Abstract
After the initial mechanical insult of spinal cord injury (SCI), secondary mediators propagate a massive loss of oligodendrocytes. We previously showed that following SCI both the total phospholipases activity and cytosolic PLA2-IVα protein expression increased. However, the expression of secreted isoforms of PLA2 (sPLA2) and their possible roles in oligodendrocyte death following SCI remains unclear. Here we report that mRNAs extracted 15 min, 4 hr, 1 day, or 1 month after cervical SCI show marked upregulation of sPLA2-IIA and IIE at 4 hr after injury. In contrast, SCI induced down regulation of sPLA2-X, and no change in sPLA2-IB, IIC, V, and XIIA expression. At the lesion site, sPLA2-IIA and IIE expression were localized to oligodendrocytes. Recombinant human sPLA2-IIA (0.01, 0.1, or 2 μM) induced a dose-dependent cytotoxicity in differentiated adult oligodendrocyte precursor cells but not primary astrocytes or Schwann cells in vitro. Most importantly, pretreatment with S3319, a sPLA2-IIA inhibitor, before a 30 min H2O2 injury (1 or 10 mM) significantly reduced oligodendrocyte cell death at 48 hr. Similarly, pretreatment with S3319 before injury with IL-1β and TNFα prevented cell death and loss of oligodendrocyte processes at 72 hr. Collectively, these findings suggest that sPLA2-IIA and IIE are increased following SCI, that increased sPLA2-IIA can be cytotoxic to oligodendrocytes, and that in vitro blockade of sPLA2 can create sparing of oligodendrocytes in two distinct injury models. Therefore sPLA2-IIA may be an important mediator of oligodendrocyte death and a novel target for therapeutic intervention following SCI.
Keywords: Astrocytes, Axons, Neurons, Phospholipases A, IL-1β, TNF-α, H2O2, Reactive Oxygen Species
INTRODUCTION
Spinal cord injury (SCI) results from an initial mechanical damage to the cord tissue followed by a cascade of “secondary injury”, leading to widespread neuronal and glial cell death as well as demyelination (Cao et al. 2005b; McTigue et al. 2001; Totoiu and Keirstead 2005). Notably, oligodendrocytes are particularly sensitive to apoptosis during secondary injury, which results in a loss of myelin around surviving axons peripheral to the lesion epicenter (Blight 1985; Crowe et al. 1997; Totoiu and Keirstead 2005). By seven days post injury, 93% of the oligodendrocytes at the impact site are lost (McTigue et al., 2001). Therefore, therapeutic manipulation of oligodendrocyte survival after neurotrauma represents a viable approach to restore functional conduction of intact but demyelinated axons.
To date, many mediators of secondary injury have been suggested such as free radicals (Liu et al. 2004a; Park et al. 2004) and cytokines including TNFα an IL-1β (Demjen et al. 2004; Hostettler and Carlson 2002; Wang et al. 2006). Previously we proposed that phospholipases A2 (PLA2) might function as both a secondary mediator of SCI as well as a convergence molecule that mediates the cytotoxicity of other injurious agents (Liu et al. 2006). Since the CNS is predominantly composed of lipids and 44% exclusively phospholipids, it could be particularly susceptible to phospholipases A2 (Morell 1984). PLA2 are a group of enzymes that hydrolyze the ester bond at the sn-2 position of membrane phospholipids producing a free fatty acid, such as arachidonic acid (AA), and a lyso-phospholipid, such as lysolechithin (a.k.a lysophosphatidyl choline, L-PC).
Our previous work demonstrated that both total PLA2 activity and cPLA2α (PLA2-IVα) protein expression increased following SCI (Liu et al. 2006). However, total PLA2 activity peaked at 4 hr while cPLA2α (PLA2-IVα) protein did not significantly increase until 7 days post injury. This paradox suggests that another isoform of PLA2 might be responsible for the increase in total phospholipases activity after SCI. The PLA2 isoforms are divided into either secreted (sPLA2), Ca2+-dependent cytosolic (cPLA2; group IV), or Ca2+-independent cytosolic (iPLA2; group VI) (Six and Dennis 2000). To date, eleven mammalian sPLA2s, i.e., groups IB, IIA, IIC, IID, IIE, IIF, III, V, X, XII, and XIII, have been identified. Normally, many of the sPLA2s are present in the mammalian brain (Kolko et al. 2006; Molloy et al. 1998) and spinal cord at low levels (Svensson et al. 2005). More clinically relevant, sPLA2 has recently immerged as a mediating factor in cerebral ischemia and neuronal apoptosis (Adibhatla and Hatcher 2007; Estevez and Phillis 1997; Lin et al. 2004; Yagami et al. 2002). However, any role that sPLA2 might play in oligodendrocyte death following neurotrauma is unknown.
One rational for investigating sPLA2’s role in SCI-induced oligodendrocyte death is that many mediators of secondary SCI are both activators of sPLA2 and cytotoxic to oligodendrocytes. For example, hydrogen peroxide injury triggers phospholipid metabolism and AA release in various cell types (Cane et al. 1998; Meyer et al. 1996; Tournier et al. 1997) and H2O2 induced AA release is mediated, at least in part, by sPLA2-IIA (Han et al. 2003). Likewise, IL-1β and TNFα trigger AA release from cultured cells via a sPLA2-IIA and cPLA2-IVα dependent mechanism (Kuwata et al. 2005; Mounier et al. 2004). Finally H2O2 (Mronga et al. 2004; Richter-Landsberg and Vollgraf 1998), IL-1β (Takahashi et al. 2003), TNFα (Lee et al. 2000; Selmaj and Raine 1988), and AA (Wang et al. 2004) have all been shown to damage cultured oligodendrocytes.
Until now, the expression of sPLA2 isoforms after SCI and their possible role in oligodendrocyte death has not been directly studied. Here we provide cellular and molecular evidence that sPLA2-IIA and IIE are the two major sPLA2 isoforms that are induced in the hours following SCI and that both isoforms are present within oligodendrocytes. Moreover, exogenous administration of sPLA2-IIA in vitro can induce oligodendrocyte cell death but has no affect on astrocytes or Schwann cells. Finally, blockade of sPLA2 can partially ameliorate cultured oligodendrocyte cell death induced by either H2O2 or IL-1β and TNFα injury. Thus, sPLA2-IIA may serve as a mediator of oligodendrocyte death and a target for therapeutic intervention against injury-induced oligodendrocyte cell death following SCI.
MATERIALS AND METHODS
Animals
A total of 82 female Sprague–Dawley rats (Harlan, Indianapolis, IN), 200 to 220 g, were used (Table 1). All surgeries and animal care were performed in accordance with the Guide for the Care and Use of Laboratory Animals and the Guidelines of the University of Louisville Institutional Animal Care and Use Committee.
Table 1.
Animal Usage and Experimental Groups
| Sham | 15 min | 1 hr | 4 hr | 1 day | 1 week | |
|---|---|---|---|---|---|---|
| Contusion | − | + | + | + | + | + |
|
| ||||||
| mRNA | n=3 | n=3 | n=3 | n=3 | n=3 | n=3 |
| Protein | n=4 | n=4 | - | n=4 | n=4 | n=4 |
| Subcellular Protein | n=4 | - | - | n=4 | - | - |
| Histology | n=5 | n=5 | - | n=5 | n=5 | n=5 |
| Cell Culture | n=5 | - | - | - | - | - |
Spinal Cord Injury
Rats were anesthetized with sodium pentobarbital (50 mg/kg), given preoperative gentamicin (5 mg/kg, s.c.), 5 ml of normal saline, and placed on a homeothermic blanket. Skin was incised and the underlying muscles were dissected to expose the C2-C7 vertebrae. The exposed vertebral column was stabilized using bilateral transverse supports placed underneath the spinal column which were developed at the University of Louisville (Cao et al. 2005b; Liu et al. 2007; Zhang et al. 2004). A dorsal laminectomy was performed at the C4-5 level to expose the spinal cord. The body was immobilized with a horizontal brace gently secured over the middle of the back to prevent it from moving upward during the SCI. Rats received either a 200 kDyn injury (measured force = 210±7 kDyn, Cv = 3.28%) inflicted via an Infinite Horizons (IH) impactor with an enlarged head (3.4 mm vs. 2.5 mm), or sham laminectomy (Onifer et al. 2007; Scheff et al. 2003). Displacements were measured to insure lesion uniformity (1086±93 μm; Cv = 8.56%). Muscle and skin incisions were closed with silk sutures and wound clips, respectively. Histological quantification of the injury is provided in Sup.1 using methods previously described (Titsworth et al. 2007).
Special post-operative care was given as previously described (Onifer et al. 2007). Briefly, injured rats were universally quadriplegic but showed no signs of respiratory distress. Each rat was returned to its cage with clean bedding. Half of the cage was placed on a water circulating heating pad for 24 hr. Water bottles with straight spouts were used and standard rat chow, rat chow softened with water, and ENSURE® were placed on the bedding when necessary. The state of hydration and gastrointestinal function were monitored daily. Bladders were manually voided twice daily until bladder function returned. Breathing was monitored every 10 min for the first hr, every hr for the first 12 hrs, and twice daily thereafter. Antibiotics were injected at 2 day intervals for 6 days. The wound clips were removed 7–10 days post surgery.
RNA Extraction and RT-PCR
A 1.5 cm long spinal cord segment containing the injury epicenter or equivalent (shams) was removed 4 hr post injury, frozen in liquid nitrogen, and later homogenized in STAT-60 solution (TelTest, Friendswood, TX) according to the manufacturer’s instructions. Purify RNA was quantified by spectrophotometric analysis at 260 nm. Primers used for end point RT-PCR are listed in Table 2.
Table 2.
End point RT-PCR primers.
| Gene | Accession No. | Primer sequence 5′-3′ | Product (bp) | Reference |
|---|---|---|---|---|
| PLA2-IB | NM_031585 | 232–251: ACA ATC AGG CCA AGA AGC TG 462–481: ACG GCA TAG ACA GGA AGT GG |
250 | (Kolko, 2004) |
| PLA2-IIA | NM_031598 | 687-707: TTGCCATTGTGGTGTGGGTGG 965-986: CAACTGGGCGTCTTCCCTTTGC |
300 | (Molloy, 1998) |
| PLA2-IIC | NM_019202 | 1-20: CCTCCACCTCTCAAATGCTG 231-250: CATTGCTGTTCCAGCCTTTT |
250 | (Molloy, 1998) |
| PLA2-IID | NM_001013428 | 1–20: CTGCCTTGCTCTGTGCTGGA 234-253: CCATCGATCTTCAGGTGGGC |
254 | |
| PLA2-IIE | XM_238421 | 401-419: GTGGGAACCTGGTCCAGTT 667-687: GGCAGCTCTCTTGTCACACTC |
285 | (Kolko, 2004) |
| PLA2-IIF | XM_233589 | 1-20: ATGAAGGAGGTTGAGTTTGC 242-261: TGGAATATCACAGAGCTGGA |
262 | |
| PLA2-III | XM_223553 | 12-36: TATACTTGAGTATAAGACCTCGTGT 243-262: TCAGAAGAATTGAGCAGGAC |
251 | |
| PLA2-V | NM_017174 | 380-401: CCCTAAGGATGGCACTGATTGG 530-551: CCCTAAGGATGGCACTGATTGG |
172 | (Molloy, 1998) |
| PLA2-X | NM_017176 | 461–481: TCC CCT CGG TTT TAT GTG AG 640–660: GCT CCA CAG GCT CAT AGT CC |
200 | (Kolko, 2004) |
| PLA2-XIIa | XM_342340 | 144-163: CCAGGAACAGGACCAGACCA 373-393: CTTGGTCAGGGAAGGGATGC |
250 | |
Primers for sPLA2-IID, IIF, and XIIA were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA). Each primer set was validitated in mRNA extracted from the liver, lung, kidney, or spleen. Total RNA (0.5 μg) was used in a 20 μl mixture containing 4 μl of 5× reaction buffer, 0.2 mM dNTP, 1 μM of up and down stream primer, 1 mM MgSO4, 0.1 u/μl AMV Reverse Transcriptase, and 0.1 u/μl Tfl DNA Polymerase. The reverse transcription was conducted with a 45 min first strand cDNA synthesis (45 °C), 2 min denaturation (94 °C), followed by 35 cycles of synthesis and amplification consisting of 30 seconds (94 °C), 1 minute (60 °C), and 2 minutes (72 °C) (Access RT-PCR system, Promega, Madison, WI). Amplified samples were separated on a 1% agarose gel containing ethidium bromide in 1× TBE buffer. After electrophoresis, gels were imaged using an Image Station 4000R (Kodak, Rochester, NY).
Real-Time Quantitative PCR
Total RNA was extracted 4 hr after sham operation or 15 min, 1 hr, 4 hr, 1 day, or 1 week after SCI. Primers and a taqman probe for sPLA2-IIA were designed using Primer Express 2.0 and are as follows: IIA sense 5′-CCAAATCTCCTGCTCTACAAACC-3′, IIA antisense 5′-CTTTTCTTGTTCCGGGCAAAAC-3′, and IIA probe 5′-CGGCAGCTTTATCGCACTGGCACA-3′. MX3000P (Stratagene, La Jolla, CA) calculated the threshold cycle number (Ct) as ten-fold the standard deviation of the baseline. Primer pairs were chosen to minimize primer dimerization and secondary structure; and to generate an amplicon of 96 bp. To correct for volume differences and cap transparency the passive reference dye 5(6)-carboxy-X-rhodamine-C5-maleimide (Stratagene) was used. After a 3 min denaturation step, the samples were subjected to 40 cycles of 30 sec annealing and 30 sec extension at 72 °C. Relative expression of the PCR products was determined by using the ΔΔCt method (Gibson et al. 1996). Each sample was run in duplicate, and the mean Ct was used in the ΔΔCt equation. After PCR, reaction products were electrophoresed as before to ensure that the PCR product was the desired amplicon size.
Western Blotting
Western blotting followed a previously described procedure (Liu et al. 2007). In brief, whole cell lysis proteins were extracted from a 1.5 cm long spinal cord segment containing the injury epicenter 4 hr after sham laminectomy or 15 min, 4 hr, 1 day or 1 week after SCI. Additionally, subcellular protein isolation was performed on either sham animals or 4 hr after injury using the Focus SubCell kit (G-Biosciences, St. Louis, MO) according to manufacturer’s protocol. In brief, 100 mg of fresh tissue was homogenized and centrifuge at 700 × g for 5 minutes to pelletise nuclei. Supernatant was removed and centrifuged at 12,000 × g for 10 min to pelletise mitochondria. Supernatant was again removed and centrifuged at 14,000 × g for 60min to separate the enriched cytosolic membrane fraction from the soluble cytosol fraction. The protein content of all samples was assessed by the Bradford method (Bio-Rad Protein Assay, Hercules, CA) and normalized prior to adding sample buffer.
Similar amounts of protein (40 μg) was electrophoresed on a 12% SDS-polyacrylamide gel and immunoblotted with primary rabbit monoclonal anti-sPLA2-I antibody (1:100; “Anti-PLA2, low molecular weight” Millipore, Billerica, MA), polyclonal anti–sPLA2-IIA antibody (1:1000; Cayman Chemical, Ann Arbor, MI), polyclonal anti-sPLA2-IIE antibody (1:1000; Biovendor, Candler, NC), or goat polyclonal anti-sPLA2-X antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and a secondary horseradish peroxidase–conjugated donkey anti–rabbit or anti-goat IgG antibody (1:10,000; Amersham Pharmacia Biotech, Piscataway, NJ). Whole cell lysis membranes were stripped and reblotted with anti-β-tubulin (mouse monoclonal antibody, 1:1000, Sigma, St. Louis, MO). Subcellular fraction membranes were stripped and reblotted with anti-β-tubulin, caveolin (rabbit polyclonal antibody 1:100, Santa Cruz), or histone H1 (rabbit polyclonal antibody, 1:200, Santa Cruz) for the cytosol, membrane, and nuclear fractions respectively. The primary antibody was omitted for negative controls. Splenic protein served as positive control for sPLA2-IIA. Densitometry allowed for relative comparison of signal strength by Image J software.
Immunohistochemistry
Spinal cords were removed 4 hrs after sham operation or 15 min, 1 hr, or 4 hr after SCI. After perfusion with PBS and 4% paraformaldehyde, a 2 cm-long spinal cord segment containing the injury epicenter of each rat was removed, cryoprotected in 30% sucrose buffer, sectioned transversely at 40 μm, and mounted on charged slides in eight identical sets. Three sections were taken from each of five sample sites within the tissue, every 0.5cm, and were incubated with either anti-sPLA2-IB (1:200), anti-sPLA2-IIA (1:300), anti-sPLA2-IIE (1:200), or anti-sPLA2-X antibody (1:200) overnight at 4°C and subsequently with secondary biotinylated IgG antibody (1:400; Vector Laboratories, Burlingame, CA) for 1 hr at room temperature. The reaction product was shown by incubation with 0.02% diaminobenzidine tetrahydrochloride (DAB) and 0.003% H2O2 in 0.05M Tris-HCl. Negative controls of pooled normal antibodies (Vector Laboratories) were used simultaneously.
Using standard light and aperture settings, images were captured at 2 × and staining intensities were determined using Image J software (NIH) by first inverting the image and then measuring the mean intensity. The intensity of the negative control animal was subtracted from the recorded intensities to account for unintentional secondary antibody binding and natural tissue coloration.
Immunofluorescence Labeling
Immunofluorescence double labeling at the injury epicenter was performed on different tissue sets using previously described methods (Liu et al. 2004b). A mixture of anti-sPLA2-IB (1:100), anti-sPLA2-IIA (1:100), anti-sPLA2-IIE (1:50), or anti-sPLA2-X antibody (1:50), and mouse anti-CC1 (1;100; Chemicon), anti–glial fibrillary acidic protein (1:300; Sigma), and anti-O4 (1:1; hybridoma), anti-NeuN (1:100; Chemicon, Temecula, CA), and anti-SMI-31 (1:2,000; Sigma) antibodies were used to examine neurons, axons, oligodendrocytes, or astrocytes in vivo or mature oligodendrocytes in vitro respectively. The following day, sections were incubated with fluorescein-conjugated goat anti–mouse (1:100) and Texas red-conjugated goat anti–rabbit antibodies (1:100; Jackson Immunoresearch; West Grove, PA). Controls were similar to immunohistochemistry. Images were taken using a Nikon Eclipse 90i confocal microscopy (Nikon Instruments; Melville, NY).
sPLA2-IIA signal within oligodendrocytes was quantified using StereoInvestigator software (Microbrightfield,Williston, VT) under 100× oil immersion. Under standard exposure times contour tracing was begun around the cells of interest while viewed through an FITC filter. After switching to Texas Red filter, luminescence data was acquired for the cell of interest by closing the contour. Twenty oligodendrocytes were chosen from each of three different tissue sections, by systematic random sampling from the ventral funiculus of the lesion epicenter. The intensities of these cells were averaged to create mean florescence for oligodendrocytes in each animal (n=5, per group). Background intensity was gathered in a similar manner from primary antibody omission control section and subtracted from the mean intensity for that given animal. This was done to control for non-specific binding of secondary antibody following contusion.
Cell Culture
Adult oligodendrocyte precursor cells (aOPCs) were obtained from adult rat spinal cords using protocols modified from (Cao et al. 2002). Minced spinal cords were incubated in HBSS containing 0.1% papain, 0.1% neutral protease, and 0.01% DNase. Following the addition of DMEM containing 10% fetal bovine serum (FBS) tissues were dissociated and incubated on an anti-RAN-2 antibody (ATCC, Rockville, MA) coated dish to deplete type-1 astrocytes and meningeal cells and then an anti-O4 antibody-coated dish to select for adult OPCs. The purified aOPCs were cultured in DMEM/F12 medium containing N2 and B27 supplements, FGF2 (20 ng/ml), PDGF-aa (10 ng/ml), Insulin (5 μg/ml) and BSA (0.1%). Only those cell preparations in which >95% A2B5+ cells were used.
The cells were seeded onto either PDL/laminin coated culture dishes or chamber slides. Two days after seeding or once cells reached 70-80% confluence, the FGF-2 and PDGF-aa were removed, and CNTF (0.001 μg/ml) was added to the OPC medium to induce differentiation. aOPCs were induced to differentiate in vitro for 4 days prior to use.
The procedures for Schwann cell preparation and purification have been extensively used in our lab (Xu et al. 1995). Schwann cells were purified from sciatic nerve explants with 10% FBS in DMEM. Explants were passaged 5 times and treated with 1.25U/ml dispase (Boehringer Mannheim Biochemicals, Indapapolis, IN), 0.05% collagenase (Worthington Biochemical Corp., Freehold, NJ), and 15% FBS in DMEM. Explants were dissociated, plated onto poly-L-lysine coated dishes, and treated with 20 μg/ml pituitary extract (BTI, Stoughton, MA) and 2 mM forskolin (Sigma). When SC’s reached confluence they were treated with Ca2+ and Mg2+ free HBSS and briefly treated with 0.05% trypsin (Gibco) and 0.02% EDTA (Gibso) for passage and were plated in a 96-well plate at a density of 4×104 cell per well. The purity of the SCs was ascertained by s-100 staining.
Astrocytes were isolated from the cerebral cortices of postnatal 2 to 3 day old SD rat pups. Minced cortices were incubated in DMEM with 0.25% (w/v) trypsin-EDTA at 37 °C for 7 min. The suspension was filtered and centrifuged at 1000 rpm for 5 min. The cell pellet was re-suspended in DMEM plus 10% (v/v) FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and transferred to T75 culture flasks. The medium was changed twice a week until cells reached approximately 80–90% confluence, flasks were shaken at 170 rpm for 16 h at 37 °C to remove microglia. Then, cells were removed from the flasks by 0.05% (w/v) trypsin-EDTA treatment, and seeded at 1×104 cells per well in 96 well culture dishes. Astrocytes were identified by GFAP staining with purity > 95% in all cultures.
Schwann cell, astrocytes, and mature oligodendrocyte cultures were injured with recombinant human sPLA2-IIA (Biovendor, Candler, NC) and assayed at 48 hr. Cytotoxicity was measured by measuring lactate dehydrogenase (LDH) in the medium (CytoTox96 assay; Promega, Madison, WI). Data were normalized to the amount of LDH released from similarly-treated cells lysed with 9% Triton X-100 and are corrected for background from wells lacking cells. Cell viability was assessed by reduction of (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide by the mitochondria of surviving cells (MTT, CellTiter 96 Cell Proliferation Assay; Promega). Control wells were run in each plate to ensure that measurements fell within test sensitivity.
It has been previously shown that the commercially available, small (487.63 MW), lipophilic molecule 5-(4-Benzyloxyphenyl)-4S-(7-phenylheptanoylamino) pentanoic acid (henceforth referred to as S3319; Sigma) inhibits sPLA2-IIA in vitro using a standard enzyme assay (IC50 = 0.029 μm, 0.000019 mole fraction; compound 2b in (Hansford et al. 2003). Therefore, a second set of cultures were pretreated with vehicle, or 0.25, 1.25, 6.25 μM of S3319, a sPLA2-IIA inhibitor diluted in 1% DMSO (Sigma). Then cells were challenged with vehicle, 1mM, 5 mM, or 10mM H2O2, with or without sPLA2-IIA inhibitor for 30min, washed once with fresh medium, and the vehicle or S3319 was replaced. Again cytotoxicity was evaluated by measuring LDH released.
A third set of cultures were pretreated as above with S3319 but were challenged with the cytokines IL-1β (PeproTech Inc., Rocky Hill, NJ) and TNFα (PeproTech) at low (1 and 2 ng respectively) or high dose (5 and 10 ng). Again cytotoxicity was evaluated by LDH released. All cell culture experiments consisted of 4-6 separate wells and were repeated in triplicate on separate days. The results presented are the averaged of the three separate experiments. The area of process extensions was calculated using three separate images take from the center of each well. Images were converted to binary by setting a common constant threshold and then the area was determined by Image J software.
Statistical Analysis
One-way analysis of variance (ANOVA) with post hoc Tukey HSD was used to determine statistical significance of three or more groups. A multiple analysis of variance (MANOVA) with post hoc Tukey HSD was used to determine statistical significance of three or more groups when repeated measures were taken from each animal overtime or space. Two extreme outliers were excluded from the Q-PCR data using the Grubb’s extreme studentized deviate method.
RESULTS
sPLA2 mRNAs are differentially expressed following SCI
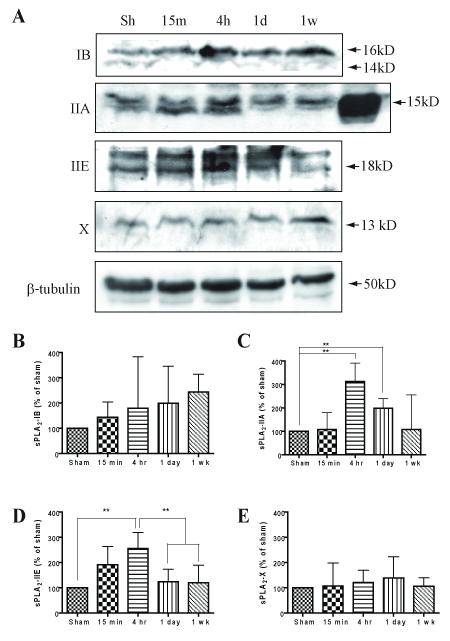
End point RT-PCR was used to scan for the expression of all the sequenced mammalian sPLA2 isoforms in sham and contused animals 4 hr following surgery (n = 3). Of the nine mammalian sPLA2 isoforms, seven were detected in naïve and contused animals (sPLA2-IB, IIA, IIC, IIE, V, X, and XIIA; Fig. 1A). Among all sPLA2 isoforms examined, sPLA2-IIA showed the most dramatic change after SCI (Fig. 1A). Similarly, sPLA2-IIE showed an increase in the injured cord. In contrast, PLA2-X showed a decrease in signal intensity (Fig. 1A). sPLA2-IB, IIC, V and XIIA were present in both the sham and contused cord in similar amounts.
Fig. 1.
Changes in secretory PLA2 mRNA following SCI. A) Representative mRNA from 3 sham (lanes 2-4) or 3 SCI rats (lanes 5-7) 4hr post surgery. Lane 1 is a 100 bp ladder. Increases were seen in sPLA2-IIA and IIE with a decrease seen in sPLA2-X. B) Relative fold increase of sPLA2-IIA mRNA expression after real time Q-PCR (mean ± SD).
Next, real-time Q-PCR was used to quantify the increase seen after RT-PCR and determine the time course of sPLA2-IIA expression following SCI. sPLA2-IIA was chosen because of its significant association with inflammation (Kolko et al. 2004) and is quantified as a fold increase from naïve animals (Fig. 1B). In agreement with the RT-PCR results, sPLA2-IIA mRNA expression had a significant 4-fold increase at 1hr following contusion and remained elevated at 4 hr (ANOVA, F6, 30 = 4.313, p = 0.0012).
sPLA2 proteins are differentially expressed following SCI
To confirm that mRNA changes corresponded to changes in protein production, spinal cord homogenates were immunoblotted with antibodies to sPLA2-IB, IIA, IIE, or X. While a general increase in the mean expression of sPLA2-IB was seen, this was not statistically significant further confirming the PCR results (Fig. 2A & B; ANOVA, F4,15 = 0.8335, p = 0.52). In contrast, sPLA2-IIA showed a 3-fold increase in protein expression at 4 hr compared to sham controls (Fig. 2A & C, ANOVA, F4,15 = 4.860, p < 0.01). Significantly increased sPLA2-IIA expression was also found at both 4 hr and 1day post injury (Fig. 2C). An equal amount of splenic protein was run as a positive control for sPLA2-IIA and showed a band about 3.6 times greater than the strongest spinal cord band, confirming the lower abundance in the spinal cord as compared to the spleen (Fig. 2A, lane 6 of IIA blot). Not surprisingly, sPLA2-IIE showed similar results to IIA with a significant 2.5 fold-increase that peaked at 4 hr after SCI and returned to the baseline by 1 day (Fig. 2A & D, ANOVA, F4,15 = 5.025, p < 0.01). Finally, while sPLA2-X mRNA decreased following SCI there was little change in the protein levels compared to the sham controls (Fig. 2A & E, ANOVA, F4,15 = 0.2554, p = 0.90). Interestingly, the peak expression of the two sPLA2 isoforms coincides well with the peak activation of total PLA2, as we demonstrated previously (Liu et al. 2006). Thus, sPLA2-IIA and IIE may represent major contributors to phospholipase activity following SCI.
Fig. 2.
sPLA2 protein expression following SCI. A) Representative western blots from sham or contused animals at 15 min, 4 hr, 1 day, or 1 wk post injury. The top panel in A shows time course of sPLA2-IB (16 & 14 kDa) showing no changes following SCI. SCI induced significant increase in sPLA2-IIA (15 kDa) and IIE (18 kDa) beginning at 4 hr and 1 day post injury, but returning to baseline by 1 week. Group X (13 kDa) showed no change in expression. Note lane 6 in IIA blot is equal amount of splenic protein for control. B - E) Quantification of western blots in A (n = 4/group, mean ± SD). (**p < 0.01 versus sham)
Spatiotemporal distribution of sPLA2 isozymes following SCI
Since sPLA2 mRNA and proteins were differentially expressed post SCI, we next determined their temporal and spatial distribution using immunohistochemistry. In this study, sPLA2-IB, IIA, IIE, and X were examined at 5 mm intervals from the injury epicenter. Initial examination of whole spinal cord sections reveals significantly more immunoreactivity of IIA (Fig. 3B & F) and IIE (Fig. 3C & G) in the contused animals with little change in either IB or X isoforms (Fig. 3A, E, D & H). Quantification of staining intensity using Image J showed no change in sPLA2-IB signaling either among different time points post SCI or distance from the epicenter (MANOVA F3, 263.806 = 4.367, p > 0.05, Fig. 3I). However, a significant increase in sPLA2-IIA immunoreactivity was observed at the injury epicenter in all SCI groups over sham controls (MANOVA F18.3, 15.575 = 46.437, p < 0.001, Fig. 3J).
Fig. 3.
Immunohistochemical changes in sPLA2 expression following SCI. There was an increase in the sPLA2-IIA and IIE immunoreactivity following SCI (F & G) compare (B & C). No change in sPLA2-IB (A & E) or group X (D & H). Bar: A-H, 500 μm. Quantification of IB (I) and IIA (J) staining at the epicenter and at 5 mm increments rostrally and caudally show increases in sPLA2-IIA only and only at the epicenter. I-J; n = 5, mean ± SD; * p < 0.05; sham,  15 min
15 min  , 1 hr
, 1 hr  , 4 hr
, 4 hr  . Changes was also seen in the ventral white matter between the sham (K - N) and 4 hr SCI (O - R). SCI induced a significant increase in sPLA2-IIA (L & P) and IIE (M & Q) within the white matter. However, there was little or no staining of sPLA2-X (N & R).
. Changes was also seen in the ventral white matter between the sham (K - N) and 4 hr SCI (O - R). SCI induced a significant increase in sPLA2-IIA (L & P) and IIE (M & Q) within the white matter. However, there was little or no staining of sPLA2-X (N & R).
We chose to focus on white matter because previous results indicate that bilateral injections of sPLA2-III (0.1 μg) into the ventral grey matter/white matter interface of rat spinal cords resulted in a massive destruction of white matter with a relative sparing of grey matter at 4 weeks (Sup. 2)(Liu et al. 2006). Within the white matter sPLA2-IB showed strong expression both before and after injury (Fig. 3K & O). In contrast, sPLA2-IIA and IIE each showed a weak baseline staining in the white matter of sham controls (Fig. 3L & M respectively). However, following SCI, sPLA2-IIA and IIE immunoreactivity increased markedly (Fig. 3P & Q). Finally, sPLA2-X showed little immunoreactivity either in sham or SCI animals (Fig. 3N & R). Based solely on morphology it appears that within the white matter, a strong increase in sPLA2-IIA and IIE staining in glial cells and axons, particularly swollen axons, was seen (Fig. 3P, arrows). Subsequently these observations were confirmed with immunofluorescent double labeling of axons (SMI-31+), astrocytes (GFAP+), and neurons (NeuN+) and are provided in the Supplemental Figures 3-5. It should be noted that IIA and IIE are structurally and functionally similar and are both located at the same genetic locus (Kudo and Murakami 2002).
Cellular localization of sPLA2 isozymes following SCI
To better characterize what cell types express sPLA2 isoforms, immunofluorescent double labeling of sPLA2–IB, IIA, IIE, or X and cell specific markers was performed. As was suggested by the immunohistochemistry, we found that oligodendrocytes (CC1/APC; Fig. 4) co-localized with sPLA2-IB (Fig. 4B & C), sPLA2-IIA (Fig. 4E & F), and sPLA2-IIE (Fig. 4H & I). However, sPLA2-X did not appear to co-localize with oligodendrocytes (Fig. 4J-L). The sPLA2-X staining appeared to reside only in the extracellular space. This morphology is not surprising since sPLA2-X has the greatest secreted fraction and the lowest cytosolic fraction of any sPLA2 (Murakami et al. 2002). Additionally, previous work with stably transfected cell lines yielded similar sPLA2-X immunofluorescence (Kudo and Murakami 2002).
Fig. 4.
Co-localization of sPLA2 in oligodendrocytes post-SCI. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C), IIA (D-F), IIE (G-I), but not X (J-L) are present within oligodendrocytes (CC1/APC) 4 hr following contusion. Orthoganol views of confocal image stacks. Scale bars: 20 μm for all.
Within the white matter, astrocytes (GFAP; Sup. 3) co-localized with sPLA2-IB (Sup. 3B & C) and weakly with sPLA2-IIA (Sup. 3E & F) but showed no co-localization with sPLA2-IIE (Sup. 3H & I) or with sPLA2-X (Sup. 3J-L). Neurons within the ventral grey matter (NeuN; Sup. 4), showed similar staining to oligodendrocytes with the presence of sPLA2-IB (Sup. 4B & C), sPLA2-IIA (Sup. 4E & F), and sPLA2-IIE (Sup. 4H & I), but not sPLA2-X (Sup. 4K & L). Almost all axons (SMI-31; Sup. 5) within the ventral white matter showed co-localization with sPLA2-IB (Sup. 5B & C) and sPLA2-IIA (Sup. 5E & F). sPLA2-IIE staining was weak and confined mainly in axons that appeared to be swollen (Sup. 5H & I, arrows) and no axons appeared to co-localize with sPLA2-X (Sup. 5K & L).
To further confirm that sPLA2-IIA was not merely present in oligodendrocytes but increases following contusion, we compared sPLA2-IIA labeling intensities within oligodendrocytes at the injury epicenter 4 hr after either sham surgery (Fig. 5A-C) or SCI (Fig. 5D-F). A significant increase in sPLA2-IIA expression was found within oligodendrocytes (t(5.947) = −3.517, p = 0.01, Fig. 5G). Increased expression of sPLA2s, particularly IIA, opens a new possibility that it may play a role in the death of oligodendrocytes following SCI.
Fig. 5.
sPLA2-IIA expression increases within oligodendrocytes following SCI and within the membrane fraction. sPLA2-IIA localizes to CC1-positive oligodendrocytes in both sham animals (A-C) and 4 hr post-SCI (D-F) with expression increasing post SCI (E versus B). G) Quantification of the sPLA2-IIA staining intensity in oligodendrocytes at the injury epicenter (n = 5 animals per group, mean ± SD; ** p<0.01). H) Western blots of subcellular fractions showing the increase of sPLA2-IIA is predominantly within the membrane fraction. I) Anti-caveolin antibody a membrane marker, anti-histone H1 a nuclear marker, and β-tubulin a cytosolic marker demonstrate the purity of the subcellular fractions.
sPLA2-IIA increases in membrane fraction following SCI
sPLA2-IIA could only increased phospholipid hydrolysis following SCI if it co-localized with its phospholipid substrate, which is most abundant in the membrane fraction of cells. Therefore subcellular fractions of sham and contused spinal cords were immunoblotted for sPLA2-IIA expression. Four hours following contusion, sPLA2-IIA increased about 4-fold within the membrane fraction of the cytosol (Fig. 5H). While the non-membrane fraction of the cytosol did show a strong band for sPLA2-IIA there was little increase following contusion. Additionally, the nuclear fraction showed no sPLA2-IIA expression. The purity of subcellular fractions was confirmed by separate blotting for caveolin, histone H1, and β-tubulin (Fig. 5I). These results suggest that the increase in sPLA2-IIA protein expression observed in the whole cell lysates after SCI is within the membrane fraction which is rich in phospholipid substrates suggesting a functional role sPLA2-IIA on its phospholipid substrates.
sPLA2-IIA induces oligodendrocyte death in vitro
We next examined what effect increased sPLA2-IIA might have on oligodendrocytes directly. These studies focused on oligodendrocytes for two reasons. First, whereas sPLA2-IIA induced neuronal apoptosis has already been shown (DeCoster 2003; Yagami et al. 2002; Yagami et al. 2003), the role of sPLA2-IIA in oligodendrocyte cell death remains unexamined. Secondly, our previous observation indicates that sPLA2 is potentially more destructive to white matter than grey matter (Sup. 2).
To determine whether sPLA2-IIA can directly affect oligodendrocyte viability, recombinant human sPLA2-IIA was added to differentiated aOPC, astrocytes, and Schwann cells. In oligodendrocytes sPLA2-IIA triggered a dose-dependent increase in LDH corresponding to an increase of cell cytotoxicity from 16.0 ± 5.3 % in control wells to 34.3 ± 6.3 % in wells treated with 2 μM of sPLA2-IIA at 48 hr (F3,16 = 13.04, p < 0.001; Fig. 6E). Similarly, the MTT assay showed a corresponding change with control wells showing an optical density (OD) of 0.040 ± 0.005 and the 2 μM sPLA2-IIA dose resulting in an OD of 0.027 ± 0.005 (F3,16 = 13.04, p < 0.001; Fig. 6F). Phase contrast images of these wells confirmed oligodendrocyte damage with a decreased arbor in the 0.01 and 0.1 μM concentrations and a complete degradation of the cell soma and processes at the 2μM concentration (Fig. 6A-D).
Fig. 6.
sPLA2-IIA selectively triggers cell death in Oligodendrocytes. Low levels of exogenously added sPLA2-IIA (0.01 and 0.1 μM; B & C) result in a loss of processes extending from the soma and at higher dose (2 μM; D) triggers a complete loss of process and cell death (phase contrast images). An 18.2 % increase in cytotoxicity as measured by an increase in LDH within the media (E), and a 33.1% decreased conversion of MTT (F) suggesting decreased cell survival. In contrast, 2 μM of sPLA2-IIA had no effect on cultured Schwann cells (G-H) or astrocytes (I-J) suggesting a specific sensitivity of oligodendrocytes to sPLA2-IIA.
To determine the relative specificity of sPLA2-IIA’s effect on oligodendrocytes, identical concentrations were added to primary astrocytes and Schwann cells cultures. Interestingly, no cell death was noted in either culture suggesting that sPLA2-IIA has little effect on astrocytes (another CNS glia) or Schwann cells (PNS glia). These findings might explain why sPLA2 injections into the spinal cord white matter result in oligodendrocyte death but showed strong gliosis and delayed remyelination of spared axons by Schwann cells (Titsworth et al. 2007). Since sPLA2-IIA administration can induce oligodendrocyte and neuron death in vitro, blocking sPLA2-IIA expression or activity may prevent such cell death following injury.
sPLA2 inhibition attenuates H2O2 induced oligodendrocyte death in vitro
To address what effects injury induced sPLA2-IIA expression might have on oligodendrocytes, an in vitro Hydrogen peroxide (H2O2) injury model was developed. When aOPC were challenged with a 30 min pulse of H2O2, there was a dose dependent increase in sPLA2-IIA expression over the vehicle control 48 hr after H2O2 insult (Fig. 7A). Immunoflorescence staining confirmed the presence of sPLA2-IIA in oligodendrocytes in naïve cultures (Fig. 7B-D) and following H2O2 treatment (Fig. 7E-J). Interestingly, sPLA2-IIA staining in naïve oligodendrocytes was homogenously distributed (Fig. 7D insert) whereas in H2O2 treated cells sPLA2 aggregated into perinuclear puncta (Fig. 7G & J inserts). It should be noted that the cells showing sPLA2-IIA aggregation also show nuclear fragmentation stained by Hoechst 33342, a nuclear dye suggesting apoptosis (Fig. 7G&J inserts, blue).
Fig. 7.
H2O2 injury induces sPLA2-IIA expression in cultured oligodendrocytes. A) Western blots show a dose dependent increase in sPLA2-IIA 48 hr following a 30 min injury with vehicle, 1mM, or 10mM of H2O2. B–J) Confocal images of oligodendrocytes 48 hr following treatment with vehicle (B-D) or 10 mM of H2O2 (E – J). Dead or dying cells showed either a fragmentation of the nuclei, loss of arborization, and micro-puncta (E – G) or a round nuclei, swelling of primary processes, and larger puncta (H – J). Bar: 50 μm.
Next, differentiated oligodendrocyte cultures were challenged with either 1 mM or 10 mM H2O2 for 30 min after the administration of various doses of S3319, a small molecule inhibitor designed to block the sPLA2-IIA enzymatic site. Forty-eight hours following H2O2 injury, wells containing the sPLA2-IIA inhibitor S3319 showed reduced cytotoxicity in both H2O2 injury intensities (MANOVA F3,48 = 26.63, p < 0.0001; Fig. 8). In the 10 mM H2O2 treated wells; cells treated with vehicle showed LDH levels suggestive of 46.3 ± 9% cell death as compared to 28.4 ± 3% cell death in the S3319 treated wells (p < 0.001). These results suggest that increased sPLA2-IIA enzymatic activity partially mediates H2O2 induced oligodendrocyte cell death.
Fig. 8.

. sPLA2-IIA enzymatic inhibition by S3319 partially reverses H2O2 induced oligodendrocyte cell death. Pretreatment with 1.25 μM S3319, a sPLA2 inhibitor, decreases cytotoxicity of a 30 min, 10 mM H2O2 pulse by by 17.9 %. (n = 7 wells per group, all in vitro studies repeated in triplicate, mean ± SD; * p<0.05, ** p < 0.01, *** p < 0.001).
sPLA2 mediates IL-1β and TNFα induced oligodendrocyte injury in vitro
To investigate whether the beneficial effects of sPLA2 blockade mediate more than H2O2 induced oligodendrocyte injury, we developed a cytokine injury model for oligodendrocyte. IL-1β and TNFα were chosen because both are suggested mediators of secondary SCI (Demjen et al. 2004; Hostettler and Carlson 2002) and exogenous IL-1β and TNFα induces sPLA2-IIA dependent AA release in many cell lines (Kuwata et al. 1998; Kuwata et al. 2005; Kuwata et al. 2000). Similarly, we found that treatment with IL-1β and TNFα created a time dependent increase in sPLA2-IIA expression in cultured oligodendrocytes (Fig. 9A). When high doses of IL-1β and TNFα were added pretreatment with S3319 showed a decrease in cytotoxicity, as measured by LDH release after treatment (MANOVA F1,30 = 16.85, p < 0.0001; Fig. 9H). More dramatic than the observed sparing was the morphological changes associated with this injury model. IL-1β and TNFα treatment results in a dramatic decrease in oligodendrocyte processes extending from the soma at 48 hr (Fig. 9B-D). Interestingly, this process loss was similar to that observed following addition of 0.01 and 0.1 μM of sPLA2-IIA to oligodendrocyte cultures (Fig. 6B & C). When cultures were pretreated with S3319, this loss of oligodendrocyte processes was almost fully prevented (Fig. 9E-G). Quantification of area covered by oligodendrocyte processes confirms this observation (MANOVA F1,30 = 9.27, p < 0.01, Fig. 9I).
Fig. 9.
Blockade of sPLA2 in oligodendrocyte cultures prevents IL-1β and TNFα injury. A) Western blot showing time dependent sPLA2-IIA expression following treatment with 5 ng/ml IL-1β and 10 ng/ml TNFα. B-G) Phase contrast images showing oligodendrocyte cultures 72 hr after injury with either vehicle, low doses (1 and 2 ng/ml) or high doses (5 and 10 ng/ml) of IL-1β and TNFα. Cultures were either pretreated with vehicle (B-D) or 1.25 μM S3319 (E-G). Note the decrease in oligodendrocyte process extension. H) IL-1β and TNFα injury resulted in very mild cytotoxicity at 72 hr, but a significant sparing when pretreated with the sPLA2 inhibitor S3319. I) Quantification of process area in (B-G) showing 1.25 μM S3319 significantly prevents the destruction of oligodendrocyte processes after cytokine injury.
DISCUSSION
sPLA2 expression following SCI
We previously showed that cPLA2-IVα (cPLA2α) protein expression increased following SCI and peaked at 7 days post injury. However, measurements of total PLA2 enzymatic activity in spinal cord homogenates peaked much earlier, at 4 hr (Liu et al. 2006). This led us to believe that some other PLA2 isozyme contributed to the increase in phospholipases activity observed following SCI. We found that of the seven sPLA2 isoforms expressing mRNA, two were up regulated (IIA and IIE). These results were further confirmed at the protein level with a peak expression at 4 hr which coincides with both apoptosis following SCI (Liu et al. 1997) and the time of peak phospholipase A2 activity following SCI (Liu et al. 2006).
The presence of sPLA2-IB, IIA, IIC, IIE, V, and X within the mammalian brain is well established (Kolko et al. 2004; Molloy et al. 1998; Suzuki et al. 2000). However, only sPLA2-IIA and V protein expression has been investigated in the spinal cord and no study has looked at the effect of neurotrauma on sPLA2 expression (Svensson et al. 2005). Interestingly, sPLA2-IB has been shown to increase following KA injection and electroconvulsive shock (Kolko et al. 2005); IB, V, and X increased after retinal damage (Kolko et al. 2004); and IIA increased following cerebral ischemia (Adibhatla and Hatcher 2007; Lin et al. 2004; Yagami et al. 2002). In comparison, this study found that only the group II enzymes increased following neurotrauma. Consistent with this tight regulation, the promoter region of sPLA2-IIA gene contains TATA and CAAT boxes as well as several elements homologous with consensus sequences for binding of transcription factors such as AP-1, C/EBPs, CREB, NF-κB, STAT, and PPARγ (Touqui and Alaoui-El-Azher 2001). The differential regulation of sPLA2 groups suggests a possible injury and isoform specific induction mechanism and varying cellular functions for sPLA2 isoforms in neuropathogenesis.
This study also demonstrated an increase of sPLA2 within the membrane fraction of cells and a perinuclear compartmentalization of sPLA2-IIA following H2O2 injury. This is significant since sPLA2-IIA, while being a secreted molecule, actually displays extremely low enzymatic activity toward the phosphatidylcholine-rich external membrane of cells. sPLA2-IIA cannot bind to the zwitterionic interface, as a result it shows a marked preference for anionic phospholipids located on the inner leaflet of the bi-lipid membrane (Bezzine et al. 2000; Murakami and Kudo 2001). This is supported by the fact that μM levels of exogenously added sPLA2-IIA was cytotoxic as opposed to nM levels of sPLA2-III (unpublished observation), which shows little preference for anionic phospholipids. Recent studies have confirmed that AA release by sPLA2-IIA transfected cells occurs within the Golgi compartment following synthesis but prior to its initial secretion to the extracellular fluid or binding to anionic heparan sulfate chains on the cell surface (Mounier et al. 2004). Therefore, we believe that our observations of increased mRNA levels and increased protein within the cytosolic membrane fraction, in conjunction with a perinuclear punctuate appearance of sPLA2-IIA following injury indirectly supports the prevailing theory of sPLA2-IIA synthesis and activation. However, further enzymatic studies will be needed for conclusive confirmation.
sPLA2-IIA’s effect on oligodendrocytes
We next showed that H2O2 or IL-1β and TNFα injuries induce sPLA2-IIA expression in cultured oligodendrocytes and that blockade of sPLA2 attenuates H2O2 or IL-1β and TNFα induced cell injury. ROS damage is a pervasive injury mechanism involved not only in SCI (Liu et al. 2004a; Liu et al. 1999; Park et al. 2004) but also Multiple Sclerosis (Lev et al. 2006), Alzheimer Disease (Reddy 2006), and Huntington Disease (Rego and Oliveira 2003). It was previously shown that H2O2 (Richter-Landsberg and Vollgraf 1998) and AA (Wang et al. 2004) can trigger oligodendrocyte death and that H2O2 injury utilizes cPLA2α and sPLA2-IIA for AA release in non-CNS cell lines (Han et al. 2003). However, this was the first study to demonstrate that blockade of sPLA2-IIA could partially ameliorate the cytotoxic effects of H2O2 in oligodendrocytes.
Likewise, IL-1β and TNFα have been known to increase sPLA2-IIA expression and trigger AA release from cultured cell lines via sPLA2-IIA and cPLA2-IVα dependent mechanisms (Kuwata et al. 2005; Mounier et al. 2004). Additionally these cytokines have been shown to damage cultured oligodendrocytes (Lee et al. 2000; Selmaj and Raine 1988; Takahashi et al. 2003). However, this was the first study to demonstrate that blockade of sPLA2-IIA could partially ameliorate the cytotoxicity and morphological damage created by IL-1β and TNFα in oligodendrocytes. It must be noted that although S3319 was developed as a specific inhibitor of sPLA2-IIA, its activity against other isozymes has not been fully assessed. This being said, sPLA2 induction in oligodendrocytes following ROS and cytokine injury could be a novel target for therapeutic intervention.
sPLA2-IIA exhibited cytotoxicity at concentrations around 1 μM in cultured oligodendrocytes. Does the concentration of endogenous sPLA2-IIA reach such a high level in vivo? By comparing the signal intensity of protein extracted from the injured spinal cord to purified recombinant human sPLA2-IIA protein we determined that SCI yields roughly 5×10−3 μg of sPLA2-IIA protein per μg of SCI protein. In comparison we added 4×10−1 μg of recombinant human sPLA2-IIA per μg of oligodendrocyte protein in culture to induce cytotoxicity. In other words, approximately 80 times more sPLA2-IIA protein was used to induce cytotoxicity in vitro than in vivo. This calculation was made on the assumptions that the antibody has similar affinity for both rat and recombinant human sPLA2-IIA and that the in vivo and in vitro protein extraction methods are comparable. Other authors have found that sPLA2-IIA activity induced apoptosis in cultured neurons but only at levels 300 times that found in ischemic brain tissue (Yagami et al. 2002). This discrepancy between in vivo and in vitro toxicities can be explained by the following reports. First, sPLA2-IIA generated from cytokine-stimulated astrocytes might reach to such a high concentration within a microenvironment at the cell surfaces via attachment to heparan sulfate proteoglycan (Koduri et al., 1998). Second, sPLA2-IIA might cause neuronal and oligodendrocyte cell death at lower concentrations in the presence of cofactors (Murakami et al., 1991; Fourcade et al., 1995). Third, the sensitivity of the cells to endogenously produced sPLA2-IIA is higher than exogenously added sPLA2-IIA due to its intracellular location (Murakami et al., 1999). The latter seems likely given the preference of sPLA2-IIA for inner leaflet phospholipids (Porcellati 1983). It must be noted that the efficacy of S3319, a sPLA2 inhibitor, in systems with cytokine and peroxide induction of sPLA2-IIA suggests that its blockade can crucially affect cytotoxicity despite high levels being need to directly induce cytotoxicity.
In this study we chose to focus on the direct cytotoxicity of sPLA2 in isolated oligodendrocytes. A second, equally compelling, yet unexplored hypothesis is that sPLA2 could increase secondary SCI not merely by direct cytotoxicity but also by exacerbating the recruitment of neutrophils and macrophages to the injury site which has been previously reported (Popovich et al. 1997). Following sPLA2 hydrolysis, phospholipids generate a free fatty acid, such as arachidonic acid (AA), and a lysophospholipid such as lysophosphatidyl choline (LPC, a.k.a. lysolechithin). AA can later form epoxides via the cytochrome P450 pathway, leukotrienes via the lipoxygenase pathway, or thromboxanes or prostaglandins via the cyclooxygenase pathway. Many of these products, such as prostaglandin E2 (PGE2), can subsequently act as potent chemoattractants that increase the endogenous immune response (Resnick et al. 2001; Tonai et al. 1999). Furthermore, LPC has been shown to act as a proinflammatory chemoattractant for macrophages (Lauber et al. 2003). Therefore, sPLA2-IIA could directly induce tissue damage as we have shown here or increase inflammation following SCI and exacerbate secondary spinal cord injury, a hypothesis that is under current investigation.
One assumption made by this work is that SCI results in demyelination and that either protection of myelin or de novo production by spared oligodendrocytes could result in measurable functional gains. This assumption is based on several observations. First, traumatic SCI results in transient post-injury membrane phospholipid hydrolysis (Demediuk et al. 1989). Secondly, increased spared white matter correlated with increased functional recovery (Rosenberg et al. 1999; Wrathall et al. 1994). Third, transplantation of myelinating stem cells results in normal-appearing myelin, recovery of transcranial magnetic motor-evoked potential responses, and improvements in overground locomotion (Cao et al. 2005a; Karimi-Abdolrezaee et al. 2006; Keirstead et al. 2005). Finally, remyelinated axons are present from 14 to 450 days post injury but that remyelination was incomplete, as indicated by the presence of demyelinated axons at every time point examined (Totoiu and Keirstead 2005). However, recent studies have brought into question whether chronic demyelination persists in functionally intact axons since myelin sheaths not only ensure successful propagation of action potentials, but also participate in axonal transport and survival (Edgar and Garbern 2004; Edgar et al. 2004). Lasiene, et al., (2008) used anterograde tracing to evaluate the myelin status of functionally intact axons. They showed that virtually no “intact axon” remained unmyelinated at 12 weeks in mice suggesting that remyelination or myelin sparing strategies may be less promising than originally hypothesized. This hypothesis is supported by the fact that remyelination by transplanted cells shows efficacy but only if transplants are delivered before 3 weeks (Cao et al. 2005a; Karimi-Abdolrezaee et al. 2006; Keirstead et al. 2005) Additionatly pharmacological demyelination studies show an almost complete remyelination at chronic time points (Blakemore et al. 1977; Blakemore and Murray 1981; Jeffery and Blakemore 1995). These studies suggest that a delayed but sufficient endogenous remyelinating system exists. Finally, most of the conclusions drawn from pharmacological sparing of oligodendrocytes or white matter and on the persistence of “demyelinated” axons are based on correlations rather than true experiments.
A second assumption is that while the total destruction of phospholipids following SCI would obviously result in pathology of both axons and myelin, intrinsic phospholipase activity could potentially facilitate repair. It has been shown by several groups that myelin is a non-permissive substrate for neurite outgrowth (Niederost et al. 1999; Schwab and Caroni 1988). Most of myelin’s inhibition has been attributed to proteins within the myelin fraction binding to the Nogo receptor complex (Filbin 2003). This raises the possibility that sPLA2 activity following SCI could function as a clearance mechanism for myelin debris thus facilitating axon regeneration and functional recovery. Therefore inhibition of sPLA2 could possibly worsen SCI in vivo.
The results presented here suggest that the expression of sPLA2, particularly IIA, might participate in oligodendrocytes death mediated by several cytotoxic pathways. More importantly the blockade sPLA2 could provide a crucial therapeutic intervention for not only SCI but other CNS injuries in which H2O2, IL-1β and TNFα mediate damage. Whether inhibition of sPLA2 following SCI in vivo creates histological and functional sparing is under active investigation.
Supplementary Material
Sup. 1. Characterization of a C4/C5 200kDyne SCI. This injury yielded both consistent white matter damage (A) as well as lesion volume (B; mean ± SD). Assessments were made at 960 μm intervals through a 9.6 mm section of cord centering on the epicenter. 3-D reconstruction of a representative sham (C-D) and contused animals (I&J) show the substantial damage incurred by this injury. Sections from the epicenter of a sham (E-F) or 200kDyne contused animals (G-H;K-N) at one week. Images E, G, K, M are luxol fast blue stained sections showing white matter sparing. Images F, H, L, N are cresyl eosin stained sections.
Sup. 2. Representative image of bilateral injections of sPLA2-III (0.1 μg) into the ventral grey/white matter interface. A crestyl violet and eosin counter stained section taken from the injury epicenter, 4 weeks after injection. Note considerably more damage in the contiguous white matter. Dotted line demarcates the extent of lesion. Arrows indicate points of injection from beveled glass micropipettes.
Sup. 3. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C) and IIA (D-F); but not IIE (G-I), or X (J-L) are present within astrocytes (GFAP) 4 hr following contusion. Note the glia in B (double arrow) that is positive for sPLA2-IB but negative for GFAP, which is most likely an oligodendrocyte. Orthoganol confocal image stacks (see Figure 4 legend for explanation). Scale bars: 20 μm for all.
Sup. 4. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C), IIA (D-F), and IIE (G-I); but not X (J-L) are present within neurons (NeuN) 4 hr following contusion. Images were taken from the ventral grey matter. Scale bars: 20 μm.
Sup. 5. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C) and IIA (D-F) are present in axons (SMI-31) 4 hr following contusion. sPLA2-IIE (G-I) appears to be present in only a subset of axons that are swollen (arrows). sPLA2-X (J-L) was not detected ant axon. All images were taken from the ventral white matter. Scale bars: 20 μm for all.
ACKNOWLEDGEMENTS
We would like to thank Christine Nunn and Aaron Pucket for animal care. This work was supported by NIH NINDS (XMX: NS36350, NS52290, NS50243, NS61975; WLT: F31 S5657401), the Kentucky Spinal Cord and Head Injury Research Trust (#4-16), the Daniel Heumann Fund for Spinal Cord Research, the James R. Petersdorf and Mari Hulman George Endowments. We also appreciate the use of the Center’s Core facility supported by NIH COBRE RR15576.
REFERENCES
- Adibhatla RM, Hatcher JF. Secretory phospholipase A2 IIA is up-regulated by TNF-alpha and IL-1alpha/beta after transient focal cerebral ischemia in rat. Brain Res. 2007;1134(1):199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bezzine S, Koduri RS, Valentin E, Murakami M, Kudo I, Ghomashchi F, Sadilek M, Lambeau G, Gelb MH. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J Biol Chem. 2000;275(5):3179–91. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Eames RA, Smith KJ, McDonald WI. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J Neurol Sci. 1977;33(1-2):31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Murray JA. Quantitative examination of internodal length of remyelinated nerve fibres in the central nervous system. J Neurol Sci. 1981;49(2):273–84. doi: 10.1016/0022-510x(81)90084-8. [DOI] [PubMed] [Google Scholar]
- Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2(4):299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- Cane A, Breton M, Koumanov K, Bereziat G, Colard O. Oxidant-induced arachidonic acid release and impairment of fatty acid acylation in vascular smooth muscle cells. Am J Physiol. 1998;274(4 Pt 1):C1040–6. doi: 10.1152/ajpcell.1998.274.4.C1040. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. Journal of Neuroscience. 2005a;25(30):6947–57. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005b;191(Suppl 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177(2):349–59. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Medicine. 1997;3(1):73–6. doi: 10.1038/nm0197-73. erratum appears in Nat Med 1997 Feb;3(2):240. [DOI] [PubMed] [Google Scholar]
- DeCoster MA. Group III secreted phospholipase A2 causes apoptosis in rat primary cortical neuronal cultures. Brain Res. 2003;988(1-2):20–8. doi: 10.1016/s0006-8993(03)03326-2. [DOI] [PubMed] [Google Scholar]
- Demediuk P, Daly MP, Faden AI. Changes in free fatty acids, phospholipids, and cholesterol following impact injury to the rat spinal cord. J Neurosci Res. 1989;23(1):95–106. doi: 10.1002/jnr.490230113. [DOI] [PubMed] [Google Scholar]
- Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, Hirt UA, Walczak H, Falk W, Essig M. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10(4):389–95. doi: 10.1038/nm1007. others. [DOI] [PubMed] [Google Scholar]
- Edgar JM, Garbern J. The myelinated axon is dependent on the myelinating cell for support and maintenance: molecules involved. J Neurosci Res. 2004;76(5):593–8. doi: 10.1002/jnr.20063. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Yool D, Zhang SC, Fowler JH, Montague P, Barrie JA, McCulloch MC, Duncan ID, Garbern J. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 2004;166(1):121–31. doi: 10.1083/jcb.200312012. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AY, Phillis JW. The phospholipase A2 inhibitor, quinacrine, reduces infarct size in rats after transient middle cerebral artery occlusion. Brain Reseach. 1997;752:203–208. doi: 10.1016/s0006-8993(96)01450-3. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–13. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6(10):995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- Han WK, Sapirstein A, Hung CC, Alessandrini A, Bonventre JV. Cross-talk between cytosolic phospholipase A2α (cPLA2 α) and secretory phospholipase A2 (sPLA2) in hydrogen peroxide-induced arachidonic acid release in murine mesangial cells: sPLA2 regulates cPLA2α activity that is responsible for arachidonic acid release. J Biol Chem. 2003;278(26):24153–63. doi: 10.1074/jbc.M300424200. [DOI] [PubMed] [Google Scholar]
- Hansford KA, Reid RC, Clark CI, Tyndall JD, Whitehouse MW, Guthrie T, McGeary RP, Schafer K, Martin JL, Fairlie DP. D-Tyrosine as a chiral precusor to potent inhibitors of human nonpancreatic secretory phospholipase A2 (IIa) with antiinflammatory activity. Chembiochem. 2003;4(2-3):181–5. doi: 10.1002/cbic.200390029. [DOI] [PubMed] [Google Scholar]
- Hostettler ME, Carlson SL. PAF antagonist treatment reduces pro-inflammatory cytokine mRNA after spinal cord injury. Neuroreport. 2002;13(1):21–4. doi: 10.1097/00001756-200201210-00009. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol. 1995;24(10):775–81. doi: 10.1007/BF01191213. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–89. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolko M, Christoffersen NR, Barreiro SG, Bazan NG. Expression and location of mRNAs encoding multiple forms of secretory phospholipase A2 in the rat retina. Journal of Neuroscience Research. 2004;77(4):517–24. doi: 10.1002/jnr.20187. [DOI] [PubMed] [Google Scholar]
- Kolko M, Christoffersen NR, Barreiro SG, Miller ML, Pizza AJ, Bazan NG. Characterization and location of secretory phospholipase A2 groups IIE, V, and X in the rat brain. Journal of neuroscience research. 2006;83(5):874–82. doi: 10.1002/jnr.20773. [DOI] [PubMed] [Google Scholar]
- Kolko M, Christoffersen NR, Varoqui H, Bazan NG. Expression and induction of secretory phospholipase A2 group IB in brain. Cell Mol Neurobiol. 2005;25(7):1107–22. doi: 10.1007/s10571-005-8221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins & Other Lipid Mediators. 2002;68-69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Nakatani Y, Murakami M, Kudo I. Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3Y1 fibroblasts. J Biol Chem. 1998;273(3):1733–40. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Nonaka T, Murakami M, Kudo I. Search of factors that intermediate cytokine-induced group IIA phospholipase A2 expression through the cytosolic phospholipase A2-and 12/15-lipoxygenase-dependent pathway. J Biol Chem. 2005;280(27):25830–9. doi: 10.1074/jbc.M500168200. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Yamamoto S, Miyazaki Y, Shimbara S, Nakatani Y, Suzuki H, Ueda N, Murakami M, Kudo I. Studies on a mechanism by which cytosolic phospholipase A2 regulates the expression and function of type IIA secretory phospholipase A2. J Immunol. 2000;165(7):4024–31. doi: 10.4049/jimmunol.165.7.4024. [DOI] [PubMed] [Google Scholar]
- Lasiene J, Shupe L, Perlmutter S, Horner P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci. 2008;28(15):3887–96. doi: 10.1523/JNEUROSCI.4756-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–30. doi: 10.1016/s0092-8674(03)00422-7. others. [DOI] [PubMed] [Google Scholar]
- Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H, Lee EB, Kim YC, Shin ML, Markelonis GJ. Role of tumor necrosis factor-a in neuronal and glial apoptosis after spinal cord injury. Experimental Neurology. 2000;166(1):190–5. doi: 10.1006/exnr.2000.7494. others. [DOI] [PubMed] [Google Scholar]
- Lev N, Ickowicz D, Barhum Y, Blondheim N, Melamed E, Offen D. Experimental encephalomyelitis induces changes in DJ-1: implications for oxidative stress in multiple sclerosis. Antioxid Redox Signal. 2006;8(11-12):1987–95. doi: 10.1089/ars.2006.8.1987. [DOI] [PubMed] [Google Scholar]
- Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90(3):637–45. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction. J Neurotrauma. 2004a;21(6):805–16. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic Biol Med. 1999;27(3-4):478–82. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- Liu N, Han S, Lu PH, Xu XM. Upregulation of annexins I, II, and V after traumatic spinal cord injury in adult rats. Journal of neuroscience research. 2004b;77(3):391–401. doi: 10.1002/jnr.20167. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, Shields CB, Xu XM. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66(10):932–43. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A Novel Role of Phospholipase A2 in Mediating Spinal Cord Secondary Injury. Anal Neurology. 2006;59(4):606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF. Neuronal and glial apoptosis after traumatic spinal cord injury. Journal of Neuroscience. 1997;17(14):5395–406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. Journal of Neuroscience. 2001;21(10):3392–400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TN, Gloy J, Hug MJ, Greger R, Schollmeyer P, Pavenstadt H. Hydrogen peroxide increases the intracellular calcium activity in rat mesangial cells in primary culture. Kidney Int. 1996;49(2):388–95. doi: 10.1038/ki.1996.57. [DOI] [PubMed] [Google Scholar]
- Molloy GY, Rattray M, Williams RJ. Genes encoding multiple forms of phospholipase A2 are expressed in rat brain. Neurosci Lett. 1998;258(3):139–42. doi: 10.1016/s0304-3940(98)00838-6. [DOI] [PubMed] [Google Scholar]
- Morell P. Myelin. xx. Plenum Press; New York: 1984. p. 545. [Google Scholar]
- Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A(2) occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A(2)-alpha. J Biol Chem. 2004;279(24):25024–38. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- Mronga T, Stahnke T, Goldbaum O, Richter-Landsberg C. Mitochondrial pathway is involved in hydrogen-peroxide-induced apoptotic cell death of oligodendrocytes. Glia. 2004;46(4):446–55. doi: 10.1002/glia.20022. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv Immunol. 2001;77:163–94. doi: 10.1016/s0065-2776(01)77017-4. [DOI] [PubMed] [Google Scholar]
- Murakami M, Yoshihara K, Shimbara S, Lambeau G, Singer A, Gelb MH, Sawada M, Inagaki N, Nagai H, Kudo I. Arachidonate release and eicosanoid generation by group IIE phospholipase A2. Biochemical and biophysical research communications. 2002;292(3):689–96. doi: 10.1006/bbrc.2002.6716. [DOI] [PubMed] [Google Scholar]
- Niederost BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J Neurosci. 1999;19(20):8979–89. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol. 2007;207(2):238–47. doi: 10.1016/j.expneurol.2007.06.012. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–74. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–64. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Porcellati G. Phospholipid metabolism in neural membranes. In: Sun GY, Bazan NG, Wu YJ, Porcellati G, Sun AY, editors. Neural Membranes. Humana Press; New York: 1983. [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96(1):1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28(10):1563–74. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Resnick DK, Nguyen P, Cechvala CF. Regional and temporal changes in prostaglandin E2 and thromboxane B2 concentrations after spinal cord injury. Spine J. 2001;1(6):432–6. doi: 10.1016/s1529-9430(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Vollgraf U. Mode of cell injury and death after hydrogen peroxide exposure in cultured oligodendroglia cells. Exp Cell Res. 1998;244(1):218–29. doi: 10.1006/excr.1998.4188. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci. 1999;19(14):6122–33. doi: 10.1523/JNEUROSCI.19-14-06122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20(2):179–93. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8(7):2381–93. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Annals of Neurology. 1988;23(4):339–46. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochimica et biophysica acta. 2000;1488(1-2):1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Ishizaki J, Yokota Y, Higashino K, Ono T, Ikeda M, Fujii N, Kawamoto K, Hanasaki K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. The Journal of biological chemistry. 2000;275(8):5785–93. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Lucas KK, Hua XY, Powell HC, Dennis EA, Yaksh TL. Spinal phospholipase A2 in inflammatory hyperalgesia: role of the small, secretory phospholipase A2. Neuroscience. 2005;133(2):543–53. doi: 10.1016/j.neuroscience.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1b promotes oligodendrocyte death through glutamate excitotoxicity. Annals of Neurology. 2003;53(5):588–95. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Titsworth WL, Onifer SM, Liu NK, Xu XM. Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Exp Neurol. 2007;207(1):150–62. doi: 10.1016/j.expneurol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Tonai T, Taketani Y, Ueda N, Nishisho T, Ohmoto Y, Sakata Y, Muraguchi M, Wada K, Yamamoto S. Possible involvement of interleukin-1 in cyclooxygenase-2 induction after spinal cord injury in rats. J Neurochem. 1999;72(1):302–9. doi: 10.1046/j.1471-4159.1999.0720302.x. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486(4):373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr Mol Med. 2001;1(6):739–54. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- Tournier C, Thomas G, Pierre J, Jacquemin C, Pierre M, Saunier B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase) Eur J Biochem. 1997;244(2):587–95. doi: 10.1111/j.1432-1033.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur J Neurosci. 2004;20(8):2049–58. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- Wang XF, Huang LD, Yu PP, Hu JG, Yin L, Wang L, Xu XM, Lu PH. Upregulation of type I interleukin-1 receptor after traumatic spinal cord injury in adult rats. Acta Neuropathol (Berl) 2006;111(3):220–8. doi: 10.1007/s00401-005-0016-x. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci. 1994;14(11 Pt 1):6598–607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134(2):261–72. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Yagami T, Ueda K, Asakura K, Hata S, Kuroda T, Sakaeda T, Takasu N, Tanaka K, Gemba T, Hori Y. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Molecular Pharmacology. 2002;61(1):114–26. doi: 10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- Yagami T, Ueda K, Asakura K, Nakazato H, Hata S, Kuroda T, Sakaeda T, Sakaguchi G, Itoh N, Hashimoto Y. Human group IIA secretory phospholipase A2 potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels in cultured rat cortical neurons. Journal of Neurochemistry. 2003;85(3):749–58. doi: 10.1046/j.1471-4159.2003.01712.x. others. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Iannotti C, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural closure, cord approximation, and clot removal: enhancement of tissue sparing in a novel laceration spinal cord injury model. J Neurosurg. 2004;100(4 Suppl Spine):343–52. doi: 10.3171/spi.2004.100.4.0343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sup. 1. Characterization of a C4/C5 200kDyne SCI. This injury yielded both consistent white matter damage (A) as well as lesion volume (B; mean ± SD). Assessments were made at 960 μm intervals through a 9.6 mm section of cord centering on the epicenter. 3-D reconstruction of a representative sham (C-D) and contused animals (I&J) show the substantial damage incurred by this injury. Sections from the epicenter of a sham (E-F) or 200kDyne contused animals (G-H;K-N) at one week. Images E, G, K, M are luxol fast blue stained sections showing white matter sparing. Images F, H, L, N are cresyl eosin stained sections.
Sup. 2. Representative image of bilateral injections of sPLA2-III (0.1 μg) into the ventral grey/white matter interface. A crestyl violet and eosin counter stained section taken from the injury epicenter, 4 weeks after injection. Note considerably more damage in the contiguous white matter. Dotted line demarcates the extent of lesion. Arrows indicate points of injection from beveled glass micropipettes.
Sup. 3. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C) and IIA (D-F); but not IIE (G-I), or X (J-L) are present within astrocytes (GFAP) 4 hr following contusion. Note the glia in B (double arrow) that is positive for sPLA2-IB but negative for GFAP, which is most likely an oligodendrocyte. Orthoganol confocal image stacks (see Figure 4 legend for explanation). Scale bars: 20 μm for all.
Sup. 4. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C), IIA (D-F), and IIE (G-I); but not X (J-L) are present within neurons (NeuN) 4 hr following contusion. Images were taken from the ventral grey matter. Scale bars: 20 μm.
Sup. 5. Confocal images of immunofluorescent double labeling indicates that sPLA2-IB (A-C) and IIA (D-F) are present in axons (SMI-31) 4 hr following contusion. sPLA2-IIE (G-I) appears to be present in only a subset of axons that are swollen (arrows). sPLA2-X (J-L) was not detected ant axon. All images were taken from the ventral white matter. Scale bars: 20 μm for all.