Abstract
Background:
The combination of artemisinin and transferrin exhibits versatile anticancer activities. In previous, we successfully prepared artemisinin and transferrin-loaded magnetic nanoliposomes and evaluated their anti-proliferative activity against MCF-7 and MDA-MB-231 cell lines in vitro. In this study, we investigate the in vivo anti-breast cancer activity of artemisinin and transferrin-loaded magnetic nanoliposome against breast transplanted tumors in BALB/c mice model.
Materials and Methods:
Artemisinin and transferrin-loaded magnetic nanoliposomes were prepared and characterized for some physiochemical properties. Pieces of tumor tissue from the breast cancer-bearing BALB/c mice were transplanted subcutaneously to the syngeneic female BALB/c mice. In the presence of the external magnet that placed at the breast tumor site, the tissue distribution and tumor-suppressing effects of prepared nanoliposomes on tumor growth was evaluated.
Results:
The prepared nanoliposomes have fine spherical shape, rough surface, nano-sized diameter and magnetic properties. At 2 h after treatment, the intravenous administration of artemisinin and transferrin-loaded magnetic nanoliposomes followed using the magnetic field approximately produced 10- and 5.5-fold higher levels of artemisinin and transferrin in the tumors, respectively, compared with free artemisinin and transferrin. Moreover, in the presence of an external magnetic field, the prepared nanoliposomes could significantly induce apoptosis in the mice breast cancer cells as well as could reduce tumor volume in tumorized mice at 15 days after treatment.
Conclusion:
The data suggested that the artemisinin and transferrin-loaded magnetic nanoliposomes would be a good choice for the breast tumor-targeted therapy, due to its high targeting efficiency.
Keywords: Artemisinin, breast cancer, in vivo, magnetic nanoliposome, transferrin
INTRODUCTION
Artemisinin [Figure 1], as a sesquiterpene lactone, found in sweet wormwood (Artemisia annua L.), and it possesses a range of biological and medicinal properties, including anti-malaria and anti-cancer activities.[1] However, the hydrophobicity of artemisinin and its nonselective targeting toward cancer cells could limit its biomedical use.[2] It has been documented that cancer cells in the presence of transferrin were more susceptible to artemisinin cytotoxicity.[3]
Figure 1.
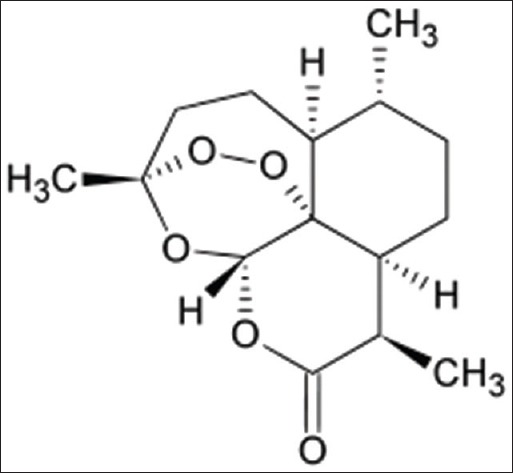
Artemisinin structure
Previous investigations showed that the covalently tagging artemisinin to transferrin and encapsulation of artemisinin in drug delivery systems could partly resolve the artemisinin water insolubility, but it could not resolve the specific targeting to tumor cells.[4,5]
As an interesting approach to drug delivery research, the co-encapsulation of artemisinin and transferrin into magnetic drug delivery nanosystems such as magnetic nanoliposomes might be able to overcome these limitations.[6]
Magnetic liposomes act as “intelligent” drug delivery systems because these carriers could congregate around the magnetic site and, therefore, could use as drug transporter to the therapeutic site in the cancer therapy.[7,8,9] Moreover, magnetic liposomes have various applications such as hyperthermia cancer therapy and image contrasting in magnetic resonance imaging.[10]
In previous, we have successfully prepared artemisinin and transferrin-loaded magnetic nanoliposomes. Moreover, our group found that prepared nanoliposomes in the presence of an external magnetic force have excellent anti-proliferation activity against MCF-7 and MDA-MB-231 cell lines. In the present study, in vivo anticancer efficiency of artemisinin and transferrin-loaded magnetic nanoliposomes against breast tumor in BALB/c mice model was evaluated.
MATERIALS AND METHODS
Chemicals
Artemisinin (purity ≥98%), distearoyl phosphatidylcholine (DSPC), dipalmitoyl phosphatidylcholine (DPPC), cholesterol (CHOL), transferrin and magnetic iron oxide were purchased from Sigma (St. Louis, USA). Acetonitrile was purchased from Merck (Darmstadt, Germany).
Mice
Seventy-eight female inbred BALB/c mice (7–9 weeks old, 20–22 g) were purchased from Pasteur Institute, Tehran, Iran. They were kept in animal houses, given sterilized water and standard mouse food throughout the study. Animal care and protocols were performed and approved by the Institutional Animals Ethics Committee of Borujerd Branch, Islamic Azad University (Number: 120, Borujerd, Iran, 4/3/2014).
Preparation of nanoliposomes
The artemisinin and transferrin-loaded magnetic nanoliposomes were prepared by our previous described method.[6] In brief, the proper molar ratio 26:4:6 of DPPC, DSPC and CHOL was determined using one variable at a time method. Then, these lipids were dissolved in chloroform and dried. The dried lipids were dispersed in artemisinin, transferrin, and magnetic iron oxide solution and sonicated. Subsequently, small magnetic nanoliposomes were obtained by extruding the dispersions through 100-nm-pore polycarbonate filters. The control magnetic nanoliposomes were prepared similarly, but PBS (pH, 7.4) was used instead of the artemisinin and transferrin solutions.
Physiochemical characterization
As described previously, the contents of the artemisinin, transferrin and magnetic iron oxide in the nanoliposomes were evaluated using high performance liquid chromatography (Perkin Elmer, USA) and spectrophotometer (Shimadzu, Japan), respectively.[6,11] Then, the loading efficiency of artemisinin, transferrin, and magnetic iron oxide was calculated.[6]
As reported previously, the magnetic properties and morphology of prepared nanoliposomes were analyzed by vibrating sample magnetometer (Meghnatis Daghigh Kavir Co., Iran) and cryo-transmission electron microscopy (cryo-TEM), respectively.[12,13]
The zeta-potential, polydispersity index and mean particle size of nanoliposomes were determined using Malvern zetasizer (Malvern instrument, Worcestershire, UK) apparatus.[14]
Mice tumor model
Spontaneous mouse mammary tumor [Figure 2], as an invasive ductal carcinoma was spontaneously developed in the female BALB/c mice.[15] The diagnosis of ductal carcinoma in tumorized mice was evaluated by the histopathological method as described previously.[16] In brief, the isolated tumors were fixed in 10% formaldehyde, passaged, and embedded in paraffin. Subsequently, prepared paraffin blocks were sectioned and stained with hematoxylin and eosin. The tumor transplantation in the mice was performed by previously described method.[17] In brief, the tumor from the breast cancer-bearing BALB/c mice was separated and then cut into pieces of <0.5 cm3. Subsequently, each piece was transplanted subcutaneously to the female BALB/c mice. These mice were studied 2 weeks after transplanted.
Figure 2.
Spontaneous mouse mammary tumor
Treatment of tumorized mice
Breast cancer bearing mice were divided into 6 groups and treated daily with different administration modalities as follows: Group 1, intravenous (i.v.) administration of control nanoliposomes (without artemisinin and transferrin) under an external magnetic force; group 2, i.v. administration of free artemisinin, transferrin and magnetic iron oxide without a magnetic force; group 3, i.v. administration of free artemisinin, transferrin and magnetic iron oxide under an external magnetic force; group 4, i.v. administration of artemisinin and transferrin-loaded magnetic nanoliposomes without a magnetic force; group 5, i.v. administration of artemisinin and transferrin-loaded magnetic nanoliposomes under an external magnetic force; the control group, i.v. administration of 1 mL PBS solution. In groups 1, 3 and 5 as an external magnetic force, a piece of magnet (1 by 1 by 0.3 cm) was placed at the breast tumor site.
In groups 1–5, the dose of artemisinin, transferrin, and magnetic iron oxide were fixed at 100, 102.4 and 79.44 μg/1 mL PBS, respectively. All preparations were administered via the tail vein as a short infusion. These mice were used for subsequent experiments.
Artemisinin, transferrin and iron oxide distribution
At 2 h after treatment, three mice in groups 1–5 were killed for collection of blood and isolation of tumor. Tumors were homogenized in a 4-fold volume of PBS, and the 20% homogenate was mixed with an equal volume of acetonitrile for the deproteinization. The homogenate mixture was centrifuged at 1000 g for 10 min to obtain a supernatant. Blood was centrifuged at 1250 g for 0.5 min to obtain plasma samples. The concentrations of artemisinin, transferrin and magnetic iron oxide in these biologic samples (supernatants of tumor homogenates and plasma) were determined in triplicate as described previously.[6,11]
Tumor volume
To evaluate the antitumor effect of i.v.-administered artemisinin, transferrin and magnetic iron oxide preparations, the remaining breast cancer bearing mice were divided into 6 groups (n = 10 for each group) and treated with different administration modalities as described above. Tumor volumes were monitored periodically over 15 days after treatment. Tumor volume was estimated by the previously described equation: 1⁄2 × Dmax × (Dmin)2, where Dmax is the maximal tumor diameter and Dmin is the corresponding perpendicular diameter.[18]
Histological examination
Fifteen days after treatment, mice were killed and apoptosis rates of tumor cells were analyzed as described previously.[19] In brief, the isolated tumors were fixed in 10% formaldehyde, embedded in paraffin. Subsequently, the paraffin blocks were sectioned and deparaffinized, and permeabilized with proteinase K. Finally, the TdT mediated dUTP Nick End Labeling staining was done to visualize the fragmented DNA directly by fluorescence microscopy.
Data analysis
All data were expressed as means ± standard deviation. The One-way ANOVA was performed to determine the significance levels among the tested groups and tumor volumes and the P < 0.05 were considered statistically significant.
RESULTS
Physicochemical properties of nanoliposomes
The entrapment rate of artemisinin, transferrin and magnetic iron oxide in the nanoliposomes was 83.06% ± 0.53%, 80.12% ± 0.12% and 66.14% ± 0.42%, respectively. The average size, zeta-potential and polydispersity index of nanoliposomes were 95.06 ± 0.15 nm, −1.40 ± 0.22 mv and 0.19 ± 0.09, respectively. In this study, the results from nanoliposomes size distribution showed a monomodal pattern. The magnetic properties of the nanoliposomes were analyzed by vibrating sample magnetometer at room temperature. The saturation magnetizations for nanoliposomes were 30.5 electromagnetic units per gram (emu/g) and the cryo-TEM analysis showed that the nanoliposomes have a fine spherical shape and rough surface [Figure 3].
Figure 3.
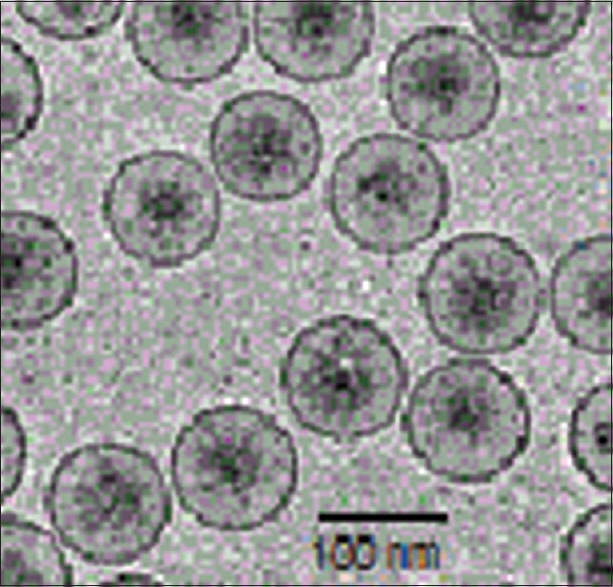
Cryo-transmission electron micrographs of the artemisinin and transferrin-loaded magnetic nanoliposomes
Tumor distribution of artemisinin, transferrin, and magnetic iron oxide
The concentration of artemisinin, transferrin and magnetic iron oxide in the plasma and tumors at 2 h after treatment is shown in Figure 4a and b. The plasma stability of artemisinin, transferrin, and magnetic iron oxide in the loaded form without an external magnetic force was significantly higher than those of free form. When compared to the free artemisinin and transferrin, the i.v administration of artemisinin and transferrin-loaded magnetic nanoliposomes followed using the magnetic field approximately produced 10- and 5.5-fold higher levels of artemisinin and transferrin in the tumors, respectively [Figure 4b]. Specifically, administration of magnetic artemisinin and transferrin nanoliposomes followed using the magnetic field produced about 4-and 3.8-fold higher concentrations of artemisinin and transferrin in the tumors, respectively, compared to magnetic artemisinin and transferrin nanoliposomes without magnetic field application [Figure 4b].
Figure 4.
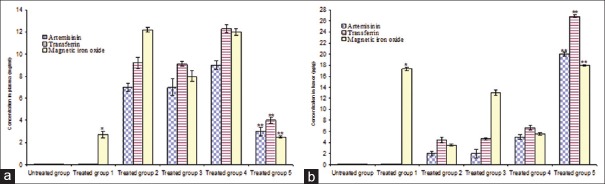
The artemisinin, transferrin and magnetic iron oxide concentrations in the plasma (a) and tumors (b) of treated mice. *Significant difference between treated group 1 versus control and treated groups 2, 3 and 4 (P < 0.01); **Significant difference between treated group 5 versus control and treated groups 2, 3 and 4 (P < 0.01)
Suppressive effects on primary tumor growth
All combined artemisinin and transferrin preparations could significantly suppress primary tumor growth compared to the control group [Figure 5]. At 15 days after treatment, the artemisinin and transferrin-loaded magnetic nanoliposomes combined with an external magnetic force not only completely suppressed the growth of primary tumor but also reduced the tumor volume in tumorized mice [Figure 5]. In the presence of an external magnetic force, a significant difference was observed between antitumor effects of magnetic artemisinin and transferrin nanoliposomes and other examined groups (P < 0.01).
Figure 5.
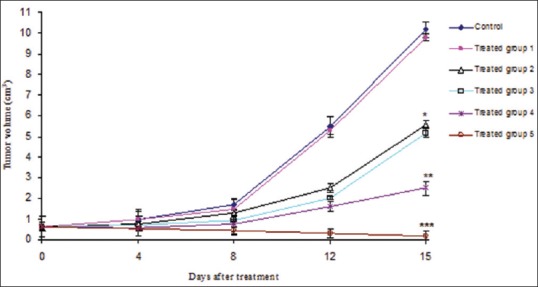
Antitumor effects of artemisinin, transferrin and magnetic iron oxide in the free and loaded forms as well as in the presence or absence of an external magnetic force. *Significant difference between treated groups 2 and 3 versus control and treated group 1 (P < 0.01); **Significant difference between treated group 4 versus treated groups 2 and 3 (P < 0.01); ***Significant difference between treated group 5 versus other groups (P < 0.01)
However, there was no significance in the effects of free artemisinin, transferrin and magnetic iron oxide with and without external magnetic force [Figure 5]. Altogether, the results indicate that the magnetic force significantly increased the therapeutic efficiency of magnetic nanoliposomal artemisinin and transferrin.
Histologic examination
Histologic examination of primary tumors at 15 days after treatment with magnetic artemisinin and transferrin nanoliposomes under external magnetic force revealed a significant apoptosis rates in the tumor cells [Figure 6]. Fifteen days after treatment, the central region of the tumor mass treated with artemisinin and transferrin-loaded magnetic nanoliposomes under the magnetic force was found with many apoptotic cells and some viable tumor cells [Figure 6]. The mean apoptosis rates of breast cancer cells for mice treated with artemisinin and transferrin-loaded magnetic nanoliposomes in the presence of magnetic force were higher than those of other groups.
Figure 6.
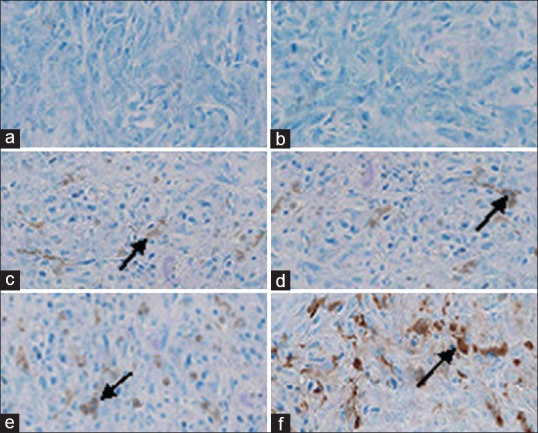
TdT mediated dUTP Nick End Labeling staining of breast cancer cells. The arrows show apoptotic cells. (a) Control group, (b) treated group with empty magnetic nanoliposomes (without artemisinin and transferrin), (c) treated group with free artemisinin, transferrin and magnetic iron oxide in the presence of an external magnetic force, (d) treated group with free artemisinin, transferrin and magnetic iron oxide in the absence of an external magnetic force, (e) treated group with artemisinin and transferrin-loaded magnetic nanoliposomes in the absence of an external magnetic force, and (f) treated group with artemisinin and transferrin-loaded magnetic nanoliposomes in the presence of an external magnetic force
DISCUSSION
Artemisinin is a sesquiterpene lactone and phytochemical found naturally in A. annua L.[1] Evidence for artemisinin's benefit was strongest for anti-malaria, anti-oxidative, anti-inflammatory, and anti-cancer effects.[4,5] It is reported that the anti-cancer effect of artemisinin in the presence of iron sources such as transferrin was increased several fold.[3]
The use of tagged and nanoparticulate forms of artemisinin for cancer therapy has been investigated.[20,21] However, the main problem associated with the application of such artemisinin formulations is insufficient delivery to the target site.[22]
In previous study, we prepared magnetic nanoliposomes containing artemisinin and transferrin with acceptable size homogeneity, polydispersity index, as well as the stability in pH 7.4 citrate-phosphate buffers within 12 h at 37°C.[6] Moreover, the in vitro studies showed that prepared nanoliposomes in the presence of a magnetic field could increase MCF-7 and MDA-MB-231 cells apoptosis, so that after 12 h application of magnetic force the majority of MCF-7 and MDA-MB-231 cells easily eliminated.[6]
In the present study, in vivo anticancer efficiency of artemisinin and transferrin-loaded magnetic nanoliposomes against breast tumor in BALB/c mice model was evaluated.
The results showed that the application of an external magnetic force for 2 h and elicited the highest artemisinin and transferrin concentration in the tumor [Figure 3b]. Moreover, our data showed that the antitumor effect of artemisinin and transferrin-loaded magnetic nanoliposomes was greater than those of free artemisinin and transferrin treatments.
In similar studies showed that magnetic nanoliposomes loaded with doxorubicin or adriamycin were tailored to target cancer cells, and the application of a magnetic field could increase their concentration in the tumors.[18,23]
Some hypotheses, including increasing the delivery and penetration of mentioned drugs into cancer cells, may explain the mechanism of the enhanced anticancer activities of these liposomal formulations.[24,25]
Magnetic artemisinin and transferrin nanoliposomes alone (without magnetic force) elicited slightly higher levels of artemisinin and transferrin in the plasma than free artemisinin and transferrin. In this case, it has been reported that encapsulating of drugs in nanocarriers could increase their stability in the bloodstream as well as enhanced their bioactivity.[26,27]
In the present study, histologic examination revealed high apoptosis rates in the breast cancer cells treated by artemisinin and transferrin-loaded magnetic nanoliposomes in the presence of an external magnetic force. These results showed that prepared nanoliposomes have acceptable performance, because it is reported that the survival rates of cancer patients was related to the percentage of apoptotic cancer cells as well as higher concentrations of antitumor agents in the location of tumors.[28,29]
No significant difference was observed between tumor volumes, and apoptotic cells rate groups treated with control liposomes (without artemisinin and transferrin) and PBS. Moreover, our results indicate that systemic chemotherapy with artemisinin and transferrin-loaded magnetic nanoliposomes and an external magnetic force could reduce tumor volume in the treated mice. This finding is consistent with previous studies, which showed that targeted drug delivery to the tumors could enhance drug performance.[30,31]
CONCLUSION
In the presence of a magnetic force, we successfully investigate the in vivo anti-breast cancer activity of artemisinin and transferrin-loaded magnetic nanoliposomes.
Altogether, in the presence of an external magnetic field, the systemic chemotherapy with magnetic nanoliposomes containing artemisinin and transferrin could effectively reduce the breast tumor mass in the treated mice. This is the first report regarding to breast cancer tumor targeted therapy by artemisinin and transferrin-loaded magnetic nanoliposomes and more research about efficacy of this formulation will be evaluated in future investigations.
ACKNOWLEDGMENT
The author gratefully acknowledges financial support from the Iran National Science Foundation (INSF) under grant agreement no: 91001200.
Footnotes
Source of Support: The author gratefully acknowledges financial support from the Iran National Science Foundation (INSF) under grant agreement no: 91001200.
Conflict of Interest: None declared.
REFERENCES
- 1.Oh YC, Jeong YH, Kim T, Cho WK, Ma JY. Anti-inflammatory effect of Artemisiae annuae herba in lipopolysaccharide-stimulated RAW 264.7 Cells. Pharmacogn Mag. 2014;10:S588–95. doi: 10.4103/0973-1296.139793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh NP, Lai HC. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004;24:2277–80. [PubMed] [Google Scholar]
- 3.Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Rustaiyan A, Nahrevanian H, Kazemi M. A new antimalarial agent; effect of extracts of Artemisia diffusa against Plasmodium berghei. Pharmacogn Mag. 2009;5:1–7. [Google Scholar]
- 5.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–8. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Gharib A, Faezizadeh Z, Mesbah-Namin SA, Saravani R. Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. DARU J Pharm Sci. 2014;22:44. doi: 10.1186/2008-2231-22-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva AK, Silva EL, Carriço AS, Egito ES. Magnetic carriers: A promising device for targeting drugs into the human body. Curr Pharm Des. 2007;13:1179–85. doi: 10.2174/138161207780618993. [DOI] [PubMed] [Google Scholar]
- 8.Chen GJ, Wang LF. Design of magnetic nanoparticles-assisted drug delivery system. Curr Pharm Des. 2011;17:2331–51. doi: 10.2174/138161211797052574. [DOI] [PubMed] [Google Scholar]
- 9.Wang YX, Xuan S, Port M, Idee JM. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr Pharm Des. 2013;19:6575–93. doi: 10.2174/1381612811319370003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu D, An X. Controllable release from magnetoliposomes by magnetic stimulation and thermal stimulation. Colloids Surf B Biointerfaces. 2013;104:326–9. doi: 10.1016/j.colsurfb.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Liu XM, Zhang Y, Chen F, Khutsishvili I, Fehringer EV, Marky LA, et al. Prevention of orthopedic device-associated osteomyelitis using oxacillin-containing biomineral-binding liposomes. Pharm Res. 2012;29:3169–79. doi: 10.1007/s11095-012-0812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai H, Sasaki T, Singh NP, Messay A. Effects of artemisinin-tagged holotransferrin on cancer cells. Life Sci. 2005;76:1267–79. doi: 10.1016/j.lfs.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Silva-Freitas EL, Carvalho JF, Pontes TR, Araújo-Neto RP, Carriço AS, Egito ES. Magnetite content evaluation on magnetic drug delivery systems by spectrophotometry: A technical note. AAPS PharmSciTech. 2011;12:521–4. doi: 10.1208/s12249-011-9610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharib A, Faezizadeh Z. In vitro anti-telomerase activity of novel lycopene-loaded nanospheres in the human leukemia cell line K562. Pharmacogn Mag. 2014;10:S157–63. doi: 10.4103/0973-1296.127368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan ZM, Yaraee R, Zare N, Ghazanfari T, Sarraf Nejad AH, Nazori B. Immunomodulatory affect of R10 fraction of garlic extract on natural killer activity. Int Immunopharmacol. 2003;3:1483–9. doi: 10.1016/S1567-5769(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 16.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajimoradi M, Hassan ZM, Pourfathollah AA, Daneshmandi S, Pakravan N. The effect of shark liver oil on the tumor infiltrating lymphocytes and cytokine pattern in mice. J Ethnopharmacol. 2009;126:565–70. doi: 10.1016/j.jep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Nobuto H, Sugita T, Kubo T, Shimose S, Yasunaga Y, Murakami T, et al. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int J Cancer. 2004;109:627–35. doi: 10.1002/ijc.20035. [DOI] [PubMed] [Google Scholar]
- 19.Chougule MB, Patel AR, Jackson T, Singh M. Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PLoS One. 2011;6:e17733. doi: 10.1371/journal.pone.0017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai H, Nakase I, Lacoste E, Singh NP, Sasaki T. Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res. 2009;29:3807–10. [PubMed] [Google Scholar]
- 21.Isacchi B, Arrigucci S, la Marca G, Bergonzi MC, Vannucchi MG, Novelli A, et al. Conventional and long-circulating liposomes of artemisinin: Preparation, characterization, and pharmacokinetic profile in mice. J Liposome Res. 2011;21:237–44. doi: 10.3109/08982104.2010.539185. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZY, Wang L, Zhang J, Li YT, Zhang DS. A study on the preparation and characterization of plasmid DNA and drug-containing magnetic nanoliposomes for the treatment of tumors. Int J Nanomedicine. 2011;6:871–5. doi: 10.2147/IJN.S16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo T, Sugita T, Shimose S, Nitta Y, Ikuta Y, Murakami T. Targeted systemic chemotherapy using magnetic liposomes with incorporated adriamycin for osteosarcoma in hamsters. Int J Oncol. 2001;18:121–5. doi: 10.3892/ijo.18.1.121. [DOI] [PubMed] [Google Scholar]
- 24.Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces. 2014;114:349–56. doi: 10.1016/j.colsurfb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, et al. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8:440–51. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokhtarieh AA, Davarpanah SJ, Lee MK. Ethanol treatment a non-extrusion method for asymmetric liposome size optimization. DARU J Pharm Sci. 2013;21:32. doi: 10.1186/2008-2231-21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levacheva I, Samsonova O, Tazina E, Beck-Broichsitter M, Levachev S, Strehlow B, et al. Optimized thermosensitive liposomes for selective doxorubicin delivery: Formulation development, quality analysis and bioactivity proof. Colloids Surf B Biointerfaces. 2014;121:248–56. doi: 10.1016/j.colsurfb.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Ichihara H, Nakagawa S, Matsuoka Y, Yoshida K, Matsumoto Y, Ueoka R. Nanotherapy with hybrid liposomes for colorectal cancer along with apoptosis in vitro and in vivo. Anticancer Res. 2014;34:4701–8. [PubMed] [Google Scholar]
- 29.Yang C, Fu ZX. PEG-liposomal oxaliplatin combined with nuclear factor-κB inhibitor (PDTC) induces apoptosis in human colorectal cancer cells. Oncol Rep. 2014;32:1617–21. doi: 10.3892/or.2014.3336. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Zhang L, Zhu D, Song C. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int J Nanomedicine. 2014;9:4055–66. doi: 10.2147/IJN.S61880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang M, Yoon YI, Kwon YS, Yoon TJ, Lee HJ, Hwang SI, et al. Trastuzumab-conjugated liposome-coated fluorescent magnetic nanoparticles to target breast cancer. Korean J Radiol. 2014;15:411–22. doi: 10.3348/kjr.2014.15.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]


