Abstract
T-helper type 17 cytokines have been implicated in epithelial cancer progression at mucosal sites. In this issue of the European Journal of Immunology, Nardinocchi, et al. show that the Th17 cytokines IL-17 and IL-22 can both signal to non-melanoma skin cancer cells, inducing both cellular proliferation and enhanced migration of human basal-cell carcinoma (BCC) and squamous cell carcinoma (SCC) cell lines in vitro. These cytokines were also shown to exacerbate tumor growth in mice injected with the SCC line, CAL27. Thus, IL-17 and IL-22 may be key factors in skin cancer progression and may provide novel prognostic markers in non-melanoma skin cancer.
Keywords: Cancer, IL-17, IL-22, skin, T cells
T helper 17 (TH17) cells represent a CD4+ T-cell lineage that develop from naïve CD4+ T cells in the presence of specific factors, such as TGF-β, IL-6 and IL-1β (reviewed in [1]), and that are maintained in the presence of IL-23 [2]. TH17 cells are characterized by their capacity to secrete IL-17A, IL-17F, IL-21, IL-22 and CCL20 (reviewed in [3]) and their development is regulated by the transcription factors RORγt, RORα, interferon regulatory factor 4 (IRF4) and the aryl hydrocarbon receptor (AHR) (reviewed in [4]). The induction of RORγt is dependent on STAT3, which is preferentially activated by IL-6, IL-21 and IL-23, and plays a key role in the regulation of IL-17 production by TH17 cells [5]. Although CD4+T cells are a key source of IL-17, it should be noted that IL-17 can also be produced by CD8+T cells, γδ T cells, NKT cells, neutrophils, eosinophils, and mast cells.
The role of TH17 cells and their effector cytokines in inflammation and autoimmunity has been well established but their role in tumor immunity has been considered “controversial” and highly influenced by the context, the type of inflammation surrounding the developing tumor, as well as the type of tumor these inflammatory cells infiltrate (reviewed in [6]). However, it is important to note that a clear distinction must be made between the role of TH17 cells and their signature cytokine, IL-17, in tumor immunity and development. As an example of this, Muranski et al. have reported [7] that TH17 cells have an anti-tumoral effect against melanoma, but the effect was not affected by IL-17 antibodies and instead, was completely inhibited by the inhibition of IFN-γ. Secondly, TH17 cells have a dynamic phenotype and differentiation into IL-17-producing FOXP3+ regulatory T cells [8] or into T helper 1 (TH1) [9] cells have been described.
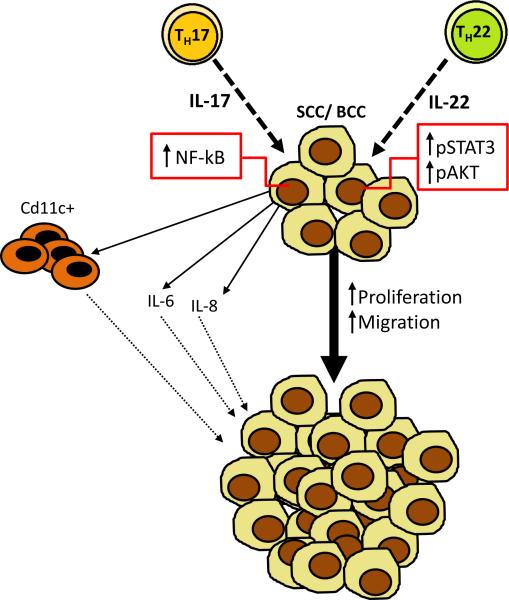
Several human cancers, including gastric cancer [10], hepatocarcinoma [11], non-small cell lung cancer (NSCLC) [12], and breast cancer [13], are characterized by a strong infiltration by TH17 cells and in many cases, the degree of infiltration had a prognostic value. In colorectal cancer, the detection of a TH17 gene over-expression signature was also associated with a worse prognosis following the primary tumor resection in stages I and II [14]. Non-melanoma skin cancers (NMSCs) are comprised mainly by basal-cell carcinomas (BCCs) and squamous-cell carcinomas (SCCs) which represent the most common human cancers. Both UVB and UVA radiation cause DNA damage and immunosuppression, two of the leading etiologies for NMSC [15]. The estimated incidence of non-melanoma skin cancer in USA is higher than 1,000,000 per year, and despite the public awareness about the harmful effect of sun exposure, the incidence only continues to increase [16]. In this issue of the European Journal of Immunology, Nardinocchi et al. [17] show that IL-17+ and IL-22+ T cells infiltrate non melanoma-skin carcinomas. The authors showed that IL-17 and IL-22 directly promote the proliferation of BCC and SCC cell lines in vitro (Figure 1). Using scratch assays, the authors demonstrated that both IL-17 and IL-22 also increase the migration of BCC and SCC cell lines (Figure 1). This response was associated with the induction of IL-6 and IL-8, factors associated with tumor proliferation and migration [17]. Moreover, the authors demonstrated that IL-17 also induces tumor proliferation in an in vivo xenograft model, in which nude mice are injected with the SCC line CAL27. IL-17 stimulation of BCC and SCC cell lines was shown to result in activation of NF-kB, and IL-22 stimulation was shown to result in activation of Erk1/2, STAT3 and AKT. These data suggest that these signaling pathways may be responsible for the proliferative effects observed in both the in vitro and in vivo assays.
Figure 1. Roles of IL-17 and IL-22 play roles in non-melanoma skin cancer.
Non-melanoma skin cells, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), express receptors for IL-17 and IL-22 and Nardinocchi et al. [16] show that these factors can increase tumor cell proliferation and migration in vitro. The enhanced proliferation and migration of BCC and SCC cell lines in response to IL-17 and IL-22 is associated with the induction of IL-6 and IL-8. The recruitment of myeloid cells (CD11c+) may constitute one of the pro-tumorigenic mechanisms of action of Th17 cells. Th17 and Th22 cells are depicted in the figure, but the cellular source of their signature cytokines in skin cancer remains to be determined. Using xenograft assays, Nardinocchi et al. show that these cytokines also accelerate tumor growth in vivo. IL-17 treatment of the SCC cell line CAL 27 induced NF-κB signaling in these cells and IL-22 treatment activated the Erk1/2 and STAT3 signaling pathway, which may be responsible for tumor progression.
Several preclinical studies utilizing genetically engineered mouse models have attempted to define the role of TH17 cells in tumor initiation and development [17-21]. Most of these studies have actually presented evidence of a pro-tumorigenic role for TH17 cells, which is mostly dependent on the effects of the signature cytokine, IL-17A; but it may also depend on the effect of other TH17-secreted cytokines such as IL-17F [18]. In 2009, using the APCMin mouse model of colon cancer, Wu et al. demonstrated that Bacteroides fragilis (ETBF) colonization can induce colorectal tumors through a TH17-type immune response to the bacteria, and that the effect was blocked with IL-17A neutralization [19]. McAllister et al. [20] has recently investigated the role of TH17/IL-17 using a mouse model in which an oncogenic form of Kras (KrasG12D), the most common mutation in pancreatic cancer, is restricted to pancreatic acinar cells. Pancreatic tumorigenesis was shown to accelerate in these mice upon induction of chronic pancreatitis and this effect was accompanied by TH17-cell infiltration into the pancreas [20]. Overexpression of IL-17, achieved by pancreatic adenoviral overexpression, also induced tumor progression. Conversely, by using antibodies that neutralize the IL-17 pathway and transplanting mice which had pancreatic Kras activation with IL-17KO bone marrow, pancreatic tumorigenesis was significantly delayed. The same group also showed that murine and human oncogenic Kras activation of pancreatic epithelial cells can induce the expression of IL-17RA [20]. Chang et al. [21] have reported similar pro-tumorigenic results using mice in which KRAS G12D, also frequently found in human lung cancer, is specifically expressed in lung epithelium (through Club Cell secretory protein (CCSPCre)); the authors showed that repeatedly challenging these mice with Haemophilus influenza (HI) induces a COPD-type inflammation [21]. In this model, similar to that shown in the pancreatic tumor model [20], neutralization of IL-17 showed a significant reduction in lung tumor numbers and the effect was mostly dependent on the recruitment of Gr1+CD11b+ myeloid cells, as depletion of these cells caused suppression of tumor growth [21]. Furthermore, Wang et al. [22] have recently confirmed the requirement of IL-17R expression on the oncogenic epithelium for the tumorigenic effects of IL-17 by deleting the IL-17RA specifically from the colon epithelium in a CPC-APC model (carriers of a CDX2PNLS Cre recombinase transgene and a loxP-targeted Apc allele). Using bone marrow chimerism studies, this group also showed that colon tumorigenesis was unaffected when IL-17RA was deleted in bone marrow cells. Taken together, these data suggest that a direct interaction between IL-17 ligands and IL-17R-bearing epithelial cells is required for the observed effects on tumorigenesis.
The study by Nardinocchi et al. also suggests that IL-17 and IL-22 can have direct effects on non-melanoma skin cancer, and that this immune pathway may be an important regulator of tumor progression. The cellular sources of these cytokines in the skin need to be determined as several subsets of cells can produce these cytokines in skin, including Th22 cells (Figure 1). The principal treatment for skin cancer is surgery but the pathway identified by the authors may be important factors in gauging prognosis or may represent novel therapeutic targets in advanced disease. Future studies are needed to determine the prognostic or therapeutic value of this pathway in skin cancer.
Acknowledgments
F.M. is supported by the Pancreatic Cancer Action Network- AACR Career Development Award (Grant number: 14-20-25-MCAL) and by funds from the Sheikh Ahmed Center for Pancreatic Cancer Research at The University of Texas M. D. Anderson Cancer Center. JKK was supported by a grant from NHLBI (R37HL079142).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, Radbruch A, et al. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. 2010;40:3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012;178:685–691. doi: 10.1016/j.jss.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL- 17A expression and invasiveness of the tumor. Eur J Immunol. 2013;43:1518–1528. doi: 10.1002/eji.201242951. [DOI] [PubMed] [Google Scholar]
- 14.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 15.Chen AC, Halliday GM, Damian DL. Non-melanoma skin cancer: carcinogenesis and chemoprevention. Pathology. 2013;45:331–341. doi: 10.1097/PAT.0b013e32835f515c. [DOI] [PubMed] [Google Scholar]
- 16.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 17.Lavinia Nardinocchi GS. Francesca Passarelli, Simona Avitabile, Claudia Scarponi, Cristina Maria Failla, Stefano Simoni, Cristina Albanesi and Andrea Cavani, Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. European Journal of Immunology. 2015 doi: 10.1002/eji.201445052. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Chae WJ, Bothwell AL. IL-17F deficiency inhibits small intestinal tumorigenesis in ApcMin/+ mice. Biochem Biophys Res Commun. 2011;414:31–36. doi: 10.1016/j.bbrc.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111:5664–5669. doi: 10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–1063. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]