Abstract
Most animal studies using methylphenidate (MP) do not administer it the same way it is administered clinically (orally), but rather by injection, resulting in an altered pharmacokinetic profile (i.e. quicker and higher peak concentrations). Here, we evaluated several oral-dosing regimens in rats, including dual-dose drinking, to mimic the clinical drug delivery profile. Using an 8-hour-limited-access-drinking-paradigm, MP solutions were delivered at different doses (20, 30, or 60 mg/kg/day; as well as dual-dosages of 4 and 10 mg/kg/day, 20 and 30 mg/kg/day, or 30 and 60 mg/kg/day, in which the low dose was administered in the first hour of drinking followed by 7 h of drinking the high dose). Blood was sampled and plasma was assayed for MP levels at many time points. Results showed that an 8-hour limited drinking of a dual-dosage 30/60 mg/kg MP solution achieved a pharmacokinetic profile similar to clinically administered doses of MP at the high end of the spectrum (peaking at ~30 ng/mL), while the 4/10 mg/kg MP dual-dosage produced plasma levels in the range produced by typically prescribed clinical doses of MP (peaking at ~8 ng/mL). Treatment with the higher dual-dosage (HD: 30/60 mg/kg) resulted in hyperactivity, while the lower (LD: 4/10 mg/kg) had no effect. Next, chronic effects of these dual-dosages were assessed on behavior throughout three months of treatment and one month of abstinence, beginning in adolescence. MP dose-dependently decreased body weight, which remained attenuated throughout abstinence. MP decreased food intake during early treatment, suggesting that MP may be an appetite suppressant and may also speed metabolism and/or suppress growth. Chronic HD MP resulted in hyperactivity limited during the dark cycle; decreased exploratory behavior; and increased anxiolytic behavior. These findings suggest that this dual-dosage-drinking-paradigm can be used to examine the effects of clinically relevant pharmacokinetic doses of MP, and that chronic treatment with such dosages can result in long-lasting developmental and behavioral changes.
Keywords: Methylphenidate, Ritalin, Attention deficit hyperactivity disorder, Psychostimulant, Dopamine transporter
1. Introduction
Methylphenidate (MP) remains one of the most widely prescribed drugs for the treatment of attention deficit hyperactivity disorder (ADHD) (Swanson and Volkow, 2008; Swanson and Volkow, 2009). In the last decade, the diagnosis rate of ADHD for youth aged 4 to 17 increased 41%, jumping to a national average in the United States of 11%, with two-thirds of diagnosed children being treated with psychostimulant medications (Bloom et al., 2012). Lifetime diagnosis (10% in girls and 19% in boys) and stimulant prescription rates (~10% in boys) in high-school aged youth are even higher (Bloom et al., 2012). The new DSM-5 increasing the maximum age of symptom onset from 7 to 12, and reducing the number of criteria needed from six to five for adults (APA, 2013), will likely result in greater diagnosis rates across all age groups. MP is also used illegally as a study aid among high school and college students and is abused recreationally (McCabe et al., 2006; Wilens et al., 2008). Among college students in the United States, self-reported rates range from 1.5% to 31%, with the most nationally representative study estimating annual illicit stimulant use at ~4% (McCabe et al., 2005; Teter et al., 2006; Bogle and Smith, 2009; Garnier-Dykstra et al., 2012).
The increasing use and abuse of MP, particularly during critical stages of neurodevelopment, presents great concerns of subsequent neurobiological, developmental, and behavioral effects. Also of concern is the capability of MP to produce cross-sensitization to the effects of other stimulant drugs (Pierce and Kalivas, 1997), as this phenomenon of cross-sensitization is hypothesized as a mechanism that increases vulnerability to polysubstance abuse later in life (Robinson and Berridge, 2001). These concerns raise the need for preclinical studies that assess possible consequences of MP treatment at doses that are clinically relevant. Preclinical studies have found significant effects of MP on neurochemistry (Brandon et al., 2003; Brandon and Steiner, 2003; Grund et al., 2006; Thanos et al., 2007; Robison et al., 2012), development (Robison et al., 2010; Komatsu et al., 2012), behavior (Kuczenski and Segal, 2001; Thanos et al., 2009; Robison et al., 2010; Zhu et al., 2010), and psychostimulant cross-sensitization and self-administration (Kuczenski and Segal, 2002; Torres-Reveron and Dow-Edwards, 2005; Thanos et al., 2007).
A major limitation of animal studies, however, is that the route of administration of MP is typically by injection and not oral as is used clinically (Volkow and Insel, 2003). Humans being treated for ADHD receive MP orally, either in the immediate release (IR) formulation administered two (b.i.d.) or three (t.i.d.) times daily, or in the extended release (ER) formulation administered once daily (q.d.) (Volkow and Swanson, 2003). In most animal studies, MP is administered intravenously (IV), intraperitoneally (IP), or subcutaneously. Studies have shown that these routes of MP administration differ significantly from oral administration, specifically with respect to magnitude of and time to peak serum concentration, half-life, and rate of elimination (Kuczenski and Segal, 2005), as well as absolute magnitude and time course of increases in extracellular DA and locomotor responses (Gerasimov et al., 2000; Kuczenski and Segal, 2001). Since these are key factors in the abuse liability of drugs (Volkow and Swanson, 2003), it is likely that administering MP in a fashion that leads to rapid peak serum and brain DA levels (such as IP or IV) might preferentially induce sensitization or other adaptations of the neural substrate in ways that oral MP (with its more gradual onset and reduced bioavailability), might not.
Doses of 0.5 to 5 mg/kg IP have been used in most rodent studies, and it has been reported that even an IP injection of 0.5 mg/kg would result in plasma concentrations at the highest end of the clinically-relevant spectrum (~40 ng/mL; equivalent to a 1.0 mg/kg dose in humans) and would peak within minutes post-injection rather than hours post-oral administration (Kuczenski and Segal, 2005). Additionally, many studies that have aimed to explore the effects of oral MP utilize the gavage method (Kuczenski and Segal, 2002; Justo et al., 2010), which can result in a significant stress response, as well as aspiration, and/or pulmonary injury in rats (Brown et al., 2000; Balcombe et al., 2004). Other studies have utilized voluntary oral consumption of MP (administered on oyster crackers or mixed with chow) to avoid these issues (LeBlanc-Duchin and Taukulis, 2007; Zhu et al., 2010); however, these methods also have some limitations. Oral administration results in peak serum concentration 15 min post-administration, and this concentration has been shown to drop by half within an additional 5 min (Patrick et al., 1984). The faster metabolism and shorter half-life of MP in rats compared to humans would therefore necessitate nearly constant dosing to maintain clinically relevant plasma concentrations. Therefore, the challenge addressed in the present study was to develop a method of administering MP to rodents that would produce a drug delivery profile similar to that achieved by clinical administration of MP. This means that the route of administration must be oral, and that plasma levels and profiles should resemble the patterns of dosing used in clinical practice (Swanson and Volkow, 2002). In the present study, we tested several oral dosing paradigms and chose two (a clinically-relevant low and high dose) for further examination of effects of chronic treatment (three months) on development and behavior in rats. Rats were also assessed following a one month abstinence period to determine whether any effects persisted beyond the cessation of treatment.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats were obtained from Taconic Farms (Germantown, NY). On arrival, rats were single housed in a temperature- and humidity-controlled room on a reverse 12 hour light cycle (lights off 0800 h). Food access was provided ad libitum at all times during the experiment and consisted of standard laboratory rat chow (Purina). Food intake and body weight were recorded daily during chronic exposure and abstinence. Experiments were conducted in conformity with the National Academy of Science’s Guide for the Care and Use of Laboratory Animals (NAS and NRC, 1996) and approved by the Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
2.2. Drugs
Methylphenidate hydrochloride (Sigma-Aldrich, St Louis, MO) was mixed with distilled water to deliver respective experimental doses in the rats’ daily drinking water.
2.3. Procedures
2.3.1. Determination of clinically relevant dosing regimens of MP
2.3.1.1. Drug administration
One week after arrival, rats were given limited access to water 8 h per day (8:00 h–16:00 h) in their home cages. This restricted access continued throughout the length of the experiment, except for the five days following an experimental blood draw when water access was ad libitum.
Different MP total daily doses were examined in this experiment (n = 12/group): 20, 30, and 60 mg/kg/day, as well as dual dosages of 4/10 mg/kg/day, 20/30 mg/kg/day, and 30/60 mg/kg/day, which were administered in daily drinking water. Specifically, in the dual dose groups, rats received the low dose of the MP solution for the first hour, followed by the higher dose of MP solution for the remaining 7 h. Concentrations of MP solution were calculated daily and individually for each rat based on the animal’s weight and the average volume of the last three days’ fluid consumption.
2.3.2. Blood sampling and MP assay
On each blood-sampling day, animals were given 8-hour access to their respectively dosed MP drinking solutions. Rats were sampled at various times (T = 1, 2, 4, 6, 8, and 10 h post-initiation of drinking). The MP solution was withdrawn from all rats at T = 8.
Blood was collected in two ways: a) venipuncture from the lateral tail vein while the animal was awake and lightly restrained (this usually took less than 5 min); and b) terminal cardiac puncture under deep anesthesia. Blood obtained by either method was immediately placed in K2EDTA-coated tubes and centrifuged. The plasma was drawn off and stored at −80 °C until analysis occurred. A minimum of two weeks were allowed for recovery after each tail venipuncture, and no animal underwent more than two tail vein sampling procedures.
2.3.3. Locomotor activity
Rats were tested for locomotor responses to MP treatment, which was measured for three consecutive days in cages similar to their home cages (50 cm × 25 cm ×30 cm high) (Mini Mitter VitalView software; Bend, Oregon). The first day was used for habituation to the experimental room; this data was discarded, and the remaining two days of locomotor data were averaged for each animal. Data was binned so as to measure activity at T = 0, 1, 2, 4, 6, 8, and 10 h post-initiation of drinking. Food was provided ad libitum, and the 8 h limited access drinking paradigm was kept in place during these tests.
2.3.4. Determination of developmental and behavioral effects of chronic MP and abstinence
2.3.4.1. Drug administration
Beginning at 4 weeks of age, rats were given limited access to their respective drinking solution for 8 h per day (9:00 h–17:00 h) in their home cages. This restricted access continued throughout the length of the experiment. Rats received either water (control), 4 mg/kg MP (low dose; LD) or 30 mg/kg MP (high dose; HD) during the first hour (09:00–10:00), and water (control), 10 mg/kg (LD) or 60 mg/kg MP (HD) for the remaining 7 h (10:00–17:00). Concentrations of MP solution were calculated daily and individually for each rat based on the animal’s weight and the average volume of the last three days’ fluid consumption. Rats were treated for three months with their respective treatment (n = 24/group), following which half of the rats in each treatment group underwent a one month abstinence period (n = 12/group), during which they were given only water to drink for the entire 8 h limited access drinking period daily.
2.3.4.2. Open field locomotor activity
Animals were run in an open-field arena photo beam activity monitoring system (Coulbourn Instruments, Allentown, PA) (dimensions 40.64 cm × 40.64 cm × 40.64 cm, 2.54 cm beam space and 1.27 cm spatial resolution) for 90 min to test locomotor activity weekly, during treatment weeks 1–11 and abstinence weeks 1–5. Tests were performed during the dark cycle between the hours of 11:00 and 17:00. Open field locomotor data was acquired with Tru Scan v2.0 software, and activity measures tested included: a) floor plane (FP) moves (the total number of start to stop movements in the X–Y plane, regardless of length or distance of movement); b) floor plane (FP) distance traveled; c) floor plane (FP) velocity; d) vertical plane (VP) entries (the total number of times the rat enters the vertical plane); e) vertical plane (VP) time (the total time the rat spends in the vertical plane); f) center entries (the number of times the rat enters the center of the arena); g) relative center distance traveled (distance traveled in the center of the arena in relation to distance traveled in the 1.9 cm margin of the arena); and h) relative center time (time spent in the center of the arena in relation to time spent in the 1.9 cm margin of the arena).
2.3.4.3. Circadian activity
Rats were tested for circadian locomotor activity: a) towards the end of chronic MP treatment (treatment weeks 12–13), and b) during the last week of the abstinence period, which was preceded by chronic MP treatment. Circadian activity was measured for three consecutive days in cages similar to their home cages (50 cm × 25 cm × 30 cm high) (Mini Mitter VitalView software; Bend, Oregon). The first day was used for habituation to the experimental room; this data was discarded, and the remaining two days of data were averaged for each animal to obtain activity levels over a 24-hour period. Throughout the three day experiment, food was provided ad libitum, and the 8 h limited access drinking paradigm was kept in place.
2.3.4.4. Statistical analysis
For the first experiment (determination of clinically relevant dosing regimens of MP), differences in the consumption of MP solutions were analyzed using a two-way ANOVA [between-subjects factors: drug; time (hour post-initiation of drinking)], and differences in locomotor activity were assessed with a two-way repeated measures ANOVA [between-subjects factor: drug; within-subjects factor: time (hour post-initiation of drinking)]. For the second experiment (determination of developmental and behavioral effects of chronic MP and abstinence), differences in body weight, food intake, and open field measures were assessed with two-way repeated measures ANOVA [between-subjects factor: drug; within-subjects factor: time (week of treatment or abstinence)]. Hourly circadian activity was assessed during treatment and abstinence separately with two-way repeated measures ANOVA [between-subjects factor: drug; within-subjects factor: time (hour of the day)]. Additionally, light and dark cycle circadian activity was assessed during treatment and abstinence with three-way repeated measures ANOVA [between-subjects factor: drug; within-subjects factors: cycle (light vs. dark cycle) and time (treatment vs. abstinence)]. When appropriate, post-hoc tests were performed to assess pairwise comparisons using the Holm–Sidak method. Statistical significance was set at α = 0.05 for all tests.
3. Results
3.1. Determination of clinically relevant dosing regimens of MP
3.1.1. Consumption of MP solutions
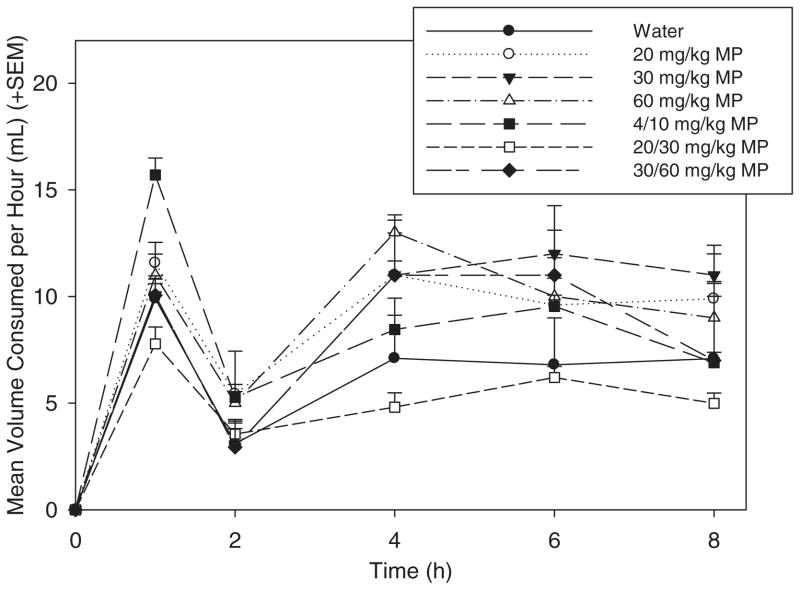
All groups of rats were tested for their fluid consumption throughout the 8 hour drinking period (Fig. 1). A two-way ANOVA showed that there was a significant effect of drug [F(6,378) = 3.713; p <0.01]. While none of the MP treatment doses resulted in decreased consumption compared to water, rats drinking the 20/30 mg/kg MP solution drank less than some of the other MP groups [20 mg/kg MP (p <0.01), 30 mg/kg MP (p <0.01), and 60 mg/kg MP (p <0.001)].
Fig. 1.
Mean (+SEM) volume consumption (mL) across treatment groups. Overall, no MP treatment group drank significantly different volumes compared to water-treated rats.
3.1.2. MP plasma levels
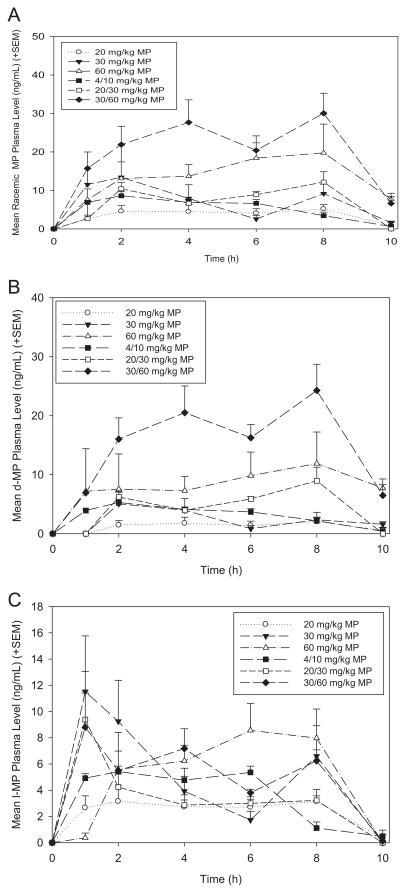
Racemic (D + L) MP plasma levels for all of the groups were tested over time (Fig. 2A). Rats treated with the 30/60 mg/kg MP dual dosage exhibited the highest plasma levels which peaked at ~30 ng/mL. Plasma levels of rats treated with 4/10 mg/kg peaked at ~8 ng/mL. The 20 mg/kg MP group delivered the lowest plasma levels, averaging less than 5 ng/mL. Other dosages peaked between ~10 and 20 ng/mL. In addition to racemic MP levels, the concentrations of the D- and L-isomers of MP were assayed and plotted separately (Fig. 2B–C).
Fig. 2.
A. Mean (+SEM) racemic (D + L) MP plasma levels (ng/mL) across treatment groups. The 30/60 mg/kg dual dosage produced the highest racemic MP plasma concentration (~30 ng/mL) within the clinically-relevant spectrum, while the 4/10 mg/kg dual dosage produced a peak concentration of ~8 ng/mL, which corresponds to the optimal plasma concentration produced in the clinical scenario. B. Mean (+SEM) D-isomer MP plasma levels (ng/mL) across treatment groups. C. Mean (+SEM) L-isomer MP plasma levels (ng/mL) across treatment groups.
3.1.3. Locomotor activity
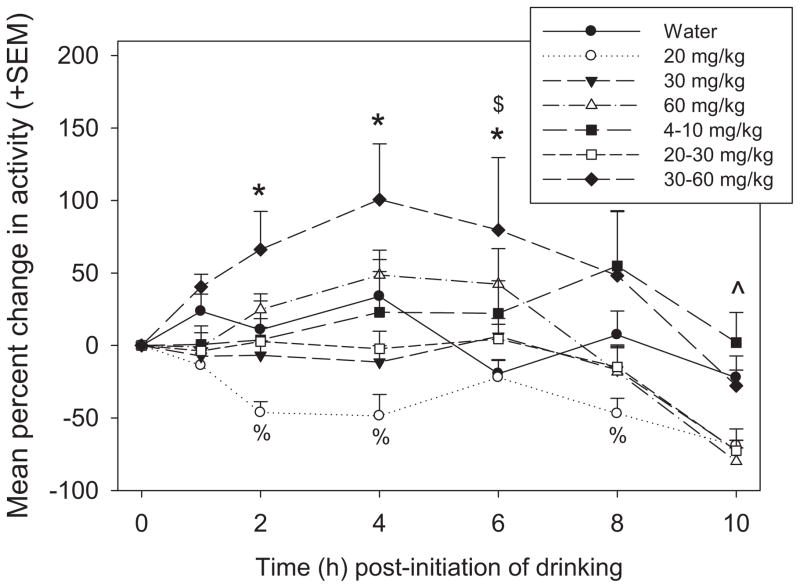
Pharmacodynamic effects of MP treatment were assessed by measuring change in locomotor activity from baseline (t = 0 h post-initiation of drinking) across the 8 hour drinking period and beyond (Fig. 3). A two-way repeated measures ANOVA revealed a significant main effect of drug [F(6,312) = 5.297, p <0.001], with pairwise comparisons showing that overall the 30/60 mg/kg group was more active than the water group (p <0.05), and the 20 mg/kg MP group was less active than the water group (p <0.01). The main effect of time was also significant [F(6,312) = 18.346, p <0.001], such that overall, rats were less active at t = 10 compared to all other time points (p <0.001 for all), and that rats were more active at t = 4 and t = 6 compared to t = 0 and t = 8 (p <0.05 for all). Additionally, the treatment × time interaction was significant [F(36,312) = 2.565, p <0.001]. Rats on 30/60 mg/kg MP were more active than rats treated with water at t = 2, 4, and 6, and 60 mg/kg rats were more active than water treated rats at t = 6 (p <0.05 for all). Treatment with 20 mg/kg resulted in hypoactivity compared to water treated rats at t = 2, 4, and 8 (p <0.05 for all). At t = 10, animals treated with 20, 30, 20/30, and 60 mg/kg MP doses were less active than rats treated with water (p <0.05 for all).
Fig. 3.
Mean (+SEM) percent change in locomotor activity (beam breaks) from baseline across all treatment groups. There were significant treatment effects at various time points after the initiation of drinking. *, 30/60 mg/kg <water; $, 60 mg/kg <water; %, 20 mg/kg <water; ^, water <20, 30, 60, and 20/30 mg/kg.
3.1.4. Determination of developmental and behavioral effects of chronic MP and abstinence
3.1.4.1. Body weight
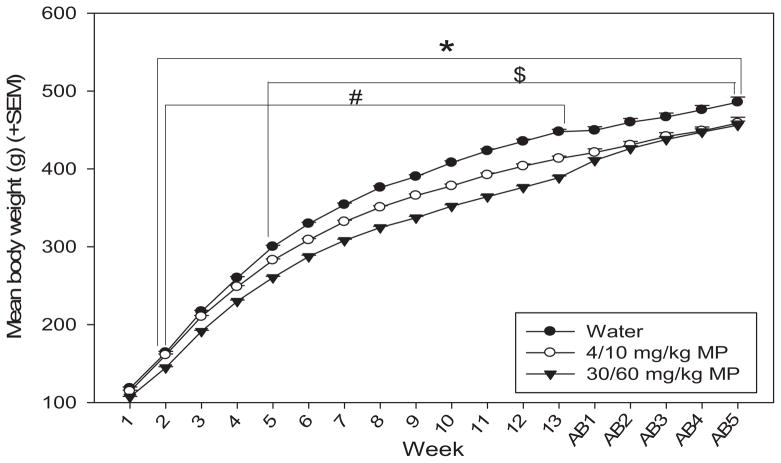
Body weight was measured daily throughout treatment and abstinence periods, and weekly averages were computed (Fig. 4). A two-way repeated measures ANOVA revealed that there was a significant main effect of time on body weight [F(17,993) = 3323.437; p <0.001], such that rats gained weight as they grew from adolescents to adults. There was also a significant main effect of drug [F(2,993) = 16.188; p <0.001], with MP dose-dependently decreasing body weight [water <LD and HD MP (p <0.01 for both), LD <HD MP (p <0.05)]. The drug × time interaction produced a significant effect on body weight as well [F(34,993) = 6.606; p <0.001]. Water treated rats weighed significantly more than both LD (treatment weeks 5–13) and HD (treatment weeks 2–13) MP rats (p <0.05 for all). Water treated rats also weighed significantly more than both MP treated groups throughout all weeks of abstinence (p <0.05 for all). HD MP rats also weighed less than LD MP rats during treatment weeks 2–13 (p <0.05 for all).
Fig. 4.
Mean (+SEM) body weight by treatment group during MP treatment and abstinence periods. Rats expectedly gained weight as they grew from adolescents to adults. MP treatment dose-dependently attenuated body weight through most of the treatment period. Control rats weighed significantly more than both LD ($p <0.05) and HD (*p <0.05) MP rats in treatment weeks 5–13 and 2–13, respectively, and throughout all weeks of abstinence. HD MP rats also weighed less than LD MP rats during treatment weeks 2–13 (#p <0.05).
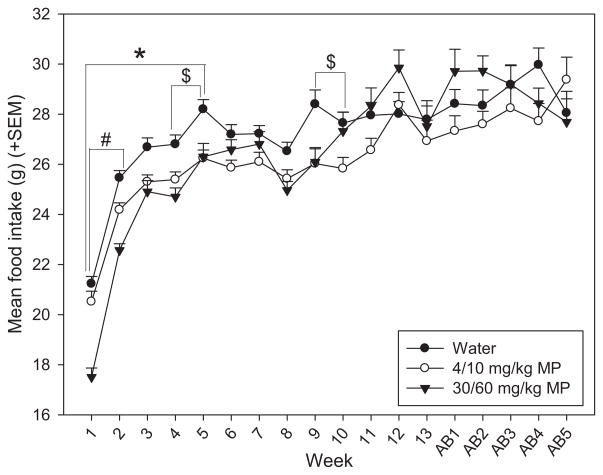
3.1.4.2. Food intake
Food intake was measured daily throughout treatment and abstinence periods, average weekly intake was computed (Fig. 5). A two-way repeated measures ANOVA found that time had a significant main effect on food intake [F(17,993) = 68.573; p <0.001], with food intake generally increasing during the treatment period as rats grew from adolescents to adults, and reaching a plateau during the abstinence period in adulthood. There was also a significant drug × time interaction effect on food intake [F(34,993) = 3.983; p <0.001]. Water treated rats ate significantly more than both LD (treatment weeks 1–5) and HD (treatment weeks 4–5 and 9–10) MP rats during early treatment (p <0.05 for all). HD MP rats also ate less than LD MP rats during treatment weeks 1–2 (p <0.05 for both).
Fig. 5.
Mean (+SEM) daily food intake by treatment group during MP treatment and abstinence periods. Generally, food intake increased over time as rats grew from adolescents to adults. MP treatment decreased food intake during some of the treatment period, particularly the first few weeks. Control rats ate significantly more than both LD ($p <0.05) and HD (*p <0.05) MP rats in treatment weeks 1–5, and 4–5 and 9–10, respectively. HD MP rats also ate less than LD MP rats during treatment weeks 1–2 (#p <0.05).
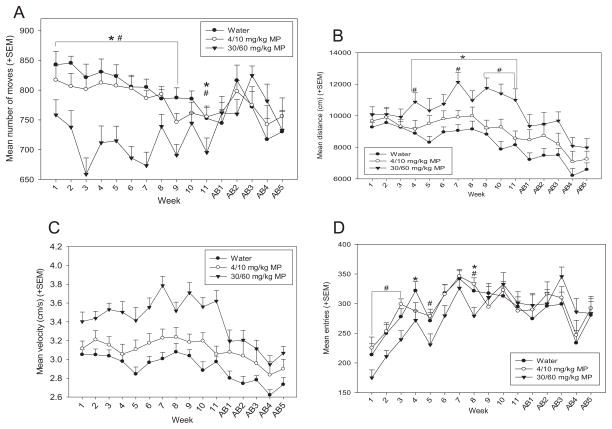
3.1.4.3. Open field
Rats were run in the open field for 90 min once per week during treatment and abstinence periods. A two-way repeated measures ANOVA found that there was a main effect of time on floor plane (FP) moves [F(15,855) = 3.433; p <0.001; Fig. 6A], and the effect of drug was significant as well [F(2,855) = 12.284; p <0.001], such that HD MP rats exhibited fewer FP moves than both LD MP and water treated rats (p <0.001 for both). The drug × time interaction was also significant [F(30,855) = 3.476; p <0.001]. HD MP treated rats performed a greater number of moves than both LD MP and water treated rats in treatment weeks 1–9 and 11 (p <0.05 for all).
Fig. 6.
A: Mean (+SEM) floor plane moves performed in the open field by treatment group during MP treatment and abstinence periods. HD MP treated rats performed a greater number of moves than both LD MP and water treated rats overall (p <0.001 for both), and in treatment weeks 1–9 and 11 (p <0.05 for all). *HD <water, #HD <LD. B: Mean (+SEM) floor plane distance traveled in the open field by treatment group during MP treatment and abstinence periods. HD MP treated rats traveled a greater distance than both LD MP (p <0.05) and water (p <0.001) treated rats overall, and in treatment weeks 4, 7, and 9–11 (#p <0.05) and 4–11 (*p <0.05), respectively. C: Mean (+SEM) floor plane (FP) velocity in the open field by treatment group during MP treatment and abstinence periods. Overall, HD MP treated rats traveled at a greater velocity than both LD MP (p <0.05) and water (p <0.001) treated rats. D: Mean (+SEM) vertical plane entries in the open field by treatment group during MP treatment and abstinence periods. HD MP rats exhibited significantly fewer rearing events than both LD MP and water treated rats in treatment weeks 1–3, 5, and 8 (#p <0.05) and 4 and 8 (*p <0.05), respectively. E: Mean (+SEM) vertical plane time in the open field by treatment group during MP treatment and abstinence periods. HD MP rats spent significantly less time rearing compared to both LD MP and water treated rats overall (p <0.01) and in treatment weeks 2, 4–9, and abstinence week 2 (#p <0.05) and treatment weeks 2, 4–9, and 11 (*p <0.05), respectively. F: Mean (+SEM) center entries in the open field by treatment group during MP treatment and abstinence periods. Overall, HD (p <0.01) and LD (p <0.05) MP rats entered the center of the arena more than water treated rats during open field runs. G: Mean (+SEM) distance traveled in the center vs. margin of the open field arena by treatment group (center distance/margin distance) during MP treatment and abstinence periods. HD rats traveled a greater distance in the center of the arena compared to LD MP (#p <0.01) and water (*p <0.01) treated rats in treatment weeks 10–11 and in the second and fifth weeks of abstinence. H: Mean (+SEM) time spent in the center vs. margin of the open field arena by treatment group (center time/margin time) during MP treatment and abstinence periods. HD rats spent more time in the center of the arena compared to water treated rats in treatment weeks 10–11 and the second week of abstinence (*p <0.01), and compared to LD rats in treatment weeks 9–11 and the second week of abstinence (#p <0.05).
A two-way repeated measures ANOVA found that there was a significant effect of drug on floor plane (FP) distance traveled [F(2,855) = 7.936; p <0.001; Fig. 6B]: HD MP rats traveled a greater distance in the open field than both LD MP (p <0.05) and water (p <0.001) treated rats. The main effect of time was also significant [F(15,971) = 11.775; p <0.001], with activity decreasing throughout the treatment and abstinence periods. The drug × time interaction also reached significance for FP distance traveled [F(30,855) = 1.7366; p <0.01]. HD MP treated rats traveled a greater distance than both LD MP (treatment weeks 4, 7, and 9–11) and water (treatment weeks 4–11) treated rats (p <0.05 for all).
A two-way repeated measures ANOVA found that the main effect of drug on floor plane (FP) velocity was significant [F(2,855) = 14.010; p <0.001; Fig. 6C], with HD MP rats moving at a greater velocity than both LD MP (p <0.01) and water (p <0.001) treated rats. Time also had a significant main effect on velocity [F(15,855) = 3.464; p <0.001], such that rats moved with decreasing speed during abstinence weeks compared to treatment weeks.
A two-way repeated measures ANOVA revealed a significant main effect of time on vertical plane entries [F(15,855) = 20.452; p <0.001; Fig. 6D], with an increase in behavior from weeks 1 through 7, and remaining steady thereafter through the abstinence period. The drug × time interaction also had a significant effect on vertical plane entries [F(30,855) = 1.773; p <0.05]. HD MP rats exhibited significantly fewer rearing events than both LD MP (treatment weeks 1–3, 5, and 8) and water (treatment weeks 4 and 8) treated rats (p <0.05 for all).
A two-way repeated measures ANOVA revealed a significant main effect of drug on vertical plane time [F(2,855) = 6.529; p <0.01; Fig. 6E], such that HD MP rats displayed less rearing time than both water and LD MP treated rats (p <0.01 for both). The main effect of time was also significant [F(15,855) = 44.447; p <0.001], with an increase in rearing from weeks 1 through 7, and remaining steady thereafter through the abstinence period. The drug × time interaction also produced significant effects [F(30,971) = 1.928; p <0.05]. HD MP rats spent significantly less time rearing compared to both LD MP (treatment weeks 2, 4–9, and abstinence week 2) and water (treatment weeks 2, 4–9, and 11) treated rats (p <0.05 for all).
A two way repeated measures ANOVA found that drug had a significant main effect on center entries [F(2,855) = 5.884; p <0.01; Fig. 6F], such that HD (p <0.01) and LD (p <0.05) MP rats entered the center of the arena more than water treated rats during open field runs. Time also had a significant effect [F(15,855) = 13.085; p <0.001], with a pattern of rats increasing center entries through treatment weeks, followed by a subsequent decrease in abstinence weeks.
It could be speculated that the MP rats appeared to display increased center activity simply because they exhibited greater general floor plane activity. Therefore, additional two way repeated measures ANOVAs were performed to assess time spent in the center of the arena compared to the margin of the arena (Fig. 6G), as well as distance traveled in the center vs. margin of the arena (Fig. 6H), to determine relative center activity. There was a significant main effect of drug on both relative center distance [F(2,855) = 6.882; p <0.01] and time [F(2,855) = 3.576; p <0.05]. While HD rats expressed greater relative center distance compared to both LD MP and water treated rats (p <0.01 for both), pairwise comparisons showed no significant differences between groups on relative center time. The main effect of time was significant as well for relative center distance [F(15,855) = 38.373; p <0.001] and time [F(15,855) = 32.580; p <0.001], with a general pattern of increasing relative center activity for both measures. The drug × time interaction effects were also significant for relative center distance [F(30,855) = 2.440; p <0.001] and relative center time [F(30,855) = 2.440; p <0.001]. HD rats traveled a greater relative distance in the center of the arena compared to LD MP and water treated rats in treatment weeks 10–11 and in the second and fifth weeks of abstinence (p <0.01 for all). HD rats spent more time in the center of the arena compared to water treated rats in treatment weeks 10–11 and the second week of abstinence (*p <0.01), and compared to LD rats in treatment weeks 9–11 and the second week of abstinence (#p <0.05).
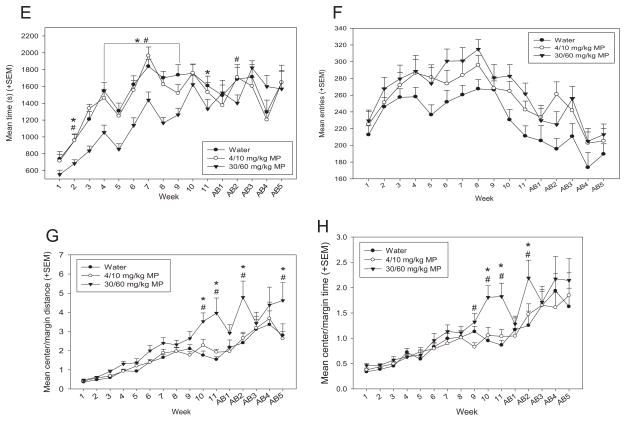
3.1.4.4. Circadian activity
Circadian locomotor activity was measured for three consecutive days; the first day was used for habituation (data discarded), and data for last two days were averaged for each animal (30 minute bins over the 24 hour period). This test was performed during the last two weeks of MP treatment (Fig. 7A) and during the last week of the abstinence period (Fig. 7B) on different cohorts of rats. Separate two-way repeated measures ANOVAs were conducted for treatment and abstinence. The main effect of drug had significant effects during MP treatment [F(2,987) = 20.459; p <0.001] and abstinence [F(2,1551) = 4.738; p <0.02]: HD MP rats were hyperactive compared to both water and LD MP treated rats at both times of testing (p <0.05 for all). During both MP treatment [F(47,987) = 28.490; p <0.001] and abstinence [F(47,1551) = 42.694; p <0.001], time had a significant main effect on circadian activity, such that rats displayed normal variation in activity levels characteristic of rodents throughout the circadian cycle. The drug × time interaction produced significant effects on circadian activity during MP treatment [F(94,987) = 4.309; p <0.001]. HD MP treatment resulted in increased activity compared to both LD MP (09:30–19:00 h) and water (09:00–17:30 h) treatment at specific times during the dark cycle. LD MP treatment decreased activity compared to water treated rats at a few time points during the dark cycle, 10:00–10:30 and 13:00–13:30 (p <0.05 for both).
Fig. 7.
A: Mean (+SEM) activity over the circadian cycle by treatment group during MP treatment. A normal circadian cycle was exhibited by all groups, with no apparent shift in cycle. HD MP treatment resulted in hyperactivity compared to both LD MP and water treatment overall (p <0.05), and at specific times during the dark cycle: 09:00–17:30 (#p <0.05) and 09:30–19:00 (*p <0.05), respectively. LD MP treatment decreased activity compared to controls at a few time points during the dark cycle, 10:00–10:30 and 13:00–13:30 ($p <0.05). B: Mean (+SEM) activity over the circadian cycle by treatment group during abstinence. A normal circadian cycle was exhibited by all groups, with no apparent shift in cycle. HD MP treatment resulted in hyperactivity compared to both LD MP and water treatment overall (p <0.05). C: Mean (+SEM) total activity during the dark and light cycles by treatment group during treatment (TX) and abstinence (AB). During treatment, HD MP resulted in increased activity (vs. water *p <0.001; vs. LD MP #p <0.001) and LD MP resulted in decreased activity during the dark compared to water ($p <0.05). During abstinence, previously-treated HD rats were more active during the dark cycle than water (*p <0.01) and previously-treated LD MP rats (#p <0.01). Dark cycle activity of HD MP rats was greater during treatment than during abstinence (^p <0.001).
Additionally, total activity was calculated during the dark and light cycles during MP treatment and abstinence. A three-way repeated measures ANOVA revealed a significant main effect of drug [F(2,54) = 37.847; p <0.001], such that HD rats were more active than LD and water treated rats (p <0.001 for both). There was also a significant drug × time interaction [F(2,54) = 8.289; p <0.001], with HD rats on MP treatment being more active than any other treatment groups at either time point (treatment or abstinence) (p <0.01 for all). Additionally, previously treated HD rats were more active than previously treated LD rats during abstinence (p = 0.01). The main effect of cycle was significant [F(1,54) = 797.610; p <0.001], with rats being more active during the dark cycle compared to the light cycle (p <0.001). The cycle × drug interaction was also significant [F(2,54) = 50.001; p <0.001]. All treatment groups were more active in the dark cycle compared to the light cycle (p <0.001 for all), and dark cycle activity exhibited by HD rats was greater compared to both water and LD rats (p <0.001 for both). The cycle × time interaction was significant as well [F(2,54) = 16.856; p <0.001]. During both treatment and abstinence, rats were more active during the dark cycle than the light cycle (p <0.001 for both). Additionally, rats were more active during treatment compared to abstinence during the dark cycle only (p <0.05). Lastly, the drug × cycle × time interaction was significant [F(2,54) = 18.681; p <0.001]. During treatment, HD MP resulted in increased activity and LD MP resulted in decreased activity during the dark cycle (p <0.05 for both). During abstinence, previously treated HD rats were more active during the dark cycle than water and previously treated LD MP rats (p <0.05 for both). Dark cycle activity of HD MP rats was attenuated following abstinence compared to treatment levels (p <0.05) (Table 1).
Table 1.
SPM results.
| Cluster-level
|
Peak-level
|
||||||
|---|---|---|---|---|---|---|---|
| PFWE-corr | qFDR-corr | KE | Puncorr | T | Z | mm mm mm | Region |
| RYGB (chow < bacon) | |||||||
| 0.01 | 0.01 | 1390 | <0.001 | 41.32 | 4.75 | 1.8 5.6 –12.8 | Right cerebellum (lob. 8) |
| <0.001 | 19.33 | 4.1 | 2.0 7.4 –8.6 | Right MPB, DMTg | |||
| <0.001 | 18.53 | 4.05 | 0.5 5.5 –13.0 | Midline cerebellum (lob. 8) | |||
| High-fat diet (AL) (chow <bacon) | |||||||
| 0.022 | 0.015 | 1499 | <0.001 | 16.02 | 4.3 | 2.2 1.4 –7.4 | Retrosplenial cortex (RS)/left primary visual cortex (V1M) |
| <0.001 | 15.9 | 4.29 | 3.4 2.5 –9.8 | Left cerebellum (Sim) | |||
| <0.001 | 14.8 | 4.21 | 4.0 3.5 –10.2 | Left cerebellum (SimB) | |||
Statistical parametric mapping (SPM) results showing significant clusters and statistical parameters for the contrast bacon <chow in rats that underwent gastric bypass surgery (RYGB) and sham-operated controls (AL). The contrast chow <bacon did not yield any significant clusters. (MPB) medial parabrachial nucleus, (DMTg) dorsomedial tegmental area, (Sim) simple lobule.
4. Discussion
The increasing use and abuse of MP, particularly during critical stages of neurodevelopment, presents concerns of subsequent neurobiological, developmental, and behavioral effects and makes necessary preclinical studies that can better assess the extent and mechanism of these effects. Although rodent studies have been conducted, a vast majority of these studies administer MP in a way that is not relevant to clinical applications [e.g. intravenously (IV), intraperitoneally (IP), or subcutaneously], and even studies using oral dosing are inconsistent in achieving and maintaining clinically-relevant plasma MP concentrations (Kuczenski and Segal, 2005) (Wargin et al., 1983; Gerasimov et al., 2000; Kuczenski and Segal, 2001; Ding et al., 2004). Therefore, the aim of the current study was to establish and describe a paradigm for the oral administration of MP in rats that would better mimic the clinical scenario and determine developmental and behavioral consequences of chronic treatment and abstinence.
4.1. Determination of clinically relevant dosing regimens of MP
MP plasma levels of rats from each treatment group were assessed between 1 and 10 h post-initiation of drinking to determine which treatments best model the pharmacokinetic profile of MP used in clinical applications. When MP is used to treat ADHD in children, oral doses of 0.25–1 mg/kg MP are prescribed, which result in plasma concentrations of 8–40 ng/mL (Swanson et al., 1999; Swanson and Volkow, 2002). The highest dual bottle dosage (30/60 mg/kg; HD) produced the greatest racemic MP plasma concentration, with the mean plasma concentration peaking at just over 30 ng/mL by the end of the eight-hour drinking period, which is near the higher range of the clinical spectrum. Studies suggest that the full pharmacodynamic and therapeutic effects of MP are a result of the D-isomer (Srinivas et al., 1992; Davids et al., 2002; Ding et al., 2004; Quinn, 2008), while the L-MP isomer seems to have little to no effect on the behavioral effects of MP (Markowitz and Patrick, 2008). Therefore, assaying the D- and L-isomers of MP separately was necessary to fully understand the pharmacokinetic profiles of these dosages. The HD treatment also produced the highest plasma concentration of the functional D-isomer, peaking at nearly 25 ng/mL by hour eight, which is at the high range of concentrations that has been seen to be produced in clinical studies (Teicher et al., 2006). Additionally, HD treatment produced the greatest increase in locomotor activity over the drinking period. Locomotor activity exhibited by this group was stably increased over controls between hours two and six post-initiation of drinking and peaked at hour four, indicating a robust and long-lasting pharmacodynamic effect. Taken together, these findings suggest that this 30/60 mg/kg dual dosage would be useful in future MP experiments as a clinically relevant high dose.
The lowest dual bottle dosage of 4/10 mg/kg (LD) resulted in a racemic MP plasma concentration that peaks at about 8 ng/mL. This concentration is comparable to clinically-used oral doses of approximately 0.3 mg/kg, which lead to plasma concentrations of approximately 8–10 ng/mL in children (Swanson et al., 1999). This dual dosage produced a peak D-MP concentration between 4 and 5 ng/mL in plasma, which in humans has been shown to block about 50% of striatal dopamine transporter (Volkow et al., 1998), while having no significant effect on locomotor activity. Therefore, in the paradigm reported here, pharmacokinetic profiles are produced in the rat with oral dosing of MP that mimics those formulations now in clinical use to treat ADHD and thus present a valuable animal model of studying the effects of MP treatments in rodent models.
We observed a trend such that the drinking behavior of rats on an 8 hour restricted drinking regimen were marked by high consumption in the first hour, followed by a steady consumption of smaller amounts over the next few hours with a smaller peak later, in agreement with one of our previous studies (Thanos et al., 2004). Due to this consumption pattern, an initial bolus-like dosage could be delivered, followed by steady and then slightly increased intake to maintain this peak effect, which is similar to the delivery design of commonly prescribed drugs shown to be effective in treating ADHD (Swanson et al., 2002). Additionally, we found that administering MP in rats’ drinking water does not appear to significantly alter fluid consumption, which is in agreement with both early preclinical (Barone et al., 1979) and clinical (Conners, 1975) studies.
4.2. Determination of developmental and behavioral effects of chronic MP and abstinence
We then tested these two clinically relevant dual dosage paradigms [4/10 mg/kg (LD) and 30/60 mg/kg (HD) MP] to assess their effects on development and behavior following chronic treatment and abstinence.
Methylphenidate (MP) decreased food intake during early weeks of treatment, suggesting that MP is an appetite suppressant, particularly in the short-term. MP treatment also dose-dependently decreased body weight compared to the water group. During abstinence, body weight of HD MP rats rebounded to that of LD MP rats; however, both groups still weighed significantly less than water treated rats. These findings agree with previous studies that have shown that MP treatment reduces appetite and food intake, and results in weight loss in rodents and humans (Heffner et al., 1977; Vanina et al., 2002; Leddy et al., 2004; Gray et al., 2007). Our study found that the effects of MP on body weight, however, far outlast its effects on appetite, which suggests that MP may reduce body weight by increasing energy expenditure, speeding metabolism and/or suppressing growth. Additionally, the attenuation of body weight persisted throughout abstinence. Clinical studies have found that stimulant treatment of ADHD results in decreased height and body weight, though these effects were ameliorated with the cessation of treatment, and ultimate growth parameters were not affected (Safer et al., 1972; Mattes and Gittelman, 1983; Faraone et al., 2008). It is also possible that our treatment did affect ultimate growth parameters or that the abstinence period was not long enough to see a rebound effect. It appears that the clinical effects of MP on reducing body weight may be less drastic than that observed here, possibly due to the drug’s locomotor-attenuating effect (reduced energy expenditure) in treated patients.
Open field activity was recorded once per week during treatment and abstinence periods. During most of the treatment period, HD MP rats displayed hyperactivity compared to controls, as measured by distance traveled. These effects were greatest during later weeks, suggesting sensitization to the drug, which is in agreement with previous studies (Kuczenski and Segal, 2001; Yang et al., 2003). Displays of behavioral sensitization to a psychostimulant present concerns, as it provides evidence for persistent neurological changes in circuitry involved in motivation and reward (Robinson, 1993). While open field velocity was also increased by HD MP treatment, the number of floor plane moves performed by this treatment group was reduced. These results suggest that HD MP treatment likely results in increased ambulation rather than stereotypic-like behavior.
HD MP treatment also reduced rearing activity in the open field (vertical plane entries and time), with behaviors normalizing during abstinence. Attenuated rearing during MP treatment is in agreement with a previous study (Wultz et al., 1990), and it is possible that the MP-induced hyperactivity in the horizontal plane hindered vertical plane activity, though MP has been shown to increase both measures in some cases (Izenwasser et al., 1999). Rearing can also be an indicator of exploratory behavior, and interpreting this behavior as such is in agreement with previous findings that MP treatment diminishes exploration, as well as preference for novelty (Hughes, 1972; Misslin and Ropartz, 1981; Heyser et al., 2004).
It was also seen that HD MP treated rats displayed increased center activity (center entries, relative center distance, and relative center time) compared to the water group during the treatment period, specifically during later weeks of treatment. Increased center activity is an indicator of an anxiolytic effect (Fernández-Teruel et al., 1992). This is in agreement with previous studies on rats that have found that MP treatment decreases anxiety in other tests, such as the elevated plus maze (Zhu et al., 2010). It has also been reported that clinically treated ADHD patients taking MP report decreased anxiety (Barrickman et al., 1995; Bouffard et al., 2003). It is also possible that cognitive processes (e.g. attention) were negatively affected by MP treatment in these animals, leading to poor discrimination of “safe” versus “unsafe” areas. MP’s deleterious effects on cognitive processes in non-ADHD rodent models have been demonstrated previously (Ferguson et al., 2007; Thanos et al., 2010). Therefore, it would be beneficial to assess additional aspects of anxiety (e.g. social anxiety) in the future.
Circadian activity testing showed that all MP groups, during treatment and abstinence, exhibited a generally normal pattern of circadian activity, with rats being more active during the dark cycle than the light cycle. MP treatment did, however, affect activity levels during the dark phase. During treatment, LD MP decreased activity at a few time points in the early to mid-dark phases, as well as total activity in the dark phase. This is in agreement with a previous study that found that low doses of oral MP have been shown to decrease activity in rodents when given at a dosage that produces comparable plasma concentrations (Kuczenski and Segal, 2002). We did not see this effect in the open field, possibly because circadian tests were performed in a home cage-like setting, while open field tests were performed in a different environment. In contrast, HD MP treatment resulted in hyperlocomotion throughout most of the dark phase, corroborating open field results. Activity levels of LD MP rats returned to normal following the abstinence period, while those of HD MP rats were reduced but remained significantly elevated over controls in the dark phase. These results suggest that chronic MP treatment increases the magnitude of activity during the dark cycle, but does not alter or shift the pattern of circadian activity. Light cycle activity remained unaffected, suggesting that these doses of MP do not inhibit normal sleep. Despite concern over MP-induced sleep disturbances (Schwartz et al., 2004; Sangal et al., 2006), our findings are in agreement with previous clinical studies, which found that MP had no significant effect on multiple sleep parameters (Tirosh et al., 1993; Kent et al., 1995). It is possible that sleep disturbances are not seen due to our dosing schedule (dosing ended at 17:00 h and the light cycle began at 20:00 h) and the speed of MP’s me tabolism in rats compared to humans (~1 h vs. ~3 h, respectively) (Patrick et al., 1984; Aoyama et al., 1990; Patrick and Markowitz, 1997; Thai et al., 1999).
5. Conclusion
The impetus for this study was the concern about the widespread prescribed or illicit use of MP by both children and adults. Concerns have arisen regarding chronic MP exposure, since it may produce long-term developmental or behavioral effects, as well as sensitization to the effects of other psychostimulants such as cocaine or metham phetamine, leading to an increased vulnerability to stimulant abuse later in life (Volkow et al., 1999; Thanos et al., 2007). The current study found that chronic MP exposure leads to alterations in body weight, food consumption, locomotor activity, and measures of explora tion and anxiety, with some of these measures being affected even after an extended period of abstinence. Results suggest the need for studies with longer treatment length, as many observed effects took several weeks to appear, and most prior studies on MP have only dosed for ~1–2 weeks or less. Additional pharmacokinetic studies of MP metabo lism in females and in different strains of rats will need to be performed, as first-pass hepatic metabolism of MP may vary. In conclusion, these results and model provide a critical foundation for further animal studies to examine the effects of acute or chronic MP administration.
Acknowledgments
This work was supported by NIAAA and NICHD.
References
- Aoyama T, Kotaki H, Iga T. Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. J Pharmacobiodyn. 1990;13:647. doi: 10.1248/bpb1978.13.647. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Barone F, Wayner M, Lee H, Tsai W, Dehaven D, Woodson WJ. Effects of methylphenidate on food and water consumption at different body weights. Pharmacol Biochem Behav. 1979;10:591–5. doi: 10.1016/0091-3057(79)90238-7. [DOI] [PubMed] [Google Scholar]
- Barrickman LL, Perry PJ, Allen AJ, Kuperman S, Arndt SV, Herrmann KJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:649–57. doi: 10.1097/00004583-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Stat. 2012:10. [PubMed] [Google Scholar]
- Bogle KE, Smith BH. Illicit methylphenidate use: a review of prevalence, availability, pharmacology, and consequences. Curr Drug Abuse Rev. 2009;2:157–76. doi: 10.2174/1874473710902020157. [DOI] [PubMed] [Google Scholar]
- Bouffard R, Hechtman L, Minde K, Iaboni-Kassab F. The efficacy of 2 different dosages of methylphenidate in treating adults with attention-deficit hyperactivity disorder. Can J Psychiatry. 2003;48:546–54. doi: 10.1177/070674370304800806. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the ac tivity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–44. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur J Neurosci. 2003;18:1584–92. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17–21. [PubMed] [Google Scholar]
- Conners CK. Controlled trial of methylphenidate in preschool children with minimal brain dysfunction. Int J Ment Health. 1975:61–74. [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl) 2002;160:92–8. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- Ding YS, Gatley SJ, Thanos PK, Shea C, Garza V, Xu Y, et al. Brain kinetics of methylpheni date (Ritalin) enantiomers after oral administration. Synapse. 2004;53:168–75. doi: 10.1002/syn.20046. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994–1009. doi: 10.1097/CHI.ObO13e31817eOea7. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Paule MG, Cada A, Fogle CM, Gray EP, Berry KJ. Baseline behavior, but not sensitivity to stimulant drugs, differs among spontaneously hypertensive, Wistar–Kyoto, and Sprague–Dawley rat strains. Neurotoxicol Teratol. 2007;29:547–61. doi: 10.1016/j.ntt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Fernández-Teruel A, Escorihuela R, Driscoll P, Tobeña A, Bättig K. Differential effects of early stimulation and/or perinatal flumazenil treatment in young Roman low- and high-avoidance rats. Psychopharmacology (Berl) 1992;108:170–6. doi: 10.1007/BF02245303. [DOI] [PubMed] [Google Scholar]
- Garnier-Dykstra LM, Caldeira KM, Vincent KB, O’Grady KE, Arria AM. Nonmedical use of prescription stimulants during college: four-year trends in exposure opportunity, use, motives, and sources. J Am Coll Health. 2012;60:226–34. doi: 10.1080/07448481.2011.589876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, et al. Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–7. [PubMed] [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, et al. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci. 2007;27:7196–207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund T, Lehmann K, Bock N, Rothenberger A, Teuchert-Noodt G. Influence of methylphenidate on brain development—an update of recent animal experiments. Behav Brain Funct. 2006;2:2. doi: 10.1186/1744-9081-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner TG, Zigmond MJ, Stricker EM. Effects of dopaminergic agonists and antagonists of feeding in intact and 6-hydroxydopamine-treated rats. J Pharmacol Exp Ther. 1977;201:386–99. [PubMed] [Google Scholar]
- Heyser CJ, Pelletier M, Ferris JS. The effects of methylphenidate on novel object exploration in weanling and periadolescent rats. Ann N Y Acad Sci. 2004;1021:465–9. doi: 10.1196/annals.1308.066. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Methylphenidate induced inhibition of exploratory behaviour in rats. Life Sci I. 1972;11:161–7. doi: 10.1016/0024-3205(72)90257-3. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Coy A, Ladenheim B, Loeloff R, Cadet J, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol. 1999;373:187–93. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Justo CC, Carneirode-Oliveira PE, Delucia R, Aizenstein ML, Planeta CS. Repeated exposure of adolescent rats to oral methylphenidate does not induce behavioral sensitization or cross-sensitization to nicotine. Braz J Med Biol Res. 2010;43:651–6. doi: 10.1590/s0100-879x2010007500042. [DOI] [PubMed] [Google Scholar]
- Kent JD, Blader JC, Koplewicz HS, Abikoff H, Foley CA. Effects of late-afternoon methylphenidate administration on behavior and sleep in attention-deficit hyperactivity disorder. Pediatrics. 1995;96:320–5. [PubMed] [Google Scholar]
- Komatsu DE, Thanos PK, Mary MN, Janda HA, John CM, Robison L, et al. Chronic exposure to methylphenidate impairs appendicular bone quality in young rats. Bone. 2012;50:1214–22. doi: 10.1016/j.bone.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–83. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–71. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/ hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–6. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- LeBlanc-Duchin D, Taukulis HK. Chronic oral methylphenidate administration to periadolescent rats yields prolonged impairment of memory for objects. Neurobiol Learn Mem. 2007;88:312–20. doi: 10.1016/j.nlm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Epstein LH, Jaroni JL, Roemmich JN, Paluch RA, Goldfield GS, et al. Influence of methylphenidate on eating in obese men. Obes Res. 2004;12:224–32. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol. 2008;28:S54–61. doi: 10.1097/JCP.0b013e3181733560. [DOI] [PubMed] [Google Scholar]
- Mattes JA, Gittelman R. Growth of hyperactive children on maintenance regimen of methylphenidate. Arch Gen Psychiatry. 1983;40:317–21. doi: 10.1001/archpsyc.1983.01790030087011. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Knight JR, Wechsler H. Nonmedical use of prescription opioids among U.S. college students: prevalence and correlates from a national survey. Addict Behav. 2005;30:789–805. doi: 10.1016/j.addbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Misslin R, Ropartz P. Effects of methamphetamine on novelty-seeking behaviour by mice. Psychopharmacology (Berl) 1981;75:39–43. doi: 10.1007/BF00433499. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Ellington KR, Breese GR. Distribution of methylphenidate and p-hydroxymethylphenidate in rats. J Pharmacol Exp Ther. 1984;231:61–5. [PubMed] [Google Scholar]
- Patrick KS, Markowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol Clin Exp. 1997;12:527–46. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Quinn D. Does chirality matter? Pharmacodynamics of enantiomers of methylphenidate in patients with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2008;28:S62–6. doi: 10.1097/JCP.0b013e3181744aa6. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Persistent sensitizing effects on drugs on brain dopamine systems and behavior: implications for addiction and relapse. In: Korenman SG, Barchas JD, editors. Biological basis of substance abuse. NY: Oxford University Press; 1993. [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robison L, Ananth M, Swanson J, Robinson J, Wang G-J, Volkow N, et al. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Effect of chronic oral methylphenidate treatment and abstinence on dopamine transporter (DAT), D1R, and D2R binding in young rats. Program No. 871.07. Online; 2012. [Google Scholar]
- Robison LS, Ananth M, Johnson S, Clark J, Murmello M, Malitsis NG, et al. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. Effects of chronic oral methylphenidate exposure on body weight, food intake, circadian activity, open field activity and novel object recognition in rats. Program No. 367.4. Online; 2010. [Google Scholar]
- Safer D, Allen R, Barr E. Depression of growth in hyperactive children on stimulant drugs. N Engl J Med. 1972;287:217–20. doi: 10.1056/NEJM197208032870503. [DOI] [PubMed] [Google Scholar]
- Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006;29:1573–85. doi: 10.1093/sleep/29.12.1573. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Amor LB, Grizenko N, Lageix P, Baron C, Boivin DB, et al. Actigraphic monitoring during sleep of children with ADHD on methylphenidate and placebo. J Am Acad Child Adolesc Psychiatry. 2004;43:1276–82. doi: 10.1097/01.chi.0000135802.94090.93. [DOI] [PubMed] [Google Scholar]
- Srinivas NR, Hubbard JW, Quinn D, Midha KK. Enantioselective pharmacokinetics and pharmacodynamics of DL-threomethylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52:561–8. doi: 10.1038/clpt.1992.185. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Lerner M, Wigal T, Steinhoff K, Greenhill L, Posner K, et al. The use of a laboratory school protocol to evaluate concepts about efficacy and side effects of new formulations of stimulant medications. J Atten Disord. 2002;6(Suppl 1):S73–88. doi: 10.1177/070674370200601s10. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–8. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Psychopharmacology: concepts and opinions about the use of stimulant medications. J Child Psychol Psychiatry. 2009;50:180–93. doi: 10.1111/j.1469-7610.2008.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, Foley M, Valente E, McGreenery CE, Chang WW, et al. Methylphenidate blood levels and therapeutic response in children with attention-deficit hyperactivity disorder: I. Effects of different dosing regimens. J Child Adolesc Psychopharmacol. 2006;16:416–31. doi: 10.1089/cap.2006.16.416. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–10. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai DL, Yurasits LN, Rudolph GR, Perel JM. Comparative pharmacokinetics and tissue distribution of the D-enantiomers of para-substituted methylphenidate analogs. Drug Metab Dispos. 1999;27:645–50. [PubMed] [Google Scholar]
- Thanos P, Bermeo C, Rubinstein M, Suchland K, Wang G, Grandy D, et al. Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J Psychopharmacol. 2009 doi: 10.1177/0269881109102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang G-J, Swanson JM, et al. Dissociation between spontaneously hypertensive (SHR) and Wistar–Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a visual stimulus position discrimination task. Pharmacol Biochem Behav. 2010;94:374–9. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–33. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Taintor N, Rivera SN, Umegaki H, Ikari H, Roth G, et al. DRD2 gene transfer into the nucleus accumbens of the alcohol preferring (P) and non preferring (NP) rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–8. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Tirosh E, Sadeh A, Munvez R, Lavie P. Effects of methylphenidate on sleep in children with attention-deficit hyperactivity disorder: an activity monitor study. Arch Pediatr Adolesc Med. 1993;147:1313. doi: 10.1001/archpedi.1993.02160360055018. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- Vanina Y, Podolskaya A, Sedky K, Shahab H, Siddiqui A, Munshi F, et al. Body weight changes associated with psychopharmacology. Psychiatr Serv. 2002;53:842–7. doi: 10.1176/appi.ps.53.7.842. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increase in brain dopamine and occupancy of D2 receptors. J Pharmacol Exp Ther. 1999;291:409–15. [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiatry. 2003;54:1307–9. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–31. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, et al. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226:382–6. [PubMed] [Google Scholar]
- Wilens T, Adler L, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylphenidate on exploratory behavior. Behav Neural Biol. 1990;53:88–102. doi: 10.1016/0163-1047(90)90848-z. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–52. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. The multifaceted effects of oral administration of methylphenidate in juvenile rats: anxiety, activity, and attention. Eur Neuropsychopharmacol. 2010;20:236–44. doi: 10.1016/j.euroneuro.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]