Abstract
Background:
Rhamnus alaternus (Rhamnaceae) L. has been traditionally used for treatment of many diseases.
Objective:
In this study, we determined the antioxidant/free radical scavenger properties, the flavonoid profile and the cytotoxicity of aqueous and methanolic extracts obtained by maceration from Algerian R. alaternus bark, like also of aqueous extract prepared by decoction according to the traditional method. This to estimate the usefulness of the drug traditional preparation and compare it with those made in the laboratory.
Materials and Methods:
The antioxidant activity of the extracts was evaluated using five different redox-based assays, all involving one redox reaction with the oxidant. High-performance liquid chromatography/diode array detection/electrospray ionization mass spectrometry analysis was used to identify and quantify the flavonoids content. Cytotoxicity on human monocytic leukemia cells (U937) was also carried out.
Results:
All the extracts tested showed a good antioxidant/free radical scavenger activity and a similar flavonoid fingerprint. However, the methanolic one presented the best antioxidant activity that can be due to the highest flavonoid amount and significantly reduced the proliferation of leukemia cells. The results confirm that the extract prepared by decoction contains efficient antioxidant compounds and this justifies in part the therapeutic and preventive usefulness. Moreover, the methanolic extract exerted excellent cytotoxicity on U937 that could be attributed to kaempferol and rhamnocitrin glycosides.
Keywords: Antioxidant activity, cytotoxicity, folk medicine, Rhamnus alaternus L, U937 cancer cells
INTRODUCTION
The genus Rhamnus (Rhamnaceae) is widely used in folk medicine[1] and the decoction of aerial parts of Rhamnus alaternus L., are used for the treatment of some hepatic disease.[2]
Rhamnus alaternus leaves contain many active compounds,[3,4,5] and experimental studies have demonstrated that extracts of R. alaternus leaves possess different biological activities.[6,7,8,9,10,11,12,13,14,15]
The purpose of the present paper was to characterize the flavonoid profile, the antioxidant properties, and the cytotoxic activity of two extracts obtained from R. algerian alaternus bark by maceration in water or in methanol, in comparison with that of an aqueous extract obtained by decoction according to the traditional method used in Algeria.
MATERIALS AND METHODS
Chemicals
Folin-Ciocalteau phenol reagent, analysis grade methanol, High-performance liquid chromatography (HPLC) grade methanol, fluorescein (FL) sodium nitrite, and hydrochloride acid were purchased from Carlo Erba (Milan, Italy).
High-performance liquid chromatography grade water and acetonitrile, aluminum chloride anhydrous and potassium peroxodisulfate were from VWR. All other reagents, if not specified, were purchased from Sigma-Aldrich (Milan, Italy).
Plant preparation
The bark of R. alaternus was collected in October 2012 from Teniet En Nasr (Algeria). The identification of the plant was based on the work of Quezel and Santa,[16] and validated by botanists in the Department of Botany and Ecology at Sétif1 University (Algeria). The plant drug was dried, packed in paper bags, and stored at room temperature until analysis.
Extraction procedure
The methanolic and aqueous extracts from R. alaternus bark were prepared by maceration. Briefly, drug samples were powdered by a mill and 50 g of the powdered drugs were mixed with 500 ml of methanol or water and then left for 24 h under occasional stirring. Furthermore, a traditional aqueous extract was prepared by decoction: Boiling 10 g of the powdered drug in 500 ml of water for 10 min,[17] then to obtain the dried extracts, the mixtures were filtered, and the filtrates were evaporated to dryness under vacuum by using a rotavapor (Büchi). The residues were kept into brown vial until use.[18,19] The yields of the extractions, expressed as a percentage, were 6.30%, 16.60%, and 27.75% for methanolic, aqueous and traditional extracts respectively.
For the chemical and biochemical assays, the appropriate volumes of methanol or water were used to redissolve the methanolic extract, and the aqueous and traditional extracts respectively. For the experiments carried out on cells, the extracts as well as the reference compound taxol, were redissolved in the appropriate volume of dimethylsulfoxide (DMSO), so that the final concentration of DMSO in the cell culture medium was <0.1% v/v. All solutions were freshly prepared before experiments and used immediately.
Total flavonol content
The content of flavonols was determined by the method described by Tomaino et al.[20] Aliquots (125 μl) of the solutions containing the R. alaternus extracts to be tested were mixed with 125 μl of AlCl3 (2 mg/ml), and 750 μl of sodium acetate (50 mg/ml). The absorbance at 440 nm was detected by a spectrophotometer after 2.5 h. Total flavonol content was expressed as μg of quercetin equivalents (QE) per mg of dried extract. All determinations were carried out in duplicate and repeated at least two times.
Flavonoid analysis by high-performance liquid chromatography-photo diode array/electrospray ionisation mass spectrometry
The solutions containing the R. alaternus extracts to be tested (10 mg/ml) were filtered through a 0.45 μm membrane filter (Whatman, Clifton, NJ, USA). HPLC-photo diode array-electrospray ionisation mass spectrometry (HPLC-PDA/ESI-MS) analyses of flavonoids were performed on a Prominence LC system (Shimadzu, Milan, Italy) consisting of a CBM-20A controller, two LC-20AD pumps, a CTO-20AC, a DGU-20A3 degasser, a SIL-20AC autosampler, a SPD-M20A PDA detector, and a quadrupolar mass analyzer (LCMS-2020, Shimadzu, Japan), equipped with an electrospray ionization (ESI) interface, operated in the negative mode. Data acquisition was performed by Shimadzu Lab Solution software ver. 5.10.153 (Shimadzu, Kyoto, Japan).
Separation was carried out on an Ascentis Express C18 column, 15 cm × 4.6 mm i.d. packed with 2.7 m partially porous particles (Supelco, Bellefonte, PA, USA). The injection volume was 5 μl, and the mobile phase consisted of water/acetic acid (0.075%) at pH = 3 (solvent A) and acetonitrile/acetic acid (0.075%) (solvent B), respectively in the following linear gradient mode: 0 min, 0% B; 60 min, 40% B; 70 min, 100% B; 71 min, 0% B. The mobile phase flow rate was 1.0 ml/min, and it was splitted to 0.2 ml/min prior to MS detection.
Photo diode array wavelength range was 190–400 nm and the chromatograms were extracted at 280 nm (time constant: 0.025 s; sample frequency: 40 Hz). MS acquisition was performed using ESI, in the negative mode, under the following conditions: Mass spectral range 100–800 m/z; interval: 0.5 s; scan speed: 1500 amu/s; nebulizing gas (N2) flow: 1.5 L/min; interface temperature: 350°C, heat block: 300°C, DL temperature: 300°C; DL voltage: −34 V; interface voltage: −4.5 kV; Qarray DC voltage: 1.0 V; Qarray RF voltage: 60 V.
The quantitative determination of each compound was carried out by means of the external standard method using kaempferol (λ = 365) and quercetin (λ = 370) as reference compounds in a concentration range of 1–100 ppm. The results were obtained from the average of three determinations and are expressed as mg/g dried extract ± percent relative standard deviation (%RSD).
Folin-Ciocalteau colorimetric method
The antioxidant capacity (expressed as total phenols content) of the R. alaternus extracts was determined by means of the Folin-Ciocalteu reagent.[21,22] Fifty microliter of the solutions containing different concentrations of the extracts to be tested were added to 450 μL of deionized water, 500 μl of Folin-Ciocalteu reagent, and 500 μl of 10% aqueous sodium carbonate solution; samples were then maintained at room temperature for 1 h. Absorbance was measured at 786 nm ultraviolet (UV-Vis Spectrophotometer, Shimadzu Japan) against the blank containing 50 μl of the same solvent used to dissolve the extracts. Total phenol content was expressed as μg of gallic acid equivalents per mg of extract, using the calibration curve prepared with gallic acid standard solutions. Each determination was performed in duplicate and repeated at least two times.
2,2-diphenyl-1-picrylhydrazyl test
The free radical-scavenging capacity of R. alaternus extracts was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay,[23,24] a method based on the reduction of the stable radical DPPH•. The reagent mixture consisted in 1.5 ml of 100 mM DPPH• in methanol, to which 37.5 μl of various concentrations of the extracts to be tested were added; an equal volume of the solvent employed to dissolve the extracts was added to control tubes. After 20 min of incubation at room temperature, the absorbance was recorded at 517 nm in a UV-Vis spectrophotometer (Shimadzu, Japan). Results were expressed as mmol trolox equivalents/gram of dried extract, using the calibration curve prepared with trolox as standard solutions. Each determination was performed in duplicate and repeated at least two times.
2,2’- azinobis-(3-ethylbenzothiazine-6-sulfonic acid radical assay
The ability of the R. alaternus extracts under study, to scavenge the 2,2’- azinobis-(3-ethylbenzothiazine-6-sulfonic acid (ABTS+) radical was evaluated as described by Morabito et al.[24] Briefly, this method determines the capacity of antioxidants to quench the ABTS + radical. The ABTS + radical cation was produced by the oxidation of 1.7 mM ABTS + with potassium persulfate (4.3 mM final concentration) in water. The mixture was allowed to stand in the dark at room temperature for 12–16 h before use, and then the ABTS + solution was diluted with phosphate buffered saline (PBS) at pH 7.4 to give an absorbance of 0.7 ± 0.02 at 734 nm. Fifty microliter of a solution containing different concentrations of the extracts to be tested or of the vehicle alone (methanol or water) were added to 2 ml of the ABTS + solution, and the absorbance was recorded at 734 nm in a UV-Vis spectrophotometer (Shimadzu, Japan) after allowing the reaction to stand for 6 min in the dark at room temperature. Each determination was performed in duplicate and repeated at least two times. Results were expressed as mmol trolox equivalents/gram of dried extract, using calibration curve prepared with trolox standard solutions.
Ferric reducing/antioxidant power
The ferric reducing ability of the extracts under study was evaluated according to the method described by Cimino et al.[25] with some modifications. The method is based on the reduction of a ferric-tripyridyltriazine complex to its colored ferrous form in the presence of antioxidants. The fresh working solution Ferric reducing/antioxidant power (FRAP reagent) was prepared by mixing 2.5 ml of 10 mM 2, 4, 6-tripyridyl-s-triazine solution (prepared in 40 mM HCl), with 25 ml of 0.3 M acetate buffer (pH 3.6), and 2.5 ml of 20 mM FeCl3 ∙ 6H2O solution, and then warmed (preheated) at 37°C before use. Fifty μl of a methanolic or water solution containing different concentrations of the extracts to be tested, or of the vehicle alone (methanol or water), were added to 1 ml of FRAP reagent, and the absorbance was measured at 593 nm in a spectrophotometer (Shimadzu, Japan) after incubation at 20°C for 4 min. A standard curve was prepared using various concentrations of FeSO4·7H2O. Each determination was performed in duplicate and repeated at least two times, and results were expressed as mmol Fe2+/g of dried extract.
Oxygen radical absorbance capacity assay using fluorescein assay
The oxygen radical absorbance capacity assay using FL (ORAC-FL) assay was performed as described by Dávalos et al.[26] This assay is based on the effect of peroxyl radicals generated from the thermal decomposition of 2,2’-azobis (2-methylpropionamidine) dihydrochloride (AAPH) on the signal intensity from the fluorescent probe FL in the presence of an oxygen-radical absorbing substance. The reaction was carried out in 75 mM phosphate buffer (pH 7.4), and the final reaction mixture was 2 ml. An aliquot of the solution containing different concentrations of the extracts to be tested (200 μl), and 1.2 ml of FL solution (70 nM, final concentration) were placed in the cuvette. The mixture was preincubated for 15 min at 37°C in the spectrofluorimeter. Freshly prepared AAPH solution (600 μL; 12 mM, final concentration) was rapidly added. Fluorescence was recorded every minute for 80 min at 37°C; the fluorescence conditions were as follows: Excitation at 485 nm and emission at 520 nm. A blank containing FL and AAPH but only the solvent employed to dissolve the extracts, and different calibration solutions using Trolox (1–7.5 μM, final concentration) as reference antioxidant, were also carried out in each assay. All the reaction mixtures were prepared in triplicate and at least three independent assays were performed for each sample. The area under the curve (AUC) was calculated for each sample by integrating the relative fluorescence curve. The net AUC was calculated by subtracting the AUC of the blank. The final ORAC values were determined by linear regression equation of Trolox concentrations, and expressed as mmol Trolox equivalents/gram dried extract.
Cell culture assay
Human monocytic leukemia cells U937 (ATCC® CRL1593.2) and normal peripheral blood mononuclear cells (PBMCs), obtained from healthy donors peripheral blood upon centrifugation on Ficoll-Hypaque (Sigma-Aldrich) gradient, were cultured in RPMI-1640 medium supplemented with 10% FBS (Gibco), 1% L-glutamine, and 1% penicillin-streptomycin. The cultures were maintained in a humidified incubator with 5% CO2 at 37°C.
Both PBMCs and U937 were cultured for 24 h in 48-well cell culture plates in complete medium containing various concentrations of R. alaternus bark extracts (from 0.5 to 300 μg/ml) or taxol (from 0.5 to 30 μg/ml). Control cells were treated with 0.1% DMSO. Taxol was used as reference cytotoxic compound. After 24 h cells were collected and cell number assessed by trypan blue exclusion assay.[27] The 50% inhibition concentration (IC50) of R. alaternus extracts or of taxol was determined as the drug concentration that reduced cell number by 50% in treated cells compared to untreated cultures.
RESULTS
Flavonoid content and profile
Characterization of the flavonoid content in the R. alaternus bark samples was accomplished by HPLC–diode array detection (DAD)–ESI–MS. A linear gradient of increasing ACN percentages allowed to attain baseline separation of all the components on a C18 fused-core stationary phase (2.7 μm d.p.), operated at 1.0 mL/min, in roughly 45 min. On-line coupling to DAD (extracted at a 280 nm wavelength) and MS detection provided complementary information for reliable identification of all the separated compounds. The list of identified flavonoids is reported in Table 1. MS identification was achieved by inspection of both m/z values of the [M − H]− ions and secondary fragment ions resulting from further fragmentation due to the loss of the sugar moieties. Quantitative determination of the flavonoid content was performed by interpolation of the apigenin, daidzein, genistein, kaempferol, luteolin, quercetin calibration curves. All the three extracts showed a pretty similar flavonoid fingerprint [Figure 1] with rhamnetin hexoside as the main compound, whereas they differed for the quantitative content [Table 1]. In fact, the methanolic extract presented a higher flavonoid content with respect to the aqueous (17.88 mg/g extract) and the traditional (14.21 mg/g extract) extracts. These data confirm those obtained by the spectrophotometric assay based on AlCl3 -flavonol complexation, showing that the flavonol content found in the methanolic extract (51.17 mg QE/g extract) was significantly higher than that measured in the aqueous extract (24.09 mg QE/g extract) and in the traditional one (12.03 mg QE/g extract).
Table 1.
HPLC-PDA/ESI-MS identification and quantification of flavonoids contained in methanolic, aqueous and traditional extracts obtained from Rhamnus alaternus bark
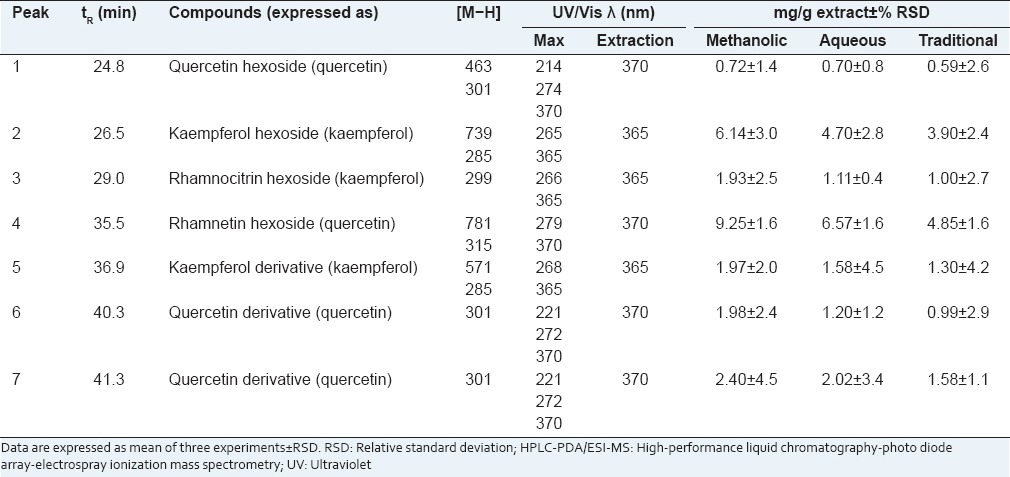
Figure 1.
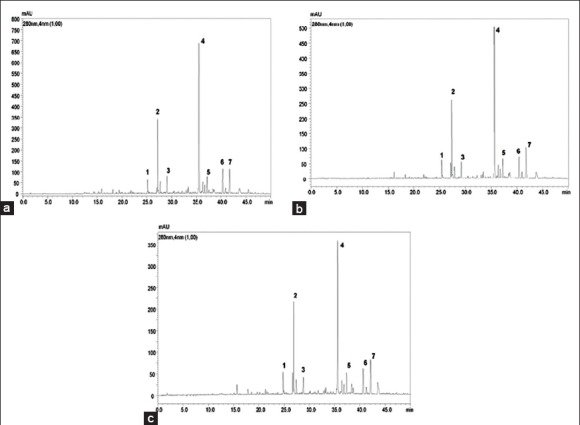
High-performance liquid chromatography-photo diode array representative chromatogram of flavonoids (extracted at 280 nm) of (a) Methanolic extract, (b) Aqueous extract, and (c) Traditional extract from Rhamnus alaternus bark
Antioxidant/radical scavenging activity
The extracts from R. alaternus bark were evaluated for their antioxidant/radical scavenging capacity. The selected electron transfer (ET)-based methods were the DPPH assay, ABTS-based assay in order to measure the TEAC (Trolox Equivalents Antioxidant Capacity), and the FRAP method, while the selected hydrogen atom transfer (HAT)-based method was the ORAC assay. It is widely accepted that to characterize the properties of antioxidant agents, different validated benchmark methods are needed. One has to point out that the antioxidant activity measured by an individual assay reflects only the chemical reactivity under the specific conditions applied in that assay. In our study we used a battery of different cell-free chemical assays for measuring the reducing capacity of the test extracts. Among these assays, ORAC is a HAT reaction-based assay, while FRAP and total phenols assay by Folin-Ciocalteu reagent are single ET reaction based assays; furthermore, we used the TEAC and DPPH assays which are usually classified as ET reactions, although the two indicator radicals employed may be neutralized either by direct reduction via electron transfers or by radical quenching via H atom transfer. Concerning the oxidant employed in the assays, only ORAC is an antioxidant capacity assay involving oxidants that are pro-oxidants; in fact, ORAC is a competitive method since it evaluates the ability of a particular sample to inhibit the consumption of a target molecule mediated by physiologically-relevant peroxyl radicals. One has to point out that, to date, ORAC is the only method that takes free radical action to completion and uses an AUC technique for quantitation, and thus, combines both the inhibition percentage and the length of inhibition time of the free radical action by antioxidants into a single quantity; because of the very high molar ratio (more than 2000) of AAPH to antioxidants used in the ORAC assay, this procedure has high specificity and thus, measures the capacity of antioxidants to directly quench free radicals. The weakest point of ORAC, as well as of FRAP and Folin-Ciocalteu–based assay, is that they are not suitable for measuring lipid-soluble antioxidants; conversely the strong point of TEAC is that it can be applied for both water-soluble and lipid soluble antioxidants, while DPPH• is soluble only in organic solvents. FRAP, TEAC, and total phenols assay by FCR were carried out under acidic, neutral, or basic conditions respectively. This is important because the pH values have an important effect on the reducing capacity of antioxidants: At acidic conditions, the reducing capacity may be suppressed due to protonation on antioxidant compounds, whereas in basic conditions, proton dissociation of phenolic compounds would enhance sample's reducing capacity.
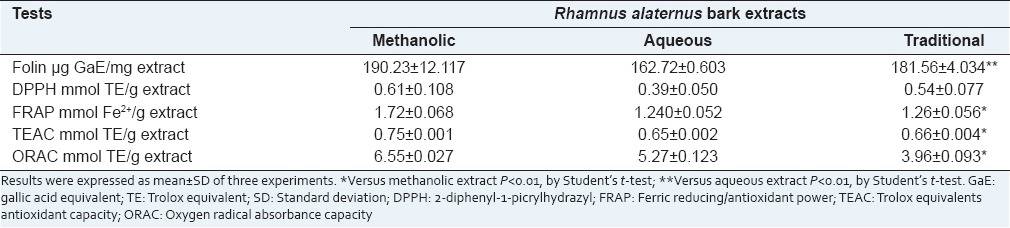
The full results are summarized in Table 2. Briefly, the three extracts showed significant antioxidant capacities, with few differences between them in ET-based assay. Conversely in ORAC assay the methanolic extract appeared significantly more active than the other two extracts.
Table 2.
Antioxidant activity of Rhamnus alaternus bark extracts measured by means of different in vitro tests
Cytotoxicity of Rhamnus alaternus bark extracts on peripheral blood mononuclear cells and U937 cells
The viability of PBMCs treated with increasing concentrations of R. alaternus bark extracts for 24 h was examined using the trypan blue assay as described in the materials and methods section. In normal PBMCs, the traditional extract significantly induced cell death in a dose-dependent manner so that the concentration inducing the 50% of cell death (IC50) was 29.35 μg/ml [Table 3]. When the aqueous extract was tested, the concentration inducing the 50% of cell death was similar (IC50 38.81 μg/ml) to that of the traditional extract, even if at less extend. Conversely, the methanolic extract produced less cell death with the highest IC50 (220.35 μg/ml) after 24 h.
Table 3.
IC50 values (concentration eliciting 50% inhibition) for Rhamnus alaternus extracts and taxol in PBMC cells and U937 cells. Cells were treated with various concentrations of the drugs, and the cell number was counted after 24 h of exposure
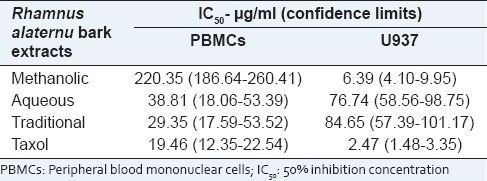
Data analysis of trypan blue assay, as shown in Table 3, also revealed IC50 values of the R. alaternus bark extracts on human leukemic monocyte lymphoma cell line (U937) after 24 h of exposure. Proliferation was most potently suppressed by the methanolic extract with the lowest IC50 value (6.39 μg/ml). Proliferation of U937 cells exposed to traditional and aqueous extracts was not significantly reduced resulting in higher IC50 values (84.65 μg/ml and 76.74 μg/ml respectively).
The criteria of cytotoxicity for the crude extract, established by the U.S. National Cancer Institute (NCI) in the preliminary assays, report IC50 values of 10–20 μg/ml of a total/crude extract as cytotoxic, 20 to ≤50 μg/ml as moderately cytotoxic, and <10 μg/ml as strongly cytotoxic.[28] The IC50 value for the methanolic extract of R. alaternus bark on U937 cells was found to be lower than that specified by NCI for categorization as anticancer agent. On the contrary, the traditional and aqueous extracts can be considered as poorly cytotoxic for U937 cells. Furthermore, these data are also interesting as they suggest that the methanolic extract possesses a cytotoxic activity specifically on cancer cells (U937), while the traditional and aqueous extracts are moderately toxic on normal PBMCs.
DISCUSSION
The aim of this preliminary study was to characterize the flavonoid content, the antioxidant properties and the cytotoxic activity of different extracts obtained from Algerian R. alaternus bark, a drug used in North African folk medicine.
The three extracts under investigation show a similar flavonoid profile, but the methanolic extract has a value of flavonol content (51.17 ± 0.409 μg QE/mg) significantly higher than that of two extracts obtained by water (24.096 ± 0.852 and 12.03 ± 0.88 μg QE/mg of extract respectively). This may be due to the low water solubility of flavonoids, needing an organic solvent to be extracted from the vegetable matrix. Furthermore, the traditional extract obtained by decoction contains, despite the heating, an amount of flavonoids lower than that of the aqueous one obtained by maceration, very likely because according to the traditional methodology this extract was obtained from 10 g of raw drug, thus an amount lower than that (50 g) employed to obtain the other two extracts.
As to the antioxidant power, in all the usually classified as ET assays (DPPH, Folin, FRAP, TEAC), the three extracts show a similar free-radical scavenging power, being the methanolic extract more effective than the others. Conversely when the HAT based assay ORAC was employed, the effectiveness order was: Methanolic extract > aqueous extract > traditional extract.
It is generally considered that the primary mechanism of the radical scavenging activity of flavonoids is hydrogen atom donation, although they may also act by single-electron transfer. Thus our findings, taken together, suggest that the antioxidant activity of R. alaternus bark extracts may be due, at least partially, to their content in flavonols but also it demonstrate that the aqueous extract and especially the traditional one contains a significant amount of compounds, different from flavonoids, acting by single-electron transfer, which contribute (independently on the characteristics of chemical environment) to the antioxidant activity measured in the ET-based assays. Furthermore, it is evident that, also with regard to the antioxidant power, the traditional methodology (decoction) allows to obtain an extract which (although starting from a less drug amount) retains a good efficacy, similar to that of an aqueous extract produced without heating, and very likely due to the contribution of compounds released from the plant matrix by heating.
Cytotoxicity of R. alaternus extracts was evaluated in a leukemic cancer cell line (U937) and in normal PBMCs. This method gives an indication of potential usefulness in a clinical setting, for which selectivity in favor of the cancer cell line being the more susceptible is required.[29] The aqueous extract and at a higher degree, the traditional one produced a marked decrease in the viability of PBMCs, with IC50 of 38.81 μg/ml (C.L. 18.06–53.39) and 29.35 μg/ml (C.L. 17.59–53.52), respectively. Conversely, the methanolic extract exerted only a weak cytotoxic effect on normal PBMCs, with IC50 of 220.35 μg/ml (C.L. 186.64–260.41), which is a clear indication that normal human cells may be more resistant to this extract.
As to the experiments on leukemia U937 cells, the methanolic extract (richer in kaempferol, rhamnocitrin, and quercetin derivatives), but not the other two extracts, decreased significantly the viability of these cells, showing an IC50 of 6.39 μg/ml (C.L. 4.10–9.95), with the suggested effective doses for a 50% inhibition in cell viability for plant extracts being <20 μg/ml and thus to be considered active according to the NCI guidelines.[28]
Kaempferol and rhamnocitrin glycosides from R. alaternus have been demonstrated to induce apoptosis in human lymphoblastoid cells TK6,[12] and quercetin can induce caspase-dependent cell death in U937 cells.[30] Thus, we can suppose that the cytotoxic effect of the methanolic extract under study is due to kaempferol, rhamnocitrin, and quercetin derivatives contained in it. As to the other two extracts, they do not contain a sufficient amount of these compounds to obtain an evident cytotoxic effect on leukemia cells and on the other hand, they contain other hydrophilic compounds toxic for normal PBMCs.
CONCLUSION
These preliminary data evidence that R. alaternus bark can be considered as a good source of bioactive compounds possessing significant antioxidant and cytotoxic activity, in particular kaempferol, rhamnocitrin, and quercetin derivatives. However, only the methanolic extract is able to decrease specifically the growth of cancer cells, such as the human leukemia cells U937, while the water extracts obtained by maceration or by decoction are moderately cytotoxic for normal human cells. Further studies are warranted to optimize the conditions needed to obtain from R. alaternus bark an extract enriched in anticancer flavonols but lacking of compounds toxic for normal cells.
ACKNOWLEDGMENT
This research was partially supported by the Erasmus Mundus programme of the European Union (PROJECT EU-MARE NOSTRUM Erasmus Mundus Action 2, Strand 1, lot 1).
Footnotes
Source of Support: This research was partially supported by the Erasmus Mundus programme of the European Union (PROJECT EU-MARE NOSTRUM Erasmus Mundus Action 2, Strand 1, lot 1)
Conflict of Interest: None declared.
REFERENCES
- 1.Mai LP, Guéritte F, Dumontet V, Tri MV, Hill B, Thoison O, et al. Cytotoxicity of Rhamnosylanthraquinones and Rhamnosylanthrones from Rhamnus nepalensis. J Nat Prod. 2001;64:1162–8. doi: 10.1021/np010030v. [DOI] [PubMed] [Google Scholar]
- 2.Boukef K. Rhamnus alaternus. Essaydali. 2001;81:34–5. [Google Scholar]
- 3.Wei BL, Lin CN, Won SJ. Nakahalene and cytotoxic principles of Formosan Rhamnus species. J Nat Prod. 1992;55:967–9. [Google Scholar]
- 4.Alemayu G, Abegaz B, Snatzke G, Duddeck H. Bianthrones from Senna longiracemosa. Phytochemistry. 1993;32:1273–7. [Google Scholar]
- 5.Boussahel S, Dahamna S, Ruberto G, Siracusa L, Harzallah D. Phytochemical Study and antioxidant activities of leaves extracts from Rhamnus alaternus L. Pharmacogn Commun. 2013;3:46–53. [Google Scholar]
- 6.Ammar RB, Bouhlel I, Valenti K, Sghaier MB, Kilani S, Mariotte AM, et al. Transcriptional response of genes involved in cell defense system in human cells stressed by H2O2 and pre-treated with (Tunisian) Rhamnus alaternus extracts: Combination with polyphenolic compounds and classic in vitro assays. Chem Biol Interact. 2007;168:171–83. doi: 10.1016/j.cbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Ammar RB, Kilani S, Bouhel I, Skandrani I, Naffeti A, Boubaker J, et al. Antibacterial and cytotoxic activities of extracts from (Tunisian) Rhamnus alaternus (Rhamnaceae) Ann Microbiol. 2007;57:453–60. [Google Scholar]
- 8.Ammar RB, Sghaier MB, Boubaker J, Bhouri W, Naffeti AI, Skandrani I, et al. Antioxidant activity and inhibition of aflatoxin B1-, nifuroxazide-, and sodium azide-induced mutagenicity by extracts from Rhamnus alaternus L. Chem Biol Interact. 2008;174:1–10. doi: 10.1016/j.cbi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Ammar RB, Kilani S, Bouhlel I, Ezzi L, Skandrani I, Boubaker J, et al. Antiproliferative, antioxidant, and antimutagenic activities of flavonoid-enriched extracts from (Tunisian) Rhamnus alaternus L.: Combination with the phytochemical composition. Drug Chem Toxicol. 2008;31:61–80. doi: 10.1080/01480540701688725. [DOI] [PubMed] [Google Scholar]
- 10.Ammar RB, Bhouri W, Sghaier MB, Boubaker J, Skandrani I, Naffeti A, et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L.(Rhamnaceae): A structure-activity relationship study. Food Chem. 2009;116:258–64. [Google Scholar]
- 11.Ammar RB, Neffati A, Skandrani I, Sghaier MB, Bhouri W, Ghedira K, et al. Anti-lipid peroxidation and induction of apoptosis in the erythroleukaemic cell line K562 by extracts from (Tunisian) Rhamnus alaternus L.(Rhamnaceae) Nat Prod Res. 2011;25:1047–58. doi: 10.1080/14786419.2010.490783. [DOI] [PubMed] [Google Scholar]
- 12.Bhouri W, Sghaier MB, Kilani S, Bouhlel I, Dijoux-Franca MG, Ghedira K, et al. Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L.(Rhamnaceae): Kaempferol 3-O-ß-isorhamninoside and rhamnocitrin 3-O-ß-isorhamninoside. Food Chem Toxicol. 2011;49:1167–73. doi: 10.1016/j.fct.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Bhouri W, Bouhlel I, Boubaker J, Kilani S, Ghedira K, Ghedira LC. Induction of apoptosis in human lymphoblastoid cells by kaempferol 3-O-ß-isorhamninoside and rhamnocitrin 3-O-ß-isorhamninoside from Rhamnus alaternus L.(Rhamnaceae) Cell Prolif. 2011;44:283–90. doi: 10.1111/j.1365-2184.2011.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhouri W, Boubaker J, Kilani S, Ghedira K, Ghedira LC. Flavonoids from Rhamnus alaternus L.(Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside protect against DNA damage in human lymphoblastoid cell and enhance antioxidant activity. S Afr J Bot. 2012;80:57–62. [Google Scholar]
- 15.Kosalec I, Kremer D, Locatelli M, Epifano F, Genovese S, Carlucci G, et al. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013;136:335–41. doi: 10.1016/j.foodchem.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Quezel P, Santa S. Vol. 63. Paris: National Centre Editions of Scientific Research; 1962. New Flora of Algeria and the southern desert regions; pp. 475–6. [Google Scholar]
- 17.Beloued A. Medicinal plants of Algeria. Center place of Ben-Aknoun Algiers (Algeria): Office of University Publications. 2005:142. [Google Scholar]
- 18.Gnanaprakash K, Madhusudhana CC, Ramkanth S, Alagusundaram M, Tiruvengadarajan VS, AngalaParameswari S, et al. Aqueous Extract of Flacourtia indica Prevents Carbon Tetrachloride Induced Hepatotoxicity in Rat. Int J Biol Life Sci. 2010;6:51–5. [Google Scholar]
- 19.Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A. Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. J Ethnopharmacol. 2006;107:370–3. doi: 10.1016/j.jep.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L. variety Bronte) seeds and skins. Biochimie. 2010;92:1115–22. doi: 10.1016/j.biochi.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Spagna G, Tomaino A, Cimino F, Barbagallo R, Ventura D, Bonina F, et al. Chemical analysis and photoprotective effect of an extract of wine from Jacquez grapes. J Sci Food Agric. 2002;82:1867–74. [Google Scholar]
- 22.Cimino F, Cristani M, Saija A, Bonina FP, Virgili F. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors. 2007;30:129–38. doi: 10.1002/biof.5520300206. [DOI] [PubMed] [Google Scholar]
- 23.Rapisarda P, Tomaino A, Lo Cascio R, Bonina F, De Pasquale A, Saija A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J Agric Food Chem. 1999;47:4718–23. doi: 10.1021/jf990111l. [DOI] [PubMed] [Google Scholar]
- 24.Morabito G, Trombetta D, Singh Brajendra K, Prasad Ashok K, Parmar Virinder S, Naccari C, et al. Antioxidant properties of 4-methylcoumarins in in vitro cell-free systems. Biochimie. 2010;92:1101–7. doi: 10.1016/j.biochi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, et al. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–9. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dávalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 27.Cimino F, Speciale A, Siracusa L, Naccari C, Saija A, Mancari F, et al. Cytotoxic effects induced in vitro by organic extracts from urban air particulate matter in human leukocytes. Drug Chem Toxicol. 2014;37:32–9. doi: 10.3109/01480545.2013.806529. [DOI] [PubMed] [Google Scholar]
- 28.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep Part 3. 1972;3:1–103. [Google Scholar]
- 29.Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–87. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee TJ, Kim OH, Kim YH, Lim JH, Kim S, Park JW, et al. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006;240:234–42. doi: 10.1016/j.canlet.2005.09.013. [DOI] [PubMed] [Google Scholar]


