Abstract
The purpose of this study was to analyze the inhibitory action of quercetin glycosides by computational docking studies. For this, natural metabolite quercetin glycosides isolated from buckwheat and onions were used as ligand for molecular interaction. The crystallographic structure of molecular target angiotensin-converting enzyme (ACE) (peptidyl-dipeptidase A) was obtained from PDB database (PDB ID: 1O86). Enalapril, a well-known brand of ACE inhibitor was taken as the standard for comparative analysis. Computational docking analysis was performed using PyRx, AutoDock Vina option based on scoring functions. The quercetin showed optimum binding affinity with a molecular target (angiotensin-converting-enzyme) with the binding energy of −8.5 kcal/mol as compared to the standard (−7.0 kcal/mol). These results indicated that quercetin glycosides could be one of the potential ligands to treat hypertension, myocardial infarction, and congestive heart failure.
Keywords: Angiotensin-converting enzyme inhibition, binding energy, in silico analysis, quercetin glycosides
INTRODUCTION
Hypertension and congestive heart failures are becoming epidemic throughout the world. In 2000, nearly one billion cases of the adult population of the world had hypertension[1] and more than 20 million people were affected with heart failure.[2] In the last three decades, several intensive efforts have been conducted into researching the antihypertensive therapeutic values of local plants and as compared to allopathic treatment, medicinal and bioactive plants have become a vital resource for the treatment of heart problems. Almost 80% of the world population use natural medicines, largely in developing countries, because of their better acceptability with human physiology and lesser side effects.[3] Therefore, to control blood pressure and heart failure diseases without any side effects is increasing demand for natural products in health care system.
Quercetin glycosides [Figure 1] are found in citrus fruit, buckwheat and onions, and preliminary studies proved its potential therapeutic qualities and lesser side effects.[4,5,6,7] The use of this glycoside to inhibit the function of angiotensin-converting enzyme (ACE) by virtual screening is an important consideration. Computational and bioinformatics tools have become very important resource to identify the potential targets for various ligands.[8] Using these virtual tools, we analyzed to find quercetin as a good ACE inhibitor.
Figure 1.
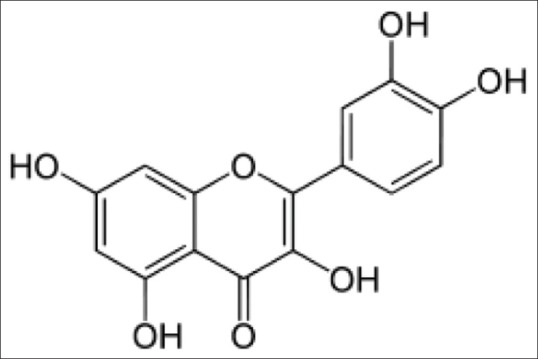
Quercetin glycosides
Angiotensin-converting enzyme is secreted in the lungs and kidneys by cells in the endothelium of blood vessels, and it is the part of the renin-angiotensin system (RAS). It indirectly increases blood pressure by causing blood vessels to constrict by converting angiotensin-I to angiotensin-II. For this reason, ACE is an ideal target for controlling blood pressures and heart failures,[9] and synthetic compounds are being used as ACE inhibitors to treat heart problems. These inhibitors inhibit the conversion (angiotensin-I to angiotensin-II), dilate the blood vessels and control the blood pressures.
It has been found that there are no reports of ACE inhibition of quercetin glycosides and the conformation of binding of these glycosides with ACE has not yet been observed. In this study, the structural model of the quercetin in ACE has been performed, which may expedite further development of more natural ACE-inhibitors to control heart problems.
MATERIALS AND METHODS
Accession of target protein
The three-dimensional structure of ACE (PDB: 1O86) was downloaded from the RCSB protein Data Bank.[10]
Ligand selection
The chemical structure of quercetin glycoside was obtained from PubChem compound database. It was prepared by ChemBioDraw and MOL SDF format of this ligand was converted to PDBQT file using PyRx tool to generate atomic coordinates.
Target and ligand optimization
For docking analysis, PDB coordinates of the target protein and quercetin molecule were optimized by Drug Discovery Studio version 3.0 software and UCSF Chimera tool, respectively (adding missing residues). These coordinates had minimum energy and stable conformation.
Analysis of target active binding sites
The active sites are the coordinates of the ligand in the original target protein grids, and these active binding sites of target protein were analyzed using the Drug Discovery Studio version 3.0 and 3D LigandSite virtual tools.[11]
Molecular docking analysis
A computational ligand-target docking approach was used to analyze structural complexes of the ACE (target) with quercetin glycoside (ligand) in order to understand the structural basis of this protein target specificity. Initially, protein–ligand attraction was investigated for hydrophobic/hydrophilic properties of these complexes by Platinum software web server.[12] Finally, docking was carried out by PyRx, AutoDock Vina option based on scoring functions. The energy of interaction of quercetin glycoside with the ACE is assigned “grid point.” At each step of the simulation, the energy of interaction of ligand and protein was evaluated using atomic affinity potentials computed on a grid. The remaining parameters were set as default.
RESULTS AND DISCUSSION
The minimum binding energy indicated that the ACE protein (target enzyme) was successfully docked with quercetin glycosides [Table 1]. The possible binding modes of quercetin at ACE active sites have been shown in Figure 2. ACE protein residues Arg 124, Tyr 135, Ile 204, Ala 208, Glu 216, Tyr 215, Glu 96 was formed H-bond with quercetin ligand molecule. Quercetin showed relatively good binding affinity as compared to enalapril as standard which showed minimum binding energy of −7.0 kcal/mol.
Table 1.
Energy and RMSD values obtained during docking analysis of quercetin as ligand molecule and ACE as target protein
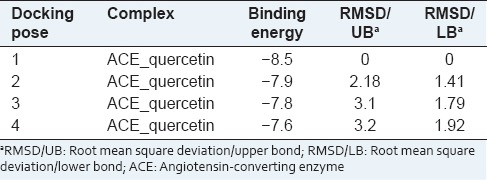
Figure 2.
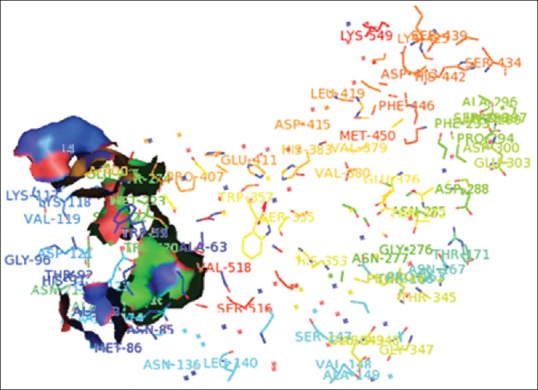
Potential binding sites of angiotensin-converting enzyme: Arg 124, Tyr 135, Ile 204, Ala 208, Glu 216, Tyr 215, Glu 96
Angiotensin-converting enzyme, angiotensin I and angiotensin II are part of the RAS, which controls blood pressure by regulating the volume of fluids in the body. ACE is secreted in the lungs and kidneys by cells in the endothelium (inner layer) of blood vessels.[9]
It is responsible for conversion of angiotensin I to angiotensin II that is a potent vasoconstrictor and its inhibition results in the decreased formation of angiotensin II and decreased metabolism of bradykinin, leading to systematic dilation of the arteries and veins and a decrease in arterial blood pressure.[13,14]
Angiotensin-converting enzyme inhibitors reduce the activity of the renin-angiotensin-aldosterone system as the primary etiologic (causal) event in the development of hypertension.[15] The research on potential ACE inhibitors is expanding broadly, and most are focused on natural product derivatives such as peptides, polyphenolics, glycosides, and terpenes.
Angiotensin-converting enzyme inhibitory drugs are first-class therapeutics since decades. Captopril, lisinopril, enalapril, and ramipril are some examples for drugs targeted as ACE inhibitors. However, the prolong use of the drugs could initiate adverse side effects such as dizziness, coughing, and angioneurotic edema. New alternatives have been explored extensively as replacements of these drugs. Most of the researches have been targeted at bioactive compounds from natural resources.[16] Anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa was studied for ACE inhibition.[17] In 2009, Charles et al. reported naturally occurring glycosides as ACE inhibitors.[18] Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively.[19]
The distribution of hydrophobic/hydrophilic properties of ACE and its binding site projected onto the surface of the quercetin, and it has been observed that aromatic groups are less hydrophilic. These stacking interactions are used to rank the molecular docking and matching between target and drugs. Considering hydrophobic properties of these interacting molecules (ACE protein target and quercetin) using the concepts of the molecular hydrophobicity potential was calculated.[20] These hydrophobicity maps are used to analyze the progress of hydrophobicity clusters on the membrane surface along the molecular dynamics run and the features of interface between the membrane and membrane-binding molecule [Figure 3].
Figure 3.
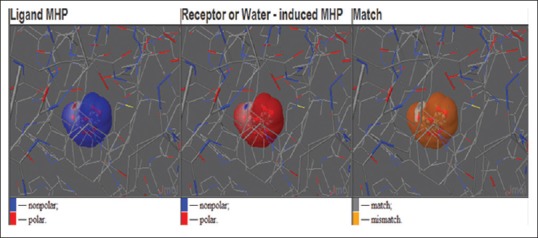
Visuals where 3-windows show the distribution of hydrophobic/hydrophilic properties of angiotensin-converting enzyme in X-ray position (left) and exerted by protein surroundings (middle), and their complementarity (right). The screenshot was produced using the online visualization Jmol applet as implemented in the PLATINUM web-service
The docking of ACE target with quercetin using docking procedure revealed that all the computationally predicted lowest energy complexes of ACE are stabilized by intermolecular hydrogen bonds and stacking interactions. It is found that A, SA, OA, HD, N are the ligand atoms involved in docking with the enzymes. The AutoGrid model presented the most energetically favorable binding mode of quercetin to ACE. The quercetin as ligand is docked into the generated combined grids and the root mean square deviation (RMSD) from native pose and the binding energy are evaluated, and it is observed that the weight averaged grids performs the best. The ligand showed the best interaction with target proteins based on the RMSD values as compared to standard. Beside RMSD clustering, AutoDock Vina has also calculated the binding free energies of these interactive molecules to find the best binding mode. The calculated final docked energies for quercetin is −8.5 kcal/mol [Figure 4] and for enalapril (standard) is −7.0 kcal/mol [Figure 5]. Docking results revealed that this quercetin compound can enter the substrate-binding region of the active site. Finally, the results demonstrated clearly that quercetin accurately interact with ACE protein target.
Figure 4.
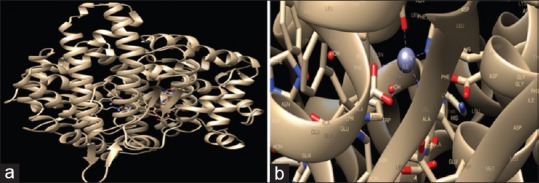
Visuals of docking interaction steps of quercetin ligand molecule. (a) The AutoGrid dimensions between ligand and angiotensin-converting enzyme target protein atoms (A, HD, OA, and N) are: grid center X: 18.0175, Y: 23.2912, Z: 13.5997 with dimension (Angstrom) X: Y: Z: 25.000. (b) Confirmation and pose of quercetin ligand molecule with a protein target and binding site atoms of angiotensin-converting enzyme amino acids residues around ligand
Figure 5.
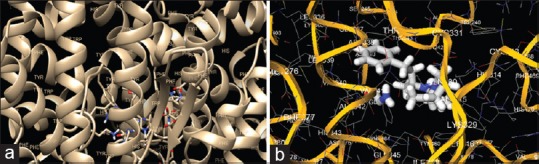
Docking procedure of enalapril as a standard ligand. (a) The AutoGrid dimensions between ligand and angiotensin-converting enzyme (ACE) target protein are: grid center X: 13.0161, Y: 19.2901, Z: 11.0499. (b) Confirmation and pose of enalapril with ACE protein target with binding affinity of − 7.0 kcal/mol
CONCLUSION
Docking studies of the quercetin glycosides with ACE showed that this ligand is good molecule which docks well with ACE target. Therefore, quercetin molecule plays an important play role in inhibiting the conversion of angiotensin I to angiotensin II to regulate the blood pressure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabassum N, Ahmad F. Role of natural herbs in the treatment of hypertension. Pharmacogn Rev. 2011;5:30–40. doi: 10.4103/0973-7847.79097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LL, Yang XB, Huang ZM, Liu HZ, Wu GX. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin. 2007;28:404–9. doi: 10.1111/j.1745-7254.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 5.Verschoyle RD, Steward WP, Gescher AJ. Putative cancer chemopreventive agents of dietary origin-how safe are they? Nutr Cancer. 2007;59:152–62. doi: 10.1080/01635580701458186. [DOI] [PubMed] [Google Scholar]
- 6.Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, et al. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HJ, Lee CM, Jung ID, Lee JS, Jeong YI, Chang JH, et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. 2009;9:261–7. doi: 10.1016/j.intimp.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Osguthorpe DJ, Sherman W, Hagler AT. Generation of receptor structural ensembles for virtual screening using binding site shape analysis and clustering. Chem Biol Drug Des. 2012;80:182–93. doi: 10.1111/j.1747-0285.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 9.Kierszenbaum AL. Histology and Cell Biology: An Introduction to Pathology. Mosby Elsevier. 2007 [Google Scholar]
- 10. [Last accessed on 2014 Aug 15]. Available from: http://www.rcsb.org/pdb/home/home.do .
- 11.Wass MN, Kelley LA, Sternberg MJ. 3DLigandSite: Predicting ligand-binding sites using similar structures. Nucleic Acids Res. 2010;38:W469–73. doi: 10.1093/nar/gkq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyrkov TV, Chugunov AO, Krylov NA, Nolde DE, Efremov RG. PLATINUM: A web tool for analysis of hydrophobic/hydrophilic organization of biomolecular complexes. Bioinformatics. 2009;25:1201–2. doi: 10.1093/bioinformatics/btp111. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Xu X, Chen T, Li L, Rao P. An assay for angiotensin-converting enzyme using capillary zone electrophoresis. Anal Biochem. 2000;280:286–90. doi: 10.1006/abio.2000.4535. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde RE. ACE-inhibitors. Cardiovascular Pharmacol Concepts. [Last retrieved on 2014 Aug 18]. Available from: http://www.cvpharmacology.com .
- 15.Jandeleit-Dahm K, Cooper ME. Hypertension and diabetes: Role of the renin-angiotensin system. Endocrinol Metab Clin North Am. 2006;35:469–90. doi: 10.1016/j.ecl.2006.06.007. vii. [DOI] [PubMed] [Google Scholar]
- 16.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–42. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 17.Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 18.Charles RE, Ponrasu T, Sivakumar R, Divakar S. Angiotensin-converting enzyme inhibitory and antioxidant activities of enzymatically synthesized phenolic and vitamin glycosides. Biotechnol Appl Biochem. 2009;52:177–84. doi: 10.1042/BA20080041. [DOI] [PubMed] [Google Scholar]
- 19.Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A. In vitro studies indicate that miquelianin (quercetin 3-O-beta-D-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 2003;69:1013–7. doi: 10.1055/s-2003-45148. [DOI] [PubMed] [Google Scholar]
- 20.Muhammad SA, Ali A, Ismail T, Zafar R, Ilyas U, Ahmad J. Insilico study of anti-carcinogenic lysyl oxidase-like 2 inhibitors. Comput Biol Chem. 2014;51:71–82. doi: 10.1016/j.compbiolchem.2014.03.002. [DOI] [PubMed] [Google Scholar]


