Abstract
Transient gamma-band (40–80 Hz) spatiotemporal patterns are hypothesized to play important roles in cortical function. Here we report the direct observation of gamma oscillations as spatiotemporal waves induced by targeted optogenetic stimulation, recorded by intracortical multichannel extracellular techniques in macaque monkeys during their awake resting states. Microelectrode arrays integrating an optical fiber at their center were chronically implanted in primary motor (M1) and ventral premotor (PMv) cortices of two subjects. Targeted brain tissue was transduced with the red-shifted opsin C1V1(T/T). Constant (1-s square pulses) and ramp stimulation induced narrowband gamma oscillations during awake resting states. Recordings across 95 microelectrodes (4 × 4-mm array) enabled us to track the transient gamma spatiotemporal patterns manifested, e.g., as concentric expanding and spiral waves. Gamma oscillations were induced well beyond the light stimulation volume, via network interactions at distal electrode sites, depending on optical power. Despite stimulation-related modulation in spiking rates, neuronal spiking remained highly asynchronous during induced gamma oscillations. In one subject we examined stimulation effects during preparation and execution of a motor task and observed that movement execution largely attenuated optically induced gamma oscillations. Our findings demonstrate that, beyond previously reported induced gamma activity under periodic drive, a prolonged constant stimulus above a certain threshold may carry primate motor cortex network dynamics into gamma oscillations, likely via a Hopf bifurcation. More broadly, the experimental capability in combining microelectrode array recordings and optogenetic stimulation provides an important approach for probing spatiotemporal dynamics in primate cortical networks during various physiological and behavioral conditions.
Keywords: gamma oscillations, collective dynamics, cortical waves, neocortex
spatiotemporal gamma oscillations in neocortex are hypothesized to play important roles in neural representation, computation, and the shaping of communication among cortical neurons (Bressler 1990; Bressler et al. 1993; Bressler and Freeman 1980; Fries 2009; Gray et al. 1989; Gray and Singer 1989; Singer 2011). In addition, disruption of these spatiotemporal oscillations may play a role in neurological and neuropsychiatric disorders (Bressler 2003; Traub et al. 2005; Uhlhass and Singer 2012). Recent studies have used optogenetic stimulation to probe the mechanisms and neural circuits underlying the generation of gamma oscillations in neocortex (e.g., Cardin et al. 2009; Sohal et al. 2009). In particular, mouse models have been used to demonstrate the generation of gamma oscillations in the somatosensory cortex, by activation of either fast-spiking interneurons (Cardin et al. 2009) or layer 2–3 pyramidal cells with continuously incrementing light stimuli (Adesnik and Scanziani 2010). Despite these advances, most optogenetic studies have focused on mouse/rodent models and little is known about the generation of transient gamma oscillations in primate neocortex. In addition, rather than utilizing microelectrode arrays to cover an area over extended cortical regions, most previous studies have been focused on independent electrode recordings of a few neurons and local field potentials (LFPs) around the optical stimulus sites, thereby limiting the assessment of the spatiotemporal features of transient gamma oscillations, especially regarding their propagation as cortical waves and the roles of network activity through cortical circuits.
Here we studied how transient gamma oscillation spatiotemporal patterns can be generated in primate large-scale neocortical networks, using a novel technique to record neuronal ensemble spiking activity and high-density LFPs during simultaneous optogenetic stimulation. We developed an optogenetic probe specifically designed for primate chronic implantation based on the framework of our previously reported device (Wang et al. 2012). Specifically, the new probe used a microelectrode array for ensemble recordings, integrated with a plastic optical fiber in the center, whose flexibility was critical for chronic implantation into the primate brain. We expressed the red-shifted opsin C1V1(T/T) under the control of the CaMKIIα promoter in either primary motor cortex (MI) or ventral premotor cortex (PMv) in two macaque monkeys. Gamma oscillations were generated reliably and could be reproduced in both cortical areas with different peak frequencies. Under two time profiles of optical stimulation (constant and ramp) during subjects' awake resting states, we investigated the generation of these transient gamma oscillations both within the direct light-stimulated area and outside the area via network interaction. Our findings showed that these oscillations were induced above a critical excitation threshold, while spiking activity remained mostly asynchronous, despite the induced narrowband gamma oscillations. These optically induced gamma oscillations tended to propagate in stereotypical spatiotemporal patterns consisting mostly of concentric expanding waves as demonstrated by vector field (optical flow) and time delay analyses. Additionally, in subject T we were able to examine optically induced gamma oscillations during preparation and execution of a reach-and-grasp task. We found that the oscillations were attenuated upon movement execution at cortical sites distal to the stimulation, demonstrating how the intrinsic motor neuronal dynamics overrides external optogenetically mediated perturbations.
METHODS
Polymer optical fiber microelectrode array.
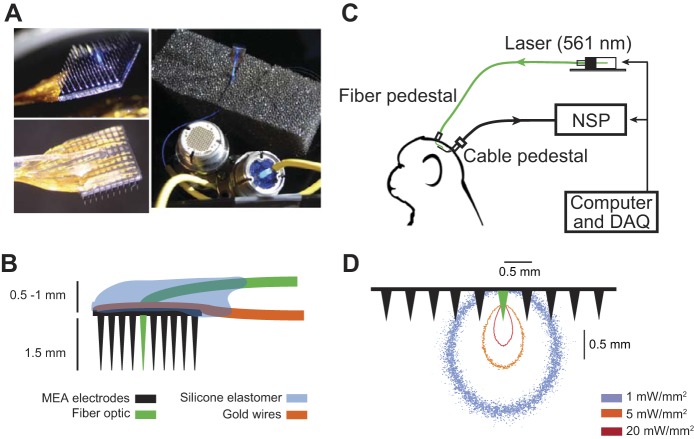
A 4 × 4-mm microelectrode array (Blackrock Microsystems) with 96 silicon-based electrodes that protrude 1.5 mm from the platform and have a 400-μm interelectrode distance was used to fabricate the polymer optical fiber microelectrode array (POF-MEA). The center electrode of the array was removed by laser drilling (Blackrock Microsystems). The remaining surrounding 95 microelectrodes were used for simultaneous recordings of neural activity. A polymer optical fiber with a diameter of 260 μm (core diameter of 250 μm) was subjected to heat-assisted pulling to make a tapered tip. At 2 mm away from the tip, the polymer optical fiber was bent around 90°, placed into the laser drilled hole to replace the center electrode of the array, and affixed with silicon elastomer (NuSil R-2188). Two pedestals were used to receive optical and electrical connection, respectively (Fig. 1). During optical stimulation trials, a 561-nm green laser (Opto Engine) was used to deliver light through the polymer optical fiber. The light intensity on the intracortical side of the array at the tapered optical fiber tip was measured to be one-third of the input power before being coupled to the pedestal. The input power was calibrated to be 18 mW before recording sessions, resulting in an ∼6-mW power delivered into cortex. Monte Carlo simulations of intracortical light distribution were implemented as in previously reported work (Ozden et al. 2013).
Fig. 1.
Polymer optical fiber microelectrode array (POF-MEA). A: POF-MEA viewed from the electrode side (top left), from the pad side (bottom left), and attached to two pedestals (Cereport, Blackrock Microsystems) for optical and electrical connections, respectively (right). B: schematics of the POF-MEA; an optical fiber (green) was integrated around the center of the 10 × 10 MEA. C: neural recording and optical stimulation setup. NSP, neural signal processor; DAQ, data acquisition system. D: Monte Carlo simulations of light distribution in the brain under 6-mW laser power shows isointensity contours at 1, 5, and 20 mW/mm2, respectively. (For illustration purpose, microelectrode separation, but not length, is on scale.) For details on the simulation parameters see Wang et al. (2012) and Ozden et al. (2013).
Viral injections and array implantation.
All surgical procedures and daily care were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Brown University Institutional Animal Care and Use Committee (IACUC). Two macaque monkeys (subject T, 6 kg; subject P, 7 kg) were chronically implanted with POF-MEA arrays in the motor cortex in MI hand area (subject T) and in PMv (subject P). Injections of the viral vector [AAV5-CaMKIIα-C1V1(T/T)-eYFP] were performed at three sites (set 1–2 mm apart in the shape of an equilateral triangle approximately at the center of the array). At each site, 2 μl of viral solution was injected at three different depths (1, 2, 3 mm), resulting in a total volume of 18 μl. Despite the preferential expression of the CaMKIIα promoter in excitatory cells, previous studies using an AAV5 with various promoters in nonhuman primates have shown transduction in excitatory but also inhibitory neurons (Diester et al. 2011; Watakabe et al. 2014). Array implantation was performed immediately after viral injections to facilitate positioning of the array aligned to the injection sites.
Electrophysiological recordings and optical stimulation.
Neural recordings were done through a Cerebus multichannel data acquisition system (Blackrock Microsystems). The optical stimulation was controlled by a custom program written in LabVIEW (National Instruments), sending an analog output to both the laser and the Cerebus data acquisition system for synchronization. In the stimulation protocols during awake resting states, we used constant pulses and light ramps. In particular, 4-s-long voltage ramps were used for analog modulation of the laser output, which attained a maximum optical power of 6 mW at the end of the ramp. Measurements using a photodiode indicated that the optical output was slightly sublinear with respect to the input voltage. Although this could affect the threshold values obtained with ramps, the qualitative results reported here are unaffected. Three sessions were recorded in each subject, over nonconsecutive days during a period of ∼1 mo. At the time of experiments, subject T had been implanted and injected for ∼5 mo and subject P for ∼2 mo.
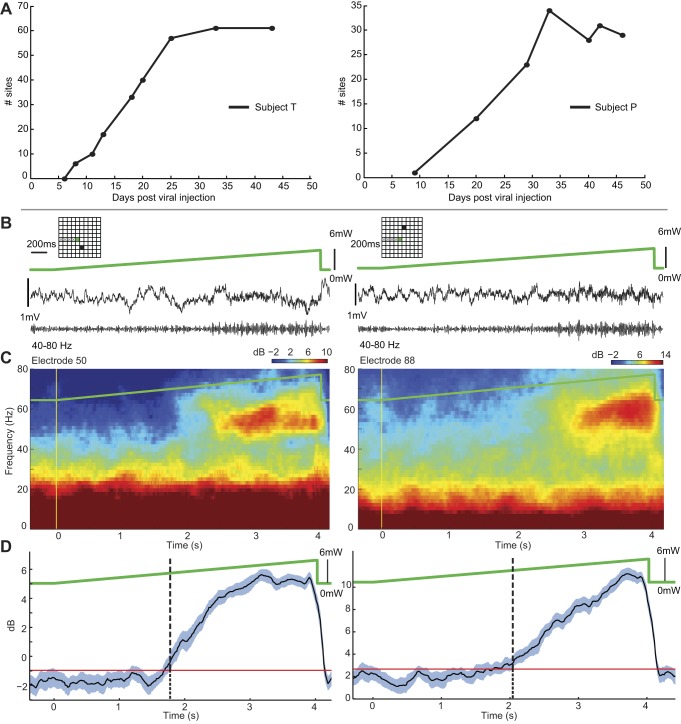
Figure 3A shows data on the modulation of spiking activity over an extended period of time following viral injections for both monkeys (with the same stimulation parameters across days, in particular the same optical power). These data showed little optical stimulation effect in terms of modulation of neuronal spiking within the first week after injection and an increase of modulation over the following weeks, reaching a plateau after ∼30 days. This result implies that the opto-modulation observed here is due to opsin-mediated effects rather than heat or other non-opsin-mediated effects that do not rely on the viral transduction process.
Fig. 3.
Gamma oscillations induced by ramp light stimulation. A: no. of recording sites that showed modulation in multiunit spiking activity (increasing and decreasing) to optical stimulation according to time elapsed since viral injection. B: LFP (<300 Hz, top; 40–80 Hz, bottom) from 2 representative microelectrodes during a 4-s-long light ramp. Insets show position of electrode. C: trial-averaged (n = 36) spectrograms showed elevated LFP power in the gamma band (40–80 Hz) during the late stage of the light ramp. D: evolution of gamma power during light ramps (blue solid line: mean across trials, shaded area: 95% CI). Onset of optogenetically induced gamma oscillations, detected as the lower CI bound crossing the 99% percentile (red line) of the baseline gamma power, occurred once a critical light stimulation level was reached (2.67 mW and 3.06 mW). Time differences in the onset of gamma oscillations in the 2 recording sites in this ramp stimulation condition could result from the progressive recruitment of more distant cortical regions, as the cortical volume affected by light increases with increasing light power.
LFP spectral analysis and pairwise phase consistency between neuronal spiking and LFPs.
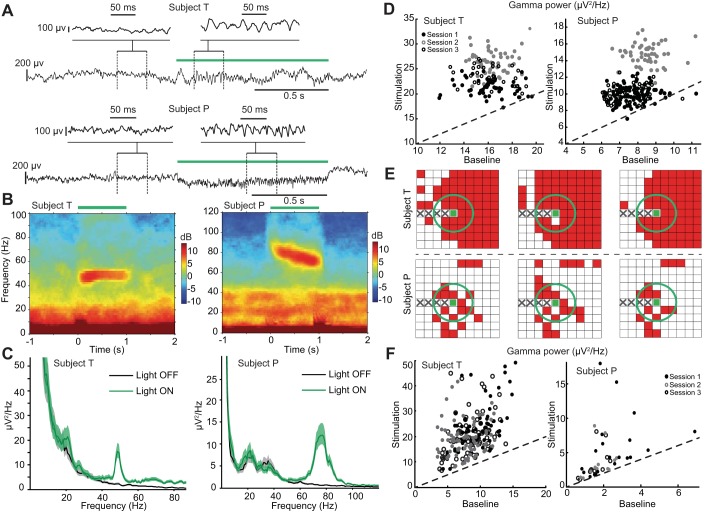
Spectral analysis was done in MATLAB (MathWorks) with the Chronux toolbox (http://chronux.org; Mitra and Bokil 2008; Mitra and Pesaran 1999). Raw data were low-pass filtered with a cutoff frequency of 300 Hz by a zero-phase (forward-backward) 9th-order Butterworth filter to yield LFPs. Time-frequency spectrograms were generated through multitaper methods with a half-bandwidth W = 5 Hz, a time window T = 300 ms, and a number of tapers K = 2TW − 1 = 2 and averaged across trials in each session. Power spectral density (PSD; Fig. 2C) was computed over a period of 900 ms during or before optical stimulation and averaged over trials. We used the power spectrum theoretical confidence intervals (Mitra and Bokil 2008) provided by the Chronux toolbox. Amplitude of gamma power was determined by averaging the spectral power in the gamma band (40–80 Hz).
Fig. 2.
Gamma oscillations induced by constant (square) pulse light stimulation. A: low-pass (<300 Hz) filtered local field potentials (LFPs) from 2 representative recording sites in subjects T (top) and P (bottom). Green bar indicates the duration of 1-s square-pulse light stimulation. Insets: zoomed-in LFP signals. B: trial-averaged (n = 54; 1 session) LFP spectrograms for the corresponding LFP channels shown in A for subjects T and P. The spectrograms show clear optogenetically induced narrowband gamma oscillations. In these 2 sessions, induced gamma had a frequency near 50 Hz in subject T, slowed from 80 Hz to 70 Hz in subject P. C: trial-averaged LFP power spectral density (PSD) based on the time interval [0.1, 0.9] s during stimulation (green curve). (The first and last 100 ms were removed to prevent the contributions of any potential artifacts.) Black curve shows PSD computed for a 0.8-s time window preceding the stimulation onset. Shaded areas: 95% confidence intervals (CIs). D: gamma power during stimulation vs. baseline period in each trial. Each data point in the scatterplot shows the gamma band power in 1 trial for the 2 electrodes as in A–C. x (y)-Axis corresponds to power during baseline (stimulation) period. Diagonal line indicates equality. (n = 54, 54, 34 trials in subject T; n = 49, 99, 50 trials in subject P). E: spatial distribution of optically induced gamma activity over the arrays in subjects T and P, all 6 sessions. Red squares indicate channels in which gamma-band LFP power (40–80 Hz) increased during the stimulation period [P < 0.01; random permutation test with false discovery rate (FDR) correction for multiple testing]. Green circle indicates estimated border for direct optical stimulation effects (threshold of 1 mW/mm2; see also Fig. 1D). The 5 electrode sites on left to the stimulation site (including it) were nonrecording sites because of the optical fiber integration. F: gamma power during stimulation vs. baseline period in each electrode. Each data point shows trial-averaged gamma-band power for 1 electrode that has optically induced gamma. Diagonal line indicates equality. Data from 3 sessions of each subject were included.
In addition to power spectrum analyses, we also examined the spectral coherence between single-neuron spiking and LFPs. Recent studies have examined the bias in spike-field coherence estimators, especially in the case of small-sample spike trains. In particular, Vinck et al. (2010) proposed an alternative measure called the pairwise phase consistency (PPC). That is the approach taken here to assess the strength of the phase-locking of neuronal spiking to LFP ongoing oscillations. Chance-level distribution for PPC values was estimated via a random resampling approach based on jittering spike times under a uniform distribution in [−50, 50] ms.
Spatiotemporal pattern analysis.
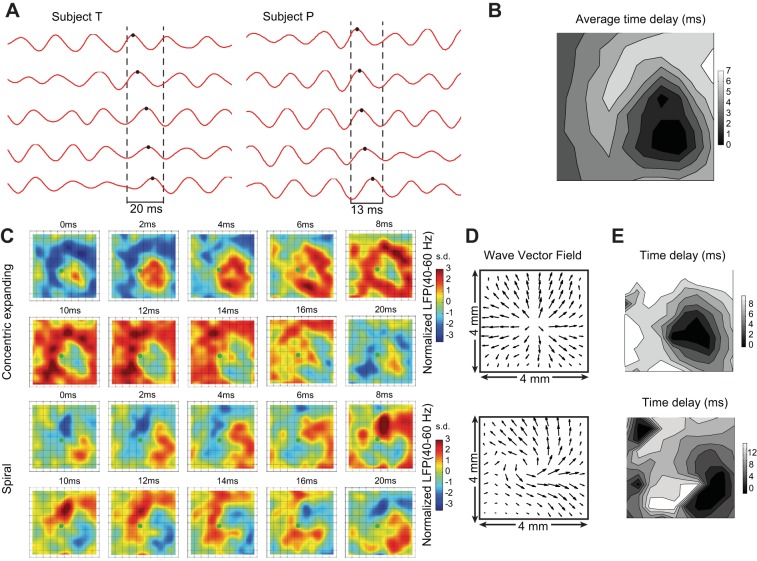
Time delays across the array were computed as time differences of the peaks in gamma-band LFPs (40–60 Hz, subject T) between each electrode and a reference electrode. The electrode on the array with the earliest peak onset was used as reference. Corresponding delay maps were computed during single 20-ms gamma cycles (see Fig. 4E) and averaged over all gamma cycles during multiple trials (n = 54; see Fig. 4B).
Fig. 4.
Spatiotemporal patterns of optogenetically induced gamma waves. A: during stimulation, gamma-band LFPs (40–60 Hz in subject T, 60–80 Hz in subject P) showed time delays between different electrodes. Black dots indicate the peaks of a gamma cycle in the period between the 2 dashed lines across 5 recording sites on the microelectrode array. B: contour map of time delay at each electrode (subject T) with respect to the electrode with the shortest latency (methods), averaged across all gamma cycles from 54 trials. Five nonrecording sites on left of and including the light stimulation site show interpolated values. C: snapshots of LFP amplitude maps (40–60 Hz, subject T; z-scored, interpolated) during a single gamma cycle of 20 ms. Top: gamma waves propagate in an expanding concentric pattern. Bottom: spiral pattern. The shift of the wave “center” to the right (with respect to the fiber optic location) could have resulted from the actual orientation of the tip of the optical fiber or from differences in opsin expression, for example. (The implantation goal was to have the fiber perpendicular to the cortical layer, but the actual orientation might have been different.) D: different patterns of wave propagation illustrated by the averaged (across frames in a 20-ms cycle) wave vector field evaluated via the Horn-Schunck method (Horn and Schunck 1981) corresponding to the same sets of data shown in C. E: contour map of transient time delay corresponding to the 2 gamma cycles shown in C and D.
Propagation patterns were identified based on the Horn-Schunck method (Horn and Schunck 1981), a computer vision algorithm classically used to extract the optical flow, or apparent motion, between two consecutive frames. Optical flow methods (Horn-Schunck or similar) have been used previously to study the spatiotemporal dynamics of neural signals (Lefèvre and Baillet 2009; Mohajerani et al. 2013; Slater et al. 2012). We used this algorithm to estimate wave vector fields from gamma band pass-filtered LFPs, specifically in two examples illustrating expanding concentric and spiral wave patterns (see Fig. 4D). First, we applied a two-dimensional linear interpolation of band-pass LFPs on a refined grid formed by repeatedly dividing the intervals three times in each dimension. Then we applied the Horn-Schunck algorithm on the interpolated signal (100 iterations; smoothing parameter α = 0.6). Finally, we averaged the obtained wave vector fields over time during each 20-ms gamma cycle. To further quantify the occurrence of the dominant wave pattern, i.e., expanding concentric waves, the estimated wave vector field was compared with the radial direction vector at each electrode (direction from the center of the array toward the electrode position) in each gamma oscillation cycle. If >60% of the sites all showed their wave vector pointing within ±35° of their radial directions in a given gamma cycle, the corresponding spatial pattern was defined as one expanding wave pattern event. Only sites where gamma oscillations were induced by light were included in the computation. The first 150 ms after light stimulation onset and the last 50 ms in a 1-s stimulus were excluded from the analyses to rule out influence of light artifacts.
Neural point process modeling.
Neuronal action potentials were initially sorted with an automated spike sorter in MATLAB (Vargas-Irwin and Donoghue 2007) and then manually examined in Offline Sorter (Plexon). Signal-to-noise ratio (SNR) was calculated as the ratio of spike amplitude compared to the 95% confidence interval for the five voltage measurements preceding each spike (Vargas-Irwin and Donoghue 2007). Sorted action potentials with a SNR > 1 were used in this study. A point process framework (Truccolo et al. 2005) was used to estimate a generalized linear model (GLM) of the intensity function (instantaneous spiking rate; 1-ms time resolution) of a given neuron conditioned on the amplitude envelope and phase of the optogenetically induced gamma oscillations. Gamma amplitude and phase were obtained via a Hilbert transform of the band pass-filtered LFPs (40–80 Hz). Given an estimated conditional intensity function, the probability of spiking in each 1-ms time bin and the related receiver operating characteristic (ROC) curves were computed (Truccolo et al. 2010). Based on the area under the curve (AUC), we estimated the power of induced gamma amplitude and phase to predict neuronal spiking. Predictive power was defined as 2 × (AUC − 0.5) and was computed under a fivefold cross-validation scheme.
Behavioral task sessions.
In the five behavioral trial sessions, subject T was trained with a visually guided reach-and-grasp task. In a typical trial, the animal began with resting his hand on a start pad next to a blocked food tray. When an opaque door was lifted to reveal treats on the food tray, the subject reached out to grasp treats. Optical stimulation was continuously administered for 2.5 s, encompassing 1 s prior to the door lifting (movement preparation period), reaction time, and movement execution period (1–1.5 s). This is to ensure that gamma oscillations were induced by light prior to the task and subject to perturbation brought on by actual physical movement execution. Forty light-on trials were performed in each session, along with an additional forty control trials without light stimulus. These five sessions were recorded under the same experimental condition on five different days within a 2-wk period.
Statistical tests.
To compute spatial distribution maps of induced gamma, in each electrode the presence of optically induced gamma oscillations was determined by comparing gamma power (averaged over 40–80 Hz) during and before the 1-s constant light stimulation. Otherwise stated, P values were calculated via random permutation tests (n = 104) and subjected to multiple testing corrections, which were implemented via the false discovery rate (FDR) approach, for dependent samples (Benjamini and Yekutieli 2001). In all hypothesis tests, we used a significance level of 0.01.
RESULTS
We recorded three sessions in the motor cortex in each of the two opsin-expressing macaque subjects (subject T, area M1; subject P, area PMv). Beyond these six passive recording sessions in which the subjects were in awake resting states, five additional sessions in subject T were recorded in which a visually guided reach-and-grasp task was performed under applied perturbation of optogenetic stimulus. Sessions were recorded on different days with the same stimulation parameter and recording settings to ensure consistency. During each session, subjects were head-fixed in a custom primate chair with a food tray blocked by a lifting door for implementation of the task. Light was delivered through the polymer optical fiber integrated on the MEA into targeted neocortical region with estimated 6-mW maximal intracortical power (Fig. 1, A–C). The modeled dependence of light spatial distribution on the particular optical stimulation power in neocortex and under MEA is shown in Fig. 1D.
Optogenetic stimulation above a critical intensity level induces narrowband gamma oscillations.
We applied two light stimulation time profiles, constant and increasing ramp, respectively, to examine the induction of gamma band activity in primate motor cortex. A constant-stimulus profile consisting of a single 1-s square pulse per trial was applied in all six recording sessions for both subjects. Single-trial raw LFP signals, average (across trials) LFP spectrograms, and PSDs showed clear induced narrowband gamma oscillations (Fig. 2, A–D). Induced oscillations in subject T had peak frequencies of 48.0, 51.2, and 46.5 Hz in the three studied sessions, respectively (75.4, 72.9, and 73.6 Hz for subject P). Induced gamma oscillations occurred immediately after stimulation onset and remained throughout the entire stimulation period, decreasing to background level after stimulation ended. The increase in the corresponding gamma power, with respect to the background gamma power measured in the 1-s period preceding stimulation onset, was significant (P < 0.01; random permutation test) in all sessions for both subjects in the examples shown in Fig. 2, A–D. Despite differences in cortical motor areas (M1 vs PMv) and peak frequencies, 1-s constant optogenetic stimulation consistently induced strong narrowband gamma LFP oscillations in the motor cortex of the two studied subjects.
To more closely examine the dependence of induced gamma oscillations on light stimulation power, the increasing ramp stimulation profile was applied in two sessions in subject T (methods). This stimulation profile was repeated in 30 trials in each session. Through examination of simultaneously recorded LFPs from the POF-MEA, we observed the onset of 40- to 80-Hz LFP oscillations after a certain optical stimulation intensity was reached (Fig. 3, B–D). Gamma-band LFP power was enhanced as the light intensity further increased. This pattern of gamma oscillation onset with increasing light intensity was reliably reproduced across sessions and trials. The onset of optogenetically induced gamma LFP activity was determined by the time at which the power in the 40–80 Hz frequency band arose above the 95% confidence interval of the corresponding power during the preceding nonstimulation period. Qualitatively similar results were obtained for the ramp stimulation in subject P.
Optogenetically induced gamma oscillations engage a larger network beyond the direct light stimulation region.
The simultaneous multichannel recording capability of the POF-MEA allowed us to track optically induced transient gamma LFP oscillations in the 4 × 4-mm neocortical region surrounding the optical stimulation site (Fig. 2E). These oscillations were observed across a wide area of the recorded neocortical patch in both subjects; 80.0% and 23.4% of recording electrodes in subject T and subject P, respectively, had a significant increase (P < 0.01; random permutation test with FDR multitest correction) in 40–80 Hz LFP power during the 1-s stimulation. Although consistent within subjects, this difference in proportion across the two subjects might reflect the extent of opsin expression, differences in local connectivity, and/or alignment between the stimulation site and viral injection.
According to previous intracortical light scattering simulations (Ozden et al. 2013; Wang et al. 2012; see also Fig. 1D), recording electrodes within ∼1 mm distance from the optical fiber are under direct light stimulation (using a threshold of 1 mW/mm2 as in Aravanis et al. 2007). Thus considering that laser light stimulation was delivered locally through a single polymer optical fiber at the center of the array, induced gamma LFP oscillations were distributed not only within, but also well outside, the volume of direct light stimulation, such as corner electrodes 2.6 mm away from the optical fiber tip. Thus the light-induced gamma oscillations from the peripheral recording electrodes on the array resulted from indirect recruitment of larger network activity rather than from direct activation of local circuits of opsin-expressing neurons.
Spatiotemporal patterns (waves) of optogenetically induced gamma oscillations.
We examined the induced gamma oscillations in the time domain at each electrode site across the array. Band pass-filtered LFPs (40–60 Hz in subject T and 60–80 Hz in subject P) in spatially separated electrodes revealed systematic differences in the time delays of induced gamma oscillations (Fig. 4A). When averaged across all trials, these delays in gamma oscillations were spatially organized across the array (Fig. 4B), indicating horizontal cortical wave propagation.
To further reveal the spatial features of the induced propagating gamma waves, amplitudes of band pass-filtered LFPs across all 95 electrodes in subject T (where a larger proportion of sites with induced gamma occurred) were mapped onto the microelectrode array site and over sequential time frames covering one gamma cycle (∼20 ms for subject T). Two wave propagation patterns were observed during the 1-s square-pulse light stimulation periods (Supplemental Movie S1).1 One of the patterns consisted of expanding concentric waves originating at the electrodes close to the light stimulation site. In the other pattern, anticlockwise spiral waves centered near the optical stimulation site were observed (Fig. 4C). The former pattern, expanding concentric waves, was clearly the dominant spatiotemporal feature of the induced gamma oscillation in this subject, appearing in >60% of the overall light stimulation period (61.9% of 660 gamma cycles in n = 54 1-s square pulses, methods; see also Supplemental Movie S1). In addition, the delay map averaged over gamma cycles and trials (Fig. 4B) resembled the transient delay maps of the expanding concentric wave (Fig. 4E, top).
Neuronal spiking during optogenetically induced gamma oscillations.
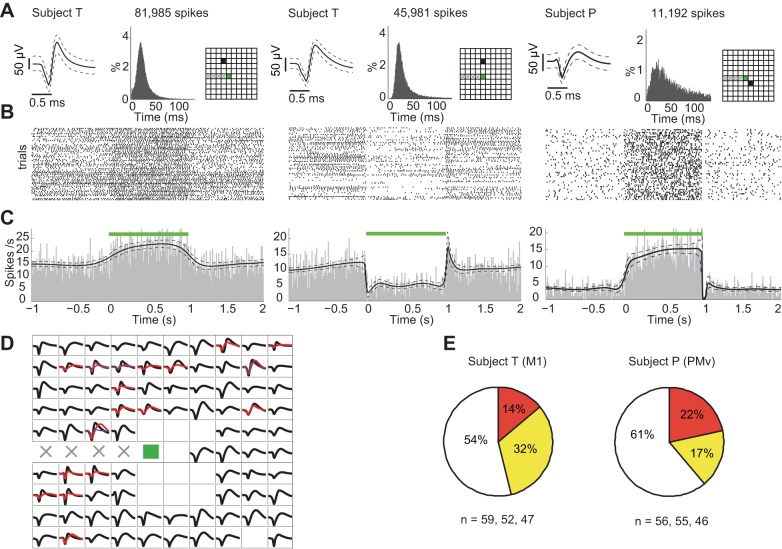
We examined recorded neuronal spiking activity in both subject T (M1) and subject P (PMv) during and outside the optogenetic stimulation period. Spiking activity of single units were sorted in the data sets of all six sessions from the two subjects (n = 59, 52, 47 in subject T; n = 56, 55, 46 in subject P). Neuronal spiking activity with both increased and decreased firing rates during the course of the 1-s square-pulse light stimulation were observed over a wide range of electrode positions on the array. Example raster plots and peristimulus time histograms (PSTHs) are shown in Fig. 5. Since optical activation of C1V1(T/T) results in neuronal excitation/depolarization, the observed decreased firing rates likely resulted from inhibitory effects mediated by network interactions. The proportion of neurons that showed increased and decreased firing rates during stimulation were determined to be 32% and 14% in subject T, respectively, while in subject P they were 17% and 22%, respectively. Again, we conjecture that these differences across the two subjects might reflect differences in local opsin expression and the recorded populations by the array. Analysis of the location on the microelectrode array where single units showed optical stimulation-related suppression or increase in firing rates showed that suppression/gain effects were not obviously related to the distance from the optical fiber. Nevertheless, given that the direct opsin-mediated effects were depolarizing in nature, suppression of spiking activity must have involved network effects via mono- or polysynaptic interactions.
Fig. 5.
Modulation of neuronal spiking rates during optogenetically induced gamma LFP oscillations. A: action potential waveforms, interspike time intervals (ISIs), and recording sites for 3 representative neurons recorded from the 2 subjects are shown. Waveform: mean waveform with dashed line envelope showing the corresponding 95% CI for observed waveform values. The modes of ISI of the 3 units are 23, 26, and 29 ms, respectively. B: corresponding raster plots for each neuron (n = 54 trials). C: corresponding peristimulus time histograms (PSTHs) estimated via Bayesian adaptive regression splines (Kass et al. 2003) showing either substantial increase or decrease in spike rates during optical stimulation. Dashed curves indicate the 95% CIs. D: average waveforms of all recorded neurons on the array (subject T, 1 session). Units on the same electrode are shown in different colors. E: proportion of neuronal recordings that showed an increase (yellow), a decrease (orange), or no change (white) in spiking rates during optical stimulation. (subject T: n = 59, 52, 47 neuronal recordings in 3 sessions; subject P: n = 56, 55, 46 neuronal recordings in 3 sessions.)
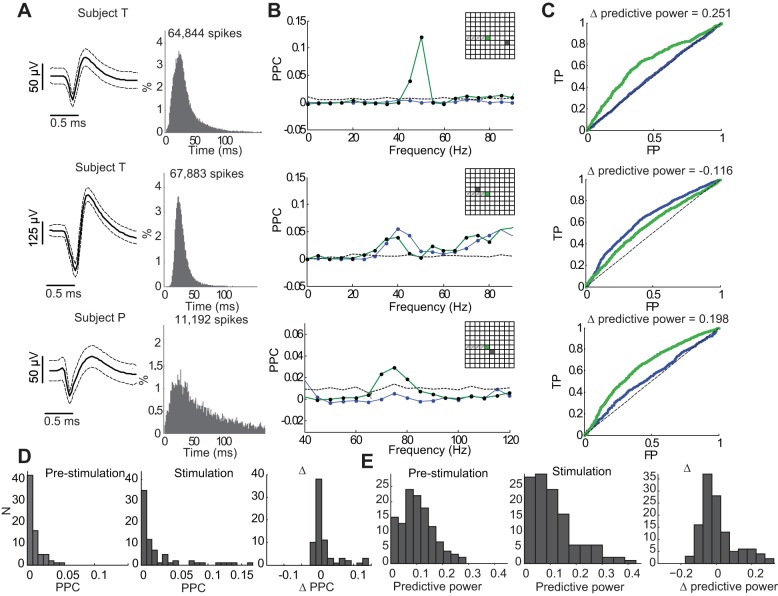
To reveal the relationship between neuronal spiking activity and the simultaneous induced gamma-band LFP oscillations on the same electrode, PPC (Vinck et al. 2010) was computed (Fig. 6B). Only electrode sites where increase in gamma power was statistically significant were included in the analysis. Despite the strong induced narrowband gamma LFP oscillations in these electrodes, only a subset of neurons showed elevated PPC values above chance levels in the specific gamma frequency band (40–80 Hz). The distribution of chance-level PPC values was estimated via random resampling based on jittering the spike times (methods). Nevertheless, even in this subset with statistically significant PPC, PPC values were relatively low, indicating weak phase-locking of neuronal spiking to induced gamma LFP oscillations. Overall, although neuronal spiking rates could be largely modulated by light stimulation and many neurons' interspike time interval (ISI) distribution showed a peak corresponding to gamma frequency (Fig. 5, Fig. 6), neuronal spiking remained highly asynchronous.
Fig. 6.
Spike-LFP pairwise phase consistency (PPC) analysis and predictive power of optogenetically induced gamma oscillations on neuronal spiking. A: waveforms and ISIs of 3 examples of recorded neuronal spiking. Top 2 neurons were recorded in subject T and bottom neuron in subject P. B: corresponding PPC analysis quantifying the phase-locking of neuronal spiking to LFP oscillations during the 1 s before light stimulation (blue) and during the 1-s optical stimulation period (green). Dashed line shows 95% CI for the PPC chance level (methods). In these 3 examples, all neurons showed some level of significant PPC, although weak (PPC = 1 indicates perfect phase-locking) in the corresponding gamma band during optical stimulation. Insets show position of electrode. C: receiver operating characteristic (ROC) curves assessing prediction of spiking for the corresponding 3 neurons using a point process model where the instantaneous rate (intensity) was modeled as a function of the amplitude envelope and phase of the optogenetically induced gamma oscillations (methods). Diagonal dashed line indicates chance-level prediction. TP (FP) denotes true (false) positive rates. Blue and green ROC curves correspond to the baseline and stimulation periods, respectively. D: summary of gamma oscillation PPC over subjects, sessions, and neuronal recordings (N = 45, 30, and 22 neuronal recordings in 3 sessions for subject T, respectively and N = 13, 5, and 5 for subject P, respectively. Only neurons where the same recording site showed induced gamma oscillations were included.) E: gamma predictive power analysis over the same set of data as in D.
To complement the PPC analysis, we fitted neural point process models to quantify the power of the instantaneous amplitude envelope and phase of the optogenetically induced gamma-band oscillations to predict neuronal spiking. Instantaneous amplitudes and phases were obtained by taking the Hilbert transform of the band pass-filtered gamma LFP in the same electrode site as the modeled neuron. A point process framework (Truccolo et al. 2005) was used to fit GLMs of the instantaneous spiking rate (conditional intensity function) conditioned on the instantaneous gamma amplitude and phase. Given an estimated GLM, the probability of the modeled neuron to spike in every 1-ms time bin and the corresponding ROC curves were computed (Fig. 6C). Based on the area under the ROC curve, the predictive power, ranging from 0 (no prediction) to 1 (perfect prediction), was computed (methods; Truccolo et al. 2010). Predictive power was computed under a fivefold cross-validation scheme. Predictive power was computed both for the 1-s period preceding stimulation onset (baseline period) and for the 1-s stimulation period. Both increase and decrease in gamma predictive power, with respect to the baseline period, were observed during the 1-s square-pulse light stimulation period. The summary of gamma predictive power of all examined neurons in both subjects (Fig. 6E) shows that before light stimulation gamma predictive power was concentrated around 0.1. During the 1-s light stimulation period, gamma predictive power peaked around 0.05 and varied over a wider range, reaching up in some cases to 0.35. Differences between predictive powers during baseline and stimulation periods further revealed that the majority of neurons (n > 20) showed a slightly decreased predictive power (Δ = ∼0–0.1) while several neurons (n = 5) showed distinctly elevated predictive power (Δ > 0.2) during optical stimulation. Overall, our analyses based on PSTHs, PPC, and GLM predictive power suggest varied and complex relationships between neuronal spiking and the optogenetically induced collective dynamics characterized by narrowband gamma oscillations.
Voluntary movement suppresses optogenetically induced gamma oscillations in motor cortex.
In subject T it was possible to examine the effect of voluntary movement execution on the optogenetically induced gamma oscillations. Optical stimulation was applied during a visually guided reach-and-grasp task in which the subject maintained his hand in a resting position and, upon the lifting (go cue) of an opaque door blocking a target treat, reached for the target (methods; Fig. 7A). Optical stimulation was applied starting before the door lifting (movement preparation) and extended over 1.5 s after the lifting (movement execution). Forty stimulation trials and forty control trials (no light stimulus) were performed in each of five sessions during different days.
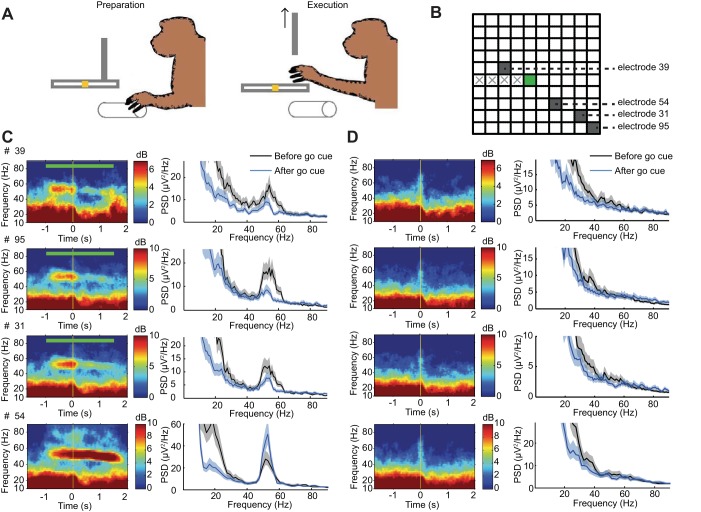
Fig. 7.
Optogenetically induced gamma oscillations during movement preparation are suppressed upon voluntary movement execution. A: cartoon of the task: the subject (subject T) rests his hand on a stationary stick in preparation for a reach-and-grasp movement until an opaque door blocking the food tray is lifted (go cue). The reach-and-grasp movement (toward a food pellet) is then performed. The movement preparation period lasted at least 1 s, and the movement execution period (terminating at the pellet grasping time) ranged from 1 to 1.5 s. Optical stimulation (constant square pulse; green line on plots) was turned ON during the preparation period and lasted 2.5 s, overlapping with the movement execution period. B: positions of the 4 recording electrodes in C and D. C: across-trial (n = 40 trials, 1 session) average LFP spectrogram and PSD for 4 microelectrode recording sites. (Of those 4 sites, 3 showed substantial attenuation of induced gamma on movement execution phase.) PSD was computed on a time window covering 0.5 s before the go cue (black) and 0.5 s starting 150 ms past (blue) go cue onset. Shaded areas indicate 95% CIs. D: same as in C but in control trial (n = 40) where no optical stimulation was presented while the subject performed the same task.
Similar to the data sets recorded during awake resting periods examined above, optical stimulation during the movement preparation stages induced strong narrowband gamma LFP oscillations as demonstrated by the power spectrograms and average PSDs (Fig. 7C). By contrast, control trials showed no such elevation of gamma power during the movement preparation period (Fig. 7D). Remarkably, movement execution was accompanied with a significant decrease of the optogenetically induced gamma LFP power for many of the recording sites. The number of recording sites that showed movement-related attenuation of optically induced gamma in the five examined sessions corresponded to 37/72, 44/68, 42/71, 48/83, and 44/79 sites, respectively. In other words, on average, 57.6% of the sites that showed optically induced gamma presented the movement-related attenuation of induced gamma oscillations. Thus it appears that M1 intrinsic network dynamics related to voluntary motor control tended to override optically induced gamma oscillations even though optical stimulation remained constant during movement execution. This attenuation effect was consistent across the five recorded sessions (example in Fig. 8A).
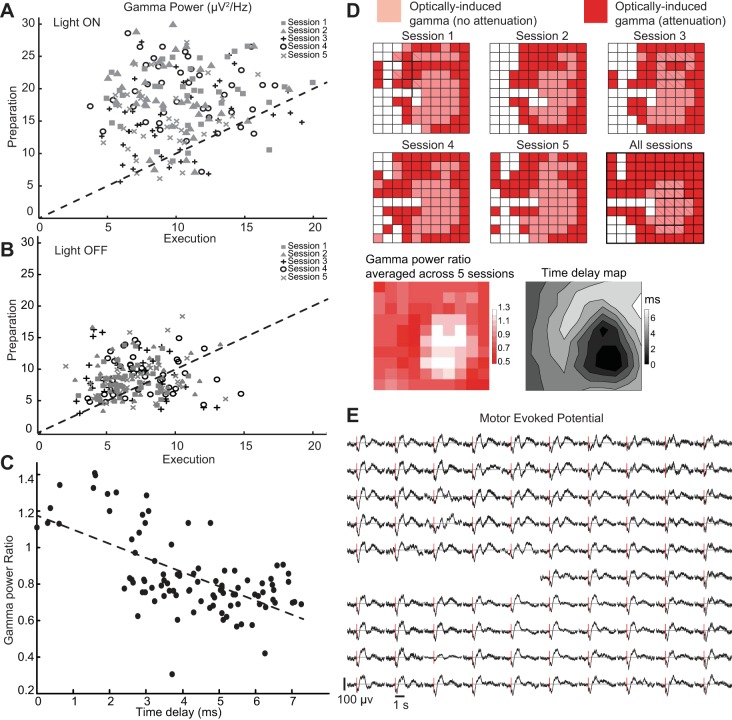
Fig. 8.
Movement execution attenuates optogenetically induced gamma oscillations: summary over trials and recording sites. A: power of optically induced gamma during movement preparation (horizontal) and execution (vertical) for recording site 95. Each dot is the power of 1 trial (n = 40 trials each session, subject T) in 5 experimental sessions. PSD was computed as described in Fig. 7. B: same scatterplot in control condition (light OFF). C: the level of attenuation in optically induced gamma oscillations during movement execution was anticorrelated with the time delay (linear regression, P = 10−10, r2 = 0.4), indicating that sites away from the directly stimulated area tended to show larger attenuation effects. D, top: recording sites over the array where induced gamma was attenuated during the movement execution phase (red) and where gamma was not affected (light red). Bottom: summary over recording sites on the microelectrode array: the heat map denotes the ratio between the gamma power during movement execution and during preparation. Values < 1 indicate attenuation of the optically induced gamma oscillation. E: average motor evoked potentials in 1 session for all of the recording sites. Motor evoked potentials were detected in all of the recording sites and did not seem to play a role in the suppression or not of optically induced gamma oscillations.
We note that the decrease of gamma power during movement execution occurred mostly in electrodes more distant from the center (Fig. 8D). This attenuation was not observed in the nearest neighborhood of the center (near optical fiber location; Fig. 8D; Fig. 7C, bottom). This spatial distribution of the movement-related attenuation of gamma oscillations resembled to some extent the pattern of expanding concentric waves examined above (Fig. 4). Sites that showed attenuation in optically induced gamma oscillations during movement execution tended to have larger time delays than sites where optically induced gamma oscillations were not attenuated (Fig. 8C; linear regression, P = 10−10, r2 = 0.4). It appears that sites where induced gamma depended more on propagation via neuronal interactions were more affected by the movement execution attenuation effects. Additionally, all electrode sites showed clear motor evoked potentials (MEPs), indicating that movement-related attenuation of optogenetically induced gamma oscillations was unlikely to result from a simple interaction between MEPs and induced gamma (Fig. 8E). Analysis of reaction times, error trials, or kinematics revealed no significant optical stimulation effects across the examined sessions.
DISCUSSION
In this study we have demonstrated that constant optogenetic stimulation based on the C1V1(T/T) opsin can induce sustained narrowband gamma LFP oscillations in nonhuman primate motor cortex. Induced gamma oscillations were observed in both M1 and PMv. These induced oscillations engaged larger cortical networks in the 4 × 4-mm recorded neocortical patches beyond the direct light stimulation domain. The larger network gamma activity was organized in spatiotemporal patterns consisting primarily of expanding concentric waves and other occasional patterns such as spiral waves. Optical stimulation modulated the firing rate of 46% and 39% of recorded neurons in subjects T and P, respectively. Rate modulation effects showed either a substantial increase or decrease in firing rates. Despite this stimulation-related modulation effect in firing rates, neuronal spiking remained largely asynchronous with respect to the optogenetically induced gamma oscillations, as assessed by measures of phase-locking (PPC) and neural point process predictive power. Gamma oscillations were induced not only during awake resting states but also during motor behavior. Interestingly, in the latter, gamma oscillations optically induced during movement preparation were attenuated during movement execution in on average 57.6% of all the electrodes that showed optically induced gamma oscillations. This finding indicates that these externally driven oscillations were overridden by the intrinsic neural dynamics controlling motor output. Movement-related attenuation of the optically induced gamma oscillations could be specific to mechanisms operating in M1 or a more general effect extending to other motor cortical areas. We hope to be able to examine this issue in the future. Overall, our study provides new findings on gamma oscillations induced via optogenetics. The fact that these gamma oscillations were generated by a very simple (constant) stimulation pattern supports the predisposition of neural circuits to generate gamma oscillations under driving inputs in primate motor cortex.
Most previous optogenetic studies of gamma oscillations have focused on oscillations induced by periodic optical stimulation (see e.g., Adesnik and Scanziani 2010). The main underlying mechanism under period drive appears to involve a gamma resonant property of the targeted neural circuits (Cardin et al. 2009; Moca et al. 2014). Here we have focused on gamma oscillations induced by above-threshold constant optical stimulation in nonhuman primates. Our light ramp stimulation data indicate that gamma oscillations were induced after a critical level (threshold) of light intensity was reached. Furthermore, amplitude (power) of gamma oscillations appeared to grow gradually from the threshold point as the optical stimulus magnitude continued to increase, rather than emerging abruptly with large magnitudes. External input-driven transitions into oscillatory dynamics in nonlinear systems typically occur in either of two different scenarios. In the first scenario, the system transitions abruptly into large-amplitude oscillations. Common general transitions (Strogatz 1994) in this case include saddle-node or subcritical Hopf bifurcations, or also perturbation of a system showing multistability (e.g., a large enough perturbation “kicks” the system from a nonoscillatory attractor into the basin of attraction of a limit cycle). In the second scenario, similar to our data, the oscillation amplitude grows gradually from the transition point. The supercritical Hopf bifurcation is the typical general mechanism in this case (Strogatz 1994; Truccolo et al. 2003). Therefore, we conjecture that the general transition mechanism underlying the induction of gamma oscillations under constant optical stimulation in our data is likely to involve a Hopf bifurcation. This finding may be relevant for the development of detailed biophysical models of how optogenetic stimulation can induce oscillatory dynamics in cortical networks. This conjecture is still consistent with the gamma resonance hypothesis under periodic drive, since one would expect these neocortical circuits to also show damped gamma oscillations in dynamical regimes below the bifurcation point. Although the peak frequency of the optically induced gamma oscillations was consistent across trials/sessions in the same subject, it varied across the two subjects. This difference may have resulted from, among other things, differences in viral diffusion/opsin expression, orientation and position of the fiber with respect to cortical layers, different effective light intensities, or potential differences in single-neuron and network properties between M1 and PMv.
Gamma oscillations in neocortex are currently considered to arise via activation of inhibitory interneuron networks [interneuron gamma (ING) mechanism], via activation of reciprocally connected pyramidal and interneuron neurons [pyramidal-interneuron gamma (PING)], or via a combination of both ING and PING mechanisms (Buzsáki and Wang 2012; Tiesinga and Sejnowski 2009). The particular optogenetic construct we used (CaMKIIα promoter and AAV5 viral vector) is known to express primarily in pyramidal neurons and to a lesser degree in inhibitory interneurons (Watakabe et al. 2014). It also seems that it might still be a challenge to disentangle the contribution of different neuronal types to optical responses that involve network even when using cell-specific optogenetic tools. That is because different neuron types, not directly targeted by optical stimulation, might be recruited (via network interactions) at very short times, e.g., 1–2 ms, after stimulation onset. We considered further analyses based on classifying the recorded neurons into putative pyramidal and inhibitory interneurons. Based on this classification, one could attempt to infer potentially distinct roles for putative pyramidal and inhibitory interneurons in the generation of optogenetically induced gamma LFP oscillations. However, any analysis based on this putative classification would remain highly questionable given the recent demonstration by Vigneswaran et al. (2011) that large pyramidal neurons in both M1 and PMv can show spiking statistics and extracellular action potential features similar to those typically attributed to fast-spiking inhibitory interneurons. We also note that optogenetics in primates is presently a less developed subject than for mouse models (transgenic lines, Cre-recombinase strategies, etc.). Progress in this area can be expected to yield better cell type-specific access to cortical circuits in primate brains. In addition, studies of optically induced gamma oscillations in rodent cortex and the fact that both M1 and PMv can show similar induced gamma oscillations under 1-s square light pulse suggest that this phenomenon is likely a general property of pyramidal-interneuron networks. These gamma oscillations can emerge under oscillatory optical drive or even constant input above a critical level as shown here.
Neuronal spiking remained largely asynchronous with respect to the induced gamma LFP oscillations, even though these oscillations were strong and narrowband. This was demonstrated by the weak phase-locking of spiking to the induced gamma LFPs, and by the low gamma amplitude and phase power to predict neuronal spiking. One could expect that under these narrowband oscillations the phase-coupling should be much stronger. However, similar observations have been made in various neural systems and different conditions. For example, Truccolo et al. (2014) have reported human focal epileptic seizures characterized by sustained narrowband (40–60 Hz) gamma oscillations where neuronal spiking also remains highly asynchronous. Furthermore, previous mathematical and computational modeling presented by Brunel and Wang (2003) has demonstrated that narrowband sustained gamma LFP oscillations can indeed emerge at the collective dynamics levels even though neuronal spiking, in both pyramidal and inhibitory interneurons, remains highly irregular and asynchronous.
Optical stimulation during the examined reach-and-grasp task, comprising both movement preparation and execution stages, showed the same type of induced narrowband gamma oscillations as observed during the awake resting condition. Notably, however, the induced gamma oscillations, clearly present during movement preparation, were attenuated during movement execution in many recording sites. Although our data in this case included only one subject, the effect was consistently observed in all of the five recording sessions conducted over several different days. This finding indicates that intrinsic network dynamics in motor cortex controlling voluntary movement tend to override the externally driven gamma activity. We hope to investigate this issue further and contrast optogenetic stimulation of motor versus early sensory cortices in the future. In our experience, it appears to be more challenging to elicit behavioral responses in primates via optical stimulation of motor cortex than via stimulation of sensory cortices (May et al. 2014). We conjecture that part of the challenge resides in the fact that motor cortex is set, as part of its function, to directly control cortical output, while early sensory cortices need to maintain sensitivity to external inputs. One would thus expect that motor cortex intrinsic dynamics would tend to override optical stimulation during movement execution. Recent studies (e.g., Churchland et al. 2012) have provided evidence for M1 behaving as a dynamical system similar to an oscillatory pattern generator. Movement preparation would place M1’s neuronal network in the appropriate initial condition for a given target movement. In the case of stereotypical and fast movements, where feedbacks might not play a critical role, neural activity after a “go decision” would correspond to the evolution of the neural dynamics according to some attractor and the initial condition set during the preparation. In this scenario of intrinsic attractor dynamics, we conjectured that M1 activity would become robust to external perturbations originating in this case from optical stimulation. The robustness would come from the resilience of attractor dynamics to perturbations. This dominance of motor cortex intrinsic dynamics might be more noticeable for subtler types of stimulation such as optogenetics in comparison to, for example, electrical stimulation, which is known to directly affect much larger areas and axonal projections.
The enhancement of beta oscillations during movement preparation and their attenuation during movement execution are typically observed in our data during reach-and-grasp tasks independently of optical stimulation. Furthermore, as shown in Fig. 2C, beta oscillations tended to appear also during the awake resting condition examined in our study. Importantly, optical stimulation did not seem to interfere with these ongoing beta oscillations during this condition. In other words, we observed the coexistence of beta-band activity and optically induced gamma activity during the awake resting condition. This seems to be also the case during optical stimulation at the movement preparation phase (Fig. 7C) of the reach-and-grasp task. The typical attenuation of beta oscillations during movement execution is also observed in this reach-and-grasp task in all of the recording sites. By contrast, the attenuation of gamma oscillations during movement execution is restricted to sites away from the optic fiber location, i.e., outside the region of direct light effects, as noted above.
Overall, our findings have also broader relevance to motor cortex neural dynamics in three main aspects. First, narrowband (>40 Hz) gamma oscillations as shown in our study are not commonly observed in primate motor cortex recordings using microelectrode arrays (electrode length: 1.5 mm). By contrast, several previous studies based on EEG/ECoG recordings from human motor cortex have shown gamma activity (Joundi et al. 2012; Muthukumaraswamy 2010). In our recordings, we do see increase in broadband power (>40 Hz) around movement onset, which we think may reflect mostly increase in multiunit activity. It is possible that this broadband multiunit contribution masks the true narrowband gamma-band activity. As recently demonstrated by Waldert et al. (2013), multiunit activity surrounding movement onset can contaminate motor cortex LFPs over a broad range of frequencies, extending down to ∼10 Hz. Our finding that narrowband gamma oscillations can be induced in motor cortex even with a very simple type of input, i.e., constant stimulation, indicates that gamma is likely part of the dynamics repertoire of motor cortex neuronal networks. We think gamma oscillations would likely emerge in response to sustained nonlocal inputs to the area, for example. Our finding that simple nonoscillatory drive can induce a much richer output, sustained gamma oscillations, also emphasizes the subtleties involved in probing neural circuits with optogenetics, currently one of the main tools for this purpose. Second, as shown in this report, the optically induced gamma oscillations do not seem to interfere with the generation of ongoing beta oscillations during both awake resting and movement preparation. This finding indicates that these two types of LFP oscillations are likely generated by different circuits. Third, successful modulation of motor behavior via direct optogenetic stimulation of motor cortical areas remains challenging in primates. This seems to be the case not only for stimulation patterns that induce gamma oscillations (as shown here) but also for a variety of stimulation patterns that have been explored by our and other groups (Han et al. 2009). We showed in one subject that motor cortex intrinsic dynamics (i.e., activity generated by the subject's volitional movement control) tends to override externally (optically) induced gamma oscillations in sites away from the domain of the direct light stimulation (i.e., sites where induced gamma would require propagation via network interactions). We think that this finding may offer an explanation for why direct perturbation of motor behavior via optogenetic stimulation remains challenging. In contrast to sensory cortices and deeper nuclei, where optogenetic modulation of perceptual/behavioral processes has been successful in primates (e.g., Cavanaugh et al. 2012; Dai et al. 2014; May et al. 2014), the intrinsic dynamics in motor cortex seems more robust to optical perturbations as discussed above.
Additionally, the combination of optogenetic stimulation and microelectrode array recordings as presented here provides an important approach for probing spatiotemporal cortical dynamics in primates, both in healthy conditions and in neurological disorders. We are currently working on further developing the capabilities of this tool by allowing, for example, multiple optical stimulation sites on the microelectrode array. In this way, new studies can be extended to the application of complex spatiotemporal stimulation patterns at multiple recording sites, to probe neural dynamics across multiple temporal and spatial scales.
GRANTS
This research was supported by the Defense Advanced Research Projects Agency (DARPA REPAIR N66001-10-C-2010, co-principal investigators: A. V. Nurmikko, W. Truccolo); National Institute of Neurological Disorders and Stroke (NINDS) Grant R01 NS-079533 (to W. Truccolo) and K01 Career Award NS-057389 (to W. Truccolo); a Department of Veterans Affairs Merit Review Award (to W. Truccolo); the Pablo J. Salame '88 Goldman Sachs endowed Assistant Professorship in Computational Neuroscience (to W. Truccolo); National Science Foundation Grants CBET-1402803 and CBET-1264816 (to A. V. Nurmikko); and NINDS-Javits Grant NS-025074.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., W.T., C.E.V.-I., I.O., J.B.Z., and A.V.N. conception and design of research; Y.L., F.B.W., C.E.V.-I., I.O., J.B.Z., T.M., N.S.A., and J.W. performed experiments; Y.L., W.T., F.B.W., and T.M. analyzed data; Y.L., W.T., F.B.W., C.E.V.-I., I.O., J.B.Z., T.M., N.S.A., J.W., and A.V.N. interpreted results of experiments; Y.L. and W.T. prepared figures; Y.L., W.T., F.B.W., and A.V.N. drafted manuscript; Y.L., W.T., F.B.W., C.E.V.-I., I.O., J.B.Z., and A.V.N. edited and revised manuscript; Y.L., W.T., F.B.W., C.E.V.-I., I.O., J.B.Z., T.M., N.S.A., J.W., and A.V.N. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Borton, James Barrese, Corey Triebwasser, Paul Kalanithi, John Murphy, James Harper, and the personnel at the Brown University Animal Care Facility for their assistance with animal surgery and care and instrumentation design. We also thank the Deisseroth lab for sharing their optogenetic constructs.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464: 1155–1160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang L, Zhang F, Meltzer L, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29: 1165–1188, 2001. [Google Scholar]
- Bressler SL. The gamma wave: a cortical information carrier? Trends Neurosci 13: 161–162, 1990. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Cortical coordination dynamics and the disorganization syndrome in schizophrenia. Neuropsychopharmacology 28, Suppl 1: S35–S39, 2003. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature 366: 153–156, 1993. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol 50: 19–24, 1980. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol 90: 415–430, 2003. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci 35: 203–225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, Wang KH, Boyden ES, Wurtz RH. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron 76: 901–907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV. Neural population dynamics during reaching. Nature 487: 51–56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Brooks DI, Sheinberg DL. Optogenetic and electrical microstimulation systematically bias visuospatial choice in primates. Curr Biol 24: 63–69, 2014. [DOI] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci 14: 387–389, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62: 191–198, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn BK, Schunck BG. Determining optical flow. Artif Intell 17: 185–203, 1981. [Google Scholar]
- Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol 22: 403–407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RE, Ventura V, Cai C. Statistical smoothing of neuronal data. Network 14: 5–15, 2003. [DOI] [PubMed] [Google Scholar]
- Lefèvre J, Baillet S. Optical flow approaches to the identification of brain dynamics. Hum Brain Mapp 30: 1887–1897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Ozden I, Brush B, Borton DA, Wagner F, Agha N, Sheinberg DL, Nurmikko AV. Detection of optogenetic stimulation in somatosensory cortex by non-human primates—towards artificial tactile sensation. PLoS One 9: e114529, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Bokil H. Observed Brain Dynamics. New York: Oxford Univ. Press, 2008. [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J 76: 691–708, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moca VV, Nikolić D, Singer W, Mureşan RC. Membrane resonance enables stable and robust gamma oscillations. Cereb Cortex 24: 119–142, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci 16: 1426–1435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol 104: 2873–2885, 2010. [DOI] [PubMed] [Google Scholar]
- Ozden I, Wang J, Lu Y, May T, Lee J, Goo W, O'Shea DJ, Kalanithi P, Diester I, Diagne M, Deisseroth K, Shenoy KV, Nurmikko AV. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J Neurosci Methods 219: 142–154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Dynamic formation of functional networks by synchronization. Neuron 69: 191–193, 2011. [DOI] [PubMed] [Google Scholar]
- Slater JD, Khan S, Li Z, Castillo E. Characterization of interictal epileptiform discharges with time-resolved cortical current maps using the helmholtz-hodge decomposition. Front Neurol 3: 138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. Nonlinear Dynamics and Chaos. Cambridge, MA: Perseus Books, 1994. [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron 63: 727–732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Contreras D, Whittington MA. Combined experimental/simulation studies of cellular and network mechanisms of epileptogenesis in vitro and in vivo. J Clin Neurophysiol 22: 330–342, 2005. [PubMed] [Google Scholar]
- Truccolo W, Ahmed OJ, Harrison MT, Eskandar EN, Cosgrove GR, Madsen JR, Blum AS, Potter NS, Hochberg LR, Cash SS. Neuronal ensemble synchrony during human focal seizures. J Neurosci 34: 9927–9944, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J Neurophysiol 93: 1074–1089, 2005. [DOI] [PubMed] [Google Scholar]
- Truccolo W, Hochberg LR, Donoghue JP. Collective dynamics in human and monkey sensorimotor cortex: predicting single neuron spikes. Nat Neurosci 13: 105–111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo WA, Rangarajan G, Chen Y, Ding M. Analyzing stability of equilibrium points in neural networks: a general approach. Neural Netw 16: 1453–1460, 2003. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 75: 963–980, 2012. [DOI] [PubMed] [Google Scholar]
- Vargas-Irwin CE, Donoghue JP. Automated spike sorting using density grid contour clustering and subtractive waveform decomposition. J Neurosci Methods 164: 1–18, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G, Kraskov A, Lemon RN. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J Neurosci 31: 14235–14242, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, van Wingerden M, Womelsdorf T, Fries P, Pennartz CM. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage 51: 112–122, 2010. [DOI] [PubMed] [Google Scholar]
- Waldert S, Lemon RN, Kraskov A. Influence of spiking activity on cortical local field potentials. J Physiol 591: 5291–5303, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wagner F, Borton DA, Zhang J, Ozden I, Burwell RD, Nurmikko AV, van Wagenen R, Diester I, Deisseroth K. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J Neural Eng 9: 016001–016015, 2012. [DOI] [PubMed] [Google Scholar]
- Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res S0168–S0102, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.