Abstract
Hesperiidae is one of the largest families of butterflies. Our knowledge of the higher systematics on hesperiids from China is still very limited. We infer the phylogenetic relationships of the subfamilies of Chinese skippers based on three mitochondrial genes (cytochrome b (Cytb), the NADH dehydrogenase subunit 1 (ND1) and cytochrome oxidase I (COI)). In this study, 30 species in 23 genera were included in the Bayesian and maximum likelihood analyses. The subfamily Coeliadinae, Eudaminae, Pyrginae and Heteropterinae were recovered as a monophyletic clade with strong support. The subfamily Hesperiinae formed a clade, but support for monophyly was weak. Our results imply that the five subfamilies of Chinese Hesperiidae should be divided into: Coeliadinae, Eudaminae, Pyrginae, Heteropterinae and Hesperiinae. The relationships of the five subfamilies should be as follows: Coeliadinae + (Eudaminae + (Pyrginae + (Heteropterinae + Hesperiinae))).
Reconstruction of the phylogenetic relationship of organisms plays an essential role in better understanding their evolution and diversification1. Lepidoptera, as the second largest order of insects with more than 157,000 species, are of particular interest in systematic research2,3. The skipper butterfly (Hesperiidae) which include around 4000 species is one of the most diverse groups of butterflies4,5. Although Hesperidae has been well defined, historically there exists disagreement at the subfamily and tribe levels.
The higher-level classification of Hesperiidae was established in the late 19th century. Watson divided Hesperiidae into three subfamilies (Pyrrhopyginae, Hesperiinae and Pamphilinae) based on the morphological characteristics of 201 genera6. The family was further arranged into six subfamilies by Evans7. Evans placed 130 genera in 4 subfamilies and 13 generic groups (equivalent to the current tribes), which shaped the current system for higher-level classification and interrelationships of the Hesperiidae. Studies on more detailed morphological characteristics have further advanced our knowledge important for classification and construction of phylogenetic relationships of the members in Hesperiidae8,9,10. Chou11,12 proposed three families in Hesperioidea (Euschemonidae, Megathymidae and Hesperiidae) and added three subfamilies (Coediadinae, Pyrginae and Hesperiinae) of Hesperiidae identified in China.
With information from molecular systematics studies in the past two decades, Warren et al.13 proposed the recent classification of Hesperiidae, to include five subfamilies: Coeliadinae, Pyrginae, Heteropterinae, Trapezitinae and Hesperiinae. With combined molecular and morphological data, Warren et al.14 subsequently revised the classification of Hesperiidae to include seven subfamilies: Coeliadinae, Euschemoninae, Eudaminae, Pyrginae, Heteropterinae, Trapezitinae and Hesperiinae. Warren’s molecular phylogeny included approximately 200 genera, representing about 35% of the skipper genera in the world. The skipper butterflies distributed in the Palaearctic and Oriental fauna were only partially covered. Less than half of skipper butterfly genera distributed in China are not known. The family Hesperiidae contains approximately 370 described species in 83 genera in China15 and so far there has been no molecular study of the higher-level phylogeny of the Chinese skipper butterflies. Mitochondrial DNA (mt DNA) sequence has been widely used in phylogenetic studies of Lepidoptera16,17,18. In this study, we used DNA sequences from the mitochondrial cytochrome b (Cytb), the NADH dehydrogenase subunit 1 (ND1) and cytochrome oxidase I (COI) to analyze the phylogenetic relationships of the genera in Hesperiidae.
Materials and methods
Taxon sampling
The butterflies studied were either collected with aerial nets in the field or were specimens in the Entomological Museum of Northwest A&F University in China. The specimens sampled and their collection site are listed in Appendix 1. A total of 30 skipper butterfly species in 23 genera were used in this study. In addition, five outgroup species, Papilio protenor, Troides helena, Sericinus montelus (Papilionidae), Eurema andersoni, Pontia edusa (Pieridae), from the Papilionoidea, the putative sister clade to the Hesperioidea19, were collected and used in this study.
DNA extraction, PCR amplification and DNA sequencing
Total genomic DNA was extracted from a pair of legs of an adult specimen either dried or preserved in 95% ethanol, using the phenol-chloroform extraction protocol20,21. The genomic DNA prepared was dissolved in a 50 μL TE buffer and stored in a freezer (−20 °C).
PCR reactions were prepared in 50 μL that included 5 μL 10 × reaction buffer, 2.5 mM Mg2+, 0.6 mM primers, 4 μL of DNA template, 0.25 mM dNTPs and 1.0 U Taq polymerase. For amplification of the fragment from Cytb, the PCR amplification was performed by an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 1 min at 94 °C, 1 min at 50 °C and 2 min at 72 °C, and a final extension at 72 °C for 10 min. For amplification of the fragment from ND1, the PCR cycles included 0.5 min at 94 °C, 0.7 min at 49 °C and 2 min at 72 °C. For amplification of the fragment from COI, the PCR cycles included 0.5 min at 94 °C, 0.7 min at 50 °C and 0.5 min at 72 °C. All primers used in this study were listed in Table 1. The PCR products of the PCR reactions from individual specimens were examined by agarose gel electrophoresis to verify the specific amplification of the desired fragments and the PCR products were sequenced for strands by commercial service (GeneScript Biological Technology, Nanjing, China, and Aoke Biological Technology, Beijing, China).
Table 1. Primers for PCR amplification ofgenes used in this study.
| Gene | Primer name | Sequence (5′-3′) | references |
|---|---|---|---|
| Cytb | CB-J-10933 | TATGTACTACCATGAGGACAAATATA | Simon et al.22 |
| CB-N-11367 | ATTACACCTCCTAATTTATTAGGAAT | ||
| ND1 | 3264-J-12095 | ATCAAAAGGAGCTCGATTAGTTTC | Aubert et al.23 |
| 1957-N-12567 | CGTAAAGTCCTAGGTTATATTCAGATTCG | ||
| COI | LCO1490-J-1514 | GGTCAACAAATCATAAAGATATTGG | Folmer et al.24 |
| HCO2198-N-2175 | TAAACTTCAGGGTGACCAAAAAATCA |
Phylogenetic analysis
The DNA sequences from the individuals were aligned using MAFFT v7.03725, and the parsimony informative sites, base frequencies and Kimura-2-parameter distances (K2P distance) were calculated using MEGA v5.0526. The alignment was evaluated by substitution saturation using DAMBE v5.3.7427,28. The combined sequence datasets of Cytb, ND1 and COI were used to construct phylogenetic trees.
Phylogenetic analysis by maximum likelihood (ML) model was conducted using jModelTest v 2.1.429 using the Akaike information criterion (AICc). The best-fitting model of nucleotide substitution was GTR + I + G for all genes, and the general ratchet analysis conditions were as following: Lset base = (0.3552 0.0751 0.0866), nst = 6, rmat = (2.7601 10.2085 4.5566 7.6424 44.6148), rates = gamma, shape = 0.4670, ncat = 4, pinvar = 0.3640. PAUP* v4.0b1030 was used to calculate the ML analyses with 1000 bootstraps. Bayesian inference (BI) analysis was run in MrBayes 3.1.231 using the model generated in jModelTest. The partitioned analysis comprised two runs with four Markov chain Monte Carlo simulations (MCMC) each, with flat priors, dataset partitioned by one million generations, sampling every 100 generations with 25% of samples discarded as burn-in.
Results
Sequence characterization
Alignment of the combined PCR fragment sequences from Cytb, ND1 and COI showed that in the 1458 bp combined DNA sequences there were 717 variable sites and 568 parsimony-informative characters. The base composition of the fragments showed a strong bias of A + T (Table 2) as is commonly found in insect mitochondrial genomes22. The results of the substitution saturation test showed that the index of substitution saturation (Iss) was significantly lower than the critical value of the index of substitution saturation (Iss.c).
Table 2. Summary of number of taxa and characters for the three gene regions.
| Gene region | Number of sequences | Alignment length | A (%) | T (%) | C (%) | G (%) | Variable sites | Parsimony informative sites | Iss values | Iss.c values |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytb | 34 | 408 | 30.6 | 43.5 | 16.6 | 9.4 | 215 | 182 | 0.373 | 0.692 |
| ND1 | 34 | 446 | 30.7 | 49.0 | 8.3 | 12.0 | 260 | 193 | 0.400 | 0.696 |
| COI | 30 | 604 | 30.7 | 39.7 | 16.0 | 13.7 | 242 | 193 | 0.373 | 0.692 |
| Combined | 35 | 1458 | 30.6 | 43.8 | 13.7 | 11.8 | 717 | 568 |
Genetic distances
Calculation of the K2P distances between different species showed that they ranged from 0.1% (Lobocla bifasciata/Lobocla liliana) to 27.8% (Eurema andersoni/Pontia edusa) with an average genetic distance of 17.2%. The mean out- and in-group distance was 19.6% with a range of a minimal value of 13.8% (Sericinus montelus/Choaspes hemixantha) to maximal values of 25.2% (Eurema andersoni/Daimio tethys; Eurema andersoni/Carterocephalus argyrostigma). The mean in-group distance was 16.2% with a minimal value of 0.1% (Lobocla bifasciata/Lobocla liliana) and a maximum value of 22.4% (Carterocephalus urasimataro/Satarupa nymphalis). The mean distance was 14.6% (max. 18.8%) in the subfamily Hesperiinae and 15% (max. 17.8%) in Pyrginae.
Phylogenetic relationships
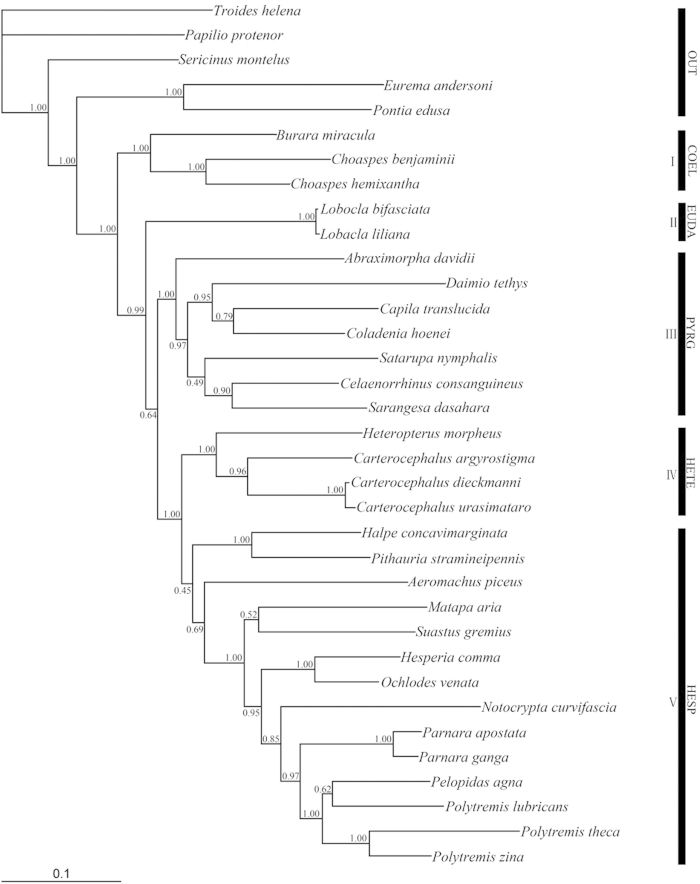
The phylogenetic trees generated from the DNA sequence dataset by BI and ML methods trees are nearly identical in major clades and patterns of branching recovered. In BI analysis (Fig. 1), five of seven currently subfamilies of Hesperiidae were recovered as monophyletic clades with the following relationships: Coeliadinae + (Eudaminae + (Pyrginae + (Heteropterinae + Hesperiinae))).
Figure 1. BI tree based on combined data of Cytb, ND1 and COI partial gene sequences.
Values above the branches indicate clade posterior probabilities. OUT, Outgroup; COEL, Coeliadinae; EUDA, Eudaminae; PYRG, Pyrginae; HETE, Heteropterinae; HESP, Hesperiinae.
The subfamily Coeliadinae (Clade I) was recovered as a monophyletic clade with strong support, although there were only three taxa (Burara miracula, Choaspes benjaminii and Choaspes hemixantha) included, and was placed in the basal position as the sister to the rest of the clades of the Hesperiidae. Although only two taxa within geneus Lobocla were included in our analysis from Eudaminae, its monophyly (Clade II) received strong support. The seven genera from Pyrginae formed a clade (Clade III) also with strong support. Furthermore, this clade split into three subclades: Abraximorpha + ((Daimio + (Capila + Coladenia)) + (Satarupa + (Celaenorrhinus + Sarangesa))).
The subfamily Heteropterinae (Clade IV) was monophyletic and strongly supported, although only two genera, Heteropterus and Carterocephalus, from this group were included in our analysis. In this clade, the geneus Carterocephalus included C. argyrostigma, C. dieckmanni and C. urasimataro, which were also recovered as a monophyletic group with strong support. As sister to Heteropterinae, the eleven genera from the Hesperiinae (Halpe, Pithauria, Aeromachus, Matapa, Suastus, Hesperia, Ochlodes, Notocrypta, Parnara, Pelopidas and Polytremis) appeared to form a clade (Clade V), but support for their monophyly is weak (<0.50). Within the Hesperiinae, the monophyly of Baorini (Parnara, Pelopidas and Polytremis), Ancistroidini (Notocrypta), Hesperiini (Hesperia, Ochlodes) and Isoteinonini (Matapa, Suastus) were recovered with strong or moderate support. However the Aeromacini (Halpe, Pithauria, Aeromachus) were not recovered as a monophyletic group.
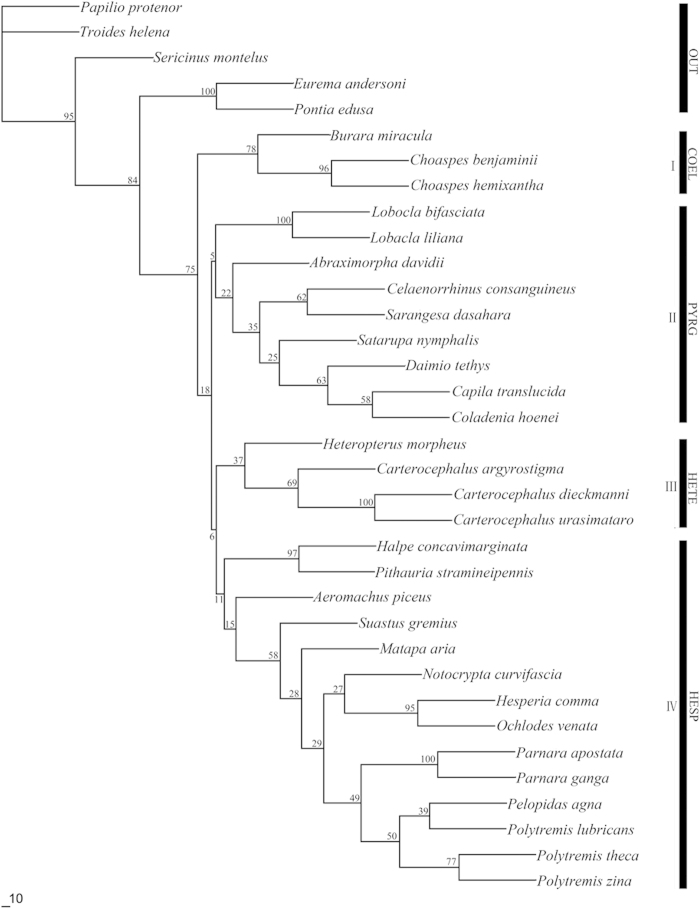
The phylogenetic tree by ML analysis showed that four major clades of Hesperiidae were recovered, although the relationships between some nodes were not strongly supported (<50) (Fig. 2). Compared with the topology of the tree by BI method, the major difference in the tree by ML is that the genus Lobocla was placed into the Pyrginae group (Clade II in Fig. 2), but the support was weak.
Figure 2. ML tree based on combined data of Cytb, ND1 and COI partial gene sequences.
Values above the branches indicate clade bootstrap support. OUT, Outgroup; COEL, Coeliadinae; PYRG, Pyrginae; HETE, Heteropterinae; HESP, Hesperiinae.
Discussion
In the family Hesperiidae, Coeliadinae with morphological synapomorphies is relatively unique and easy to be distinguished from the remaining subfamilies5,7. The five genera (Bibasis, Burara, Hasora, Badamia, Choaspes) in this subfamily are distributed in China. In this phylogenetic study with two of these genera, the monophyly of Coeliadinae and its status as the sister of the rest of the Hesperiidae were confirmed, which is consistent with previous studies based on morphological and molecular data13,14,32,33. The morphological synapomorphy for Coeliadinae is the 3rd segment of labial palpi which is long, slender, cylindrical or awl-like14. Larvae generally feed on the plants of the class Dicotyledonopsida in China15.
The genus Lobocla was placed in the Celaenorrhinus group by Evans7; while, Warren et al.13 assigned it to the tribe Eudamini, which was then promoted to the subfamily of Eudaminae14. The result from the ML analysis of the mitochondrial DNA sequences in this study placed Lobocla in the subfamily of Pyrginae with weak support (Fig. 2). However, Lobocla became separated from the Pyrginae and formed an independent clade by BI analysis (Fig. 1). Given the higher value of posterior probabilities, we support the status of Eudaminae and that Lobocla as the only genus of Eudaminae occurring in the Oriental, Neotropical and Nearctic regions.
The subfamily Pyrginae has long been treated as a paraphyletic group5,7,13,32. Warren et al.14 recovered the monophyly of Pyrginae with moderate support. Pyrginae has been divided into seven tribes (Pyrrhopygini, Achlyodini, Tagiadini, Celaenorrhinini, Carcharodini, Erynnini and Pyrgini), but no morphological synapomorphies have been known for the subfamily. In this study, seven genera were included in the analysis and they appeared to form a monophyletic group with moderate support. However, the status of many other tribes and genera and their relationships within Pyrginae (e.g., Caprona, Mooreana, Muschampia in China) remain unknown. Additional taxa with additional molecular makers will be needed to elucidate their phylogenetic positions at the level of tribe and genera. Morphological characters for this subfamily are the 3rd segment of labial palpi which is short and stout, and that the larvae generally feed on plants of the class Dicotyledonopsida in China15.
The monophyly of the subfamily Heteropterinae was recovered with strong support (PP = 1.00) in the BI analysis (Fig. 1), which is consistent with the Warren et al.14 study. Heteropterinae is grouped under two tribes (Heteropterini and Carterocephalini), but morphological synapomorphies could be difficult to identify. Morphological characters for Heteropterinae are that the abdomens are distinctly elongated, longer than the length of the hindwing dorsum. Female bursa copulatrix has an appendix bursa. Larvae feed on plants of the class Monocotyledonopsida in China15. Within this subfamily, our results indicate that the genus Carterocephalus is a monophyletic clade in both analyses (PP = 0.96, BS = 69). However, the taxonomic status of this group remains to be resolved.
The subfamily Hesperiinae, as the largest subfamily of Hesperiidae, has long been a controversial subfamily in the Hesperiidae. The monophyly Hesperiinae has been reasserted13,14 and is also supported by the analysis in this study. Evans7 split the subfamily Hesperiinae into eight groups, and Inoué and Kawazoé34 reviewed Evans’s system, i.e., defining the Halpe group to include Evans’s Astictopterus group except for the genus Astictopterus. Chou11,12 divided the Chinese Hesperiinae into ten tribes based on Evans’s classification system. Warren et al.14 reviewed and recognized eight tribes of Hesperiinae (Aeromachini, Baorini, Taractrocerini, Thymelicini, Calpodini, Anthoptini, Moncini, Hesperiini). The results form this study support the monophyly of Baorini and Hesperiini. However, the classification status of other tribes has yet to be established with more taxa to be added to the phylogenetic analysis. The morphological character for Hesperiinae is the terminal part of lower margin of discal cell in hindwing which is oblique upwards. Larvae of this subfamily generally feed on plants of the class Monocotyledonopsida in China15.
In this comprehensive phylogenetic analysis of Hesperiidae members from China at subfamily-level with 30 species in 23 genera, the monophyly of this family was demonstrated with strong support. This result is in agreement with the previous reports by Wahlberg et al.19 and Warren et al.13,14, although higher level phylogenetic relationships remain challenging to decipher in Lepidoptera3. With strong posterior probability values, the results from BI analysis (Fig. 1) imply that the five subfamilies of Chinese Hesperiidae are under Coeliadinae, Eudaminae, Pyrginae, Heteropterinae and Hesperiinae. The relationships of the five subfamilies are Coeliadinae + (Eudaminae + (Pyrginae + (Heteropterinae + Hesperiinae))).
Additional Information
How to cite this article: Xiangqun, Y. et al. Phylogenetic relationships of subfamilies in the family Hesperiidae (Lepidoptera: Hesperioidea) from China. Sci. Rep. 5, 11140; doi: 10.1038/srep11140 (2015).
Supplementary Material
Acknowledgments
We are grateful to Dr. J. R. Schrock, Emporia State University, Kansas, USA for reviewing the manuscript. This study is supported by the National Natural Science Foundation of China (31272345, 31071693), The Ministry of Science and Technology of China (2011FY120200), the Fundamental Research Funds for the Central Universities (QN2011093) and the Chinese Scholarship Council Funds.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.X.Q. and Z.Y.L. designed and performed the research; G.K. and Y.F. analyzed the phylogenetic trees; G.K. and Y.X.Q. wrote the manuscript; Y.F. and W.P. revised the paper.
References
- Trautwein M. D., Wiegrmann B. M., Beutel R., Kjer K. M. & Yeates D. K. Advances in insect phylogeny at the dawn of the postgenomic era. Annu. Rev. Entomol. 57, 449–468 (2012). [DOI] [PubMed] [Google Scholar]
- Wahlberg N., Weingartner E. & Nylin S. Towards a better understanding of the higher systematics of Nymphalidae (Lepidoptera: Papilionoidea). Mol. Phylogenet. Evol. 28, 473–484 (2003). [DOI] [PubMed] [Google Scholar]
- Regier J. C. et al. A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS One 8, 1–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. A. Catalogue of the Family-Group, Genus-Group and Species-Group Names of the Hesperiidae (Lepidoptera) of the World (Urbana, 1994). [Google Scholar]
- Ackery P. R., De Jong R. & Vane-Wright R. I. in Lepidoptera: Moths and Butterflies, Vol. 1 (eds Kristensen N. P. ), Ch.16, 263–300 (Walter de Gruyter, 1999). [Google Scholar]
- Watson E. Y. A proposed classification of the Hesperiidae, with a revision of the genera. Proc. Zool. Soc. London 1, 3–132 (1893). [Google Scholar]
- Evans W. H. A Catalogue of the Hesperiidae from Europe, Asia, and Australia in the British Museum (Natural History) (British Museum, 1949). [Google Scholar]
- Ackery P. R. in The Biology of Butterflies. (eds. Vane-Wright R. I. & Ackery P. R. ) 9–21 (Academic Press, 1984). [Google Scholar]
- Scott J. A. The phylogeny of butterflies (Papilionoidea and Hesperioidea). J. Res. Lepidoptera 23, 241–281 (1985). [Google Scholar]
- Scott J. A. & Wright D. M. in Butterflies of Europe (eds Kudrna O. ) (Aula-Verlag, 1990). [Google Scholar]
- Chou I. Monographia Rhopalocerorum Sinensium. (Henan Scientific and Technological Publishing House, 1994). [Google Scholar]
- Chou I. Classification and Identification of Chinese Butterflies. (Henan Scientific and Technological Publishing House, 1999). [Google Scholar]
- Warren A. D., Ogawa J. R. & Brower A. V. Z. Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics 24, 1–35 (2008). [Google Scholar]
- Warren A. D., Ogawa J. R. & Brower A. V. Z. Revised classification of the family Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Syst. Entomol. 34, 467–525 (2009). [Google Scholar]
- Yuan F., Yuan X. Q. & Xue G. X. Fauna Sinica (Insecta: Lepidoptera: Hesperiidae) (Science Press, 2015). [Google Scholar]
- Brower V. Z. A. Phylogeny of Heliconius butterflies inferred from mitochondrial DNA sequences (Lepidoptera: Nymphalidae). Mol. Biol. Evol. 3, 159–174 (1994). [DOI] [PubMed] [Google Scholar]
- Caterino M. S., Chol S. & Sperling F. A. H. The current state of insect molecular systematics: a thriving Tower of Babel. Annu. Rev. Entomol. 45, 1–54 (2000). [DOI] [PubMed] [Google Scholar]
- Morinaka S., Miyata T. & Tanaka K. Molecular phylogeny of the Eichhorni group of Delias Hübner, 1819 (Lepidoptera: Pieridae). Mol. Phylogenet. Evol. 23, 267–287 (2002). [DOI] [PubMed] [Google Scholar]
- Wahlberg N. et al. Synergistic effects of combining morphological and molecular data in resolving the phylogeny of butterflies and skippers. Proc. R. Soc. B 272, 1577–1586 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M., Wahlberg N. & Descimon H. Phylogeny of Euphydryas checkerspot butterflies (Lepidoptera: Nymphalidae) based on mitochondrial DNA sequences data. Ann. Entomol. Soc. Amer. 93, 347–355 (2000). [Google Scholar]
- Wang R. J., Wan H., Long Y., Lei G. C. & Li S. W. Phylogenetic analysis of Polyura in China inferred from mitochondrial COII sequences (Lepidoptera: Nymphalidae). Acta Entomol. Sin. 47, 243–247 (2004). [Google Scholar]
- Simon C. et al. Evolution, weighting, and phylogenetic utility of mitochondrial polymerase chain reaction primers. Ann. Entomol. Soc. Amer. 87, 651–701 (1994). [Google Scholar]
- Aubert J., Barascud B., Descimon H. & Michel F. Systématique moléculaire des Argynnis (Lepidoptera: Nymphalidae). C. R. Acad. Sci. 319, 647–651 (1996). [Google Scholar]
- Folmer O., Black M. B., Hoch W., Lutz R. A. & Vrijehock R. C. DNA primers for amplification of mitochondrial cytochrome coxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Bio. Biotechnol. 3, 294–299 (1994). [PubMed] [Google Scholar]
- Katoh K. & Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. & Lemey P. In The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny, 2nd edn. (eds. Salemi M., & Vandamme A. M. ), 615–630 (Cambridge University Press, 2009). [Google Scholar]
- Xia X. & Xie Z. DAMBE: data analysis in molecular biology and evolution. J. Hered. 92, 371–373 (2001). [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R. & Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. L. PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4. (Sinauer Associates, 2003). [Google Scholar]
- Huelsenbeck J. P. & Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinfor. 17, 754–755 (2001). [DOI] [PubMed] [Google Scholar]
- Dodo Y. T. et al. Molecular phylogeny of Japanese skippers (Lepidoptera, Hesperiidae) based on mitochondrial ND5 and COI gene sequences. Trans. Lep. Soc. Jap. 59, 29–41 (2008). [Google Scholar]
- Xue G. X. Taxonomy and Phylogeny of Hesperiidae (Lepidoptera: Hesperioidea) from China. (Dissertation for Doctoral Degree, 2009). [Google Scholar]
- Inoué S. & Kawazoé A. Hesperiid Butterflies from South Vietnam. Tyô to Ga 16, 84–103 (1966). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.