
- Fritz DL, Dubey JP. Pathology of Sarcocystis neurona in interferon-gamma gene knockout mice. Vet Pathol. 2002;39:137–140. doi: 10.1354/vp.39-1-137. [DOI] [PubMed] [Google Scholar]
- Furr M, Kennedy T. Cerebrospinal fluid and serum concentrations of ponazuril in horses. Veterinary Therapeutics. 2001;2:232–237. [PubMed] [Google Scholar]
- Furr M, Pontzer C. Transforming growth factor beta concentrations and interferon gamma responses in cerebrospinal fluid of horses with equine protozoal myeloencephalitis. Equine Vet J. 2001;33:721–725. doi: 10.2746/042516401776249408. [DOI] [PubMed] [Google Scholar]
- Furr M, Pontzer C, Gasper P. Lymphocyte phenotype subsets in the cerebrospinal fluid of normal horses and horses with equine protozoal myeloencephalitis. Veterinary Therapeutics. 2001a;2:317–324. [PubMed] [Google Scholar]
- Furr M, Kennedy T, MacKay R, Reed S, Andrews F, Bernard B, Bain F, Byars D. Efficacy of ponazuril 15% oral paste as a treatment for equine protozoal myeloencephalitis. Veterinary Therapeutics. 2001b;2:215–222. [PubMed] [Google Scholar]
- Furr M, MacKay R, Granstrom D, Schott H, Andrews F. Clinical diagnosis of equine protozoal myeloencephalitis (EPM) J Vet Intern Med. 2002;16:618–621. doi: 10.1892/0891-6640(2002)016<0618:cdoepm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Furr M, McKenzie H, Saville WJA, Dubey JP, Reed SM, Davis W. Prophylactic administration of ponazuril reduces clinical signs and delays seroconversion in horses challenged with Sarcocystis neurona. J Parasitol. 2006;92:637–643. doi: 10.1645/0022-3395(2006)92[637:PAOPRC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Furr M, Howe D, Reed S, Yeargan M. Antibody coefficients for the diagnosis of equine protozoal myeloencephalitis. J Vet Intern Med. 2011;25:138–142. doi: 10.1111/j.1939-1676.2010.0658.x. [DOI] [PubMed] [Google Scholar]
- Gaji RY, Zhang D, Breathnach CC, Vaishnava S, Striepen B, Howe DK. Molecular genetic transfection of the coccidian parasite Sarcocystis neurona. Mol Biochem Parasitol. 2006;150:1–9. doi: 10.1016/j.molbiopara.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Gaji RY, Howe DK. The heptanucleotide motif GAGACGC is a key component of a cis-acting promoter element that is critical for SnSAG1 expression in Sarcocystis neurona. Mol Biochem Parasitol. 2009;166:85–88. doi: 10.1016/j.molbiopara.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Gargala G, Baishanbo A, Favennec L, Francois A, Ballet JJ, Rossignol JF. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob Agents Chemother. 2005;49:4628–4634. doi: 10.1128/AAC.49.11.4628-4634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargala G, Le Goff L, Ballet JJ, Favennec L, Stachulski AV, Rossignol JF. In vitro efficacy of nitro- and halogeno-thiazolide/thiadiazolide derivatives against Sarcocystis neurona. Vet Parasitol. 2009;162:230–235. doi: 10.1016/j.vetpar.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Gautam A, Dubey JP, Saville WJ, Howe DK. The SnSAG merozoite surface antigens of Sarcocystis neurona are expressed differentially during the bradyzoite and sporozoite life cycle stages. Vet Parasitol. 2011;183:37–42. doi: 10.1016/j.vetpar.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Gerhold RW, Howerth EW, Lindsay DS. Sarcocystis neurona-associated meningoencephalitis and description of intramuscular sarcocysts in a fisher (Martes pennanti) J Wildl Dis. 2005;41:224–230. doi: 10.7589/0090-3558-41.1.224. [DOI] [PubMed] [Google Scholar]
- Gerhold R, Newman SJ, Grunenwald GM, Crews A, Hodshon A, Su C. Acute onset of encephalomyelitis with atypical lesions associaed with dual infection of Sarcocystis neurona and Toxoplasma gondii. Vet Parasitol. 2014;205:697–701. doi: 10.1016/j.vetpar.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Gibson AK, Raverty S, Lambourn DM, Huggins J, Magargal SL, Grigg ME. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Negl Trop Dis. 2011;5(5):e1142. doi: 10.1371/journal.pntd.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis KD, MacKay RJ, Yowell CA, Levy JK, Greiner EC, Dame JB, Cheadle MA, Hernandez J, Massey ET. Naturally occurring Sarcocystis infection in domestic cats (Felis catus) Int J Parasitol. 2003;33:877–883. doi: 10.1016/s0020-7519(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Goldstein T, Gill VA, Tuomi P, Monson D, Burdin A, Conrad PA, Dunn JL, Field C, Johnson C, Jessup DA, Bodkin J, Doroff AM. Assessment of clinical pathology and pathogen exposure in sea ottters (Enhydra lutris) bordering the threatened population in Alaska. J Wildl Dis. 2011;47:579–592. doi: 10.7589/0090-3558-47.3.579. [DOI] [PubMed] [Google Scholar]
- Granstrom DE, Alvarez O, Dubey JP, Comer PF, Williams NM. Equine protozoal myelitis in Panamanian horses and isolation of Sarcocystis neurona. J Parasitol. 1992;78:909–912. [PubMed] [Google Scholar]
- Granstrom DE, Dubey JP, Davis SW, Fayer R, Fox JC, Poonacha KB, Giles RC, Comer PF. Equine protozoal myeloencephalitis: antigen analysis of cultured Sarcocystis neurona merozoites. J Vet Diagn Invest. 1993;5:88–90. doi: 10.1177/104063879300500118. [DOI] [PubMed] [Google Scholar]
- Granstrom DE, MacPherson JM, Gajadhar AA, Dubey JP, Tramontin R, Stamper S. Differentiation of Sarcocystis neurona from eight related coccidia by random amplified polymorphic DNA assay. Mol Cell Probes. 1994;8:353–356. doi: 10.1006/mcpr.1994.1051. [DOI] [PubMed] [Google Scholar]
- Gray LC, Magdesian KG, Sturges BK, Madigan JE. Suspected protozoal myeloencephalitis in a two-month-old colt. Vet Rec. 2001;149:269–273. doi: 10.1136/vr.149.9.269. [DOI] [PubMed] [Google Scholar]
- Gupta GD, Lakritz J, Kim JH, Kim DY, Kim JK, Marsh AE. Seroprevalence of Neospora, Toxoplasma gondii, and Sarcocystis neurona antibodies in horses from Jeju island, South Korea. Vet Parasitol. 2002;106:193–201. doi: 10.1016/s0304-4017(02)00064-x. [DOI] [PubMed] [Google Scholar]
- Gupta GD, Lakritz J, Saville WJ, Livingston RS, Dubey JP, Middleton JR, Marsh AE. Antigenic evaluation of a recombinant Baculovirus-expressed Sarcocystis neurona SAG1 antigen. J Parasitol. 2004;90:1027–1033. doi: 10.1645/0022-3395(2004)090[1027:AEOARB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hamir AN, Dubey JP. Myocarditis and encephalitis associated with Sarcocystis neurona infection in raccoons (Procyon lotor) Vet Parasitol. 2001;95:335–340. doi: 10.1016/s0304-4017(00)00400-3. [DOI] [PubMed] [Google Scholar]
- Hancock K, Zajac AM, Elvinger F, Lindsay DS. Prevalence of agglutinating antibodies to Sarcocystis neurona in raccoons (Procyon lotor) from an urban area of Virginia. J Parasitol. 2004;90:881–882. doi: 10.1645/GE-302R. [DOI] [PubMed] [Google Scholar]
- Harder A, Haberkorn A. Possible mode of action of toltrazuril: studies on two Eimeria species and mammalian and Ascaris suum enzymes. Parasitol Res. 1989;76:8–12. doi: 10.1007/BF00931064. [DOI] [PubMed] [Google Scholar]
- Heskett KA, MacKay RJ. Antibody index and specific antibody quotient in horses after intragastric administration of Sarcocystis neurona sporocysts. Am J Vet Res. 2008;69:403–409. doi: 10.2460/ajvr.69.3.403. [DOI] [PubMed] [Google Scholar]
- Hoane JS, Carruthers VB, Striepen B, Morrison DP, Entzeroth R, Howe DK. Analysis of the Sarcocystis neurona microneme protein SnMIC10: protein characteristics and expression during intracellular development. Int J Parasitol. 2003;33:671–679. doi: 10.1016/s0020-7519(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Hoane JS, Morrow JK, Saville WJ, Dubey JP, Granstrom DE, Howe DK. Enzyme-linked immunosorbent assays for the detection of equine antibodies specific to Sarcocystis neurona surface antigens. Clin Diagn Lab Immunol. 2005a;12:1050–1056. doi: 10.1128/CDLI.12.9.1050-1056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane JS, Yeargan MR, Stamper S, Saville WJ, Morrow JK, Lindsay DS, Howe DK. Recombinant NhSAG1 ELISA: a sensitive and specific assay for detecting antibodies against Neospora hughesi in equine serum. J Parasitol. 2005b;91:446–452. doi: 10.1645/GE-395R. [DOI] [PubMed] [Google Scholar]
- Hoane JS, Gennari SM, Dubey JP, Ribeiro MG, Borges AS, Yai LEO, Aguiar DM, Cavalcante GT, Bonesi GL, Howe DK. Prevalence of Sarcocystis neurona and Neospora spp. infection in horses from Brazil based on presence of serum antibodies to parasite surface antigen. Vet Parasitol. 2006;136:155–159. doi: 10.1016/j.vetpar.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Houk AE, Goodwin DG, Zajac AM, Barr SC, Dubey JP, Lindsay DS. Prevalence of antibodies to Trypanosoma cruzi, Toxoplasma gondii, Encephalitozoon cuniculi, Sarcocystis neurona, Besnoitia darlingi, and Neospora caninum in Noarth American opossums, Didelphis virginiana, from Southern Louisiana. J Parasitol. 2010;96:1119–1122. doi: 10.1645/GE-2515.1. [DOI] [PubMed] [Google Scholar]
- Howe DK. Initiation of a Sarcocystis neurona expressed sequence tag (EST) sequencing project: a preliminary report. Vet Parasitol. 2001;95:233–239. doi: 10.1016/s0304-4017(00)00418-0. [DOI] [PubMed] [Google Scholar]
- Howe DK, Gaji RY, Mroz-Barrett M, Gubbels MJ, Striepen B, Stamper S. Sarcocystis neurona merozoites express a family of immunogenic surface antigen that are orthologues of the Toxoplasma gondii surface antigens (SAGs) and SAG-related sequences. Infect Immun. 2005;73:1023–1033. doi: 10.1128/IAI.73.2.1023-1033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK, Gaji RY, Marsh AE, Patil BA, Saville WJ, Lindsay DS, Dubey JP, Granstrom DE. Strains of Sarcocystis neurona exhibit differences in their surface antigens, including the absence of the major surface antigen SnSAG1. Int J Parasitol. 2008;38:623–631. doi: 10.1016/j.ijpara.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Hsu V, Grant DC, Dubey JP, Zajac AM, Lindsay DS. Prevalence of antibodies to Sarcocystis neurona in cats from Virginia and Pennsylvania. J Parasitol. 2010;96:800–801. doi: 10.1645/GE-2449.1. [DOI] [PubMed] [Google Scholar]
- Hunyadi L, Papich MG, Pusterla N. Pharmacokinetics of a low dose and FDA-labeled dose of diclazuril administered orally as a pelleted topdressing in adult horses. J Vet Pharmacol Ther. 2014 doi: 10.1111/jvp.12176. [DOI] [PubMed] [Google Scholar]
- Hyun C, Gupta GD, Marsh AE. Sequence comparison of Sarcocystis neurona surface antigen from multiple isolates. Vet Parasitol. 2003;112:11–20. doi: 10.1016/s0304-4017(02)00392-8. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Burton AJ, Sweeney RW. Utility of 2 immunological tests for antemortem diagnosis of equine protozoal myeloencephalitis (Sarcocystis neurona infection) in naturally occurring cases. J Vet Intern Med. 2010;24:1184–1189. doi: 10.1111/j.1939-1676.2010.0576.x. [DOI] [PubMed] [Google Scholar]
- Jordan CN, Kaur T, Koenen K, DeStefano S, Zajac AM, Lindsay DS. Prevalence of agglutinating antibodies to Toxoplasma gondii and Sarcocystis neurona in beavers (Castor canadensis) from Massachusetts. J Parasitol. 2005;91:1228–1229. doi: 10.1645/GE-543R.1. [DOI] [PubMed] [Google Scholar]
- Jung C, Lee CYF, Grigg ME. The SRS superfamily of Toxoplasma surface proteins. Int J Parasitol. 2004;34:285–296. doi: 10.1016/j.ijpara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Wada R, Kanemaru T, Sasagawa T, Uchiyama T, Matsumura T, Anzai T. First case report of Sarcocystis neurona-induced equine protozoal myeloencephalitis in Japan. J Vet Med Sci. 2003;65:757–759. doi: 10.1292/jvms.65.757. [DOI] [PubMed] [Google Scholar]
- Kennedy T, Campbell J, Selzer V. Safety of ponazuril 15% oral past in horses. Veterinary Therapeutics. 2001;2:223–231. [PubMed] [Google Scholar]
- Kim SK, Boothroyd JC. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J Immunol. 2005;174:8038–8048. doi: 10.4049/jimmunol.174.12.8038. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, Carpenter TE, Cibrad PA, Mazet JAK. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. J Wildl Dis. 2003;39:495–509. doi: 10.7589/0090-3558-39.3.495. [DOI] [PubMed] [Google Scholar]
- Kruttlin EA, Rossano MG, Murphy AJ, Vrable RA, Kaneene JB, Schott HC, Mansfield LS. The effects of pyrantel tartrate on Sarcocystis neurona merozoite viability. Veterinary Therapeutics. 2001;2:268–276. [PubMed] [Google Scholar]
- Lapointe JM, Duignan PJ, Marsh AE, Gulland FM, Barr BC, Naydan DK, King DP, Farman CA, Huntingdon KAB, Lowenstine LJ. Meningoencephalitis due to a Sarcocystis neurona-like protozoan in Pacific harbor seals (Phoca vitulina richardsi) J Parasitol. 1998;84:1184–1189. [PubMed] [Google Scholar]
- Lekutis C, Ferguson DJP, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol. 2001;31:1285–1292. doi: 10.1016/s0020-7519(01)00261-2. [DOI] [PubMed] [Google Scholar]
- Li L, Brunk BP, Kissinger JC, Pape D, Tang K, Cole RH, Martin J, Wylie T, Dante M, Fogarty SJ, Howe DK, Liberator P, Diaz C, Anderson J, Radke JA, Stoeckert CJ, Waterston RH, Clifton SW, Roos DS, Sibley LD. Gene discovery in the Apicomplexa as revealed by EST sequencing and assembly of a comparative gene database. Genome Res. 2003;13:443–454. doi: 10.1101/gr.693203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Crabtree J, Fischer S, Pinney D, Stoeckert CJ, Jr, Sibley LD, Roos DS. ApiEST-DB: analyzing clustered EST data of the apicomplexan parasites. Nucleic Acids Res. 2004;32:D326–D328. doi: 10.1093/nar/gkh112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Granstrom DE, Zhao XM, Timoney JF. Evidence that surface proteins Sn14 and Sn16 of Sarcocystis neurona merozoites are involved in infection and immunity. Infect Immun. 1998;66:1834–1838. doi: 10.1128/iai.66.5.1834-1838.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Determination of the activity of diclazuril against Sarcocystis neurona and Sarcocystis falcatula in cell cultures. J Parasitol. 2000;86:164–166. doi: 10.1645/0022-3395(2000)086[0164:DOTAOD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP, Kennedy TJ. Determination of the activity of ponazuril against Sarcocystis neurona in cell cultures. Vet Parasitol. 2000a;92:165–169. doi: 10.1016/s0304-4017(00)00280-6. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Thomas NJ, Dubey JP. Biological characterization of Sarcocystis neurona from a Southern sea otter (Enhydra lutris nereis) Int J Parasitol. 2000b;30:617–624. doi: 10.1016/s0020-7519(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dykstra CC, Williams A, Spencer JA, Lenz SD, Palma K, Dubey JP, Blagburn BL. Inoculation of Sarcocystis neurona merozoites into the central nervous system of horses. Vet Parasitol. 2000c;92:157–163. doi: 10.1016/s0304-4017(00)00281-8. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Direct agglutination test for the detection of antibodies to Sarcocystis neurona in experimentally infected animals. Vet Parasitol. 2001a;95:179–186. doi: 10.1016/s0304-4017(00)00389-7. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Determination of the activity of pyrantel tartrate against Sarcocystis neurona in gamma-interferon gene knockout mice. Vet Parasitol. 2001b;97:141–144. doi: 10.1016/s0304-4017(01)00405-8. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Thomas NJ, Rosypal AC, Dubey JP. Dual Sarcocystis neurona and Toxoplasma gondii infection in a Northern sea otter from Washington state, USA. Vet Parasitol. 2001a;97:319–327. doi: 10.1016/s0304-4017(01)00411-3. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Rosypal AC, Spencer JA, Cheadle MA, Zajac AM, Rupprecht C, Dubey JP, Blagburn BL. Prevalence of agglutinating antibodies to Sarcocystis neurona in raccoons, Procyon lotor, from the United States. Vet Parasitol. 2001b;100:131–134. doi: 10.1016/s0304-4017(01)00494-0. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Mitchell SM, Vianna MC, Dubey JP. Sarcocystis neurona (Protozoa: Apicomplexa): description of oocysts, sporocysts, sporozoites, excystation, and early development. J Parasitol. 2004;90:461–465. doi: 10.1645/GE-230R. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Mitchell SM, Yang J, Dubey JP, Gogal RM, Witonsky SG. Penetration of equine leukocytes by merozoites of Sarcocystis neurona. Vet Parasitol. 2006;138:371–376. doi: 10.1016/j.vetpar.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Nazir MM, Maqbool A, Ellison SP, Strobl JS. Efficacy of decoquinate against Sarcocystis neurona in cell cultures. Vet Parasitol. 2013;196:21–23. doi: 10.1016/j.vetpar.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Long MT, Mines MT, Knowles DP, Tanhauser SM, Dame JB, Cutler TJ, MacKay RJ, Sellon DC. Sarcocystis neurona: parasitemia in a severe combined immunodeficient (SCID) horse fed sporocysts. Exp Parasitol. 2002;100:150–154. doi: 10.1016/s0014-4894(02)00012-7. [DOI] [PubMed] [Google Scholar]
- MacKay RJ, Tanhauser ST, Gillis KD, Mayhew IG, Kennedy TJ. Effect of intermittent oral administration of ponazuril on experimental Sarcocystis neurona infection of horses. Am J Vet Res. 2008;69:396–402. doi: 10.2460/ajvr.69.3.396. [DOI] [PubMed] [Google Scholar]
- Mansfield LS, Schott HC, Murphy AJ, Rossano MG, Tanhauser SM, Patterson JS, Nelson K, Ewart SL, Marteniuk JV, Bowman DD, Kaneene JB. Comparison of Sarcocystis neurona isolates derived from horse neural tissue. Vet Parasitol. 2001;95:167–178. doi: 10.1016/s0304-4017(00)00388-5. [DOI] [PubMed] [Google Scholar]
- Mansfield LS, Mehler S, Nelson K, Elsheikha HM, Murphy AJ, Knust B, Tanhauser SM, Gearhart PM, Rossano MG, Bowman DD, Schott HC, Patterson JS. Brown-headed cowbirds (Molothrus ater) harbor Sarcocystis neurona and act as intermediate hosts. Vet Parasitol. 2008;153:24–43. doi: 10.1016/j.vetpar.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Madigan J, Lakritz J, Conrad PA. Sequence analysis and polymerase chain reaction amplification of small subunit ribosomal DNA from Sarcocystis neurona. Am J Vet Res. 1996;57:975–981. [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Lakritz J, Nordhausen R, Madigan JE, Conrad PA. Experimental infection of nude mice as a model for Sarcocystis neurona-associated encephalitis. Parasitol Res. 1997a;83:706–711. doi: 10.1007/s004360050323. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Tell L, Koski M, Greiner E, Dame J, Conrad PA. In vitro cultivation and experimental inoculation of Sarcocystis falcatula and Sarcocystis neurona merozoites into budgerigars (Melopsittacus undulatus) J Parasitol. 1997b;83:1189–1192. [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Tell L, Bowman DD, Conrad PA, Ketcherside C, Green T. Comparison of the internal transcribed spacer, ITS-1, from Sarcocystis falcatula isolates and Sarcocystis neurona. J Parasitol. 1999;85:750–757. [PubMed] [Google Scholar]
- Marsh AE, Denver M, Hill FI, McElhaney MR, Trupkiewicz JG, Stewart J, Tell L. Detection of Sarcocystis neurona in the brain of a Grant’s zebra (Equus burchelli bohmi) J Zoo Wildlife Med. 2000;31:82–86. doi: 10.1638/1042-7260(2000)031[0082:DOSNIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Mullins AL, Lakritz J. In vitro quantitative analysis of 3H-uracil incorporation by Sarcocystis neurona to determine efficacy of anti-protozoal agents. Vet Parasitol. 2001a;95:241–249. doi: 10.1016/s0304-4017(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Johnson PJ, Ramos-Vara J, Johnson GC. Characterization of a Sarcocystis neurona isolate from a Missouri horse with equine protozoal myeloencephalitis. Vet Parasitol. 2001b;95:143–154. doi: 10.1016/s0304-4017(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Hyun C, Barr BC, Tindall R, Lakritz J. Characterization of monoclonal antibodies developed against Sarcocystis neurona. Parasitol Res. 2002;88:501–506. doi: 10.1007/s00436-002-0602-y. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Lakritz J, Johnson PJ, Miller MA, Chiang YW, Chu HJ. Evaluation of immune responses in horses immunized using a killed Sarcocystis neurona vaccine. Veterinary Therapeutics. 2004;5:34–42. [PubMed] [Google Scholar]
- Miller MA, Crosbie PR, Sverlow K, Hanni K, Barr BC, Kock N, Murray MJ, Lowenstine LJ, Conrad PA. Isolation and characterization of Sarcocystis from brain tissue of a free-living southern sea otter (Enhydra lutris nereis) with fatal meningoencephalitis. Parasitol Res. 2001a;87:252–257. doi: 10.1007/s004360000340. [DOI] [PubMed] [Google Scholar]
- Miller MA, Sverlow K, Crosbie PR, Barr BC, Lowenstine LJ, Gulland FM, Packham A, Conrad PA. Isolation and characterization of two parasitic protozoa from a pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J Parasitol. 2001b;87:816–822. doi: 10.1645/0022-3395(2001)087[0816:IACOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller MA, Barr BC, Nordhausen R, James ER, Magargal SL, Murray M, Conrad PA, Toy-Choutka S, Jessup DA, Grigg ME. Ultrastructural and molecular confirmation of the development of Sarcocystis neurona tissue cysts in the central nervous system of southern sea otters (Enhydra lutris nereis) Int J Parasitol. 2009;39:1363–1372. doi: 10.1016/j.ijpara.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Conrad PA, Harris M, Hatfield B, Langlois G, Jessup DA, Magargal SL, Packham AE, Toy-Choutka S, Melli AC, Murray MA, Gulland FM, Grigg ME. A protozoal-associated epizootic impacting marine wildlife: Mass-mortality of southern sea otters (Enhydra lutris nereis) due to Sarcocystis neurona infection. Vet Parasitol. 2010;172:183–194. doi: 10.1016/j.vetpar.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SM, Richardson DJ, Cheadle MA, Zajac AM, Lindsay DS. Prevalence of agglutinating antibodies to Sarcocystis neurona in skunks (Mephitis mephitis ), raccoons (Procyon lotor). and opossums (Didelphis virginiana) from Connecticut. J Parasitol. 2002;88:1027–1029. doi: 10.1645/0022-3395(2002)088[1027:POAATS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mitchell SM, Zajac AM, Davis WL, Kennedy TJ, Lindsay DS. The effects of ponazuril on development of apicomplexans in vitro. J Eukaryot Microbiol. 2005;52:231–235. doi: 10.1111/j.1550-7408.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Moré G, Vissani A, Pardini L, Monina M, Muriel M, Howe D, Barrandeguy M, Venturini MC. Seroprevalence of Sarcocystis neurona and its association with neurologic disorders in Argentinean horses. J Equine Vet Sci. 2014;34:1051–1054. [Google Scholar]
- Morley PS, Traub-Dargatz JL, Benedict KM, Saville WJA, Voelker LD, Wagner BA. Risk factors for owner-reported occurrence of equine protozoal myeloencephalitis in the US equine population. J Vet Intern Med. 2008;22:616–629. doi: 10.1111/j.1939-1676.2008.0082.x. [DOI] [PubMed] [Google Scholar]
- Morley S, Traub-Dargatz J, Saville W, Wagner B, Garber L, Hillberg-Seitzinger A. Equine protozoal myeloencephalitis. J Equine Vet Sci. 2001;21:262–270. [Google Scholar]
- Mullaney T, Murphy AJ, Kiupel M, Bell JA, Rossano MG, Mansfield LS. Evidence to support horses as natural intermediate hosts for Sarcocystis neurona. Vet Parasitol. 2005;133:27–36. doi: 10.1016/j.vetpar.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Mansfield LS. Simplified technique for isolation, excystation, and culture of Sarcocystis species from opossums. J Parasitol. 1999;85:979–981. [PubMed] [Google Scholar]
- Murphy JE, Marsh AE, Reed SM, Meadows C, Bolten K, Saville WJ. Development and evaluation of a Sarcocystis neurona-specific IgM capture enzyme-linked immunosorbent assay. J Vet Intern Med. 2006;20:322–328. doi: 10.1892/0891-6640(2006)20[322:daeoas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mylniczenko ND, Kearns KS, Melli AC. Diagnosis and treatment of Sarcocystis neurona in a captive harbor seal (Phoca vitulina) J Zoo Wildlife Med. 2008;39:228–235. doi: 10.1638/2007-0141R.1. [DOI] [PubMed] [Google Scholar]
- Nakaar V, Samuel BU, Ngo EO, Joiner KA. Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J Biol Chem. 1999;274:5083–5087. doi: 10.1074/jbc.274.8.5083. [DOI] [PubMed] [Google Scholar]
- Njoku CJ, Saville WJA, Reed SM, Oglesbee MJ, Rajala-Schultz PJ, Stich RW. Reduced levels of nitric oxide metabolites in cerebrospinal fluid are associated with equine protozoal myeloencephalitis. Clin Diagn Lab Immunol. 2002;9:605–610. doi: 10.1128/CDLI.9.3.605-610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias P, Gruber AD, Kohls A, Hafez HM, Heydorn AO, Mehlhorn H, Lierz M. Sarcocystis species lethal for domestic pigeons. Emerg Infect Dis. 2010;16:497–499. doi: 10.3201/eid1603.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EJ, Wünschmann A, Dubey JP. Sarcocystis sp.-associated meningoencephalitis in a bald eagle (Haliaeetus leucocephalus) J Vet Diagn Invest. 2007;19:564–568. doi: 10.1177/104063870701900519. [DOI] [PubMed] [Google Scholar]
- Pitel PH, Pronost S, Gargala G, Anrioud D, Toquet MP, Foucher N, Collobert-Laugier C, Fortier G, Ballet JJ. Detection of Sarcocystis neurona antibodies in French horses with neurological signs. Int J Parasitol. 2002;32:481–485. doi: 10.1016/s0020-7519(01)00370-8. [DOI] [PubMed] [Google Scholar]
- Pitel PH, Lindsay DS, Caure S, Romand S, Pronost S, Gargala G, Mitchell SM, Hary C, Thulliez P, Fortier G, Ballet JJ. Reactivity against Sarcocystis neurona and Neospora by serum antibodies in healthy French horses from two farms with previous equine protozoal myeloencephalitis-like cases. Vet Parasitol. 2003;111:1–7. doi: 10.1016/s0304-4017(02)00346-1. [DOI] [PubMed] [Google Scholar]
- Pivoto FL, de Macêdo AG, Junior, da Silva MV, Ferreira FB, Silva DAO, Pompermayer E, Sangioni LA, Mineo TWP, Vogel FSF. Serological status of mares in parturition and the levels of antibodies (IgG) against protozoan family Sarcocystidae from their pre colostral foals. Vet Parasitol. 2014;199:107–111. doi: 10.1016/j.vetpar.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Pusterla N, Wilson WD, Conrad PA, Barr BC, Ferraro GL, Daft BM, Leutenegger CM. Cytokine gene signatures in neural tissue of horses with equine protozoal myeloencephalitis or equine herpes type 1 myeloencephalopathy. Vet Rec. 2006;159:341–346. doi: 10.1136/vr.159.11.341. [DOI] [PubMed] [Google Scholar]
- Pusterla N, Packham A, Wilson WD, White A, Bellamy P, Renier AC, Conrad PA. Short communication: evaluation of the kinetics of antibodies against Sarcocystis neurona in serum from seropositive healthy horses without neurological deficits treated with ponazuril paste. Vet Rec. 2013;173:249. doi: 10.1136/vr.101714. [DOI] [PubMed] [Google Scholar]
- Pusterla N, Tamez-Trevino E, White A, VanGeem J, Packham A, Conrad PA, Kass P. Comparison of prevalence factors in horses with and without seropositivity to Neospora hughesi and/or Sarcocystis neurona. Vet J. 2014;200:332–334. doi: 10.1016/j.tvjl.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Pusterla N, Packham A, Mackie S, Kass PH, Hunyadi L, Conrad PA. Use of daily diclazuaril pelleted for dress for the prevention of Sarcocystis neurona infection in foals. J Vet Intern Med 2015 [Google Scholar]
- Rachinel N, Buzoni-Gatel D, Dutta C, Mennechet FJD, Luangsay S, Minns LA, Grigg ME, Tomavo S, Boothroyd JC, Kasper LH. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J Immunol. 2004;173:2725–2735. doi: 10.4049/jimmunol.173.4.2725. [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Dubey JP, Watson GL, Winn-Elliot M, Patterson JS, Yamini B. Sarcocystosis in mink (Mustela vison) J Parasitol. 1997;83:1198–1201. [PubMed] [Google Scholar]
- Reid AJ, Vermont SJ, Cotton JA, Harris D, Hill-Cawthorne GA, Könen-Waisman S, Latham SM, Mourier T, Norton R, Quail MA, Sanders M, Shanmugam D, Sohal A, Wasmuth JD, Brunk B, Grigg ME, Howard JC, Parkinson J, Roos DS, Trees AJ, Berriman M, Pain A, Wastling JM. Comparative genomics of the Apicomplexan parasites Toxoplasma gondii and Neospora caninum: coccida differing in host range and transmission strategy. PLoS Pathog. 2012;8:e1002567. doi: 10.1371/journal.ppat.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SM, Howe DK, Morrow JK, Graves A, Yeargan MR, Johnson AL, MacKay RJ, Furr M, Saville WJ, Williams NM. Accurate antemortem diagnosis of equine protozoal myeloencephalitis (EPM) based on detecting intrathecal antibodies against Sarcocystis neurona using the SnSAG2 and SnSAG4/3 ELISAs. J Vet Intern Med. 2013;27:1193–1200. doi: 10.1111/jvim.12158. [DOI] [PubMed] [Google Scholar]
- Rejmanek D, VanWormer E, Miller MA, Mazet JAK, Nichelason AE, Melli AC, Packham AE, Jessup DA, Conrad PA. Prevalence and risk factors associated with Sarcocystis neurona infections in opossums (Dedelphis virginiana) from central California. Vet Parasitol. 2009;166:8–14. doi: 10.1016/j.vetpar.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rejmanek D, Miller MA, Grigg ME, Crosbie PR, Conrad PA. Molecular characaterization of Sarcocystis neurona strains from opossums (Didelphis virginiana) and intermediate hosts from Central California. Vet Parasitol. 2010;170:20–29. doi: 10.1016/j.vetpar.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard LG, Black SS, Rashmir-Raven A, Hurst G, Dubey JP. Risk factors associated with the presence of Sarcocystis neurona sporocysts in opossums (Didelphis virginiana) Vet Parasitol. 2001;102:179–184. doi: 10.1016/s0304-4017(01)00549-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal BM, Lindsay DS, Dubey JP. Relationships among Sarcocystis species transmitted by New World opossums (Didelphis spp. ) Vet Parasitol. 2001;95:133–142. doi: 10.1016/s0304-4017(00)00385-x. [DOI] [PubMed] [Google Scholar]
- Rosonke BJ, Brown SR, Tornquist SJ, Snyder SP, Garner MM, Blythe LL. Encephalomyelitis associated with a Sarcocystis neurona-like organism in a sea otter. J Am Vet Med Assoc. 1999;215:1839–1842. [PubMed] [Google Scholar]
- Rossano MG, Mansfield LS, Kaneene JB, Murphy AJ, Brown CM, Schott HC, Fox JC. Imporvement of western blot test specificity for detecting equine serum antibodies to Sarcocystis neurona. J Vet Diagn Invest. 2000;12:28–32. doi: 10.1177/104063870001200105. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Kaneene JB, Marteniuk JV, Banks BD, Schott HC, Mansfield LS. The seroprevalence of antibodies to Sarcocystis neurona in Michigan equids. Prev Vet Med. 2001;48:113–128. doi: 10.1016/s0167-5877(00)00190-2. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Murphy AJ, Vrable RA, Vanzo NE, Lewis SK, Sheline KD, Kaneene JB, Mansfield LS. Cross-sectional study of serum antibodies against Sarcocystis neurona in cats tested for antibodies against Toxoplasma gondii. Journal of American Veterinary Medical Association. 2002;221:511–514. doi: 10.2460/javma.2002.221.511. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Kaneene JB, Schott HC, Sheline KD, Mansfield LS. Assessing the agreement of Western blot test results for paired serum and cerebrospinal fluid samples from horses tested for antibodies to Sarcocystis neurona. Vet Parasitol. 2003a;115:233–238. doi: 10.1016/s0304-4017(03)00224-3. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Kaneene JB, Marteniuk JV, Banks BD, Schott HC, Mansfield LS. A herd-level analysis of risk factors for antibodies to Sarcocystis neurona in Michigan equids. Prev Vet Med. 2003b;57:7–13. doi: 10.1016/s0167-5877(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Schott HC, Kaneene JB, Murphy AJ, Kruttlin EA, Hines MT, Sellon DC, Patterson JS, Elsheikha HM, Dubey JP, Mansfield LS. Effect of daily administration of pyrantel tartrate in preventing infection in horses experimentally challenged with Sarcocystis neurona. Am J Vet Res. 2005a;66:846–852. doi: 10.2460/ajvr.2005.66.846. [DOI] [PubMed] [Google Scholar]
- Rossano MG, Schott HC, Murphy AJ, Kaneene JB, Sellon DC, Hines MT, Hochstatter T, Bell JA, Mansfield LS. Parasitemia in an immunocompetent horse experimentally challenged with Sarcocystis neurona sporocysts. Vet Parasitol. 2005b;127:3–8. doi: 10.1016/j.vetpar.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Rosypal AC, Lindsay DS, Duncan R, Ahmed SA, Zajac AM, Dubey JP. Mice lacking the gene for inducible or endothelial nitric oxide are resistant to sporocyst induced Sarcocystis neurona infections. Vet Parasitol. 2002;103:315–321. doi: 10.1016/s0304-4017(01)00555-6. [DOI] [PubMed] [Google Scholar]
- Saville WJ, Reed SM, Granstrom DE, Hinchcliff KW, Kohn CW, Wittum TE, Stamper S. Seroprevalence of antibodies to Sarcocystis neurona in horses residing in Ohio. J Am Vet Med Assoc. 1997;210:519–524. [PubMed] [Google Scholar]
- Saville WJ, Morley PS, Reed SM, Granstrom DE, Kohn CW, Hinchcliff KW, Wittum TE. Evaluation of risk factors associated with clinical improvement and survival of horses with equine protozoal myeloencephalitis. J Am Vet Med Assoc. 2000;217:1181–1185. doi: 10.2460/javma.2000.217.1181. [DOI] [PubMed] [Google Scholar]
- Saville WJA, Stich RW, Reed SM, Njoku CJ, Oglesbee MJ, Wunschmann A, Grover DL, Larew-Naugle AL, Stanek JF, Granstrom DE, Dubey JP. Utilization of stress in the development of an equine model for equine protozoal myeloencephalitis. Vet Parasitol. 2001;95:211–222. doi: 10.1016/s0304-4017(00)00421-0. [DOI] [PubMed] [Google Scholar]
- Saville WJA, Sofaly CD, Reed SM, Dubey JP, Oglesbee MJ, Lacombe VA, Keene RO, Gugisberg KM, Swensen SW, Shipley RD, Chiang YW, Chu HJ, Ng T. An equine protozoal myeloencephalitis challenge model testing a second transport after inoculation with Sarcocystis neurona sporocysts. J Parasitol. 2004a;90:1406–1410. doi: 10.1645/GE-128R. [DOI] [PubMed] [Google Scholar]
- Saville WJA, Dubey JP, Oglesbee MJ, Sofaly CD, Marsh AE, Elitsur E, Vianna MC, Lindsay DS, Reed SM. Experimental infection of ponies with Sarcocystis fayeri and differentiation from Sarcocystis neurona infections in horses. J Parasitol. 2004b;90:1487–1491. doi: 10.1645/GE-313. [DOI] [PubMed] [Google Scholar]
- Scott P, Witonsky S, Robertson J, Daft B. Increased presence of T lymphocytes in central nervous system of EPM affected horses. J Parasitol. 2005;91:1499–1502. doi: 10.1645/GE-519R.1. [DOI] [PubMed] [Google Scholar]
- Sellon DC, Knowles DP, Greiner EC, Long MT, Hines MT, Hochstatter T, Hasel KM, Ueti M, Gillis K, Dame JB. Depletion of natural killer cells does not result in neurologic disease due to Sarcocystis neurona in mice with severe combined imunodeficiency. J Parasitol. 2004a;90:782–788. doi: 10.1645/GE-205R. [DOI] [PubMed] [Google Scholar]
- Sellon DC, Knowles DP, Greiner EC, Long MT, Hines MT, Hochstatter T, Tibary A, Dame JB. Infection of immunodeficient horses with Sarcocystis neurona does not result in neurologic disease. Clin Diagn Lab Immunol. 2004b;11:1134–1139. doi: 10.1128/CDLI.11.6.1134-1139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon DC, Dubey JP. Equine protozoal myeloencephalitis. In: Sellon D, Long M, editors. Equine Infectious Diseases. Chapter 59. Elsevier; 2007. pp. 453–464. [Google Scholar]
- Shapiro K, Miller M, Mazet J. Temporal assocation between land-based runoff events and California sea otter (Enhydra lutirs nereis) protozoal mortalities. J Wildl Dis. 2012;48:394–404. doi: 10.7589/0090-3558-48.2.394. [DOI] [PubMed] [Google Scholar]
- Sofaly CD, Reed SM, Gordon JC, Dubey JP, Oglesbee MJ, Njoku CJ, Grover DL, Saville WJA. Experimental induction of equine protozoan myeloencephalitis (EPM) in the horse: effect of Sarcocystis neurona sporocyst inoculation dose on the development of clinical neurologic disease. J Parasitol. 2002;88:1164–1170. doi: 10.1645/0022-3395(2002)088[1164:EIOEPM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Speer CA, Dubey JP. Ultrastructure of schizonts and merozoites of Sarcocystis neurona. Vet Parasitol. 2001;95:263–271. doi: 10.1016/s0304-4017(00)00392-7. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Ellison SE, Guarino AJ, Blagburn BL. Cell-mediated immune responses in horses with equine protozoal myeloencephalitis. J Parasitol. 2004;90:428–430. doi: 10.1645/GE-3289RN. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Deinnocentes P, Moyana EM, Guarino AJ, Ellison SE, Bird RC, Blagburn BL. Cytokine gene expression in response to SnSAG1 in horses with equine protozoal myeloencephalitis. Clin Diagn Lab Immunol. 2005;12:644–646. doi: 10.1128/CDLI.12.5.644-646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek JF, Dubey JP, Oglesbee MJ, Reed SM, Lindsay DS, Capitini LA, Njoku CJ, Vittitow KL, Saville WJA. Life cycle of Sarcocystis neurona in its natural intermediate host, the racoon, Procyon lotor. J Parasitol. 2002;88:1151–1158. doi: 10.1645/0022-3395(2002)088[1151:LCOSNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Stanek JF, Stich RW, Dubey JP, Reed SM, Njoku CJ, Lindsay DS, Schmall LM, Johnson GK, LaFave BM, Saville WJA. Epidemiology of Sarcocystis neurona infections in domestic cats (Felis domesticus) and its association with equine protozoal myeloencephalitis (EPM) case farms and feral cats from a mobile spay and neuter clinic. Vet Parasitol. 2003;117:239–249. doi: 10.1016/j.vetpar.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Stoffregen DA, Dubey JP. A Sarcocystis sp.-like protozoan and concurrent canine distemper virus infection associated with encephalitis in a raccoon (Procyon lotor) J Wildl Dis. 1991;27:688–692. doi: 10.7589/0090-3558-27.4.688. [DOI] [PubMed] [Google Scholar]
- Sundar N, Asmundsson IM, Thomas NJ, Samuel MD, Dubey JP, Rosenthal BM. Modest genetic differentiation among North American populations of Sarcocystis neurona may reflect expansion in its geographical range. Vet Parasitol. 2008;152:8–15. doi: 10.1016/j.vetpar.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Tanhauser SM, Yowell CA, Cutler TJ, Greiner EC, MacKay RJ, Dame JB. Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula. J Parasitol. 1999a;85:221–228. [PubMed] [Google Scholar]
- Tanhauser SM, Yowell CA, Cutler TJ, Greiner EC, MacKay RJ, Dame JB. Multiple DNA markers differentiate Sarcocystis neurona and S. falcatula and identify two additional Sarcocystis spp. shed by opossums. Proc. 44th Ann. Mtg. Am. Assoc. Vet. Parasitol; 1999b. p. Abstract No. 27. [Google Scholar]
- Tanhauser SM, Cheadle MA, Massey ET, Mayer BA, Schroeder DE, Dame JB, Greiner EC, MacKay RJ. The nine-banded armadillo (Dasypus novemcinctus) is naturally infected with Sarcocystis neurona. Int J Parasitol. 2001;31:325–329. doi: 10.1016/s0020-7519(01)00178-3. [DOI] [PubMed] [Google Scholar]
- Thomas NJ, Dubey JP, Lindsay DS, Cole RA, Meteyer CU. Protozoal meningoencephalitis in sea otters (Enhydra lutris): a histopathological and immunohistochemical study of naturally occurring cases. J Comp Pathol. 2007:137. doi: 10.1016/j.jcpa.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Thulin JD, Granstrom DE, Gelberg HB, Morton DG, French RA, Giles RC. Concurrent protozoal encephalitis and canine distemper virus infection in a raccoon (Procyon lotor) Vet Rec. 1992;130:162–164. doi: 10.1136/vr.130.8.162. [DOI] [PubMed] [Google Scholar]
- Tillotson K, McCue PM, Granstrom DE, Dargatz DA, Smith MO, Traub-Dargatz JL. Seroprevalence of antibodies to Sarcocystis neurona in horses residing in northern Colorado. J Equine Vet Sci. 1999;19:122–126. [Google Scholar]
- Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, Kim K, Weiss LM. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornquist SJ, Boeder LJ, Mattson DE, Cebra CK, Bildfell RJ, Hamir AN. Lymphocyte responses and immunophenotypes in horses with Sarcocystis neurona infection. Equine Vet J. 2001;33:726–729. doi: 10.2746/042516401776249255. [DOI] [PubMed] [Google Scholar]
- Trasti SL, Dubey JP, Webb DM, Blanchard TW, Britt J, Fritz D, Lewis RM. Fatal visceral and neural sarcocystosis in dogs. J Comp Pathol. 1999;121:179–184. doi: 10.1053/jcpa.1999.0307. [DOI] [PubMed] [Google Scholar]
- Turay HO, Barr BC, Caldwell A, Branson KR, Cockrell MKR, Marsh AE. Sarcocystis neurona reacting antibodies in Missoure feral domestic cats (Felis domesticus) and their role as an intermediate host. Parasitol Res. 2002a;88:38–43. doi: 10.1007/s004360100503. [DOI] [PubMed] [Google Scholar]
- Turay HO, Barr BC, Caldwell A, Branson KR, Cockrell MKR, Marsh AE. Sarcocystis neurona reacting antibodies in Missouri feral domestic cats (Felis domesticus) and their role as intermediate host. Parasitol Res. 2002b;88:38–43. doi: 10.1007/s004360100503. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Morrison DP, Gaji RY, Murray JM, Entzeroth R, Howe DK, Striepen B. Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. J Cell Sci. 2005;118:3397–3407. doi: 10.1242/jcs.02458. [DOI] [PubMed] [Google Scholar]
- Valadas S, Gennari SM, Yai LEO, Rosypal AC, Lindsay DS. Prevalence of antibodies to Trypanosoma cruzi, Leishmania infantum, Encephalitozoon cuniculi, Sarcocystis neurona, and Neospora caninum in capybara, Hydrochoerus hydrochaeris, from São Paulo State, Brazil. J Parasitol. 2010;96:521–524. doi: 10.1645/GE-2368.1. [DOI] [PubMed] [Google Scholar]
- Vardeleon D, Marsh AE, Thorne JG, Loch W, Young R, Johnson PJ. Prevalence of Neospora hughesi and Sarcocystis neurona antibodies in horses from various geographical locations. Vet Parasitol. 2001;95:273–282. doi: 10.1016/s0304-4017(00)00393-9. [DOI] [PubMed] [Google Scholar]
- Vashisht K, Lichtensteiger CA, Miller LA, Gondim LFP, McAllister MM. Naturally occurring Sarcocystis neurona-like infection in a dog with myositis. Vet Parasitol. 2005;133:19–25. doi: 10.1016/j.vetpar.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Verma SK, Calero-Bernal R, Lovallo MJ, Sweeny A, Grigg ME, Dubey JP. Bobcat (Felis rufus) as a new host for Sarcocystis neurona: Microscopic and molecular identification, and differention from Sarcocystis felis. 2015 In review. [Google Scholar]
- Wasmuth JD, Pszenny V, Haile S, Jansen EM, Gast AT, Sher A, Boyle JP, Boulanger MJ, Parkinson J, Grigg ME. Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence. mBio. 2012;3:e00321–12. doi: 10.1128/mBio.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh TH, Bryan TM, Johnson L, Brinsko SP, Rigby SL, Love CC, Varner DD, Ing NH, Forrest DW, Blanchard TL. Characaterization of sperm and androgen production by testes from control and ponazuril-treated stallions. Theriogenology. 2002;58:389–392. [Google Scholar]
- Wendte JM, Miller MA, Lambourn DM, Magargal SL, Jessup DA, Grigg ME. Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii. PLoS Genet. 2010a;6:e1001261. doi: 10.1371/journal.pgen.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendte JM, Miller MA, Nandra AK, Peat SM, Crosbie PR, Conrad PA, Grigg ME. Limited genetic diversity among Sarcocystis neurona strains infecting southern sea otters precludes distinction between marine and terrestrial isolates. Vet Parasitol. 2010b;169:37–44. doi: 10.1016/j.vetpar.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AP, Thelen JJ, Lakritz J, Brown CR, Marsh AE. The identification of a sequence related to apicomplexan enolase from Sarcocystis neurona. Parasitol Res. 2004;94:354–360. doi: 10.1007/s00436-004-1224-3. [DOI] [PubMed] [Google Scholar]
- Witonsky S, Morrow JK, Leger C, Dascanio J, Buechner-Maxwell V, Palmer W, Kline K, Cook A. Sarcocystis neurona-specific immunoglobulin G in the serum and cerebrospinal fluid of horses administered S. neurona vaccine. J Vet Intern Med. 2004;18:98–103. doi: 10.1892/0891-6640(2004)18<98:snigit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Lindsay DS. Immunopathologic effects associated with Sarcocystis neurona-infected interferon-gamma knockout mice. J Parasitol. 2003a;89:932–940. doi: 10.1645/GE-72R. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Lindsay DS. Protective immune response to experimental infection with Sarcocystis neurona in C57BL/6 mice. J Parasitol. 2003b;89:924–931. doi: 10.1645/GE-67R. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Norton H, Ward D, Lindsay DS. Prevention of meningo/encephalomyelitis due to Sarcocystis neurona infection in mice is mediated by CD8 cells. Int J Parasitol. 2005a;35:113–123. doi: 10.1016/j.ijpara.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Norton H, Ward D, Yang J, Lindsay DS. Humoral immunity is not critical for protection against experimental infection with Sarcocystis neurona in B-cell-deficient mice. J Parasitol. 2005b;91:830–837. doi: 10.1645/GE-488R.1. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Ellison S, Yang J, Gogal RM, Lawler H, Suzuki Y, Sriranganathan N, Andrews F, Ward D, Lindsay DS. Horses experimentally infected with Sarcocystis neurona develop altered immune responses in vitro. J Parasitol. 2008;94:1047–1054. doi: 10.1645/GE-1441.1. [DOI] [PubMed] [Google Scholar]
- Wünschmann A, Armien AG, Reed L, Gruber AD, Olias P. Sarcocystis calchasi-associated neurologic disease in a domestic pigeon in North America. Transbound Emerg Dis. 2011;58:526–530. doi: 10.1111/j.1865-1682.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Jordan CN, Mitchell SM, Norton TM, Lindsay DS. Seroprevalence of Toxoplasma gondii, Sarcocystis neurona, and Encephalitozoon cuniculi in three species of lemurs from St. Catherines Island, GA, USA. Vet Parasitol. 2007;144:28–32. doi: 10.1016/j.vetpar.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Yang J, Ellison S, Gogal R, Norton H, Lindsay DS, Andrews F, Ward D, Witonsky S. Immune response to Sarcocystis neurona infection in naturally infected horses with equine protozoal myeloencephalitis. Vet Parasitol. 2006;138:200–210. doi: 10.1016/j.vetpar.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Yeargan MR, Howe DK. Improved detection of equine antibodies against sarcocystis neurona using polyvalent ELISAs based on the parasite SnSAG surface antigens. Vet Parasitol. 2011;176:16–22. doi: 10.1016/j.vetpar.2010.10.034. [DOI] [PubMed] [Google Scholar]
- Yeargan MR, Alvarado-Esquivel C, Dubey JP, Howe DK. Prevalence of antibodies to Sarcocystis neurona and Neospora hughesi in horses from Mexico. Parasite. 2013;20:29. doi: 10.1051/parasite/2013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Gaji RY, Howe DK. Identification of a dithiol-dependent nucleoside triphosphate hydrolase in Sarcocystis neurona. Int J Parasitol. 2006;36:1197–1204. doi: 10.1016/j.ijpara.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Zhang D, Howe DK. Investigation of SnSPR1, a novel and abundant surface protein of Sarcocystis neurona merozoites. Vet Parasitol. 2008;152:210–219. doi: 10.1016/j.vetpar.2007.12.036. [DOI] [PubMed] [Google Scholar]