Abstract
We recorded single-neuron activity in dorsal premotor (PMd) and primary motor cortex (M1) of two monkeys in a reach-target selection task. The monkeys chose between two color-coded potential targets by determining which target's color matched the predominant color of a multicolored checkerboard-like Decision Cue (DC). Different DCs contained differing numbers of colored squares matching each target. The DCs provided evidence about the correct target ranging from unambiguous (one color only) to very ambiguous and conflicting (nearly equal number of squares of each color). Differences in choice behavior (reach response times and success rates as a function of DC ambiguity) of the monkeys suggested that each applied a different strategy for using the target-choice evidence in the DCs. Nevertheless, the appearance of the DCs evoked a transient coactivation of PMd neurons preferring both potential targets in both monkeys. Reach response time depended both on how long it took activity to increase in neurons that preferred the chosen target and on how long it took to suppress the activity of neurons that preferred the rejected target, in both correct-choice and error-choice trials. These results indicate that PMd neurons in this task are not activated exclusively by a signal proportional to the net color bias of the DCs. They are instead initially modulated by the conflicting evidence supporting both response choices; final target selection may result from a competition between representations of the alternative choices. The results also indicate a temporal overlap between action selection and action initiation processes in PMd and M1.
Keywords: decision making, primary motor cortex, reaching
an important aspect of voluntary motor control is the selection of the target for an action in a complex environment (Cisek and Kalaska 2010). Almost since it was first identified as a distinct cerebral cortical area over a century ago, the premotor cortex (PM) has been attributed a role in the higher-order control of movement (Wise 1985). Pioneering recording studies showed that many PM neurons discharged during a delay period between the appearance of a sensory cue instructing monkeys about the nature of a movement to make in the near future and a second stimulus that signaled when to make the movement (Weinrich and Wise 1982; Wise et al. 1983; Weinrich et al. 1984; Wise and Mauritz 1985). This delay-period activity signals many properties of the impending movement, including its direction, distance, speed, and trajectory (Bastian et al. 2003; Hocherman and Wise 1991; Fu et al. 1993, 1995; Johnson et al. 1999; Crammond and Kalaska 2000; Messier and Kalaska 2000; Cisek et al. 2003; Schwartz et al. 2004; Churchland et al. 2006a, 2006b; Churchland and Shenoy 2007; Churchland et al. 2010; Afshar et al. 2011).
Beyond signaling the specific parameters of a chosen movement, dorsal (PMd) and ventral (PMv) PM have also been implicated in aspects of the decision-making processes leading up to the selection of one motor action from among multiple alternatives. This has been demonstrated in tasks using arbitrary stimulus-response rules to identify the appropriate action (Brasted and Wise 2004; Buch et al. 2006; Crammond and Kalaska 1995; di Pellegrino and Wise 1992; Gail et al. 2009; Klaes et al. 2011; Kurata and Wise 1988a, 1988b; Mitz et al. 1991; Wise et al. 1983, 1992), including visual stimuli that indicate the correct arm and target location for a reach (Hoshi and Tanji 2000, 2002, 2006), or inform go/no-go decisions based on conditional match-to-sample rules (Wallis and Miller 2003; Muhammad et al. 2006) and visual categorization decisions (Cromer et al. 2011). PMd and especially PMv neurons can even encode all the sensory information needed to determine which of two buttons to push based on a differential tactile vibratory frequency discrimination (Romo et al. 2004; de Lafuente and Romo 2006; Hernandez et al. 2010).
Action target selection has been extensively studied for saccadic eye movements, often using random-dot kinematogram (RDK) or visual-search tasks. In RDK tasks, subjects choose between two or more targets based on their estimate of the net direction of noisy visual motion in the RDK stimuli (Newsome et al. 1989; Britten et al. 1993; Shadlen et al. 1996; Shadlen and Newsome 2001; Roitman and Shadlen 2002; Huk and Shadlen 2005; Churchland et al. 2008; Bennur and Gold 2011; Bollimunta and Ditterich 2012; Ding and Gold 2012). Behavioral and modeling studies suggest that the patterns of response times (RTs) and saccade choices of subjects can be explained by the temporal accumulation of the instantaneous net visual motion signal (the “net evidence”) favoring one choice over the others until the accumulating evidence supporting one target reaches a decision threshold (“drift diffusion”; Shadlen et al. 1996; Kiani et al. 2008; Mazurek et al. 2003; Palmer et al. 2005; Ditterich 2006a, 2006b, 2010; Niwa and Ditterich 2008; Gold and Shadlen 2001, 2007). Neural correlates of the accumulation process have been recorded from saccade-related cortical neurons in the form of an initial brief suppression of activity, followed by gradual reciprocal ramp-like increases and decreases in activity of neurons that prefer the selected and rejected targets, respectively, after the RDK stimuli appear. The slope of the ramp responses varies with the strength of the net motion evidence (Bollimunta and Ditterich 2012; Churchland et al. 2008; de Lafuente et al. 2015; Ding and Gold 2012; Huk and Shadlen 2005; Kiani et al. 2008; Mazurek et al. 2003; Roitman and Shadlen 2002).
In visual-search tasks, in contrast, stimuli appear simultaneously at two or more potential target locations, and the subject must select the correct saccade target and reject the other potential targets (“distractors”) on the basis of certain criteria (Basso and Wurtz 1998; Bichot and Schall 1999, 2002; Cohen et al. 2010; Hanes and Schall 1996; Kim and Basso 2008, 2010; McPeek and Keller 2002; Sato and Schall 2001, 2003; Schall and Hanes 1993; Thompson et al. 1996). The appearance of the potential target stimuli simultaneously activates several neural populations that each prefers saccades to one of the target locations. The activity of these coactivated populations then evolves rapidly in time, so that activity of the neurons preferring the selected target increases while that of the other neurons decreases. A variety of mechanisms have been proposed to explain the target selection process among the coactivated neural populations in visual-search tasks, including signal detection, probabilistic inferences, competitive interactions among the coactivated populations, and gated accumulation of evidence to a threshold (Cohen et al. 2010; Hanes and Schall 1996; Kim and Basso 2008, 2010; Purcell et al. 2012; Schall et al. 2011).
Target selection for reaching movements has not been studied as extensively as for saccades. However, studies have found a number of parallels with saccade selection, including the effects of target uncertainty, evidence quality and salience, and the presence of distractors on RTs, error rates, and reach trajectories (Chapman et al. 2010; Georgopoulos et al. 1981, 1983; McKinstry et al. 2008; Meegan and Tipper 1998; Resulaj et al. 2009; Song and Nakayama 2006; Song et al. 2008; Welsh and Elliot 2005; Wood et al. 2011). Unlike saccades, however, reach selection is also subject to such factors as the effort, biomechanical complexity, and controllability of the movements (Burke et al. 2014; Cos et al. 2011, 2012).
Cisek and Kalaska (2005) recorded PMd neural activity while monkeys performed a task in which two color-coded potential reach targets (Spatial Cues) briefly appeared in two opposite spatial locations before disappearing. At the end of a memorized Spatial-Cue delay period, a centrally located Color Cue appeared whose color matched that of one of the two Spatial Cues, thereby identifying the correct target according to a simple color-location matching rule. The appearance of the two Spatial Cues simultaneously activated two populations of PMd neurons that preferred reach movements in those directions, reminiscent of the coactivation of multiple saccade-related neural populations by the appearance of potential target stimuli in visual-search and RDK tasks. The coactivation was sustained for the duration of the Spatial-Cue delay period, while the monkeys waited for the Color Cue to provide the final information needed to select the correct target.
The appearance of the Color Cue evoked a rapid reciprocal change in the activity of PMd neurons that preferred the two opposite potential targets, reflecting the selection of the correct reach direction and the rejection of the other, yielding an unambiguous signal about the direction of reach to perform (Cisek and Kalaska 2005). The PMd activity primarily signaled the direction of the potential and final reach choices; very few PMd neurons showed a response modulation as a function of the colors of the Spatial or Color Cues, per se.
Cisek and Kalaska (2005) suggested that the PMd activity reflected the changing likelihood of the direction of the impending reach movement across time as new sensory information appeared in the form of the Spatial and Color Cues. Similarly, Klaes et al. (2011) reported the sustained coexistence of neural correlates of two reach options in parietal and PM in a task in which the appearance of a single target stimulus ambiguously signaled either a reach toward the target or in the opposite direction. The correct choice was subsequently determined by a second stimulus that signaled which stimulus-response rule to apply. Both of these studies are consistent with a competitive process of reach target selection played out among temporally coexisting representations of the different reach options (Chapman et al. 2010; Cisek 2006, 2007; Cisek and Kalaska 2010; Klaes et al. 2011, 2012; McKinstry et al. 2008; Meegan and Tipper 1998; Pastor-Bernier and Cisek 2011; Pastor-Bernier et al. 2012; Song and Nakayama 2006; Song et al. 2008; Welsh and Elliot 2005; Wood et al. 2011). Consistent with this, Song and McPeek (2010) found a simultaneous activation of multiple PMd populations when a reach target and multiple distractors appeared in a visual-search reach task, followed by a continued increase in activity of the neurons that preferred movements toward the selected target and a rapid suppression of activity in the neurons that preferred movements toward the rejected distractors, similar to that seen in saccade tasks.
The tasks used by Cisek and Kalaska (2005) and Klaes et al. (2011) were not suited to examine the temporal dynamics of the reach decision process because the sensory inputs guiding action were uniformly simple, static, and easily discriminated, and the tasks imposed arbitrary delays before movement initiation. Therefore, we revisited the Cisek and Kalaska (2005) study using a modified version of their task in which the decisive Color Cue provided variable degrees of conflicting sensory evidence for both the correct target and the opposite (incorrect) target, and the monkeys could move as soon as they had chosen a reach target, without imposed delays. We wanted to assess whether different levels of sensory evidence supporting the two potential targets would cause a graded activation of neural activity, as has been reported in saccade tasks. Moreover, we wanted to determine whether the neural activity in the populations of neurons that prefer movements in the two opposite directions would show gradual reciprocal changes proportional to the net sensory evidence favoring the correct target, or in contrast, whether the different amounts of conflicting sensory input supporting the two targets would evoke a transient coactivation of the opposing populations. The former result would be consistent with a mechanism in which a common signal about the net evidence supporting the correct target projects in parallel onto the populations of neurons preferring the two target choices. In contrast, the latter result would be more consistent with a target selection process in which sensory input supporting each option has at least some access to the neurons that are responsible for implementing the corresponding actions. A study of human choice behavior in this modified task has been published (Coallier and Kalaska 2014).
METHODS
Two male rhesus monkeys (Macaca mulatta) were trained to make arm-reaching movements to displace a lightweight pendulum-like manipulandum in the horizontal plane. Their reach trajectories were digitized by a horizontal graphics tablet that recorded the X–Y position of the pendulum handle within the workspace (±0.05 mm precision, 100 Hz). The monkeys could move the handle in every direction in the X–Y plane, and its instantaneous position was displayed as a cursor moving on a computer monitor. We recorded their reaching movements in response to sequences of visual instructional cues in several instructed-delay tasks. We also recorded neural activity in the PMd and primary motor cortex (M1) using single-microelectrode recording techniques while they performed the task, after surgical preparation of the monkeys for neurophysiological recordings using standard aseptic surgical procedures (Fig. 1; Kalaska et al. 1989; Sergio et al. 2005). The experimental protocol was approved by the institutional animal research review board (Comité de déontologie de l'expérimentation sur les animaux). All procedures followed university and national guidelines for animal care.
Fig. 1.
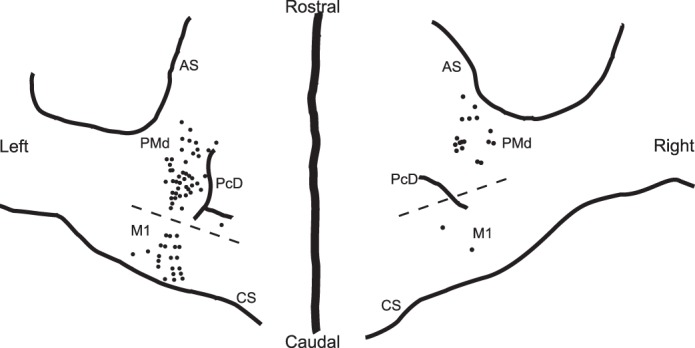
Anatomic reconstruction of the locations of penetrations in the left and right precentral cortex of monkey G in which neural data described in this report were collected. Broken line, approximate border between the primary motor cortex [M1, cortex in which intracortical microstimulation (icms) currents <20 μA often evoked movements] and premotor cortex (PMd, cortex in which icms currents <20 μA rarely evoked movements). CS, central sulcus; PCD, precentral dimple; AS, arcuate sulcus.
One-target and two-target tasks.
The monkeys were trained to perform the same one-target (1T) and two-target (2T) tasks used by Cisek and Kalaska (2005). The 1T task was a simple one-choice instructed-delay paradigm (Fig. 2). After an initial 500-ms center-hold time, a single colored (red or green) Spatial Cue appeared at one of eight potential target locations arrayed in a circle (8 cm radius) around the central start position. After 1,000 ms, it disappeared. After a 500- to 1,500-ms memorized-delay period, the central window's color changed from white to red or green (Color Cue; CC), always matching the color of the prior Spatial Cue. After 1,500–2,500 ms, the central Color Cue disappeared, and eight white circles appeared at the eight potential target locations (GO signal). The monkeys had to move the pendulum to displace the cursor from the central location to the memorized target location (minimum reaction time 150 ms, maximum reaction time 750 ms, maximum movement time 600 ms) and hold the cursor in the target window for 1,000 ms to receive drops of liquid reward. For real-time task performance control, the reach onset was measured as the time from the appearance of the GO signal to the exit of the cursor from the small central start window.
Fig. 2.
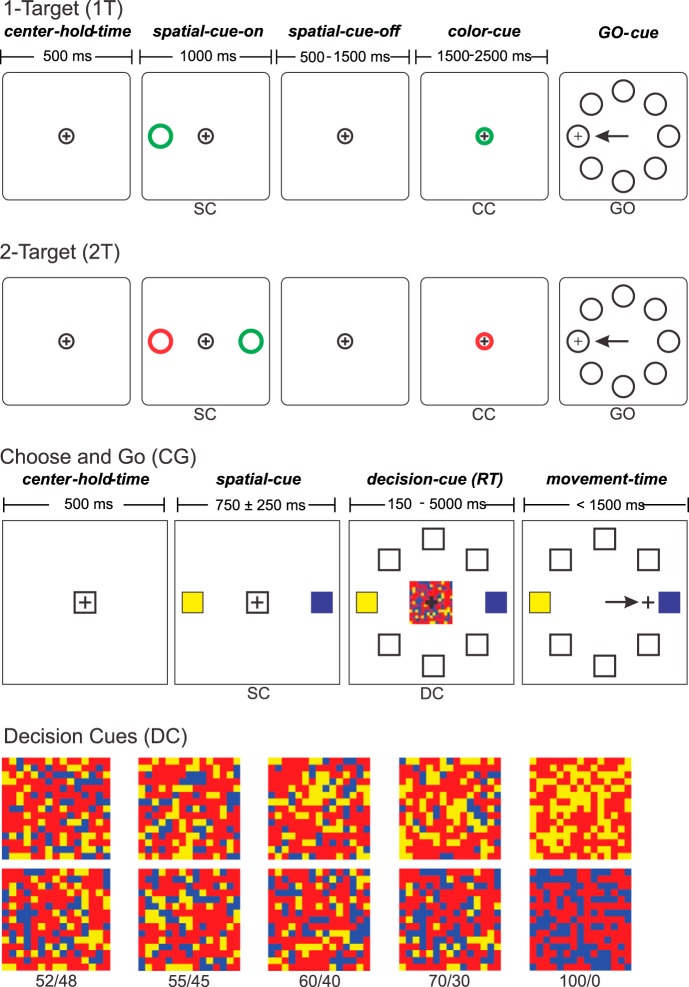
Sequences of stimulus events in trials of the three different tasks used in this study. 1-Target (1T) task: a single color-coded (green or red) Spatial Cue (SC) appears briefly at one of 8 target locations. After a memorized delay period, the central start window changes color to the same color as the previous spatial cue (Color Cue, CC). After a further delay, all 8 target locations appear, and the monkey must make a reaching movement to displace the on-screen cursor to the memorized target location. In the 1T task the target location is identified unambiguously by the spatial cue's location; its color and that of the subsequent CC are task-irrelevant. 2-Target (2T) task: two color-coded (green, red) SC appear briefly at two oppositely located target positions. After a memorized-delay period, the central window changes color to red or green (CC). After a further delay, all 8 target locations appear (GO), and the monkey must make a reaching movement to displace the on-screen cursor to the memorized target location whose color matched that of the broken. In the 2T task, the location and color of all cues is task-relevant. Choose-and-go (CG) task: two color-coded (yellow, blue) SC appear at two oppositely located target positions. After a variable delay, the central window is replaced by a checkerboard-like Decision Cue (DC) that contains different numbers of yellow and blue squares. The monkey must determine whether there are more yellow or blue squares and then reach to the corresponding target location as soon as it makes its decision about the correct target. DC, examples of the checkerboard patterns for DCs with differing amounts of color evidence for the correct target choice, ranging from 100/0 (unambiguous) to 52/48 (very ambiguous, high level of conflicting sensory evidence for both targets). The color bias of the DCs decreases from 1.0 (100/0) to 0.04 (52/48; see text for explanation).
In the 1T task, reach direction in each trial was defined unambiguously by the location of the single Spatial Cue, while its color and that of the subsequent central Color Cue provided no novel task-salient information. The 2T task (Fig. 2), in contrast, was a two-alternative forced-choice instructed-delay task in which two potential reach directions were first defined by two Spatial Cues, one red and the other green, that appeared on opposite sides of the central window. After the Spatial-Cue memorized-delay period (Fig. 2), the central circle changed color to either red or green (Color Cue), as in the 1T task. However, in the 2T task, its color provided the decisive information required to define the correct target choice in that trial according to a simple color-location matching rule, reach to the memorized location of the Spatial Cue whose color matched the Color Cue when the GO cue appears.
Choose-and-go task.
The monkeys were also trained in a modified version of the 2T task, called the “choose-and-go” (CG) task (Fig. 2; Coallier and Kalaska 2014). In this task, like the 2T task, the monkeys used a simple color-location matching rule to choose between two reaching movements to color-coded spatial targets in different directions based on the color of a centrally located color cue. However, the CG task differed from the 2T task in three important ways. First, the Spatial Cues remained on for the duration of the trial after they appeared (Fig. 2); there was no need to memorize their location and color. Second, the quality of the sensory evidence used to select the target was manipulated by altering the color composition of the central Decision Cue (DC). Finally, the monkeys could move to their chosen target at any time after the DC appeared. There was no arbitrary delay period imposed after the DC appeared, and no explicit delayed GO cue as in the 2T task. As a result, we called this the CG task.
To start each CG task trial, a white square (1.9 cm) appeared at the center of the monitor. The monkeys positioned the cursor in the square and held it there for 500 ms (center-hold period). Two peripheral square Spatial Cues (3.8 cm) then appeared on opposite sides of the central square (Fig. 2). The centers of the Spatial Cues were 15.2 cm apart. One Spatial Cue was blue and the other was yellow, and the location of the two colors changed randomly from trial to trial. After a variable Spatial-Cue delay (750 ± 250 ms), the central white square was replaced by a checkerboard-like DC (5.6-cm square), which was a square matrix of 100 randomly positioned task-relevant yellow and/or blue squares interspersed with 125 task-irrelevant red squares (Fig. 2). Pilot studies showed that the presence of the red squares encouraged the subjects to focus on the relative number of squares of the task-relevant colors rather than trying to discriminate the overall color “tone” of the stimuli. They also allowed the possibility to vary the total number of task-relevant squares, which was not exploited in this study (see Coallier and Kalaska 2014).
The predominant task-relevant color of the DC (blue or yellow) indicated which Spatial Cue was the correct target for that trial, according to a color-location matching rule similar to the 2T task. The monkeys were free to reach to the target they chose at any time within a generous time window after DC onset (acceptable RT 150-5,000 ms from DC appearance to the time the cursor exited the central window). The DC disappeared as soon as the cursor exited the central window, to avoid any effect the continued presence of the DC might have on the behavior of the monkeys, such as encouraging reversals of reach direction after exiting the central window (c.f., Resulaj et al. 2009; Coallier and Kalaska 2014). A maximum movement time of 1,500 ms was allowed from the exit of the central window to the peripheral target.
In the standard DC stimulus series that was used for this study, each stimulus always contained 100 squares of the task-relevant blue or yellow colors. Thus, the total amount of salient sensory input on which the monkeys had to base their decision was constant in every trial. We varied the quality of sensory evidence between trials by altering the relative number of the task-relevant blue and yellow squares in the DC matrix. The number of squares of the two colors used in a given trial could be 100/0, 70/30, 60/40, 55/45, or 52/48, predominantly blue or yellow, for a total series of 10 different DCs (Fig. 2).
The color ratios indicate the true composition of the DCs, that is, how much evidence each DC contained about the correct and incorrect reach target choices. The net strength of the DC evidence favoring the correct target over the incorrect choice could be quantified by its color bias, which we defined as the ratio of the difference in the number of blue and yellow squares, divided by their total number (100), and so varied from a color bias value of 1.0 to 0.04.
The color ratio and color bias measure two different but correlated attributes of the DC stimuli that reflect the challenge they present to the subjects. The strength of the sensory evidence about the predominant task-relevant color in the DCs was manipulated both by reducing the number of squares of the predominant color, i.e., the amount of sensory evidence supporting the correct choice, and by simultaneously increasing the number of squares of the other color, i.e., increasing the amount of “distractor” evidence for the wrong choice. As a result, since the color ratio changes from 100/0 to 52/48, the DCs present increasingly equal amounts of conflicting evidence for the two choices. In parallel, the color bias of the DCs decreases from 1.0 to 0.04. A DC with a low color bias is not a stimulus with little salient sensory evidence. Instead, it has nearly equal amounts of readily discriminable and competing sensory inputs favoring both choices. However, as their color bias decreases, the net evidence that supports the correct reach choice over the alternative choice decreases, and the DCs become increasingly ambiguous. We will use the term “conflicting” to describe the impact of the color ratio of each DC, and “ambiguous” to describe the amount of net evidence (the color bias) supporting the correct reach choice. A 100/0 DC has no conflicting evidence and no ambiguity (1.0 color bias), whereas a 52/48 DC is both highly conflicting and highly ambiguous (0.04 color bias).
For each of the 10 DCs, the program had a library of 100 pregenerated matrixes. In each trial, the DC was presented in one of two different formats. In “static” trials, the program randomly sampled and displayed one matrix for a particular color bias for the entire trial. In “dynamic” trials, the program randomly sampled and displayed a new matrix of the same color bias every 50 ms (every three frames of the 60-Hz liquid-crystal display monitor, synched display). Because the positions of the blue and yellow squares varied randomly in each matrix, the dynamic DCs appeared to flicker but did not elicit any sense of coherent motion. The sequence of DC matrixes presented during a given dynamic trial was always of the same color bias; we did not attempt to modify the quality of sensory evidence within single trials (c.f., Huk and Shadlen 2005).
The monkeys indicated their choice of the predominant DC color by moving the handle to displace an on-screen cursor toward the target square of the corresponding color. The trial ended if the monkeys did not begin to move within 5,000 ms after DC onset and was scored as a nonresponse. To discourage guessing or anticipation of the timing of the DC, the task program also scored as an error all trials in which the subject exited the central window too quickly (<150 ms) after DC onset. All factors (correct target location, target color, predominant DC color, DC color bias, DC format) were fully balanced in a randomized block sequence. Each trial was ∼5 s long. There was a 1,000-ms intertrial interval between trials.
Trials with all combinations of task factors in a given trial block had to be performed successfully one time each before proceeding to the next trial block of the task. When the monkeys performed a trial with a particular combination of factors incorrectly, it was reinserted into the pseudorandom sequence of trials in that block of the task. Those trial combinations were repeated until completed successfully before the program progressed to the next randomized block sequence of trials. A typical data file included five complete randomized block replications of correctly performed trials with all trial factor combinations and error choice trials. Trials in which the monkeys chose the correct target were rewarded with drops of liquid (water, fruit juice) immediately at the end of the trial. Reward size was constant for all DCs for monkey G but was often increased as DC color bias decreased for monkey Z (see results). Trials in which the monkeys made incorrect target choices or any other type of error were not rewarded.
Data analysis.
Behavioral and neural data were analyzed using custom-written routines in MATLAB (The Math Works), Borland Delphi (Borland), and Excel (Microsoft). Analysis focused on those trials in which the monkeys reached to the correct target and trials in which they moved to the incorrect target (choice errors). Other error types were not analyzed here. Before data were subjected to detailed analysis, all data files were preprocessed to identify the time of onset of movement (behavioral RT) and any changes in reach direction.
An automatic algorithm estimated the onset of movement. The X–Y coordinates of the pendulum handle measured every 10 ms were differentiated to generate an instantaneous speed profile for each trial. The speed profile was smoothed using a sliding 40-ms time window (current time step ± 2 adjacent time steps), and the mean and the SD of the speed profile were calculated during the last 500 ms before DC onset. Starting from DC onset, the algorithm advanced along the speed profile to detect the first time that the instantaneous speed exceeded the mean speed calculated during the 500-ms pre-DC period by at least three SDs and remained above the three SD threshold for at least five consecutive time intervals (Coallier and Kalaska 2014; Kalaska et al. 1989; Sergio and Kalaska 2005). This estimate of reach response onset time (RT) was used for all subsequent analyses. The algorithm also searched for changes in direction of the reaching movement from one target to the opposite target (Georgopoulos et al. 1981, 1983; Resulaj et al. 2009) by searching for a reversal of sign of the speed profile along the straight-line axis of movement from the start position to the original target, followed by four more consecutive 10-ms time steps in the reversed direction (Coallier and Kalaska 2014). After automatic processing of the trial data in each file, the estimated RT for each trial was verified visually and adjusted manually if necessary to avoid false early or late RT estimates. This occurred infrequently (typically <2% of trials in a given file).
For statistical analyses and for generating histograms of single-neuron or population responses, single-trial data were divided into time bins of different durations (5, 10, or 20 ms) or into different trial epochs. Rather than simply counting the number of spikes that fell within each bin or trial epoch in a single trial, we counted how many whole and fractional interspike intervals it contained (Georgopoulos et al. 1982; Kalaska et al. 1989; Cisek and Kalaska 2005; Sergio et al. 2005). If a particular interspike interval spanned two or more contiguous bins or epochs, each bin or epoch received a count proportional to the fraction of the interval that fell within its boundaries. This method converts the sequence of quantal events of the single-trial spike train into a continuous pseudoanalog signal. The partial-spike scores were converted to spikes per second by normalizing for the duration of each epoch or bin.
For statistical analyses of data pooled across multiple neurons, we converted binned discharge rates to z-scores to minimize the effect of the added variance resulting from the different discharge ranges of different neurons. For each neuron, the distribution of binned single-trial discharge rates was log transformed to make each distribution more symmetrical. The log-transformed data were then z-scored so that the effect of any task factor on the neuron's single-trial activity was expressed in terms of SD units away from the overall mean discharge rate of the data distribution for each single neuron. The single-neuron z-scored data were then pooled across neurons for quantitative analysis (Britten et al. 1996; Gail et al. 2009).
For statistical analyses of data from single neurons, we used a criterion statistical significance level of P < 0.01, but, for data pooled across multiple neurons, we adopted a criterion level of P < 0.001 because of the much larger data sample sizes.
We did a receiver operating characteristic (ROC) analysis (Britten et al. 1992, 1994) to estimate when an ideal observer could distinguish between correct choices for the two targets (choice probability; Celebrini and Newsome 1994) based on the pooled neural activity associated with each choice. We calculated each neuron's z-scored discharge rates in sequential 20-ms time bins in each trial in which the DCs supported each neuron's preferred direction (PD) or the opposite direction (oPD). At each 20-ms time interval, we compared the distributions of z-scored discharge rates pooled across all neurons between PD and oPD trials for each DC color bias tested on each neuron to assess the probability that binned discharge rates were higher in PD trials than oPD trials (i.e., the area under the ROC curve). This yielded a time series of ROC curve areas for PD compared with oPD trials for each DC color bias during a trial. The analysis was done after aligning the data to either the DC onset or movement onset.
To assess when the differences in binned data distributions for PD and oPD trials first became greater than chance, we did a bootstrapped permutation test (2,000 random samples of the original z-score distributions, with replacement) for each bin interval and counted the number of times the area under the original ROC curve was greater than that of the bootstrapped samples. We used a criterion threshold of 99% as a significant difference because of the large data sample sizes. The time at which the PD and oPD responses became significantly different was the first of five consecutive 20-ms bins (100 ms) that exceeded the 99% significance criterion.
To estimate when an ideal observer could reliably discriminate the time at which the monkeys made correct PD vs. oPD choices, we established a criterion of the first of five consecutive 20-ms time bins in which the area under the ROC curve for the z-scored data was greater than 0.7. We did not use a higher criterion because the peak choice probability values varied across datasets. Furthermore, many M1 neurons had a delayed phasic “braking” burst of activity in reach directions opposite to their PD (Kalaska et al. 1989; Sergio et al. 2005), which often kept their calculated choice probability values low during movement.
ANOVAs of neural activity in the CG task were performed using IBM SPSS Statistics 20 (IBM). For the three-way repeated-measures ANOVA, each trial was divided into five consecutive nonoverlapping epochs (Fig. 2): the center-hold time, the Spatial-Cue period, the DC period from the appearance of the DC to the onset of movement, the Movement-Time period, and the Target-Hold time. The basic datum for the three-way ANOVA was the mean partial spike-interval discharge rate recorded during each epoch of each trial. The three-way ANOVA was performed on the data from each epoch separately, with main factors direction (2 levels), DC predominant color (2 levels), and DC evidence strength (3–5 levels). The elapsed time of the start of each epoch in each trial from the start of collection of the data file was treated as a covariate. The three-way ANOVA was applied to the data for each neuron separately.
RESULTS
RTs and success/error rates.
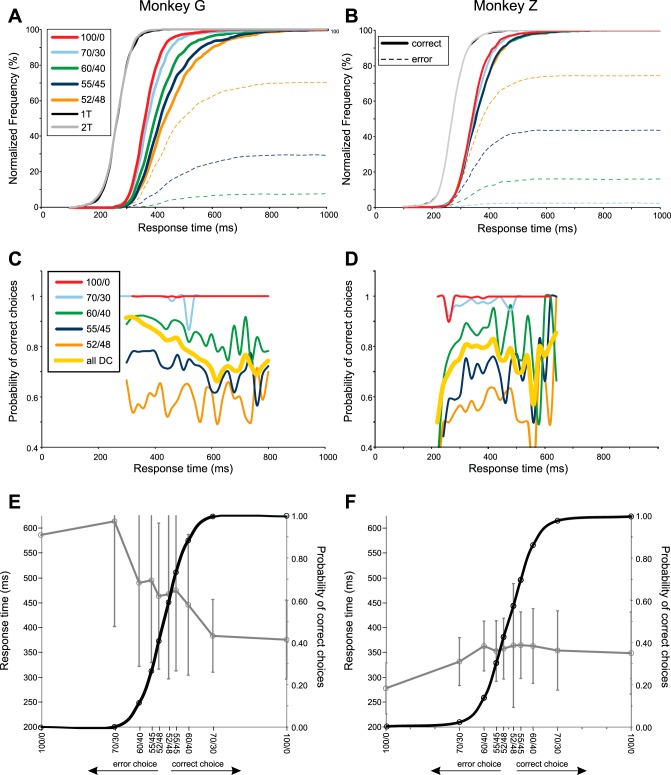
Both monkeys performed thousands of trials of the 1T, 2T, and CG tasks while we recorded neural activity in PMd and M1 (Tables 1 and 2). In the 1T and 2T tasks, the GO signal served only as a timing signal about when to start a reach movement to the memorized location of a target that had already been identified by the initial Spatial Cue (1T task) or the combination of Spatial and Color Cues (2T task). The onset of movement after the GO cue was in theory a simple visual reaction-time process in which RTs provide a measure of the minimum time required to detect the appearance of the GO signal and initiate a movement to the preselected target, and should be identical in both tasks. Consistent with that prediction, RT distributions completely overlapped in both tasks in each monkey (Table 1 and Fig. 3, A and B).
Table 1.
Number of trials and median and mean ± SD values (ms) for the RTs in the 1T and 2T tasks
| Task | 1T | 2T |
|---|---|---|
| Monkey G | ||
| Trials | 5,948 | 6,438 |
| Median | 280 | 282 |
| Mean ± SD | 290.7 ± 54.5 | 291.9 ± 55.7 |
| Monkey Z | ||
| Trials | 1,863 | 2,368 |
| Median | 270 | 270 |
| Mean ± SD | 274.0 ± 51.2 | 273.4 ± 48.1 |
RT, response time; 1T, one-target task; 2T, two-target task.
Table 2.
Number of trials, median, and mean ± SD RTs (ms) for the correct-choice and error-choice trials, and success rates (%correct) for the different DCs used in the CG task, for both monkeys
| DC |
|||||
|---|---|---|---|---|---|
| 100/0 | 70/30 | 60/40 | 55/45 | 52/48 | |
| Monkey G left (39 PMd neurons, 19 M1 neurons) | |||||
| Correct | |||||
| Trials | 5,959 | 0 | 5,889 | 5,097 | 0 |
| Median | 360 | 425 | 451 | ||
| Mean ± SD | 377.5 ± 82.1 | 451.8 ± 147.7 | 483.0 ± 169.2 | ||
| Error | |||||
| Trials | 5 | 836 | 1923 | ||
| Median | 425 | 456 | 463 | ||
| Mean ± SD | 586.4 ± 421.5 | 492.9 ± 171.7 | 499.0 ± 171.3 | ||
| Success rate | 99.99 | 87.67 | 72.61 | ||
| Monkey G right (17 PMd neurons) | |||||
| Correct | |||||
| Trials | 1,013 | 1,009 | 1,010 | 1,002 | 990 |
| Median | 362 | 373 | 398 | 413 | 435 |
| Mean ± SD | 370.3 ± 58.0 | 384.1 ± 72.9 | 414.4 ± 86.4 | 437.7 ± 108.6 | 459.2 ± 124.4 |
| Error | |||||
| Trials | 0 | 4 | 79 | 298 | 696 |
| Median | 511.5 | 429 | 449 | 437 | |
| Mean ± SD | 623.8 ± 211.0 | 462.5 ± 106.2 | 472.8 ± 113.4 | 461.3 ± 119.9 | |
| Success rate | 100.0 | 99.61 | 92.75 | 77.08 | 58.72 |
| Monkey Z (19 PMd neurons, 8 M1 neurons) | |||||
| Correct | |||||
| Trials | 1,168 | 1,166 | 1,159 | 1,146 | 1,144 |
| Median | 340 | 345 | 351 | 354 | 352 |
| Mean ± SD | 348.9 ± 67.9 | 354.3 ± 91.0 | 363.3 ± 84.4 | 364.9 ± 82.4 | 364.3 ± 79.6 |
| Error | |||||
| Trials | 5 | 29 | 188 | 500 | 851 |
| Median | 257 | 328 | 351 | 344 | 346 |
| Mean ± SD | 272.8 ± 60.8 | 332.0 ± 60.9 | 363.2 ± 124.1 | 353.1 ± 66.8 | 358.0 ± 74.9 |
| Success rate | 99.57 | 97.54 | 86.04 | 69.62 | 57.34 |
DCs, Decision Cues; CG, choose and go; PMd, dorsal premotor; M1, primary motor cortex.
Fig. 3.
Choice behavior of monkey G (left) and monkey Z (right). A and B: normalized cumulative frequency plots of the behavioral response times (RT) for trials in which the monkeys made correct (solid lines) and incorrect (broken lines) target choices in the 1T and 2T tasks, and in response to DCs with differing degrees of conflicting sensory evidence. Success rates were nearly 100% for the 100/0 and 70/30 DCs for monkey G and for the 100/0 DCs for monkey Z. As a result, the error-choice cumulative curves are not visibly distinct from the horizontal axis in the figures for those DCs. C and D: success rate as a function of time for each DC, separately (solid colored lines), as well as pooled across all DCs (thick yellow line). E and F: psychometric curves for the probability of correct and error choices as a function of DC quality (solid black line) and the chronometric curves for the corresponding RTs (mean ± SD) (gray line). Data from DCs that were predominantly yellow or blue were pooled for these psychometric and chronometric curves. Data from both hemispheres were pooled for monkey G.
In contrast, the CG task was a two-alternative response-time task in which the sequential color and GO cues were replaced by the unitary DC. The DC provided the information the monkeys needed to resolve the color/location matching rule to choose the correct target and also served as the timing signal to initiate the reach movement once it was selected. Unlike the monochromatic Color Cue of the 2T task, most DCs of the CG task contained different amounts of conflicting color evidence favoring both of the color-coded targets. Furthermore, the monkeys could not anticipate at the start of a trial whether the color evidence in the DC would be relatively strong and unambiguous (100/0, 70/30) or weak, with increasingly similar amounts of conflicting evidence for the two choices (60/40-52/48).
The RTs of the monkeys reflected these fundamental differences between the CG task and the 1T/2T tasks. First, compared with the 1T/2T tasks, the median RTs for the 100/0 DCs increased by ∼80 ms to 360–362 ms for monkey G and by ∼70 ms to 340 ms for monkey Z (Table 2 and Fig. 3, A and B). The longer RTs for the easiest DCs presumably reflected the extra time required to process the DC and select the target according to the color/location matching rule.
Second, the color composition of the DCs systematically affected monkey G's RTs and success rates (Table 2). The distributions of correct-choice RTs shifted to progressively longer values as the DCs progressed from the least (100/0) to the most conflicting and ambiguous (52/48) (Fig. 3A and Table 2). Neurons recorded in the left hemisphere of monkey G were tested with variable combinations of DCs, but most commonly with the 100/0, 60/40, and 55/45 DCs. For those DCs, median RTs for correct-choice trials increased from 360 ms (100/0 DC) to 451 ms (55/45 DC). All neurons recorded in monkey G's right hemisphere were tested with all five DC color ratios; median RTs increased from 362 ms (100/0 DC) to 435 ms (52/48 DC) (Fig. 3A and Table 2). The RT distributions for all DCs were highly significantly different [1-way ANOVA; left hemisphere: F(2,16943) = 892.1, P ≈ 0.0; right hemisphere: F(4,5019) = 156.52, P = 2.1 × 10 × 10−126]; post hoc tests showed that the RT distributions for every combination of pairs of DCs were likewise highly significantly different during recordings in both hemispheres (1-way ANOVA, P = 3.95 × 10−05 to P = 1.1 × 10−240). The variability of RTs (SD; Table 2) also increased as the color bias of DCs decreased, consistent with the increasing skew of the RTs to longer values (Fig. 3A).
Monkey G made very few target-choice errors for the 100/0 and 70/0 DCs (9/7,990 trials; Table 2 and Fig. 3, A, C, and E), indicating that it could correctly discern the predominant color of the DC and correctly apply the color/location matching rule in nearly every trial in which the DC color bias was strong. Those few “lapse” errors had long and variable RTs, suggesting that they were likely due to inattention or other factors. The number of incorrect target choices increased (Fig. 3A and Table 2) and the resulting success rate decreased (Fig. 3, C and E, and Table 2) as the color bias of the DCs decreased. Furthermore, the RTs of error-choice trials were longer than the RTs for the correct trials to the corresponding DC for all DCs except the 52/48 DC (Fig. 3E and Table 2).
Success rates were systematically lower at all RT values for the 60/40-52/48 DCs, and there was some tendency for success rates to decline for trials with longer RTs for the 60/40 and 55/45 DCs (Fig. 3C). Pooled across all DCs, there was a progressive and nearly linear decline in success rate over the range of RTs from 200 to 600 ms, which then leveled off (Fig. 3C, thick yellow line). The success rate was significantly higher during the time period from 200 to 400 ms after DC onset (89.29%) than from 420 to 800 ms (79.58%; χ2-test, P = 2.45 × 10−103). This global trend was due to a combination of factors. As time progressed, more of the trials in which a decision had not yet been made had DCs with lower color biases that had longer RTs for correct choices and a greater incidence of incorrect choices with even longer RTs (Fig. 3, A and E).
Monkey G's RTs were shorter and less variable for similar DCs during recordings in the second hemisphere compared with the first, while success rates improved modestly (Table 2), suggesting that there was a modest improvement in task performance over the course of the experiment.
The timing of monkey G's choice behavior was self-imposed, because it almost never took more than 1,000 ms to initiate a movement (Fig. 3A), which was far shorter than the 5,000 ms maximum RT limit of the paradigm.
By the same criterion, monkey Z's choice behavior in the CG task was also self-imposed, but was significantly different. Its RTs were systematically shorter than monkey G. More importantly, there were only small differences in RT durations and variability for correct-choice trials as a function of DC color bias (Fig. 3, B and F, and Table 2). Nevertheless, the small RT differences across all five levels of DC color bias were statistically significant [F(4,5778) = 8.99, P = 3.06 × 10−07]. Post hoc tests yielded mixed results. Three pairwise comparisons were highly significant (100/0 vs. 52/48, 100/0 vs. 55/45, and 100/0 vs. 60/40; P < <0.001), two were significant at a lower level (70/30 vs. 52/48 and 70/30 vs. 55/45; 0.01 > P > 0.001), and the other five comparisons were not significant (100/0 vs. 70/30, 70/30 vs. 60/40, 60/40 vs. 55/45, 60/40 vs. 52/48, and 55/45 vs. 52/48; P > 0.01).
Like monkey G, monkey Z's error rates increased as DC color bias decreased (Fig. 3, B, D, and F, and Table 2). However, its error rates were modestly higher for the 100/0-55/45 DCs than monkey G but similar for the 52/48 DCs (Table 2 and Fig. 3B, broken lines). Also unlike monkey G, the RTs for error-choice trials were either shorter than (100/0 and 70/30 DCs) or similar to (60/40-52/48 DCs) the RTs for the correct-choice trials (Fig. 3F and Table 2). Finally, monkey Z showed a trend for lower success rates for all DCs in trials with short RTs and higher success rates in trials with longer RTs (Fig. 3D), so that the cumulative success rate for the data pooled across DCs showed a progressive increase from 200 to 320 ms after DC onset (Fig. 3D, thick yellow line). The success rate was significantly lower during the time window from 200 to 300 ms after DC onset (73.46%) than from 320 to 700 ms (79.69%; χ2-test, P = 7.01 × 10−07), but not for the 200–340 vs. 360–700 ms window (P = 0.111). Finally, the success rate from 200 to 400 ms after DC onset was significantly higher for monkey G (89.29%) than monkey Z (78.82%; χ2-test; P = 5.86 × 10−80). In contrast, success rates were similar for the two monkeys from 420 to 700 ms after DC onset (79.84 vs. 77.65%; χ2-test; P = 0.045).
The higher incidence of error choices in trials with shorter RTs suggested that monkey Z occasionally initiated a movement even before it had taken enough time to make a reliable estimate of the color bias of the stimuli. Monkey Z's choice behavior trends suggest that it adopted a strategy of observing the DCs for a relatively fixed period of time independent of the DC color bias in each trial, and then making a target choice. It displayed this behavior early in training. We tried several approaches to modify its behavior to make it similar to that of monkey G, including daily sessions with only 55/45-52/48 DCs, or long time outs (5–10 s) after error choices to those DCs to encourage it to observe the stimuli for a longer time to increase its success rates, or both. We also often gave larger rewards for correct responses to DCs with low color biases. None of these approaches produced a significant change in its choice behavior.
Monkey Z's behavior might appear more “optimal” than monkey G's in that, by spending less time than monkey G observing the DCs with weaker color biases and lower success rates, this would increase the rate of occurrence of trials with DCs with strong color biases and therefore increase the overall reward rate per unit time when averaged over many trials. That strategy might work if each trial combination was presented only one time in each trial block regardless of outcome. However, we repeated error-choice trials until each one was performed correctly in each trial block so that this strategy would actually be counterproductive.
The static vs. dynamic DC format had no significant effect on the RTs and success rates to any DC for either monkey (data not shown) so results for the two formats were pooled. DC format also had very little effect in human subjects (Coallier and Kalaska 2014).
Target choice errors were the most common errors in both monkeys. Failure to respect the time constraints on RTs (0.4 and 1.7%), movement duration (1.2 and 2.6%), and target-hold duration (0.03 and 0.02%) was infrequent in monkeys G and Z, respectively. These errors were not included in the error counts and success rates presented in Fig. 3 or Table 2.
Changes in reach direction.
Human and nonhuman subjects occasionally begin to reach to one target and then change direction to a different target in reach-selection tasks (Burk et al. 2015; Coallier and Kalaska 2014; Resulaj et al. 2009; Song and Nakayama 2006; Song et al. 2008). These “changes of mind” occur more often as the quality of sensory evidence decreases and suggest that subjects may initiate a reach before the decision process had been fully resolved or can change direction as further evidence processing occurs after reach onset. Both monkeys showed similar behavior in this task.
Monkey G changed reach direction in 712/27,131 trials (2.6%), comparable to the direction change rate in the same task in human subjects (2.5%; Coallier and Kalaska 2014). The rate of direction changes increased as the color bias of the DCs decreased (100/0: 0.7%; 70/30: 0.5%; 60/40: 2.5%; 55/45: 3.4%; 52/48: 5.9%). Monkey Z changed reach direction more often (1,315/10,500 trials, 12.5%) and also showed a systematic increase in direction changes as DC color bias decreased (100/0: 4.8%; 70/30: 7.0%; 60/40: 11.1%; 55/45: 14.4%; 52/48: 17.3%).
Changes of direction had little impact (Coallier and Kalaska 2014) or improved success rates (Resulaj et al. 2009) in human subjects. Unlike those prior findings, however, trials in which the monkeys changed reach direction increased their overall error rates. Monkey G changed direction from the wrong target to the correct target in 445/22,758 correct-choice trials (1.96%) but from the correct target to the incorrect target in 267/4,373 error-choice trials (6.11%). The corresponding values were 7.24 and 30.27% for monkey Z. In monkey G, the global error rate in trials without a direction change was 4,106/26,419 trials (15.54%) but was 267/712 trials (37.5%) in direction-change trials. The corresponding values for monkey Z were 1,679/9,185 (18.28%) in nonchange trials and 729/1,315 (55.44%) in direction-change trials.
Neural database.
We recorded task-related activity from 168 cells in monkeys G and Z, 53 in M1 (33 in monkey G, 20 in monkey Z), and 115 in PMd (90 in monkey G, 25 in monkey Z). A total of 75 PMd (56 in monkey G, 19 in monkey Z) and 27 M1 cells (19 in monkey G, 8 in monkey Z) were recorded in all three tasks (Table 2). The M1 data in monkey G were collected in or near the rostral bank of the central sulcus. The majority of PMd data were collected in caudal PMd near the precentral dimple, but the most rostral penetrations in both hemispheres were in rostral PMd (Fig. 1; Cisek and Kalaska 2005). However, not enough data were collected from rostral PMd to do a comparison of results from the two parts of PMd. Anatomic localization of recording penetration sites is not yet available for monkey Z, but the implantation coordinates of the recording chambers were similar to monkey G. All neural data were collected while the monkeys made reaching movements with the arm contralateral to the hemisphere in which recordings were made (c.f., Cisek et al. 2003). All of the neurons were directionally tuned during at least one of the trial epochs in the various tasks (3-way ANOVA, P < 0.01).
Neural data collection had to be terminated prematurely in monkey Z for clinical reasons. The few M1 neurons studied in monkey Z up to that point were not strongly directional, suggesting that we had not located the primary representation of the proximal arm before data collection was stopped. As a result, those M1 data will not be presented here in detail.
1T and 2T tasks.
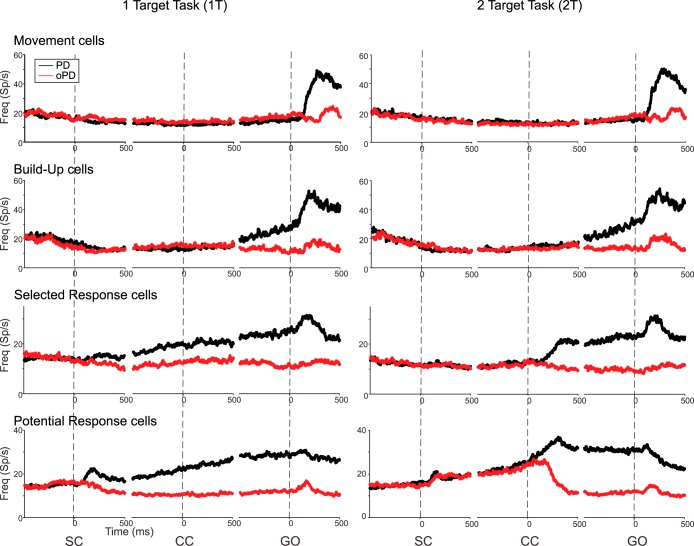
Consistent with Cisek and Kalaska (2005), the activity of PMd neurons in monkeys G and Z displayed a wide range of response properties in the 1T and 2T tasks, most of which could be assigned to one of four different cell classes (Fig. 4) on the basis of the presence or absence of statistically significant uni- or bidirectional tuning in different trial epochs [see Cisek and Kalaska (2005) for a detailed description of the criteria]. The similarity of the neural responses in the 1T and 2T tasks in the two studies confirms the robustness of the previous findings and indicates that both studies sampled PMd neurons with a similar range of response properties.
Fig. 4.
Population histograms of neurons with four different response patterns in the 1T (left) and 2T (right) tasks. In each histogram, neural activity is aligned to SC onset (left), CC onset (middle), and GO cue onset (right). Black, responses for trials toward each neuron's preferred target location; red, responses for trials toward the opposite target location.
Most relevant for this study is how the PMd neurons responded to the cues in the 1T and 2T tasks. When the single Spatial Cue of the 1T task (Fig. 4, “SC”) or the monochromatic Color Cue of the 2T task appeared (Fig. 4, “CC”), the activity of “selected-response” and “potential-response” neurons both changed abruptly. If the cue supported the target in the neurons' PD, their activity increased, but, if it supported the target in their oPD, their activity did not change or was reciprocally suppressed. Most importantly, the Color Cue of the 2T task caused a rapid reciprocal change in activity of the selected- and potential-response neurons whose PDs corresponded to the selected and rejected targets in a given trial. There was no evidence of a transient coactivation of neurons with opposing directional preferences in response to either the single Spatial Cue of the 1T task or the monochromatic Color Cue of the 2T task. Cisek and Kalaska (2005) reported similar findings, again confirming the robustness of those results.
CG task: Correct choices-neural activity as a function of DC evidence strength.
After testing the neurons in the 1T and 2T tasks, they were then tested in the CG task, using the target closest to their preferred reach direction (PD) and in the opposite direction (oPD). DCs whose color bias matched the color of the Spatial Cue at the neuron's PD in a given trial will be referred to as PD-DCs while those that matched the Spatial Cue at the oPD will be referred to as oPD-DCs. While we only studied neurons with Spatial Cues at their PD and oPD, it is highly likely that neurons whose preferred movement axes were intermediate to the tested directions also contributed to the decision process in each trial. This is consistent with the widely accepted population-coding hypothesis that neurons with diverse tuning properties make a graded contribution to the control of reaching movements as a function of the difference between the direction of intended output and the inherent directional preference of each neuron (Cisek 2006, 2007; Cisek and Kalaska 2005; Georgopoulos et al. 1982, 1983; Kalaska et al. 1989).
The static vs. dynamic format of the DCs had very modest effects on the activity of most neurons. As a result, all neural responses were pooled from both DC formats for all analyses. Because of the differences in the way the monkeys responded to the DCs, analyses of neural data in the CG task will be presented separately for the two monkeys.
PMd neural responses to the Spatial Cues were similar in the CG task to that the 2T task (data not shown). We focus here on their responses to the subsequent DCs.
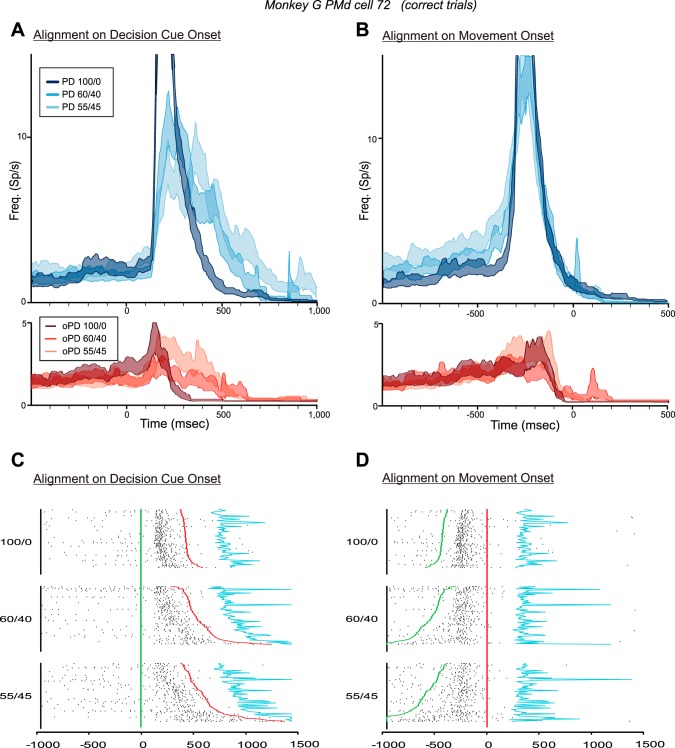
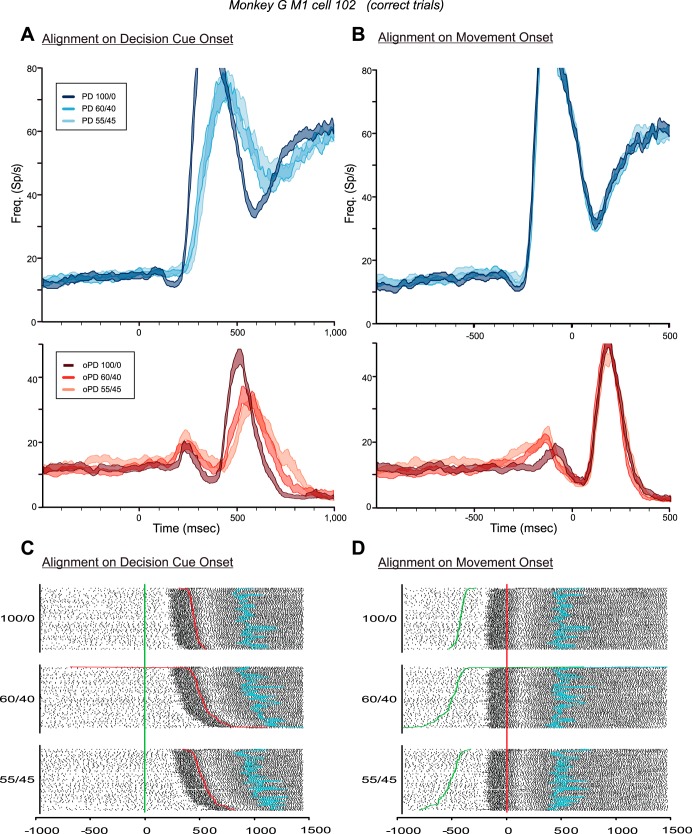
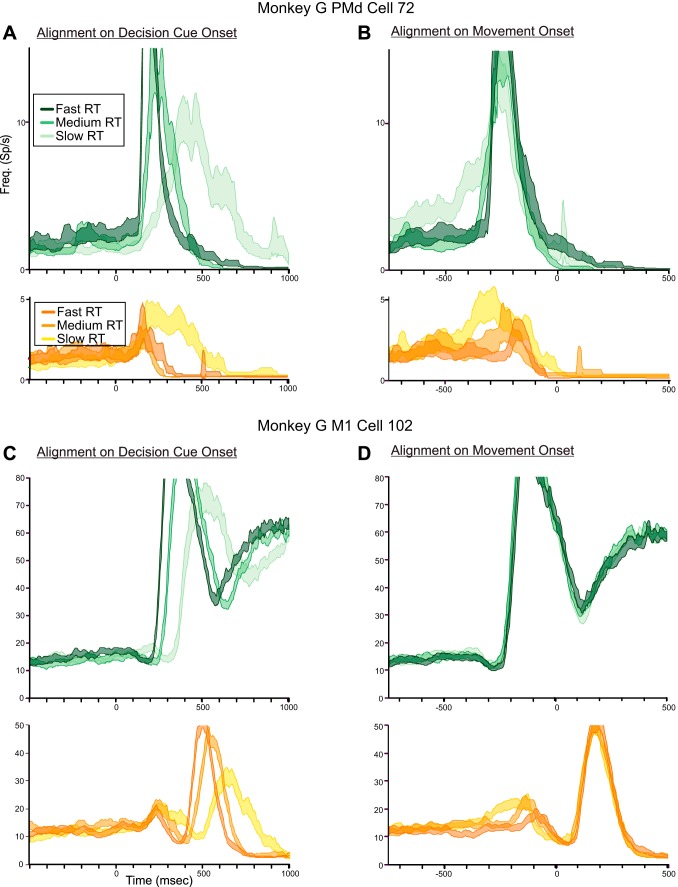
Figure 5 shows the responses of a neuron in the left PMd of monkey G in trials in which the monkey chose the correct target for 100/0, 60/40, and 55/45 DCs. The median RTs for correct-choice trials for each PD-DC (n = 80 trials/DC) increased significantly from 450 to 543.5 and 610.5 ms, respectively [1-way ANOVA, F(2,237) = 30.197, P = 2.08 × 10−12].
Fig. 5.
Responses of a PMd neuron in the CG task in trials in which the monkey made correct target choices according to the color bias of the DCs. A and B: histograms (mean ± SE) of the discharge of the neuron in response to DCs with different degrees of conflicting evidence supporting the neuron's preferred target (PD, blue histograms) and opposite target (oPD, red histograms), aligned to the onset of the DCs (A) and to the onset of movement (B). Note that in this figure and Figs. 6–12, the colors of the histograms signify the information provided by the DCs about the correct target (either in the PD or the oPD of each neuron), not the target chosen by the monkeys. C and D: rasters of the single-trial responses of the neuron in trials in which the monkey correctly chose the neuron's preferred target, sorted according to DC evidence quality and reaction-time duration and aligned to the onset of the DCs (C) and to the onset of movement (D). Green line, time of onset of the DC; red line, onset of movement; blue line, time of entry into the target window.
The neuron's responses were clearly influenced by the quality of evidence about the correct target presented in each DC. The 100/0 PD-DC evoked a brisk burst of activity that began ∼140 ms after DC onset (Fig. 5A, dark blue histogram; Fig. 5C) and ended before movement onset (Fig. 5B, dark blue histogram; Fig. 5D). As the color ratio of PD-DC evidence decreased to 60/40 and 55/45, the response of the neuron began at the same time after DC onset but built up over a longer period of time (Fig. 5A, paler blue histograms; Fig. 5C). Part of this change in response profile is due to the greater spread of onset times of the main movement-related response of the neuron as RTs increased for the 60/40 and 55/45 DCs (Fig. 5C).
However, the spread of RTs was not the sole contributing factor. When the data were aligned to movement onset (Fig. 5B, blue histograms; Fig. 5D), the main premovement burst for trials with all three PD-DCs extensively overlapped, beginning at ∼325 ms before movement onset and ending at the same time near movement onset. However, the premovement burst was preceded by a ramp-like increase in activity that extended over progressively longer times for the 60/40 and 55/45 PD-DCs compared with the 100/0 PD-DC, which was evident both in the mean activity (Fig. 5B, blue histograms) and in the single-trial rasters (Fig. 5D). The activity during the time window from 600 to 340 ms before movement onset was significantly different for the three PD-DCs [1-way ANOVA; F(2,237) = 6.379, P = 0.002]. Post hoc tests revealed that the difference in activity between the 100/0 and 55/45 PD-DCs (Fig. 5B) was highly significant [F(1,158) = 11.863, P = 0.0007], but not the 100/0-60/40 (P = 0.179) or the 60/40-55/45 (P = 0.03) comparisons. There was also a highly significant correlation between the single-trial discharge rates during the 600- to 340-ms premovement time window and the single-trial RTs pooled across all PD-DCs (r = 0.474, P = 1.443 × 10−07); trials with longer RTs had higher discharge rates during this ramp-up period. This indicated that the neuron's activity increased in intensity more slowly and variably after the appearance of PD-DCs that contained less evidence supporting the PD reach target and increasingly more conflicting distractor evidence in favor of the oPD reach (Fig. 5, C and D).
RTs also increased significantly in response to oPD-DCs with lower color biases [1-way ANOVA, F(2,237) = 29.260, P = 4.55 × 10−12]. The 100/0 oPD-DC evoked a transient burst of activity in the neuron that was rapidly suppressed (Fig. 5A, dark red histogram). This burst took much longer to suppress for the 60/40 and 55/45 oPD-DCs (Fig. 5A, paler red histograms). When aligned to movement onset, the transient response was truncated at the same time shortly before movement onset for all three oPD-DCs (Fig. 5B, red histograms). The discharge rates for the three oPD-DCs were not significantly different during the time window 100–500 ms after DC onset (Fig. 5A, red histograms; P = 0.072) nor for the time window 300-100 ms before movement onset (Fig. 5B, red histograms; P = 0.190). Nevertheless, there was a significant correlation between the single-trial discharge rates during the 100- to 500-ms time window after DC onset and the single-trial RTs, pooled across all three oPD-DCs (r = 0.355, P = 1.569 × 10−08), but not for the 300- to 100-ms window before movement onset (r = 0.144, P = 0.026).
Figure 6 shows the activity from a neuron from the left M1 of monkey G. Median RTs in trials with correct target choices increased significantly for the 100/0, 60/40, and 55/45 PD-DCs [467, 538, and 557 ms, respectively; n = 80 trials/DC; 1-way ANOVA, F(2,237) = 24.635, P = 1.90 × 10−10] and oPD-DCs [327.5, 386.5, and 410 ms, respectively; n = 80 trials/DC; F(2,237) = 12.688, P = 5.82 × 10−06]. This M1 neuron's activity increased abruptly at ∼225 ms after the 100/0 PD-DC onset (Fig. 6A, dark blue histogram) and ∼25–50 ms later for the 60/40 and 55/45 PD-DCs (Fig. 6A, paler blue histograms). When its activity was aligned to movement onset (Fig. 6B, blue histograms), the responses for all three PD-DCs overlapped completely and began at ∼220 ms before movement onset, showing that its activity was tightly coupled to movement onset (Fig. 6, C and D). There was no evidence of a gradual DC-dependent increase in discharge rate before the movement-related response of the neuron (Fig. 6B, blue histograms), and no significant difference in the neuron's activity during the time window from 800 to 240 ms before movement onset in trials with the three PD-DCs [1-way ANOVA; F(2,237) = 0.328, P = 0.721].
Fig. 6.
Responses of an M1 neuron in the CG task in trials in which the monkey made correct target choices according to the color bias of the DCs. A and B: histograms (means ± SE) of the responses of the neuron in response to DCs with different degrees of conflicting evidence supporting the neuron's PD (blue histograms) and oPD (red histograms), aligned to the onset of the DCs (A) and to the onset of movement (B). C and D: rasters of the single-trial responses of the neuron in trials in which the monkey correctly chose the neuron's preferred target, sorted according to DC evidence quality and reaction-time duration and aligned to the onset of the DCs (C) and to the onset of movement (D). Green line, time of onset of the DC; red line, onset of movement; blue line, time of entry into the target window.
Similarly, the color bias of the oPD-DCs had no influence on the strong delayed burst of the neuron from 100 to 400 ms after movement onset (Fig. 6B, red histograms). However, the oPD-DCs also evoked a small transient short-latency increase in activity peaking at ∼240 ms after DC onset (Fig. 6A, red histograms) and ending at the same time just before movement onset (Fig. 6B, red histograms). This transient early response evoked by oPD-DCs was unusual for M1 neurons (c.f., Fig. 7, C and D). For this neuron, however, the short-latency response was significantly different for the three oPD-DCs during the time window from 100 to 400 ms after DC onset [1-way ANOVA; F(2,237) = 5.611, P = 0.004] and during the time window from 300 to 100 ms before movement onset [F(2,237) = 10.008, P = 6.73 × 10−05].
Fig. 7.
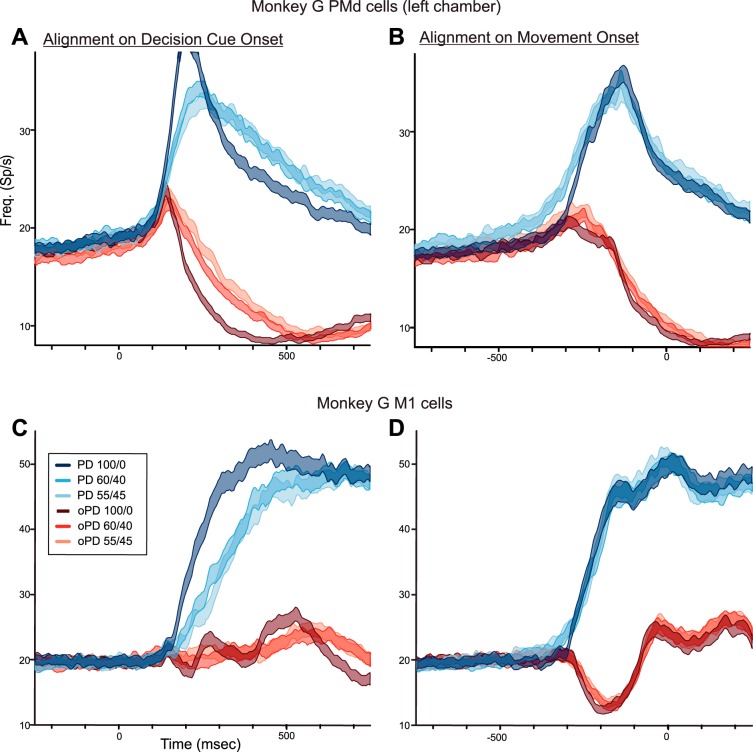
A and B: population histograms of the mean response (±SE) of the PMd neurons recorded in the left hemisphere of monkey G in trials in which the monkey chose the correct target without a change in reach direction in response to DCs of different levels of ambiguity supporting the PD of each neuron (blue histograms) and oPD (red histograms), aligned to DC onset (left) and movement onset (right). C and D: population histograms of the mean response of M1 neurons (±SE) recorded in monkey G. Same format as in A and B.
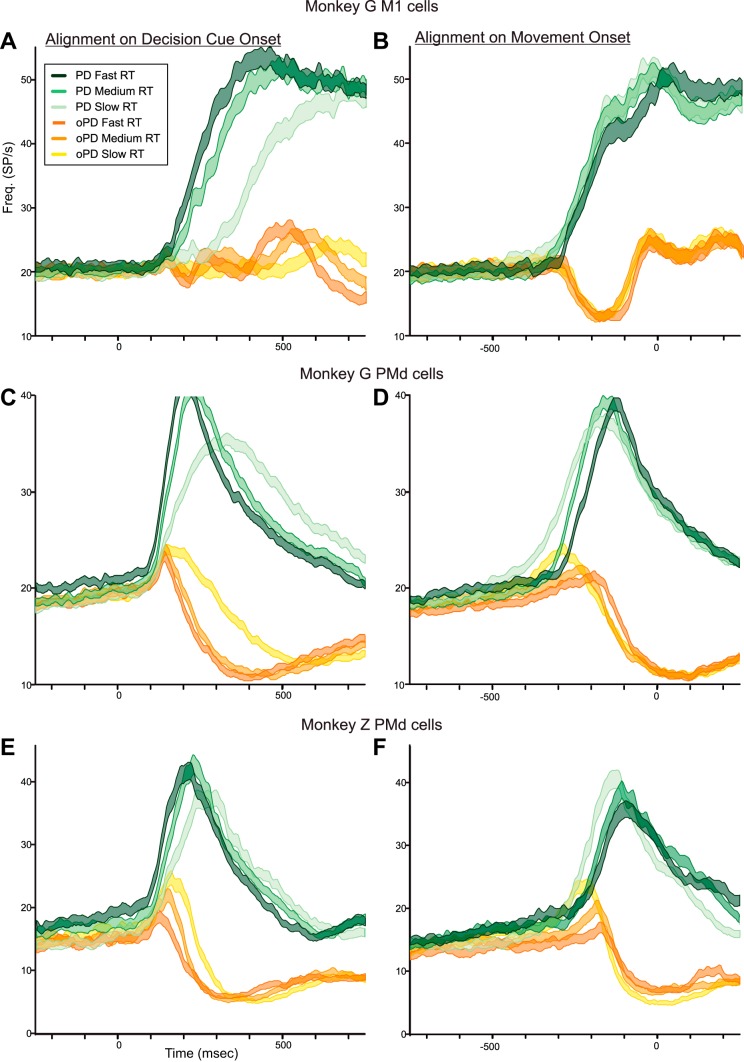
Different PMd neurons showed variable degrees of modulation of their task-related activity in trials with different levels of DC color bias, ranging from responses similar to that in Fig. 5 to almost purely movement-related responses like that of the M1 neuron in Fig. 6. When averaged over the sample population of PMd neurons tested with the 100/0, 60/40, and 55/45 DCs in the left hemisphere, graded effects of the DC color bias were still evident (Fig. 7, A and B). When a PD-DC appeared, mean neural activity began to rise at the same time (100–120 ms) after DC onset for all PD-DCs, but the rate at which the mean activity built up decreased for DCs with weaker color biases (Fig. 7A, blue histograms). When aligned to movement onset, it took longer for neural activity to build up in response to 60/40 and 55/45 PD-DCs (Fig. 7B, blue histograms). The neural activity during the time window from 600 to 300 ms before movement onset was significantly different for the three PD-DCs [1-way ANOVA, F(2,4380) = 15.237, P = 2.54 × 10−07]. Post hoc analysis revealed significant differences in activity for the 100/0 PD-DC and the 60/40 (P = 8.64 × 10−06) and 55/45 (P = 3.97 × 10−07) PD-DCs but not between the 60/40 and 55/45 PD-DCs (P = 0.608).
The oPD-DCs evoked a transient increase in PMd population activity that began at the same latency as in PD-DC trials but was abruptly truncated at 140–160 ms after DC onset and took progressively longer to suppress as the color bias decreased (Fig. 7A, red histograms). The suppression terminated at nearly the same time before movement onset (Fig. 7B, red histograms). The discharge rates during the time window from 100 to 500 ms after DC onset were highly significantly different for the three oPD-DCs [1-way ANOVA, F(2,4402) = 47.490, P ≈ 0.0] but did not quite attain statistical significance according to our P < 0.001 criterion for population data [F(2,4402) = 5.748, P = 0.003] during the time window 300-100 ms before movement onset.
The mean activity of the M1 neurons recorded in PD-DC trials in monkey G began at 160–180 ms after DC onset, later than the onset of PD-DC activity in PMd but at about the same time as the PMd PD-DC and oPD-DC responses separated (Fig. 7C, blue histograms). The M1 activity also showed decreasing rates of change as the DC color bias decreased for both PD- and oPD-DCs when aligned to DC onset (Fig. 7C, blue and red histograms, respectively). However, unlike PMd neurons, that effect was due almost entirely to the spread of RTs. When aligned to movement onset (Fig. 7D) the response profiles evoked by the three PD-DCs (Fig. 7D, blue histograms) and oPD-DCs (Fig. 7D, red histograms) almost completely overlapped. Furthermore, the PD- and oPD-DCs evoked strongly reciprocal activity that is most evident when aligned to movement onset (Fig. 7D). There was no evidence of a systematic transient coactivation of M1 neurons elicited by the oPD-DCs. The discharge rates of the M1 neurons were not significantly different during the time window 600-300 ms before movement onset in the PD (P = 0.910) (Fig. 7D, blue histograms), or for the oPD responses during the time windows 100–400 ms after DC onset (Fig. 7C, red histograms; P = 0.201) or 300-100 ms before movement onset (Fig. 7D, red histograms; P = 0.719).
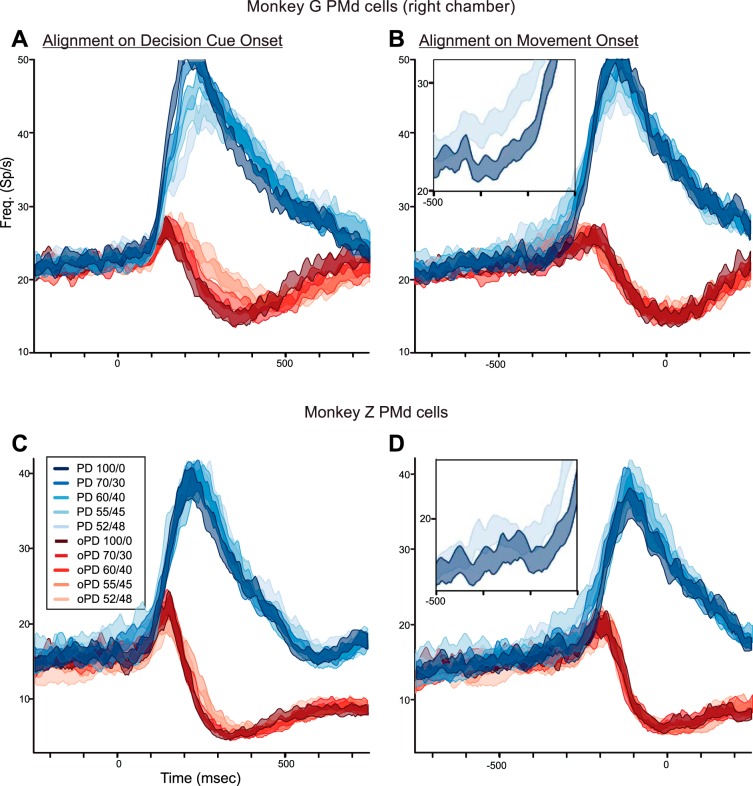
Neurons in monkey G's right hemisphere were tested with all five DC color biases. RTs increased significantly as DC color bias decreased but were shorter for the same DCs than during the left hemisphere recordings (Table 2). Correspondingly, the population responses showed similar systematically ordered but less pronounced DC-dependent response trends as in the left hemisphere (Fig. 8, A and B). When aligned to DC onset, there was an orderly decrease in the rate of rise of population activity as the color bias of PD-DCs decreased (Fig. 8A, blue histograms). Conversely, oPD-DCs evoked a progressively more sustained transient excitation as their color bias decreased (Fig. 8A, red histograms). When aligned to movement onset, neural activity took more time to ramp up when the PD-DCs were more ambiguous (Fig. 8B, blue histograms) and the mean ± SE of the population responses for the 100/0 vs. 52/48 responses were clearly separated (Fig. 8B, inset), while the transient response evoked by the oPD-DCs ended at the same latency before movement onset (Fig. 8B, red histograms). Discharge rates were significantly different both for the premovement ramps during the time window 500-260 ms before movement onset for PD-DCs [Fig. 8B, blue histograms; 1-way ANOVA, F(4,2137) = 7.755, P = 3.34 × 10−06] and for the transient responses evoked by oPD-DCs during the time window 100–500 ms after DC onset [Fig. 8A, red histograms; F(4,2137) = 7.718, P = 3.58 × 10−06]. Post hoc tests revealed significant differences in the ramp activity between the 100/0 and 55/45 PD-DCs (P = 0.0004) and the 100/0 and 52/48 PD-DCs (P = 8.25 × 10−05; Fig. 8B, inset), but not between the 100/0 and 70/30 or 60/40 PD-DCs. The activity was also significantly different between the 100/0 and 52/48 oPD-DCs (P = 6.88 × 10−06), not quite significant at P < 0.001 for the 100/0 and 55/45 oPD-DCs (P = 0.0048), and not significant for the other comparisons.
Fig. 8.
A and B: population histograms of the mean response (±SE) of the PMd neurons recorded in the right hemisphere of monkey G in trials in which the monkey chose the correct target without a change in reach direction in response to DCs of different levels of ambiguity supporting the PD of each neuron (blue histograms) and the oPD (red histograms), aligned to DC onset (left) and movement onset (right). Inset, expanded view of the mean responses for only the 100/0 and 52/48 PD-DCs, from 500 to 200 ms before movement onset. C and D: population histograms of the mean response (±SE) of the PMd neurons recorded in both hemispheres of monkey Z. Same format as in A and B.
In summary, PMd activity in monkey G took progressively longer to build up in response to PD-DCs as their color bias decreased, while oPD-DCs evoked a brief increase in activity that took longer to suppress as DC color bias decreased. These trends paralleled monkey G's choice behavior during recordings in the two hemispheres (Fig. 3A and Table 2).
In contrast to monkey G, monkey Z's RTs were only weakly modulated by DC ambiguity (Fig. 3B and Table 2). In parallel, there was very little difference in the time course of PMd population activity as a function of PD-DC evidence strength when aligned to DC onset (Fig. 8C, blue histograms). The oPD-DCs evoked a transient excitatory PMd response in monkey Z that was stronger than in monkey G (Fig. 8C, red histograms), but, unlike monkey G, there was no ordered increase in its duration as a function of oPD-DC color bias. When aligned to movement onset, there was a modest early ramp buildup of neural activity for PD-DCs with weaker color biases (Fig. 8D, blue histograms) with relatively little difference between the responses to 100/0 and 52/48 PD-DCs (Fig. 8D, inset), and the transient increase in activity evoked by oPD-DCs terminated at the same latency before movement onset (Fig. 8D, red histograms). None of the statistical tests of neural activity during the premovement and post-DC time windows were significant (1-way ANOVA, P > 0.05 for all tests). In summary, consistent with the modest effect of DC color bias on the RTs of monkey Z (Fig. 3B and Table 2), DC color bias had relatively little influence on the timing and rate of changes in PMd neural activity.
CG task: correct choices-ROC/choice probability analysis.
To further evaluate the timing of neural events, we used a ROC (Britten et al. 1992; Green and Swets 1966; Shadlen and Newsome 2001) to estimate when an ideal observer could distinguish between correct PD and oPD choices based on the pooled neural activity associated with each choice (choice probability; Celebrini and Newsome 1994). The temporal evolution of the area under the ROC curves followed the time course of the differences in the mean discharge rates for each pair of PD and oPD responses for each DC color bias. When aligned to DC onset, the ROC areas fluctuated near 50% (identical distributions of binned discharge rates) before DC onset and began to rise 140–160 ms after DC onset for all DC color biases in all three datasets from the two monkeys. This corresponded to the time at which the response histograms of activity for the PD-DC and oPD-DC trials began to separate; the ROC analysis could not detect the initial period of rising coactivation (Figs. 7 and 8). Choice probabilities rose rapidly after that, peaking at 0.722–0.774 for the left hemisphere data of monkey G, 0.772–0.840 in its right hemisphere, and 0.875-00.899 in monkey Z. In all cases, peak choice probabilities decreased as color bias decreased.
For a more quantitative analysis, we first used a bootstrapped permutation test (Britten et al. 1992) to determine when the activity for PD-DCs and oPD-DCs began to differ significantly for each DC color bias (Table 3; see methods). These occurred at a fairly consistent 140- to 160-ms latency after DC onset in all three PMd datasets. When aligned to movement onset, in contrast, the area under the ROC curve was significantly different from chance at a longer latency before movement onset as the DC color bias decreased in both datasets from monkey G (Table 3). This was due to the more gradual buildup of neural activity for 60/40 and 55/45 DCs compared with that seen for the 100/0 DCs. In contrast, the PD- and oPD-DC responses became significantly different at the same latency before movement onset in monkey Z (Table 3).
Table 3.
Latencies (20 ms resolution) of the onset of significant differences in pooled population responses between the PD-DCs and oPD-DCs (ROC bootstrapped permutation test, 99% significance level), relative to DC onset and movement onset
| DC |
|||||
|---|---|---|---|---|---|
| 100/0 | 70/30 | 60/40 | 55/45 | 52/48 | |
| DC onset | |||||
| Monkey G M1 | 180 | 200 | 220 | ||
| Monkey G left PMd | 140 | 140 | 160 | ||
| Monkey G right PMd | 160 | 140 | 160 | 160 | 140 |
| Monkey Z PMd | 160 | 160 | 160 | 140 | 140 |
| Movement onset | |||||
| Monkey G M1 | −280 | −260 | −260 | ||
| Monkey G left PMd | −300 | −500 | −400 | ||
| Monkey G right PMd | −240 | −260 | −260 | −360 | −380 |
| Monkey Z PMd | −200 | −200 | −200 | −200 | −200 |
oPD, opposite of preferred direction.
We then used the ROC time profiles to estimate when we could reliably discriminate the time at which the monkeys made correct PD vs. oPD choices (Table 4). We used a choice probability threshold of 0.7 as the criterion value for all datasets (see methods). The time to criterion increased from 240 to 340 ms after DC onset as DC color bias decreased for monkey G's left hemisphere data (Table 4) and also increased progressively as DC color bias decreased for the right hemisphere data, but by a smaller amount (Table 4). In contrast, criterion threshold was reached at nearly the same time (220–240 ms) for all five DC color biases for monkey Z (Table 4). These times occurred consistently 20–60 ms before the peak of the difference in neural activity between PD-DCs and oPD-DCs for each DC color bias in each dataset, which also corresponded to the middle of the descending phase of the oPD-DC response components for each DC color bias, shortly before the oPD activity attained its lowest level.
Table 4.
Latencies (20 ms resolution) at which the pooled population responses consistently exceeded the criterion choice probability level of 70% for correct PD versus oPD choices, relative to DC onset or movement onset
| DC |
|||||
|---|---|---|---|---|---|
| 100/0 | 70/30 | 60/40 | 55/45 | 52/48 | |
| DC onset | |||||
| Monkey G M1 | 240 | 280 | 340 | ||
| Monkey G left PMd | 220 | 280 | 360 | ||
| Monkey G right PMd | 200 | 220 | 220 | 260 | 280 |
| Monkey Z PMd | 220 | 220 | 220 | 240 | 220 |
| Movement onset | |||||
| Monkey G M1 | −200 | −180 | −180 | ||
| Monkey G left PMd | −120 | −100 | −80 | ||
| Monkey G right PMd | −180 | −180 | −200 | −200 | −200 |
| Monkey Z PMd | −140 | −140 | −140 | −140 | −140 |
When aligned to movement onset, the ROC curve area exceeded the criterion at very consistent latencies before movement onset for the PMd data of monkey Z and in the right hemisphere of monkey G, but at more variable latencies closer to movement onset in monkey G's left hemisphere (Table 4). In all cases, these times occurred just before the peak of the mean difference in neural activity between PD-DCs and oPD-DCs, near the middle of the descending phase of the transient oPD-DC activation for each DC color bias (Figs. 7 and 8).
The ROC analysis also indicated differences in timing of events in M1 vs. PMd. The first significant difference in PD vs. oPD responses occurred slightly later in M1 than in monkey G's left PMd data when aligned to DC onset and to movement onset (Table 3). When aligned to DC onset, a directionally reliable signal appeared later in the trial for each DC color bias in M1, at about the same time as a reliable signal appeared in the left PMd of monkey G (Table 4). In contrast, a directionally reliable signal is evident in M1 at an earlier latency relative to movement onset than in PMd (Table 4).
CG task: Error choices.
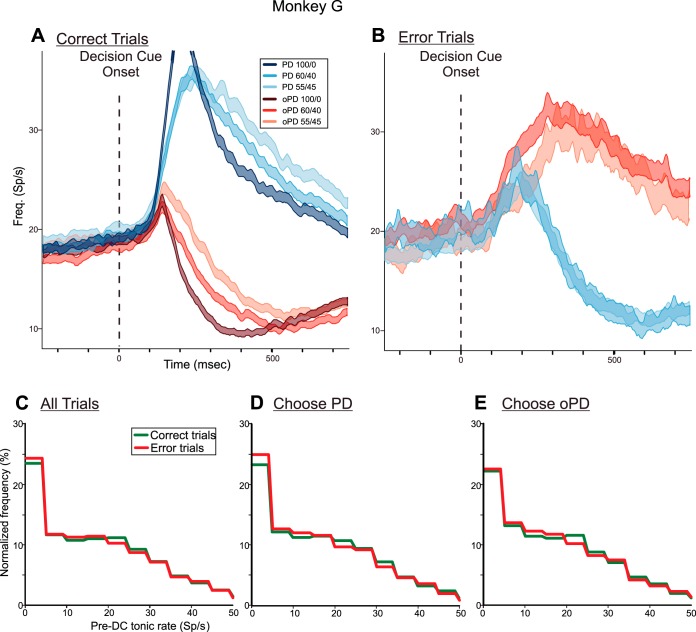
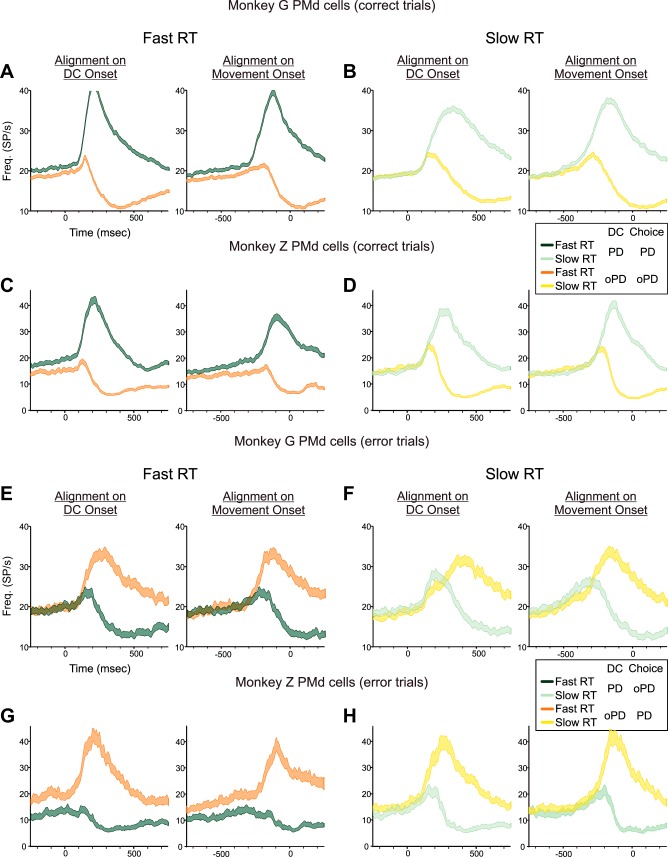
When the monkeys made an incorrect choice, neural activity reflected which reach direction the monkeys chose, not the physical properties of the DCs. Figure 9A shows the PMd population response in monkey G pooled across both hemispheres when it made correct target choices for the 100/0, 60/40, and 55/45 DCs. As already shown (Figs. 7A and 8A), PMd activity began to rise after the appearance of all DCs, and continued to rise when the monkey correctly chose a target at the neurons' PD (Fig. 9A, blue histograms), but was eventually suppressed when the monkey correctly chose the oPD (Fig. 9A, red histograms). In contrast, when the monkey erroneously chose the PD target in response to 60/40 or 55/45 oPD-DCs, the initial activation evoked by the oPD-DC continued to rise and was sustained while the monkey reached incorrectly to the target at the neurons' PD (Fig. 9B, red histograms). When the monkey chose the oPD target in response to 60/40 or 55/45 PD-DCs, neural activity increased transiently but then was suppressed before movement onset (Fig. 9B, blue histograms). Moreover, that truncated activation to PD-DCs in error-choice trials was stronger and of longer duration than the response to oPD-DCs in correct-choice trials. This indicated that the coactivation of the neural populations preferring both targets was stronger and more sustained in trials in which the monkey chose the wrong target (Fig. 9B) than when it chose the correct target (Fig. 9A). The PMd data from monkey Z showed similar trends (see Fig. 12).
Fig. 9.
A: population histograms of the mean activity (±SE) of PMd neurons in both hemispheres of monkey G in trials in which the monkey made correct target choices to the 100/0-55/45 PD-DCs (blue histograms) and oPD-DCs (red histograms). B: population histograms of the mean activity (±SE) of the same PMd neurons in trials in which the monkey incorrectly chose the oPD target in response to 60/40 and 55/45 PD-DCs (blue histograms) and incorrectly chose the PD in response to 60/40 and 55/45 oPD-DCs (red histograms). There were too few incorrect target choices for 100/0 DCs to generate a meaningful histogram. Data are aligned to DC onset (vertical broken line). As already noted in the legend of Fig. 5, the colors of the histograms signify the correct target identified by the color bias of the DC for both correct-choice (A) and error-choice (B) trials. C: normalized frequency histograms of the distribution of single-trial pre-DC tonic rates for the 500-ms period at the end of the SC epoch immediately before the appearance of the DC for all trials in which the monkeys chose the correct target (green) and the incorrect target (red). D: normalized frequency histograms of the pre-DC tonic activity in trials in which the monkeys correctly (green) and incorrectly (red) chose the target in the PD of each neuron. E: normalized frequency histograms of the pre-DC tonic activity in trials in which the monkeys correctly (green) and incorrectly (red) chose the target in the oPD of each neuron.
Fig. 12.
A–D: mean activity (±SE) of PMd neurons recorded in both hemispheres of monkeys G and Z in trials in which they chose the correct target in response to PD-DCs (green histograms) and oPD-DCs (orange histograms). Data are shown for the 1/3 of the trials that had the shortest RTs (A and C) and the 1/3 of trials with the longest RTs (B and D). E and F: mean activity (±SE) of PMd neurons recorded in both hemispheres of monkeys G and Z in trials in which they chose the incorrect target in response to PD-DCs (green histograms) and oPD-DCs (orange histograms). Data are shown for the 1/3 of the trials that had the shortest RTs (E and G) and the 1/3 of trials with the longest RTs (F and H). As in all previous population histogram figures, the colors of the histograms signify the correct targets supported by the DC color bias, not the response choices of the monkeys.
A valid question is whether the monkeys were more likely to choose the wrong target in response to the more ambiguous DCs in part because they were biased toward choosing one target over the other before the DC appeared or were even overtly guessing which would be the correct target during the Spatial-Cue period. This prior bias could be expressed by a difference in neural tonic activity in the Spatial-Cue period. However, there was no systematic difference in the tonic rates during the last 500 ms of the Spatial-Cue period before either correct (Fig. 9A) or incorrect (Fig. 9B) PD vs. oPD choices. This is also illustrated in Fig. 9, C–E, which shows the normalized frequency distributions of the single-trial tonic rates of all neurons in the last 500 ms of the Spatial-Cue epoch in trials with correct (green lines) or incorrect (red lines) reach choices. The normalized frequency distributions for all correct vs. incorrect trials (Fig. 9C), and when the monkeys correctly or incorrectly chose to reach in each neuron's PD (Fig. 9D) and oPD (Fig. 9E), were statistically identical (1-way ANOVA; P > 0.01 in all cases). There was no compelling evidence for a bimodal distribution of tonic rates at the end of the Spatial-Cue epoch before error choices toward the PD vs. oPD of the neurons that would support a strong prior bias toward the wrong target before the DC appeared. The neural events leading to correct vs. error choices were primarily expressed in PMd after the appearance of the DCs (Fig. 9, A vs. B).
CG task: Neural activity as a function of RT duration.
Because the choice behavior of the monkeys suggested that they responded to the evidence in the DCs in different ways, we searched for neural correlates the monkeys might share. To this end, we pooled the data from each neuron across all DCs and then sorted the trials into three groups with the fastest, middle, and slowest one-third of the RTs independent of the DC color bias in each trial (c.f., Hanes and Schall, 1996). This revealed several similarities in the RT-dependent neural responses in the two monkeys.
Independent of the ultimate RT in each trial, the response of the PMd neuron from Fig. 5 still began at the same time after PD-DC onset in all three RT groups but built up more slowly in trials with progressively longer RTs (Fig. 10A, green histograms). The tonic rate before PD-DC onset was modestly but nonsignificantly higher in trials with the fastest RTs than for the other trials [Fig. 10A, green histograms, time period from 500 to 0 ms before DC onset: 1-way ANOVA, F(2,237) = 1.774, P = 0.172]. When aligned to movement onset, there was a prolonged gradual buildup of activity in that neuron in the trials with the longest RTs that was not evident for the other two RT groups (Fig. 10B, green histograms). There was a significant difference in the discharge rates in the premovement ramp of activity during the time window from 800 to 340 ms before movement onset [Fig. 10B, green histograms; 1-way ANOVA, F(2,237) = 6.889, P = 0.0012]. Post hoc tests revealed that there was a significant difference in the ramp response between the fast-RT and slow-RT (P = 1.37 × 10−06) and the mid-RT and slow-RT (P = 6.18 × 10−07) trials for PD-DCs but not between the fast- and mid-RT groups (P = 0.822). In contrast, the transient increase in activity evoked by oPD-DCs was stronger and more prolonged for the trials with the longest one-third of RTs (Fig. 10, A and B, orange histograms). There were highly significantly different discharge rates from 100 to 500 ms after DC onset [1-way ANOVA, F(2,234) = 18.958, P = 2.34 × 10−08] and 400 to 0 ms before movement onset [1-way ANOVA, F(2,234) = 9.158, P = 0.00015], and significant differences in discharge rates between the fast- and slow-RT trials (P = 6.95 × 10−05) and between the mid- and slow-RT trials (P = 1.66 × 10−06) but not the fast- and mid-RT trials (P = 0.313).
Fig. 10.
Histograms of the mean activity (±SE) of the PMd neuron from Fig. 5 (A and B) and the M1 neuron from Fig. 6 (C and D) for trials sorted into three groups with the fastest, middle, and slowest 1/3 of RTs to the preferred target (green histograms) and opposite target (orange histograms). Trials are aligned to DC onset (left) and movement onset (right).
When the trial data of the M1 neuron from Fig. 6 were resorted by RT duration and aligned to DC onset (Fig. 10C) there was a systematically longer delay in onset of both the premovement burst as RTs got longer in response to PD-DCs (Fig. 10C, green histograms) and the post-movement-onset burst during movements in the neuron's oPD (Fig. 10C, orange histograms). When realigned to movement onset, those response components completely overlapped for the three RT groups, reinforcing how the timing of this neuron's activity was primarily coupled to movement onset. There was no evidence of a premovement ramp increase in activity in the PD-DC trials that was dependent on RT duration and no difference in activity between the three RT groups during the time window from 800 to 240 ms before movement onset [1-way ANOVA, F(2,236) = 0.599, P = 0.550]. However, the small, transient premovement response of the M1 neuron in oPD trials did vary significantly during the time windows from 100 to 400 ms after DC onset [Fig. 10C, orange histograms; 1-way ANOVA, F(2,231) = 8.208, P = 0.00036] and 300-0 ms before movement onset [Fig. 10D, orange histograms; 1-way ANOVA, F(2,231) = 6.373, P = 0.002].
The M1 sample population showed the same trends in correct-choice trials (Fig. 11, A and B). Aligned to movement onset, there was nearly complete overlap of the mean responses for the three RT groups for each target choice and simultaneous reciprocal bifurcation of the histograms for correct PD and oPD choices (Fig. 11B, green and orange histograms, respectively) that occurred at the same latency before movement onset for all three RT groups. In contrast, when aligned to DC onset, there was a progressive delay of the timing of M1 neural activity as a function of RT duration for both PD and oPD choices (Fig. 11A, green and orange histograms, respectively). There were no significant differences in activity among the three RT groups during the time windows from 800 to 300 ms before movement onset for PD-DC trials [Fig. 10B, green histograms; 1-way ANOVA, F(2,3205) = 1.849, P = 0.158] or for oPD-DC trials 100–400 ms after DC onset [Fig. 10A, orange histograms; 1-way ANOVA, F(2,3204) = 0.018, P = 0.982] or 300-0 ms before movement onset [Fig. 10B, orange histograms; 1-way ANOVA, F(2,3204) = 3.123, P = 0.044].
Fig. 11.
A and B: population histograms of the mean activity (±SE) of M1 neurons recorded in monkey G for trials in which the monkey made the correct target choices, sorted into three groups with the fastest, middle, and slowest 1/3 of RTs to the preferred target of each neuron (green histograms) and the opposite target (orange histograms), aligned to DC onset (A) and movement onset (B). C and D: population histograms of the mean activity (±SE) of PMd neurons recorded in both hemispheres in monkey G for trials in which the monkey made the correct target choices. Same format as in A and B. E and F: population histograms of the mean activity (±SE) of PMd neurons recorded in both hemispheres in monkey Z for trials in which the monkey made the correct target choices. Same format as in A and B.
The same analysis showed a number of similarities in the PMd activity of both monkeys, despite the differences in their choice behavior. When aligned to DC onset, the PMd sample population response from monkeys G and Z began at the same time after DC onset in all three RT groups in each monkey (Fig. 11, C and E) for both PD-DCs (green histograms) and oPD-DCs (orange histograms). The increase in activity for PD target choices was slower in trials with longer RTs (Fig. 11, C and E, green histograms). The transient response increase evoked by oPD-DCs was stronger and sustained for a longer time period for the trials with longer RTs (Fig. 11, C and E, orange histograms). This latter effect was particularly pronounced and well-ordered for the three RT groups in monkey Z (Fig. 11E, orange histograms).
When aligned to movement onset, PMd activity in both monkeys began to rise at an earlier latency before PD movement onset for the trials with longer RTs (Fig. 11, D and F, green histograms). Similarly, the onset of the transient response in oPD-DC trials began earlier relative to movement onset as RTs got longer but was suppressed at similar times before movement onset for all three RT groups (Fig. 11, D and F, orange histograms). The premovement ramp buildup of activity in PD-DC trials during the time window 600-300 ms before movement onset was significantly different across RT groups in both monkey G [1-way ANOVA; F(2,7314) = 18.707, P = 7.88 × 10−09] and monkey Z [F(2,1929) = 12.297, P = 4.93 × 10−06]. The transient responses in oPD-DC trials were also significantly different across RT groups in both monkeys. This included the initial rise 100–220 ms after DC onset [monkey G: F(2,7239) = 9.208, P = 0.0001; monkey Z: F(2,1911) = 29.220, P = 3.16 × 10−13], during the more extended time window 100–500 ms after DC onset [monkey G: F(2,7239) = 165.383, P ≈ 0.0; monkey Z: F(2,1911) = 34.784, P = 1.44 × 10−14], and during the time window from 400 to 0 ms before movement onset [monkey G: F(2,7239) = 6.948, P = 0.0009; monkey Z: F(2,1911) = 12.623, P = 3.58 × 10−06].
Reciprocally tuned discharge began in M1 in monkey G (Fig. 11, A and B) while the neural activity continued to evolve in PMd (Fig. 11, C and D). When aligned to DC onset, M1 activity in the shortest RT trials began 40–60 ms after the response onset in the shortest RT trials in PMd, and at the same time that the oPD-DC responses stopped growing and began to decline (Fig. 11, A and C, dark green histograms). For the longest RT trials, M1 activity began ∼280 ms after DC onset or ∼180 ms after response onset in PMd in the longest RT trials (Fig. 11, A and C, pale green histograms). When aligned to movement onset, reciprocal M1 activity began 300-280 ms before movement onset in all three RT groups. This coincided with the onset of PD-DC activity for the shortest RT trials in PMd (Fig. 11, B and D, dark green histograms) but later than the onset of the gradual build-up of activity in the longer RT trial groups.
Another trend concerns the tonic activity at the end of the Spatial-Cue period before DC onset. In PMd of both monkeys, the tonic rate during the time period 500-0 ms before DC onset (left of time 0 in Fig. 11, C and E) was progressively higher as RT durations decreased in PD-DC trials [Fig. 11, C and E, green histograms; 1-way ANOVA; monkey G: F(2,7239) = 31.952, P = 1.53 × 10−14; monkey Z: F(2,1929) = 16.906, P = 5.27 × 10−08]. In contrast, tonic rates were similar for oPD-DC trials at all latencies [Fig. 11, C and D, orange histograms; 1-way ANOVA; monkey G: F(2,7329) = 1.341, P = 0.262; monkey Z: F(2,1911) = 3.978, P = 0.019]. Tonic rates in M1 neurons were not significantly modulated by RTs in either movement direction [Fig. 11A; 1-way ANOVA; PD-DCs: F(2,2301) = 3.563, P = 0.029; oPD-DCs: F(2,2304) = 0.633, P = 0.531].
These tonic rate trends are seen more clearly in Fig. 12, which shows the population histograms for the fastest (Fig. 12, A and C) and slowest (Fig. 12, B and D) RTs for correct-choice trials in both monkeys and in error-choice trials (Fig. 12, E–H). When the monkeys made rapid decisions about reach direction, tonic activity before DC onset was slightly higher when the monkeys correctly chose to reach in each neuron's PD than when they correctly reached in the oPD (Fig. 12, A and C, green and orange histograms, respectively). In contrast, there was no difference in tonic activity in trials in which the monkeys took longer to make correct PD vs. oPD choices (Fig. 12, B and D, green and orange histograms).
When monkey G chose the wrong reach direction, there was no RT-dependent difference in tonic activity before DC onset (Fig. 12, E and F). In contrast, pre-DC tonic activity was higher when monkey Z incorrectly made a short RT reach in the PD of the neurons in response to an oPD-DC than when it made a short RT incorrect reach in the oPD in response to PD-DCs (Fig. 12G, orange and green histograms, respectively). This was opposite to the tonic rate difference before correct short RT PD vs. oPD choices (Fig. 12C). This was the only case in which the tonic activity before DC onset showed a correlation with the ultimate reach choices in either monkey. Monkey Z also showed the highest error rates in trials with the shortest RTs (Fig. 3D). There was no difference in monkey Z's PMd tonic activity before long RT incorrect choices (Fig. 12H).
In summary, the level of tonic activity at the end of the spatial-cue period appeared to have an influence on the duration of the RTs in correct PD-DC trials in both monkeys and may also correlate with the correct vs. incorrect choices that monkey Z made in short RT trials.
Finally, there were some significant differences in neural responses as a function of RT in error-choice trials. In monkey G, the ramp buildup during the time window 500-260 ms before reach onset in PD error-choice trials in response to oPD-DCs (Fig. 12, E and F, orange histograms) was progressively stronger as RTs slowed [1-way ANOVA; F(2,1341) = 9.901, P = 5.39 × 10−05]. This was not seen in activity before reach onset in corresponding trials in monkey Z (Fig. 12, G and H, orange histograms; P = 0.039). In both monkeys, the transient response seen when they incorrectly chose the oPD target in response to PD-DCs (Fig. 12, E–H, green histograms) was significantly stronger in trials with progressively longer RTs during both the initial rising phase of the responses from 120 to 260 ms after DC onset [1-way ANOVA; monkey G: F(2,1488) = 8.129, P = 0.0003; monkey Z: F(2,606) = 9.283, P = 0.0001] and during the more extended time window from 120 to 500 ms after DC onset [1-way ANOVA; monkey G: F(2,1488) = 56.022, P ≈ 0.0; monkey Z: F(2,606) = 8.864, P = 0.00016].
The relative effect of direction and color information on PMd activity in the CG task.
We tested the relative effects of direction and color on PMd neural activity in the CG task in 69 PMd neurons from both monkeys. No neurons were directionally tuned during the Spatial-Cue epoch, but the large majority of neurons was significantly directionally tuned (3-way ANOVA, main effect of direction, P < 0.01) during the DC epoch between the appearance of the DC and the onset of movement (60/69, 87.0%), the movement epoch (61/69, 88.4%), and the target-hold epoch (53/69, 76.8%). A much smaller but still sizeable population showed a main effect of color (3-way ANOVA, P < 0.01) during the Spatial-Cue (2/69, 2.9%), DC (14/69, 20.3%), movement (15/69, 21.7%), and target-hold (12/69, 17.4%) epochs.
The effect of direction on activity was also substantially stronger than that of color. The mean absolute difference in discharge rate of the PMd neurons as function of reach direction was 11.23 ± 0.68 spikes/s during the DC epoch and even larger during the movement epoch (Table 5). In contrast, the mean difference in neural activity evoked by yellow vs. blue Spatial Cues or DCs was largest [2.00 ± 0.18 (SE) spikes/s] during the DC epoch and smaller at all other trial epochs (Table 5). Those differences were only modestly larger than the mean difference observed during the center-hold period before any cues appeared when those data were arbitrarily sorted into two groups according to the eventual color bias of the DC in each trial (1.10 ± 0.11 spikes/s) or according to the eventual direction of movement (0.96 ± 0.08 spikes/s). Both values provide estimates of the expected random variability in neural activity.
Table 5.
Mean ± SE of the absolute difference in mean discharge rate due to the main effect of color and of reach direction across all PMd neurons in both monkeys
| Center Hold | Spatial Cue | Decision Cue | Movement | Target Hold | |
|---|---|---|---|---|---|
| Color | 1.10 ± 0.11 | 0.85 ± 0.10 | 2.00 ± 0.18 | 1.44 ± 0.16 | 1.10 ± 0.11 |
| Direction | 0.96 ± 0.08 | 0.97 ± 0.11 | 11.23 ± 0.68 | 13.31 ± 1.05 | 8.51 ± 0.66 |
A sizable proportion of the neurons also showed a significant interaction between direction and color during the Spatial-Cue (36/69, 52.2%) and DC (35/69, 50.7%) epochs, which declined during the movement (23/69, 33.3%) and the target-hold (7/69, 10.1%) epochs. The interaction term indicated that the color of the cues had a small but significant modulatory effect on the directional tuning of about one-half of the PMd neurons during the Spatial-Cue and DC epochs, which declined during the subsequent movement and target-hold epochs. This suggested that PMd neurons were not strongly modulated by the color of the Spatial Cues and DCs per se, but that a significant portion of the PMd population showed some modulation in their directional discharge that reflected the color/location combinations of the stimuli that influenced the action choices in each trial.
DISCUSSION
Summary of results.
Neural activity was recorded in the PMd and M1 of two monkeys as they chose between two opposite directions of reaching movements on the basis of different degrees of contradictory evidence contained in a single visual DC. The choice behavior of the two monkeys showed important differences. A decrease in the color bias in the DCs led to an increase in the rate of incorrect reach choices in both monkeys, and systematic increases in RT durations in monkey G, but much less in monkey Z. Error rates increased as RT duration increased in monkey G, but monkey Z's error rates were highest in short RT trials. In parallel with these behavioral differences, PMd neural activity in response to PD-DCs that supported the preferred reach direction of neurons took longer to increase as the strength of that support (the DC color bias) decreased in monkey G. This was far less prominent in PMd of monkey Z.
Nevertheless, PMd activity in both monkeys had several features in common. Most importantly, oPD-DCs that supported the target opposite to the preferred reach direction of each neuron evoked a brief transient activation of PMd neurons that was then rapidly truncated. The duration of that transient activation increased as the DC color bias decreased in monkey G but not in monkey Z. It was also particularly prominent in both monkeys when the trial data were resorted according to RT duration rather than DC color bias; the transient activation increased in strength and duration as RTs increased. The neural activity reflected the monkeys' decision rather than the physical color bias of the DCs when they made incorrect reach choices. Thus, the activity evoked by PD-DCs was truncated before the monkeys made incorrect reaches toward the oPD target, while the activity evoked by the oPD-DCs continued to grow until the monkeys made incorrect reaches toward the PD target. When error-choice trials were sorted by RT duration, the truncated responses evoked by the PD-DCs were stronger and of longer duration in error-choice trials with longer RTs.
These results from correct- and error-choice trials suggest that sensory evidence supporting both reach choices had access to PMd neurons with corresponding reach preferences. Furthermore, the time needed to make a choice depended not only on the time required to activate the neurons preferring the chosen reach but also to suppress the activity of the neurons preferring the rejected choice. This is not consistent with a reach selection process driven by a pure net-evidence signal. Moreover, this process may continue in PMd even after M1 neurons have begun to generate reciprocally tuned activity encoding the reach choices of the monkeys.
Finally, the tonic activity at the end of the Spatial-Cue period before the appearance of the DCs was modestly but significantly higher in trials with correct PD choices than oPD choices when the RTs were short in both monkeys, but not when they were long. However, the tonic activity level did not correlate with correct vs. error response choices in monkey G at any RT duration or in monkey Z for long RT trials, but did differ between rapid correct vs. error choices in monkey Z. This suggests that momentary fluctuations in tonic activity before the DC cue appearance may facilitate faster decisions, but had a smaller effect on the action choices.
The CG task.
The CG task is a two-alternative reaction-time task in which a unitary DC provided the monkeys the information they needed to resolve a color/location matching rule to choose the correct target and reach to it as soon as they wanted. The color bias of the DCs, the net strength of the sensory evidence for the correct choice, was decreased not just by reducing the amount of color evidence supporting the correct target but also by simultaneously increasing the amount of conflicting color evidence supporting the other target. This is quite different from how evidence quality is manipulated in most RDK stimuli that have been widely used in decision-making studies of action choices based on noisy sensory evidence (Britten et al. 1992; Shadlen et al. 1996; Shadlen and Newsome 2001; Gold and Shadlen 2001, 2007; Roitman and Shadlen 2002; Mazurek et al. 2003; Palmer et al. 2005; Huk and Shadlen 2005; Ditterich 2006a, 2006b; Kiani et al. 2008; Bennur and Gold 2011; Ding and Gold 2012). In standard RDK stimuli, a different amount of coherent dot motion is presented in only one of two opposite directions in each trial, against a background of random dot motions. When the amount of coherent motion is low, the subjects must detect the presence and direction of a weak sensory signal embedded in random-motion noise.
In contrast, the most ambiguous DCs in the CG task have nearly equal amounts of easily discriminable evidence (the squares with task-relevant colors) favoring both choices, and the subjects must choose the correct target based on nearly equal amounts of conflicting evidence supporting both action choices. This is potentially quite different from the signal-detection challenge presented by unidirectional low-coherence RDK stimuli, but is more similar to the simultaneous streams of variable-strength coherent motion used in one series of studies (Bollimunta and Ditterich 2012; Bollimunta et al. 2012; Niwa and Ditterich 2008).
The decision process in the CG task may also have much in common with target selection in visual-search tasks in which subjects select a motor response using conflicting evidence provided by the correct target stimulus and multiple distractors (Bichot and Schall 1999, 2002; Sato and Schall 2001; Sato et al. 2003; Song and Nakayama 2006; Song et al. 2008; Song and McPeek 2010; Purcell et al. 2012).
In theory, the DC color bias estimate could be resolved in visual perceptual circuits, and the binary “sensory” decision could then be relayed to prefrontal and premotor areas for application of the color/location matching rule to select the reach target. This would be consistent with a strictly serial perception-cognition-action hierarchy of brain organization. However, one of the most significant advances in the field of voluntary motor control over the past two decades was the discovery that neural correlates of a nominally “perceptual” decision process are seen in movement-related cortical areas when those decisions lead to action (Bennur and Gold 2011; Bollimunta and Ditterich 2012; Cisek and Kalaska 2010; Ding and Gold 2012; Gail et al. 2009; Gold and Shadlen 2001, 2007; Hernandez et al. 2010; Hoshi and Tanji 2006; Klaes et al. 2011; Meister et al. 2013; Muhammad et al. 2006; Romo et al. 2004; Wallis and Miller 2003).
Choice behavior in the CG task.
Human subjects performing the CG task showed increasing RT durations and error rates as the color bias of DCs decreased (Coallier and Kalaska, 2014). These same general choice behavior trends have been found in a wide variety of different tasks and have been interpreted as supporting a variety of different decision mechanisms, including drift-diffusion models that accumulate noisy net sensory evidence across time in single trials (Ditterich 2006a, 2006b; Gold and Shadlen 2001, 2007; Mazurek et al. 2003; Niwa and Ditterich 2008; Palmer et al. 2005; Ratcliff and McKoon 2008; Ratcliff and Smith 2004), linear deterministic accumulation models in which sensory evidence is noise-free in single trials but varies stochastically across trials (Brown and Heathcote 2008; Carpenter and Williams 1995; Heathcote and Love 2012; Noorani 2014), gated-accumulator mechanisms driven by noisy neural activity recorded in visual-search tasks (Purcell et al. 2012: Schall et al. 2011), and urgency-gated tracking of stochastically varying single-trial sensory evidence (Cisek et al. 2009; Thura et al. 2012).
The choice behavior of monkey G was similar to that seen in human subjects performing the CG task, except that the human RT distributions were substantially slower and broader (Coallier and Kalaska 2014). Its behavior is consistent with studies cited above that suggest that subjects observe noisy sensory signals until they attain a criterion level of evidence to make a decision.
In contrast, monkey Z's RTs were nearly constant across all DC color bias levels. Its success rate decreased with DC ambiguity but also tended to be lowest for the trials with the shortest RTs at each color bias and improved in trials with longer RTs. This indicated that monkey Z's decisions were still informed by the quality of the DC evidence and by how long it observed the DCs before initiating a reach. Similar trends have been reported in monkeys in tasks in which the duration of observation of RDK stimuli was short and controlled experimentally, and was interpreted as consistent with a decision process based on accumulation of noisy sensory evidence for variably short periods of time (de Lafuente et al. 2015; Gold and Shadlen 2003; Kiani et al. 2008; Meister et al. 2013; Tsetsos et al. 2012). In the present study, DC observation time was not experimentally controlled. Instead, monkey Z appeared to adopt a strategy of observing the DCs for relatively consistent periods of time independent of DC quality and making its decision based on the evidence accumulated up to that point rather than waiting to attain a particular criterion level of evidence in each trial. Indeed, the higher incidence of choice errors for short-latency responses, even for the 100/0 and 70/30 DCs, suggested that monkey Z occasionally and impulsively initiated a reach movement before it had taken enough time to make a reliable estimate of the DC color bias.
Neural activity in the CG task.
The neural data were collected from single neurons recorded at different times. Nevertheless, they can be used as a surrogate for the neural events that presumably occurred simultaneously in each trial in separate populations of PMd neurons with opposing reach direction preferences. Furthermore, although we only tested each neuron with targets near their preferred reach direction and the opposite direction, it is reasonable to assume that nonrecorded neurons whose PDs were off-axis to the tested directions were also active in the task and contributed to the selection process (Cisek and Kalaska 2005; Cisek 2006, 2007).
PMd neurons in both monkeys began to discharge at relatively consistent latencies after the appearance of the DCs independent of the DC color bias and the duration of the RT in each trial. Differences in PMd activity between the two monkeys were consistent with the different ways in which they responded to the DCs. This was most evident in the responses to PD-DCs. In the left PMd of monkey G, neurons showed a more gradual buildup of activity at both the single-neuron and population level as the strength of support for their preferred reach direction in the DCs decreased. This effect was not as strong as has been reported in some other decision-making tasks in PMd (Pastor-Bernier and Cisek 2011; Pastor-Bernier et al. 2012; Thura et al. 2012) or in some studies in the lateral intraparietal cortex (LIP) using RDK tasks (e.g., Huk and Shadlen 2005; Kiani et al. 2008; Roitman and Shadlen 2002), but was statistically significant. It was weaker but still statistically significant and well-ordered in the neurons recorded in the right PMd of monkey G at a time when the monkey showed an improvement in the speed and precision with which it could use the DCs to make reach choices. It was far less prominent in monkey Z, which responded to DCs with RTs that were only modestly (but still significantly) longer and more variable for 52/48 DCs (364.3 ± 79.6 ms) than for 100/0 DCs (348.9 ± 67.9 ms). In both monkeys, therefore, the rate at which neural activity built up in response to PD-DCs paralleled how the monkeys responded to the sensory evidence supporting the preferred reach direction of the neurons.
A critical finding is that the appearance of the multicolored DCs that usually contained sensory evidence supporting both reach choices caused a simultaneous initial increase in the activity level in neurons with opposing preferred reach directions. This initial activation was then suppressed in the neurons that preferred the ultimately rejected target in each trial, whether correct or incorrect. Most strikingly, the strength and duration of this transient coactivation clearly increased systematically as RTs increased in both monkeys when trials were sorted into groups of short, medium, and long RTs for both correct- and error-choice trials. These transient increases in activity before reaching in the oPD of each neuron ended at about the same time before reach movement onset whether the trials were sorted by DC or RT. The suppression associated with the rejected target occurred at the same time as the neurons preferring the chosen target were attaining their peak discharge rate, 140-120 ms before movement onset (Figs. 7, 8, and 11). The choice probability analysis showed that this corresponded to the time at which an ideal observer could make a reliable prediction about the response choices of the monkeys at each DC color bias level. Thura and Cisek (2014) observed a similar coincidence of timing of activity changes in the neurons supporting opposite action choices in PMd and M1, which they identified as a neural correlate of the time of decision commitment. However, this occurred ∼280 ms before movement onset in their study, considerably earlier than in the present study.
The coactivation of the neural populations preferring both reach choices by the DCs showed that sensory evidence supporting both action choices had access to PMd neurons with corresponding action preferences. It is noteworthy that even 100/0 DCs that contained physical evidence supporting only one of the two targets nonetheless briefly activated neurons preferring the nonsupported target (Figs. 5, 7, and 8). This may reflect the statistical structure of the task. At the start of each trial, there was a 50/50 probability that the DC color bias would support the preferred reach direction of each neuron, and 9 of the 10 DCs would contain at least some sensory evidence supporting its preferred reach. This uncertainty, combined with the natural tendency of monkeys to make rapid responses to visual stimuli in response-time tasks, may have facilitated the coactivation of both populations of neurons preferring both potential reach directions, even when 100/0 DCs appeared with sensory evidence supporting only one choice. These findings are consistent with a reach selection process played out by competition between neural representations of the different action choices (Bastian et al. 2003; Chapman et al. 2010; Cisek and Kalaska 2005, 2010; Cisek 2006, 2007; Klein-Flügge and Bestmann 2012; Klaes et al. 2011, 2012; McKinstry et al. 2008; Meegan and Tipper 1998; Mitchelet et al. 2010; Pastor-Bernier et al. 2012; Welsh and Elliot 2005; Wood et al. 2011). They are not consistent with a selection process driven only by the net sensory evidence provided by the DCs, which should ideally produce a reciprocal activation and suppression of the opposing neuronal populations without any period of coactivation (Mazurek et al. 2003; Roitman and Shadlen 2002).
Indeed, the PMd activity in the CG task appears to be significantly different from that of LIP neurons while monkeys discriminate the direction of a single stream of coherent RDK motion to select a saccade target. LIP neurons typically show an initial momentary and stereotypical suppression of activity at the onset of RDK stimuli whose strength and duration are independent of the coherent motion strength, followed by smoothly and reciprocally evolving changes in activity whose rate and sign depend on the net strength of the coherent motion signal and whether it is in the neuron's preferred saccade direction or the opposite direction (Shadlen and Newsome 2001; Gold and Shadlen 2001, 2007; Roitman and Shadlen 2002; Mazurek et al. 2003; Huk and Shadlen 2005; Niwa and Ditterich 2008; Kiani et al. 2008; Bennur et al. 2011; Ding and Gold 2012). They do not show a prominent coactivation of multiple neural populations even when the RDK stimuli contain multiple streams of competing visual motions (Bollimunta and Ditterich 2012). Sensory signals about all coherent motion directions may project into LIP, but local computation effectively generates a net-evidence signal to modulate neural activity (Bollimunta and Ditterich 2012). However, a neural coactivation does occur when the targets appear at the start of RDK-task trials (Bennur and Gold 2011; Churchland et al. 2008; Kim and Shadlen 1999; Meister et al. 2013), similar to the sustained coactivation evoked by the Spatial Cues in the 2T and CG tasks (Cisek and Kalaska 2005; present study). This initial coactivation by the potential targets reflects processes other than a net-evidence accumulation mechanism; since there was no net difference in the evidence favoring one target over the other when they first appeared before RDK stimulus or DC onset, no neural activation should have occurred.
The RDK paradigm has recently been extended to reach-related neurons in the medial intraparietal (MIP) cortex (de Lafuente et al. 2015), with very similar results. Reach-related MIP activity showed gradual reciprocal changes as a function of coherent motion strength with no coactivation of neurons with opposite reach preferences at the onset of the RDK stimuli.
The PMd responses to DCs in the CG task appear to more closely resemble neural responses in the superior colliculus (Basso and Wurtz 1998; Kim and Basso 2008, 2010), frontal eye fields (Bichot and Schall 1999, 2002; Purcell et al. 2012; Sato and Schall 2001; Sato et al. 2003; Schall and Hanes 1993; Schall et al. 2011), and PMd (Song and McPeek 2010) in visual-search tasks, where the initial appearance of the target and distractors simultaneously activates neurons preferring each of the available action options. The final target choice evolves over time by an increase in activity of the neurons preferring the chosen target and simultaneous suppression of the neurons preferring the “distractor” choices. Importantly, the coactivation of the neurons preferring different saccade or reach directions in visual-search tasks was evoked by the appearance of visual stimuli in the response fields of each neuron, and all salient sensory evidence distinguishing the target from distractors is usually presented at the spatial locations of the potential targets themselves. The spatial location of each stimulus thus maps directly onto the direction of each potential saccade, and perceptual processes can identify which stimulus has the appropriate combination of stimulus features to be the target. In contrast, the Spatial Cues were already visible in the CG task before the DCs appeared. The coactivation on which we focus was evoked by the appearance of a centrally located DC, whose critical property, its color bias, has no inherent relationship to movement per se outside of the context of the color/location matching rule used in the CG task. This implies that the initial coactivation elicited by the DCs in this study may involve different and potentially more complex processes than in visual-search tasks (see also Klaes et al. 2011, 2012).
The responses of neurons in visual-search tasks can be explained by a range of different computational models, including signal detection, probabilistic inferences, and competitive interactions among coactivated populations followed by gated accumulation of evidence to a threshold (Cohen et al. 2010; Hanes and Schall 1996; Kim and Basso 2008, 2010; Purcell et al. 2012; Schall et al. 2011). The similarity of the PMd responses in the CG task suggests that one or more of these models might explain the temporal dynamics of the target selection process in this study better than an evidence-accumulation model driven only by the net sensory evidence of the DCs, i.e., its color bias. Nevertheless, a net-evidence calculation could still be involved in the CG task, if it is imperfect or incomplete before excitatory inputs begin to activate PMd, thereby allowing some leak of the signals about both action choices onto the PMd neurons (Purcell et al. 2012; Schall et al. 2011).
These present findings may also be consistent with a mechanism of urgency-gated tracking of instantaneous evidence for both action choices, rather than an explicit accumulation (i.e., integration) of sensory input across time (Cisek et al. 2009; Thura et al. 2012). For instance, the urgency signal should be kept at a low level during the initial Spatial-Cue delay period to avoid uninformed premature responses (“guesses”). Once the DC appears, the task allows the monkeys to move as soon as they wish, which could cause an abrupt increase in urgency signal gain that could transiently coactivate neurons preferring both Spatial Cues, until further processing of the DC color bias modulates the relative activation level of both populations.
This discussion emphasizes how many different decision-making models can explain a particular experimental result reasonably well. Further insight into the underlying mechanisms of target selection in the CG task will require new experiments in conditions that can test specific falsifiable predictions from each computational model.
Activity in M1 began 40–60 ms later after DC onset than in PMd, at about the time that the initial coactivation in PMd stopped growing and began to be suppressed and showed no evidence of an initial coactivation at the population level. M1 neural responses were far less sensitive to the quality of the evidence provided by the DCs than PMd, and they began to discharge at a fixed latency before reach movement onset, independent of the RT duration in each trial. These findings indicate that the M1 neurons were implicated primarily in the initiation and execution of the chosen action and were not strongly involved in the decision process. When aligned to movement onset, M1 activity that was reciprocally tuned to the chosen movement direction began 300-280 ms before movement onset. That is a longer lead time than we have seen in M1 in many tasks with single unambiguous reach targets (Crammond and Kalaska 2000; Georgopoulos et al. 1982; Kalaska and Crammond 1992; Kalaska et al. 1989; Sergio et al. 2005; Sergio and Kalaska 1998). The M1 neurons began to express that reciprocal activity at the same time that the neural representations of the two choices began to separate in PMd but before the PMd activity had fully evolved to provide a reliable signal about response choices according to the choice probability analysis. This raises the intriguing possibility that neural processes implicated in reach selection and movement initiation were not strictly serially organized in these two monkeys in this study (Klein-Flügges and Bestmann 2012; Mitchelet et al. 2010). This further suggests that reach selection processes in PMd could continue even after a motor command began to be formulated (Pastor-Bernier et al. 2012).
Changes in movement direction.
The two monkeys occasionally changed reach direction from one target to the opposite target, and these changes of direction occurred more frequently as the DC color bias decreased. Unexpectedly, however, changes of direction more often resulted in an incorrect choice in our monkeys. This is contrary to prior results by human subjects in the CG task (Coallier and Kalaska 2014), and in both human (Burk et al. 2014; Resulaj et al. 2009) and nonhuman (Song et al. 2008; Bollimunta et al. 2012; Kiani et al. 2014) primates in other tasks, in which changes of mind often led to a reduction in error rates. We have no explanation for this result.
Changes of reach direction suggest not only that processing of sensory evidence continues after movement initiation even though the stimuli are removed (Burk et al. 2014; Resulaj et al. 2009) but also that subjects may initiate reaching movements before they are fully committed to their reach choice (Coallier and Kalaska 2014; Song and Nakayama 2006; Song et al. 2008). The continued evolution of PMd activity seen in this study as a component of the motor command is being generated in M1 is consistent with this possibility and could contribute to the changes in reach direction, especially since both M1 and PMd send corticospinal projections to spinal motor circuits (Dum and Strick 2002; He et al. 1993).
Tonic activity before DC onset.
In trials in which the monkeys observed the DCs for short periods of time before reaching to the correct target, tonic activity rates at the end of the Spatial-Cue period just before the DCs appeared were significantly higher when the monkeys correctly reached in each neuron's PD than the oPD. This difference in tonic activity before the DCs appeared was not evident in trials in which the monkeys observed the DC for longer periods of time before responding.
This tonic activity bias is not necessarily evidence that the monkeys were routinely anticipating or guessing the target choice before the DC appeared (Snyder et al. 2006). There was no significant difference in tonic rates before the DC appeared in correct- vs. error-choice trials in monkey G at any RT duration. The same was also true for monkey Z in trials with longer RTs, but there was a significant difference in tonic activity before error-choice trials with short RTs.
These trends suggest that the main effect of a momentarily higher tonic rate before the DCs appeared was to facilitate more rapid reach choices in both monkeys, and may have also contributed to short RT error choices in the more impulsive monkey Z. The facilitating effect of tonic activity on RTs has been reported in M1 (Riehle and Requin 1993) and the parietal cortex (Churchland et al. 2006; Snyder et al. 2006). Tonic rate biases were also seen in the activity of orbitofrontal cortex “chosen-juice” neurons, but in that case the tonic rates appeared to influence which juice the monkeys chose in difficult-choice trials, but not in easy-choice trials (Padoa-Schioppa 2013). The tonic rate biases may reflect the effect of normal fluctuations in the dynamic activation state of the neural circuits (Padoa-Schioppa 2013), which may facilitate rapid response selection and initiation (Afshar et al. 2011; Snyder et al. 2006).
Modulation of PMd activity by cue color.
Cisek and Kalaska (2005) reported that PMd neurons were strongly modulated by the directional information provided by the Spatial and Color Cues in the 1T and 2T tasks but that only 2–3% of the neurons showed a significant main effect of the color of the cues at different times in the trial (ANOVA, P < 0.01). More PMd neurons showed modest but statistically significant effects of the colors of the task cues on their activity in the CG task compared with that reported by Cisek and Kalaska (2005), or by Song and McPeek (2010) in a reach visual-search task. Moreover, the color effects were mainly expressed as an interaction with direction and were most prominent during the Spatial-Cue and Color-Cue periods when the monkeys had to use that information and the color-location matching rule to select the target. Once the monkeys began to reach, the influence of color on PMd activity diminished. The increased prominence of the color-related effects may reflect the greater color discriminative demands of the DCs in the CG task compared with the monochromatic Color Cues of the 1T and 2T tasks. Nevertheless, the magnitude of the color-related effects on PMd activity was relatively modest.
Furthermore, most PMd neurons were not activated by the Color Cues when the order of stimuli in the 2T task was reversed so that the central Color Cue was presented first, followed by the Spatial Cues (“delayed match-to-sample” task, DMS; Cisek and Kalaska 2005). The subsequent delayed appearance of the Spatial Cues in the DMS task produced a transient coactivation of the PMd neurons preferring both reach directions, followed by a reciprocal activation and suppression of the neurons preferring the selected and rejected targets, respectively. The lack of PMd responses to the highly salient Color Cues in the DMS task is consistent with the modest responses to color reported here. The DMS task results emphasize that PMd neurons respond best to sensory signals when they provide information that can be mapped onto a motor goal. This is consistent with the findings of Gail et al. (2009), who showed that PMd activity is strongly modulated by the appearance of a green or blue cue that designated a sensorimotor mapping rule (reach toward or in the direction opposite to a spatial visual cue, respectively) only when the location of the spatial cue was known, but not if the location of the spatial cue is not known.
These PMd responses to the cues, especially the transient coactivation of the neurons that preferred the ultimately rejected target, are not “visual” responses in the usual sense. Visual stimuli generally have less direct access to arm-related premotor and parietal cortical areas (Crammond and Kalaska 2000; de Lafuente et al. 2015; di Pellegrino and Wise 1993; Kubanek et al. 2013; Song et al. 2008) than they do to saccade-related cortical areas (de Lafuente et al. 2015; Kubanek et al. 2013; Thompson et al. 1996). Instead, the short-latency responses of PMd neurons to visual cues primarily reflect the novel salient information the cues provide about action choices according to learned stimulus-response association rules (Brasted and Wise 2004; Buch et al. 2006; Crammond and Kalaska 2000; di Pellegrino and Wise 1993; Gail et al. 2009; Kalaska and Crammond 1995; Klaes et al. 2011; Kurata and Wise 1988a, 1988b; Mitz et al. 1991; Wise et al. 1983, 1992, 1996). For instance, the PMd neurons responded differently to the PD-DCs and oPD-DCs, but what constituted a PD-DC vs. an oPD-DC in a given trial depended both on the color bias of the DC and whether it matched the color of the Spatial Cue at the preferred reach direction or the opposite direction of the neuron in that trial. The PMd activity primarily signaled to what degree the DC supported a reach in each neuron's PD or oPD in each trial, and was only modestly modulated by the colors per se. This was particularly evident in error-choice trials in which the PMd activity reflected how the monkeys incorrectly interpreted the DCs as supporting the wrong target.
Transient coactivation of opposing neural populations: Limitations of interpretation.
We interpret the transient coactivation of opposing PMd neural populations as a neural correlate of a momentary competition between representations of the two action choices after the DC appears. However, the strength of the coactivation may be context dependent (Gail et al. 2009; Klaes et al. 2011; Meister et al. 2013). It was not evoked by the Color Cue in the 2T task (Fig. 4; Cisek and Kalaska 2005) when the monkeys expected to receive a monochromatic cue unambiguously supporting only one of the two color-coded targets. In contrast, the monkeys in the CG task attended a polychromatic visual stimulus that could have any one of 10 different color biases, 8 of which provided variable amounts of support for both targets. Furthermore, the delay imposed after the Color Cue appeared in the 2T task afforded the monkeys an extended period of time to process the cue and select the target before the GO signal appeared. Similarly, RDK stimuli elicited a gradual reciprocal activation of MIP reach-related neurons when the task design controlled the duration of the RDK stimuli and imposed a delay between RDK offset and the initiation of the chosen reach (de Lafuente et al. 2015). In contrast, the monkeys were free to reach as quickly as they wanted after DC onset in the CG task. This freedom, and even self-imposed urgency, to respond quickly may have facilitated a coactivation of the opposing neural populations. Another difference is that the Spatial Cues in the 2T task disappeared after a short period of time and had to be memorized but were continuously present when the DC appeared in the CG task. The mere presence or absence of a visual target can alter how neurons respond to DCs (Meister et al. 2013) and could facilitate the coactivation seen in the CG task.
The activity of the two opposing populations appears temporally coextensive when averaged across many trials in many neurons recorded at different times. However, this could be an artifact of averaging neural correlates of temporally asynchronous transient vacillations between temporally nonoverlapping representations of each option (changes of mind; Bollimunta et al. 2012; Kiani et al. 2014) before the monkeys decide on a reach direction, rather than a true coactivation. We have previously rejected rapid vacillations as an explanation for the apparent coactivation of PMd potential-response neurons during the Spatial-Cue delay period of the 2T task, using an indirect post hoc analysis (Cisek and Kalaska 2005). Nevertheless, that does not discount the possibility of neural changes of mind after the appearance of the DCs, which can only be properly assessed by simultaneous recording of multiple neurons in the CG task.
Finally, even though PMd neurons are not strongly color sensitive in the CG task, it is not clear to what degree their initial responses after DC onset are implicated in some aspect of the sensory discriminative processing of the DCs or primarily reflect an ongoing estimate of the likelihood of different response choices as the sensory discrimination evolves in other neural circuits because the two processes are temporally confounded in the CG task. Neural correlates of distinct sensory-like events vs. motor processes have been found in prefrontal (Hernandez et al. 2010; Hoshi and Tanji 2006; Muhammad et al. 2006; Romo et al. 2004; Wallis and Miller 2003), premotor (Hernandez et al. 2010; Hoshi and Tanji 2006; Muhammad et al. 2006; Romo et al. 2004; Wallis and Miller 2003), and parietal (Bennur and Gold 2011; Ding and Gold 2012; Klaes et al. 2011) cortex when they are dissociated in time by task design.
Does one decision mechanism make all decisions?
It is appealing at many levels to believe that a common evidence-assessment mechanism contributes to most or all evidence-based decisions, ranging from whether or not a sensory stimulus had occurred, to whether to turn left or right at a particular road intersection, whether to drink some coffee or tea, whether to spend money on home renovations or on the purchase of a new home, or whether or not to run the risk of being attacked by a lion while crossing an open field to drink some water from a stream. While a particular computational model may account well for the choice behavior of subjects in a wide range of decision-making tasks (Ratcliff and McKoon 2008; Ratcliff and Smith 2004), it is nevertheless legitimate to ask whether one neuronal mechanism must or can account for all decisions in all contexts.
Indeed, it seems just as reasonable to expect that details of the decision process will differ depending on the nature of the decision and the sensory evidence on which it is based. Fundamentally “economic” decisions may involve neural mechanisms that are distinct from those underlying purely perceptual or sensorimotor decisions (Padoa-Schioppa 2011, 2013). Similarly, there may be differences in action-related decisions if they depend on detection of a variable-strength signal in a noisy background (Britten et al. 1996; Gold and Shadlen 2001, 2007; Mazurek et al. 2003), or require selecting a readily visible target from among several equally visible distractors (Kim and Basso 2008, 2010; Purcell et al. 2012; Schall et al. 2011), applying different arbitrary stimulus-response rules (Chen and Wise 2006; Gail et al. 2009; Hernandez et al. 2010; Hoshi and Tanji 2006; Klaes et al. 2011; Muhammad et al. 2006; Romo et al. 2004; Wallis and Miller 2003), assessing evidence that can change stochastically across time against an impending decision deadline (Cisek et al. 2009; Thura et al. 2012), or assessing competing and mutually contradictory evidence (Coallier and Kalaska 2014; present study).
GRANTS
This work was supported by Canadian Institutes of Health Research Grants MOP-67194 and MOP-97944 (J. F. Kalaska), a Fondation Fyssen postdoctoral fellowship (T. Michelet), a Fonds de recherche du Québec-Santé (FRQS) graduate studentship (É. Coallier), and an infrastructure grant from the FRQS to the Groupe de recherche sur le système nerveux central.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: É.C., T.M., and J.F.K. conception and design of research; É.C., T.M., and J.F.K. performed experiments; É.C. and J.F.K. analyzed data; É.C., T.M., and J.F.K. interpreted results of experiments; É.C. and J.F.K. prepared figures; É.C. and J.F.K. drafted manuscript; É.C., T.M., and J.F.K. edited and revised manuscript; É.C., T.M., and J.F.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Apostolos Georgopoulos provided invaluable advice on the ANOVA analysis. Christian Valiquette, Caroline Paquet, Mélissa Latourelle, Lyne Girard, and Nadine Michaud provided expert technical support.
REFERENCES
- Afshar A, Santhanam G, Yu BM, Ryu SI, Sahani M, Shenoy KV. Single-trial neural correlates of arm movement preparation. Neuron 71: 555–564, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A, Schöner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci 18: 2047–2058, 2003. [DOI] [PubMed] [Google Scholar]
- Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci 31: 913–921, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci 2: 549–554, 1999. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J Neurosci 22: 4675–4685, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Ditterich J. Local computation of decision-relevant net sensory evidence in parietal cortex. Cereb Cortex 22: 903–917, 2012. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Totten D, Ditterich J. Neural dynamics of choice: single-trial analysis of decision-related activity in parietal cortex. J Neurosci 32: 12684–12701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci 19: 721–740, 2004. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Visual Neurosci 13: 87–100, 1996. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci 10: 1157–1169, 1993. [DOI] [PubMed] [Google Scholar]
- Brown SD, Heathcote A. The simplest complete model of choice response time: linear ballistic accumulation. Cogn Psychol 2008: 153–178, 2008. [DOI] [PubMed] [Google Scholar]
- Buch ER, Brasted PJ, Wise SP. Comparison of population activity in the dorsal premotor cortex and putamen during the learning of arbitrary visuomotor mappings. Exp Brain Res 169: 69–84, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk D, Ingram JN, Franklin DW, Shadlen MN, Wolpert DM. Motor effort alters changes of mind in sensorimotor decision making. PLoS One 9: e92681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62, 1995. [DOI] [PubMed] [Google Scholar]
- Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci 14: 4109–4124, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CS, Gallivan JP, Wood DK, Milne JL, Culham JC, Goodale MA. Reaching for the unknown: multiple target encoding and real-time decision making in a rapid reach task. Cognition 116: 168–176, 2010. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Shadlen MN. Decision-making with multiple alternatives. Nat Neurosci 11: 693–702, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 68: 387–400, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96: 3130–3146, 2006a. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol 97: 348–359, 2007. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci 26: 3697–3712, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci 26: 9761–9770, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Phil Trans R Soc B 362: 1585–1599, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89: 922–942, 2003. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45: 801–814, 2005. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010. [DOI] [PubMed] [Google Scholar]
- Cisek P, Puskas GA, El-Murr S. Decisions in changing conditions: the urgency-gating model. J Neurosci 29: 11560–11571, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coallier É, Kalaska JF. Reach target selection in humans using ambiguous decision cues containing variable amounts of conflicting sensory evidence supporting each target choice. J Neurophysiol 112: 2916–2938, 2014. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Crowder EA, Heitz RP, Subraveti CR, Thompson KG, Woodman GF, Schall JD. Cooperation and competition among frontal eye field neurons during visual target selection. J Neurosci 30: 3227–3238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos I, Bélanger N, Cisek P. The influence of predicted arm biomechanics on decision making. J Neurophysiol 105: 3022–3033, 2011. [DOI] [PubMed] [Google Scholar]
- Cos I, Medleg F, Cisek P. The modulatory influence of end-point controllability on decisions between actions. J Neurophysiol 108: 1764–1780, 2012. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84: 986–1005, 2000. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Roy JE, Buschman TJ, Miller EK. Comparison of primate prefrontal and premotor cortex neuronal activity during visual categorization. J Cogn Neurosci 23: 3355–3365, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Jazayeri M, Shadlen MN. Representation of accumulating evidence for a decision in two parietal areas. J Neurosci 35: 4306–4318, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA 103: 14266–14271, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J Neurosci 13: 1227–1243, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb Cortex 22: 1052–1067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditterich J. Stochastic models of decisions about motion direction: theory and physiology. Neural Netw 19: 981–1012, 2006a. [DOI] [PubMed] [Google Scholar]
- Ditterich J. Evidence for time-variant decision making. Eur J Neurosci 24: 3628–3641, 2006b. [DOI] [PubMed] [Google Scholar]
- Ditterich J. A comparison between mechanisms of multi-alternative perceptual decision making: ability to explain human behavior, prediction for neurophysiology, and relationship with decision theory (Abstract). Front Neurosci 4: 184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682, 2002. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol 73: 836–854, 1995. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol 70: 2097–2116, 1993. [DOI] [PubMed] [Google Scholar]
- Gail A, Klaes C, Westendorff S. Implementation of spatial transformation rules for goal-directed reaching via gain modulation in monkey parietal and premotor cortex. J Neurosci 29: 9490–9499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. Interruption of motor cortical discharge subserving aimed arm movements. Exp Brain Res 49: 327–340, 1983. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Caminiti R, Kalaska JF, Massey JT. Spatial coding of movement: a hypothesis concerning the coding of movement direction by motor cortical populations. In: Neural Coding of Motor Performance, edited by Massion J, Paillard J, Schultz W, Wiesendanger M. New York, NY: Springer, 1983, vol. 7, p. 327–336. [Google Scholar]
- Georgopoulos AP, Kalaska JF, Massey JT. Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol 46: 725–743, 1981. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci 5: 10–16, 2001. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J Neurosci 23: 632–651, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York, NY: Wiley, 1966. [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952–980, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote A, Love J. Linear deterministic accumulator models of simple choice (Abstract). Front Psychol 3: 292, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Nacher V, Luna R, Zainos A, Lemus L, Alvarez M, Vazquez Y, Camarillo L, Romo R. Decoding a perceptual decision process across cortex. Neuron 66: 300–314, 2010. [DOI] [PubMed] [Google Scholar]
- Hocherman S, Wise SP. Effects of hand movement path on motor cortical activity in awake, behaving rhesus monkeys. Exp Brain Res 83: 285–302, 1991. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Contrasting neuronal activity in the dorsal and ventral premotor areas during preparation to reach. J Neurophysiol 87: 1123–1128, 2002. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616, 2006. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature 408: 466–470, 2000. [DOI] [PubMed] [Google Scholar]
- Huk AC, Shadlen MN. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J Neurosci 25: 10420–10436, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MT, Coltz JD, Ebner TJ. Encoding of target direction and speed during visual instruction and arm tracking in dorsal premotor and primary motor cortical neurons. Eur J Neurosci 11: 4433–4445, 1999. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud'homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Cerebral cortical mechanisms of reaching movements. Science 255: 1517–1523, 1992. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex 5: 410–425, 1995. [DOI] [PubMed] [Google Scholar]
- Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. J Neurosci 28: 3017–3029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding target selection. J Neurosci 30: 2340–2355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes C, Westendorff S, Chakrabarti S, Gail A. Choosing goals, not rules: deciding among rule-based action plans. Neuron 70: 536–548, 2011. [DOI] [PubMed] [Google Scholar]
- Klaes C, Schneegans Schöner G, Gail A. Sensorimotor learning biases choice behavior: a learning field model for decision making. PLoS Comput Biol 8: e1002774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flügge MC, Bestmann S. Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J Neurosci 32: 8373–8382, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Wang C, Snyder LH. Neuronal responses to target onset in oculomotor and somatomotor parietal circuits differ markedly in a choice task. J Neurophysiol 110: 2247–2256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor cortex of rhesus monkeys: set-related activity during two conditional motor tasks. Exp Brain Res 69: 327–343, 1988a. [DOI] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor and supplementary motor cortex in rhesus monkeys: neuronal activity during externally- and internally-instructed motor tasks. Exp Brain Res 72: 237–248, 1988b. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex 13: 1257–1269, 2003. [DOI] [PubMed] [Google Scholar]
- McKinstry C, Dale R, Spivey MJ. Action dynamics reveal parallel competition in decision making. Psychol Sci 19: 22–24, 2008. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002. [DOI] [PubMed] [Google Scholar]
- Meegan DV, Tipper SP. Reaching into cluttered visual environments: spatial and temporal influences of distracting objects. Q J Exp Psychol A 51: 225–249, 1998. [DOI] [PubMed] [Google Scholar]
- Meister MLR, Hennig JA, Huk AC. Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making. J Neurosci 33: 2254–2267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol 84: 152–165, 2000. [DOI] [PubMed] [Google Scholar]
- Mitchelet T, Duncan GH, Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol 104: 119–127, 2010. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci 11: 1855–1872, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad R, Wallis JD, Miller EK. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci 18: 974–989, 2006. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature 341: 52–54, 1989. [DOI] [PubMed] [Google Scholar]
- Niwa M, Ditterich J. Perceptual decisions between multiple directions of visual motion. J Neurosci 28: 4435–4445, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorani I. LATER models of neural decision behavior in choice tasks (Abstract). Front Integr Neurosci 8: 67, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annu Rev Neurosci 34: 333–359, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neuronal origins of choice variability in economic decisions. Neuron 80: 1322–1336, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis 5: 376–404, 2005. [DOI] [PubMed] [Google Scholar]
- Pastor-Bernier A, Cisek P. Neural correlates of biased competition in premotor cortex. J Neurosci 31: 7083–7088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Bernier A, Tremblay E, Cisek P. Dorsal premotor cortex is involved in switching motor plans (Abstract). Front Neuroeng 5: 5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Schall JD, Logan GD, Palmeri TJ. From salience to saccades: multiple-alternative gated stochastic accumulator model of visual search. J Neurosci 32: 3433–3446, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20: 873–922, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL. A comparison of sequential sampling models for two-choice reaction time. Psychol Rev 111: 333–367, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature 461: 263–266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Beh Brain Res 53: 35–49, 1993. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron 41: 165–173, 2004. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron 30: 583–591, 2001. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 38: 637–548, 2003. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366: 467–469, 1993. [DOI] [PubMed] [Google Scholar]
- Schall JD, Purcell BA, Heitz RP, Logan GD, Palmeri TJ. Neural mechanisms of saccade target selection: gated accumulator model of the visual-motor cascade. Eur J Neurosci 33: 1991–2002, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AB, Moran DW, Reina GA. Differential representation of perception and action in the frontal cortex. Science 303: 380–383, 2004. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Pâquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol 94: 2353–2378, 2005. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Kalaska JF. Changes in the temporal pattern of primary motor cortex activity in a directional isometric force versus limb movement task. J Neurophysiol 80: 1577–1583, 1998. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci 16: 1486–1510, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Dickinson AR, Calton JL. Preparatory delay activity in the monkey parietal reach region predicts reach reaction times. J Neurosci 26: 10091–10099, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, McPeek RM. Roles of narrow- and broad-spiking dorsal premotor area neurons in reach target selection and movement production. J Neurophysiol 103: 2124–2138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. J Vision 6: 982–995, 2006. [DOI] [PubMed] [Google Scholar]
- Song JH, Takahashi N, McPeek RM. Target selection for visually guided reaching in macaque. J Neurophysiol 99: 14–24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bishot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Thura D, Beauregard-Racine J, Fradet CW, Cisek P. Decision making by urgency gating: theory and experimental support. J Neurophysiol 108: 2912–2930, 2012. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81: 1401–1416, 2014. [DOI] [PubMed] [Google Scholar]
- Tsetsos K, Gao J, McClelland JL, Usher M. Using time-varying evidence to test models of decision dynamics: bounded diffusion vs. the leaky competing accumulator model (Abstract). Front Neurosci 6: 79, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90: 1790–1806, 2003. [DOI] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci 2: 1329–1345, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP, Mauritz KH. A neurophysiological study of the premotor cortex in the rhesus monkey. Brain 107: 385–414, 1984. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Elliot D. The effects of response priming on the planning and execution of goal-directed movements in the presence of a distracting stimulus. Acta Psychol 119: 123–142, 2005. [DOI] [PubMed] [Google Scholar]
- Wise SP. The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci 8: 1–19, 1985. [DOI] [PubMed] [Google Scholar]
- Wise SP, di Pellegrino G, Boussaoud D. Primate premotor cortex: dissociation of visuomotor from sensory signals. J Neurophysiol 68: 969–972, 1992. [DOI] [PubMed] [Google Scholar]
- Wise SP, di Pellegrino G, Boussaoud D. The premotor cortex and nonstandard sensorimotor mapping. Can J Physiol Pharmacol 74: 469–482, 1996. [DOI] [PubMed] [Google Scholar]
- Wise SP, Mauritz KH. Set-related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci 223: 331–354, 1985. [DOI] [PubMed] [Google Scholar]
- Wise SP, Weinrich M, Mauritz KH. Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain Res 260: 301–305, 1983. [DOI] [PubMed] [Google Scholar]
- Wong KF, Wang XJ. A recurrent network mechanism of time integration in perceptual decisions. J Neurosci 26: 1314–1328, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DK, Gallivan JP, Chapman CS, Milne JL, Culham JC, Goodale MA. Visual salience dominates early visuomotor competition in reaching behavior. J Vis 11: pii: 16, 2011. [DOI] [PubMed] [Google Scholar]