Abstract
Background
Molecular characterization of cattle breeds is important for the prevention of germplasm erosion by cross breeding. The Indian zebu cattle have their significant role in evolution of present day cattle breeds and development of some of the exotic breeds. Microsatellites are the best available molecular tools for characterization of cattle breeds. The present study was carried out to characterize two Indian cattle breeds, Ongole and Deoni, using microsatellite markers.
Results
Using 5 di- and 5 tri-nucleotide repeat loci, 17 Ongole and 13 Deoni unrelated individuals were studied. Of the ten loci, eight revealed polymorphism in both the breeds. The di-nucleotide repeat loci were found to be more polymorphic (100%) than tri-nucleotide repeat loci (60%). A total of 39 polymorphic alleles were obtained at 4.5 alleles per locus in Ongole and 4.1 in Deoni. The average expected heterozygosity was 0.46 (±0.1) and 0.50 (±0.1) in Ongole and Deoni breeds, respectively. The PIC values of the polymorphic loci ranged from 0.15 to 0.79 in Ongole and 0.13 to 0.80 in Deoni breeds. Six Ongole specific and three Deoni specific alleles were identified. The two breeds showed a moderate genetic relationship between themselves with a FST value of 0.117 (P = 0.01).
Conclusions
This preliminary study shows that microsatellite markers are useful in distinguishing the two zebu breeds namely, Ongole and Deoni. Further studies of other zebu breeds using many microsatellite loci with larger sample sizes can reveal the genetic relationships of Indian breeds.
Background
The Indian cattle breeds, also known as zebu cattle (Bos indicus) are broadly categorized into dairy, draft and dual purpose breeds depending upon their utility either in dairying or in agricultural work. The dual-purpose breeds have specific qualities like disease resistance, heat tolerance, ability to survive and reproduce under stress and low feed input. Ongole and Deoni are the dual-purpose breeds from the Southern part of India. Ongole breed contributed to the development of some of the exotic breeds like 'American Brahman', 'Santa Getrudis' etc. [1] and is used extensively for beef production in Latin American countries [2]. Deoni is yet another breed serving the needs of the people in semi-arid hilly areas. Short calving interval, massive body and fairly well developed udder in cows of this breed indicate its dual-purpose nature.
Zebu cattle are used in cross breeding programs as they can adapt to hot and humid climates [3,4]. However, a number of these breeds are now being bred out because of intensive cross breeding with high milk producing exotic breeds and reduction of emphasis on draft ability due to mechanization of agriculture and transport. As a result, some of the native draft breeds are on the verge of extinction. Hence, there is an urgent need to conserve these breeds. Breed characterization is the primary step in any conservation programme. The accuracy of phenotypic characterization of domestic cattle is often affected by the influence of the environment and the underlying genetic complexity. A number of studies have been initiated to characterize the European cattle breeds using the molecular tools like microsatellite markers [5-9]. Microsatellite markers, by virtue of their codominant and multiallelic nature prove to be efficient in genetic diversity studies, pedigree evaluation and genetic mapping as compared to other molecular markers like RAPD, RFLP and ISSRs [10]. Microsatellites have become markers of choice in characterization of cattle breeds [8,11-13]. Many studies have indicated that the deepest roots of cattle phylogeny occur between Indian cattle and those of Europe [14]. In spite of the evolutionary significance of the Indian cattle breeds, the available literature on characterization of these breeds using reliable molecular markers is scanty. Recently, Kumar et al. [15] carried out admixture analysis of South Asian cattle breeds revealing the influence of Bos taurus in the Indian sub-continent. In the present study, we undertook the characterization of Ongole (n = 17) and Deoni (n = 13) cattle breeds using 5 di- and 5 tri-nucleotide repeat microsatellite markers. The two breeds showed a moderate genetic relationship (FST = 0.117). A few breed-distinguishing alleles were identified, which can be used to differentiate the two breeds.
Results and Discussion
In the present study, genetic polymorphism in the two cattle breeds, Ongole and Deoni were analyzed by using 10 microsatellite markers, which were known to be polymorphic in taurine populations [16-19]. Eight microsatellite markers showed polymorphism while the remaining two markers ARO23 and PZE46 were monomorphic in both the breeds. The polymorphic loci gave a total of 39 alleles in both the breeds, with an average of 4.5 and 4.1 alleles per locus in Ongole and Deoni breeds, respectively. The Food and Agriculture Organization (FAO) suggests that five different alleles per locus are required for estimation of genetic differences between breeds. The mean number of alleles in the present study is almost in accordance with the FAO recommendations. The monomorphic locus ARO23 showed allele size deviation in both the breeds when compared to the published data from the other exotic breeds [16]. Four alleles were reported for this locus with sizes ranging from 96 bp to 105 bp in International Bovine Reference Panel (IBRP) full-sib families. However, in the present study, the same locus revealed a monomorphic allele of 78 bp in both the breeds. If this could turn out to be a zebu specific allele, it would be very useful. However, this could be only confirmed by studies involving other 24 recognized Indian zebu breeds.
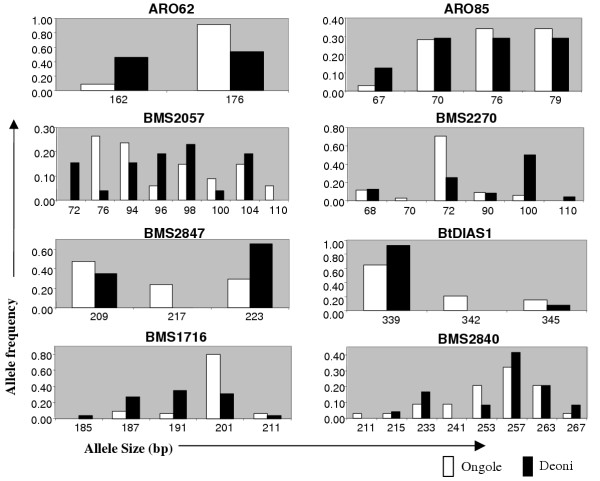
There appears to be no correlation between the number of alleles detected and the number of SSR repeats in the SSR loci used in the study. For example, the microsatellite loci containing the 'GT' repeat motifs varying from (GT)17 to (GT)22 did not show any correlation with the number of alleles they revealed. However, the di-nucleotide repeat loci were more polymorphic (100%) than the tri-nucleotide repeat loci (60%). The two monomorphic loci used in the present study (ARO23 and PZE46) are tri-nucleotide repeat loci. Three alleles specific to Deoni and six alleles specific to Ongole were observed with frequencies ranging from 3 to 24% (Table 1). Further, some alleles were more frequent in one breed than the other (Fig. 1). However, the breed specificity should be confirmed by analysing a large set of individuals from these breeds and also other zebu breeds.
Table 1.
Breed specific alleles in Ongole (n = 17) and Deoni (n = 13) breeds
| Locus | Allele Size (bp) | Frequency | Breed |
| BMS1716 | 185 | 0.04 | Deoni |
| BMS2057 | 72 | 0.15 | Deoni |
| 110 | 0.06 | Ongole | |
| BMS2270 | 70 | 0.03 | Ongole |
| 110 | 0.04 | Deoni | |
| BMS2840 | 211 | 0.03 | Ongole |
| 241 | 0.09 | Ongole | |
| BMS2847 | 217 | 0.24 | Ongole |
| BtDIAS1 | 342 | 0.21 | Ongole |
Figure 1.
Allele frequency distribution of the polymorphic microsatellite loci in Ongole and Deoni breeds
The genetic diversity in the breeds was expressed in terms of average heterozygosity. The average expected heterozygosity was 0.46 (±0.1) in Ongole breed and 0.50 (±0.1) in Deoni breed. Informativeness of the marker was expressed as Polymorphism Information Content (PIC) [20]. The PIC of the polymorphic loci ranged from 0.15 to 0.79 in Ongole breed and 0.13 to 0.80 in Deoni breed (Table 2). The FIS value, which indicates within breed genetic variation, for Ongole and Deoni breeds was 0.36 and 0.18, respectively. This suggests that our sample represented individuals of inbred population of Ongole compared to Deoni. This could also be due to the small sample size and some of the individuals considered as unrelated during collection may indeed share common parentage in the history beyond known pedigree. This is exactly the advantage of using molecular markers in revealing the actual genetic relationships. The overall genetic divergence between Ongole and Deoni, FST was 0.117, which was significant (P = 0.01). This falls in the range of moderate genetic differentiation on a scale defined by Wright [21].
Table 2.
PCR conditions and informativeness of microsatellite loci in two Indian cattle breeds, Ongole and Deoni
| Locus name | Repeat type | Tm (°C) | MgCl2 (mM) | PIC | |
| Ongole | Deoni | ||||
| ARO23 | (AGC)8 | 49 | 1.5 | 0.00 | 0.00 |
| ARO62 | (AGC)7 | 52 | 1.5 | 0.15 | 0.37 |
| ARO85 | (AGC)8 | 52 | 1.0 | 0.62 | 0.68 |
| BMS1716 | (GT)20 | 58 | 1.5 | 0.34 | 0.65 |
| BMS2057 | (GT)17 | 58 | 1.5 | 0.79 | 0.80 |
| BMS2270 | (CA)23 | 58 | 1.5 | 0.45 | 0.62 |
| BMS2840 | (GT)19 | 58 | 1.5 | 0.76 | 0.70 |
| BMS2847 | (GT)22 | 58 | 1.5 | 0.56 | 0.35 |
| BtDIAS1 | (TGC)11 | 56 | 1.0 | 0.46 | 0.13 |
| PZE46 | (CCT)8 | 52 | 1.5 | 0.00 | 0.00 |
Conclusions
The present study is a preliminary investigation on the application of SSR markers for the analysis of diversity and genetic relationships between zebu breeds. It also evaluates the polymorphism using di- and tri-nucleotide repeats in two zebu breeds. We observed a difference in inbreeding in the two breeds, with Deoni breed having less inbreeding than Ongole breed. It was also observed that the dinucleotide repeat SSRs are more polymorphic than tri-nucleotide repeats. Further studies involving large samples including samples from other zebu breeds with FAO recommended microsatellite loci are required to understand the genetic relationships among the Indian breeds.
Methods
Blood samples and DNA isolation
Blood samples were collected from 17 Ongole and 13 Deoni breed individuals maintained at two different livestock research stations managed by the A N G R Agricultural University, Hyderabad, India, in 3 ml Sodium-EDTA vacutainers. The Ongole breed individuals were tracked for 5 generations and Deoni breed for 2 generations to obtain the pedigree and select the individuals that are unrelated. These animals were produced by artificial insemination.
The genomic DNA was isolated and assessed for purity following standard molecular biology protocols [22].
Microsatellite analysis
Five di-nucleotide markers, BMS1716, BMS2057, BMS2270, BMS2840 and BMS2847 [17] and five tri-nucleotide markers, ARO23, ARO62 and ARO85 [16]; BtDIAS1 [18] and PZE46 [19] were employed in the present study. For the loci ARO62, BtDIAS1 and PZE46, primers were designed using the Amplify 1.2 program [23] as,
ARO62
F: CAGACACAACTGAAGCAACTC
R: GTAGATTCCATAACAGC
BtDIAS1
F: GTAGCATCTTAATAATGCCCTC
R: ACCCCACTCCAGCACTTTTG
PZE46
F: TTATGGCGGCTCCATATTAAC
R: GTAACTCGGGCCCTTTCTCC
For the rest, primer sequence information was obtained from GenBank [24].
PCR conditions were empirically determined (Table 2). Twenty nanograms of genomic DNA was used as template in a 10 μl PCR reaction consisting of 5 picomoles of each primers, 1 to 1.5 mM MgCl2 (MBI Fermentas), 1 μl of 10 × PCR buffer (750 mM Tris-HCl (pH 8.8 at 25°C), 200 mM (NH4)2SO4, 0.1% Tween-20), 100 μM dNTPs, 0.5 U Taq polymerase (MBI Fermentas), in Genamp 9600™ thermalcycler (Perkin Elmer). The PCR cycling conditions used were: 96°C for 1 min. (Initial Denaturation), 94°C for 30 sec. (Denaturation), 49°C–58°C for 30 sec. (Annealing), 72°C for 1 min 30 sec. (Extension) and 72°C for 10 min. (Final extension). The PCR samples were electrophoresed on 3.5% Metaphor™ agarose (BMN) gel with 1 kb plus (Invitrogen) and pUC19 DNA/MspI marker (MBI Fermentas) DNA size markers. To confirm the number of alleles and to determine the allele size, Fluorescence based SSR analysis [25] was carried out. Denatured amplified samples were separated on a 5% denaturing polyacrylamide gel containing 7 M Urea after adding 2 μl of formamide gel loading buffer and 0.3 μl of ROX-500™ Genescan ruler (Perkin Elmer).
Data analysis
The allele size variation on the Metaphor Agarose gels was studied using Quantity One software (Bio Rad). The Genescan gels were analyzed using GeneScan 3.1 and Genotyper 2.1 software. The individuals are genotyped based on allele size data. Allele frequency and heterozygosity were calculated using MS Tools v3 [26]. F-statistics were used as a measure of diversity within and between breeds respectively and were estimated using the F-STAT program [27].
The PIC was calculated by using the formula [20]:
![]()
where Pi and Pj are frequencies of ith and jth alleles.
List of Abbreviations
PIC: Polymorphism Information Content; FIS and FST: F-Statistics indices; FAO: Food and Agriculture Organization; IBRP: International Bovine Reference Panel; SSRs: Simple Sequence Repeats; n = number of individuals;
Authors' contributions
MM carried out DNA extraction, PCR assays, electrophoretic analysis, data collection and preparation of the primary manuscript. SK did the data analysis, GN carried out breed collection and JN conceived the study, participated in its design and coordination and revision of the manuscript.
Acknowledgments
Acknowledgements
We thank Dr B R Gupta and Dr K Babu Rao for providing the blood samples of Ongole and Deoni breeds. This work was supported by CDFD core grant and ICAR developmental grants.
Contributor Information
Muralidhar Metta, Email: muralidhar_metta@yahoo.com.
Sriramana Kanginakudru, Email: sriramana@cdfd.org.in.
Narasimharao Gudiseva, Email: g_narasimharao@hotmail.com.
Javaregowda Nagaraju, Email: jnagaraju@cdfd.org.in.
References
- Taneja VK. Dairy breeds and Selection. In: Falvey L, Chantalakhana C, editor. In Smallholder dairying in the tropics. International Livestock Research Institute, Nairobi, Kenya; 1999. p. 74. [Google Scholar]
- Beja-Pereira A, Alexandrino P, Bessa I, Carretero Y, Dunner S, Ferrand N, Jordana J, Laloe D, Moazami-Goudarzi K, Sanchez A, Canon J. Genetic characterization of south-western European bovine breeds: a historical and biogeographical reassessment with a set of 16 microsatellites. J Hered. 2003;94:243–250. doi: 10.1093/jhered/esg055. [DOI] [PubMed] [Google Scholar]
- Koger M. Effective crossbreeding systems utilizing zebu cattle. J Anim Sci. 1980;50:1215–1220. doi: 10.2527/jas1980.5061215x. [DOI] [PubMed] [Google Scholar]
- Turner JW. Genetic and biological aspects of zebu adaptability. J Anim Sci. 1980;50:1201–1205. doi: 10.2527/jas1980.5061201x. [DOI] [PubMed] [Google Scholar]
- Kantanen J, Olsaker I, Holm LE, Lien S, Vilkki J, Brusgaard K, Eythorsdottir E, Danell B, Adalsteinsson S. Genetic diversity and population structure of 20 North European cattle breeds. J Hered. 2000;91:446–457. doi: 10.1093/jhered/91.6.446. [DOI] [PubMed] [Google Scholar]
- Ciampolini R, Moazami-Goudarzi K, Vaiman D, Dillmann C, Mazzanti E, Foulley JL, Leveziel H, Cianci D. Individual multilocus genotypes using microsatellite polymorphisms to permit the analysis of the genetic variability within and between Italian beef cattle breeds. J Anim Sci. 1995;73:3259–3268. doi: 10.2527/1995.73113259x. [DOI] [PubMed] [Google Scholar]
- Peelman LJ, Mortiaux F, Van Zeveren A, Dansercoer A, Mommens G, Coopman F, Bouquet Y, Burny A, Renaville R, Portetelle D. Evaluation of the genetic variability of 23 bovine microsatellite markers in four Belgian cattle breeds. Anim Genet. 1998;29:161–167. doi: 10.1046/j.1365-2052.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Canon J, Alexandrino P, Bessa I, Carleos C, Carretero Y, Dunner S, Ferran N, Garcia D, Jordana J, Laloe D, Pereira A, Sanchez A, Moazami-Goudarzi K. Genetic diversity measures of local European beef cattle breeds for conservation purposes. Genet Sel Evol. 2001;33:311–332. doi: 10.1051/gse:2001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHugh DE, Loftus RT, Cunningham P, Bradley DG. Genetic structure of seven European cattle breeds assessed using 20 microsatellite markers. Anim Genet. 1998;29:333–340. doi: 10.1046/j.1365-2052.1998.295330.x. [DOI] [PubMed] [Google Scholar]
- Nagaraju J, Reddy KD, Nagaraja GM, Sethuraman BN. Comparison of multilocus RFLPs and PCR-based marker systems for genetic analysis of the silkworm Bombyx mori. Heredity. 2001;86:588–597. doi: 10.1046/j.1365-2540.2001.00861.x. [DOI] [PubMed] [Google Scholar]
- Edwards C, Bradley DG, MacHugh DE. A panel of Y-specific microsatellite markers suitable for studies of genetic differentiation in cattle and related species. Anim Genet. 2000;31:127–130. doi: 10.1046/j.1365-2052.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- Loftus RT, Ertugrul O, Harba AH, El-Barody MA, MacHugh DE, Park SD, Bradley DG. A microsatellite survey of cattle from a centre of origin: the Near East. Mol Ecol. 1999;8:2015–2022. doi: 10.1046/j.1365-294x.1999.00805.x. [DOI] [PubMed] [Google Scholar]
- MacHugh DE, Shriver MD, Loftus RT, Cunningham P, Bradley DG. Microsatellite DNA variation and the evolution domestication and phylogeography of taurine and zebu cattle (Bos taurus and Bos indicus) Genetics. 1997;146:1071–1086. doi: 10.1093/genetics/146.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DG, Loftus RT, Cunningham P, MacHugh DE. Genetics and domestic cattle origins. Evolutionary Anthropology. 1998;6:79–86. doi: 10.1002/(SICI)1520-6505(1998)6:3<79::AID-EVAN2>3.3.CO;2-2. [DOI] [Google Scholar]
- Kumar P, Freeman AR, Loftus RT, Gaillard C, Fuller DQ, Bradley DG. Admixture analysis of South Asian cattle. Heridity. 2003;91:43–50. doi: 10.1038/sj.hdy.6800277. [DOI] [PubMed] [Google Scholar]
- Band M, Ron M. Creation of a SINE enriched library for the isolation of polymorphic (AGC)n microsatellite markers in the bovine genome. Anim Genet. 1996;27:243–248. doi: 10.1111/j.1365-2052.1996.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Stone RT, Kappes SM, Keele JW, Beattie CW. Characterization of 109 bovine microsatellites. Anim Genet. 1997;28:63–65. [PubMed] [Google Scholar]
- Shukri NM, Holm LE, Brusgaard K. A polymorphic trinucleotide microsatellite in cattle: BtDIAS1. Anim Genet. 1996;27:373. doi: 10.1111/j.1365-2052.1996.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Motavalian M, Rando A, Urciuoli M, Senese C, Di Gregorio P, Masina P. PZE46 and PZE114: two bovine polymorphic microsatellite loci isolated from a placenta cDNA library. Anim Genet. 2002;33:159–160. doi: 10.1046/j.1365-2052.2002.0831b.x. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Wright S. Evaluation and the genetics of Populations Variability within and among Natural Populations. Vol. 4. University of Chicago Press, Chicago; 1978. [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York; 2001. [Google Scholar]
- Engels WR. Contributing software to the Internet: the Amplify program. Trends Biochem Sci. 1993;18:448–450. doi: 10.1016/0968-0004(93)90148-G. http://engels.genetics.wisc.edu/amplify/ [DOI] [PubMed] [Google Scholar]
- GenBank database http://www.ncbi.nlm.nih.gov/
- Nagaraju J, Kathirvel M, Ramesh Kumar R, Siddiq EA, Hasnain SE. Genetic analysis of traditional and evolved Basmati and non-Basmati rice varieties by using fluorescence-based ISSR-PCR and SSR markers. Proc Natl Acad Sci U S A. 2002;99:5836–5841. doi: 10.1073/pnas.042099099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SDE. Trypanotolerance in West African Cattle and the Population Genetic Effects of Selection [Ph.D. thesis] University of Dublin for "The Excel Microsatellite Tool Kit". 2001. http://oscar.gen.tcd.ie/~sdepark/ms-toolkit/
- Goudet J. FSTAT (Version 1.2): A computer program to calculate F-statistics. J Hered. 1995;86:485–486. http://www2.unil.ch/izea/softwares/fstat.html [Google Scholar]