Abstract
Background:
Deregs been implicated in the malignancy of cancer. Since many years investigation on the traditional herbs has been the focus to develop novel and effective drug for cancer remedies. Wheatgrass is a medicinal plant, used in folk medicine to cure various diseases. The present study was undertaken to gain insights into antiproliferative effect of methanol extract of wheatgrass.
Materials Methods:
Cell viability was assessed via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and Lactate Dehydrogenase assays. Cell cycle was analyzed by flow cytometry. Western blot was performed to determine the p53 and cyclin D1 levels. In silico docking interaction of the 14 active components (identified by high-performance liquid chromatography/gas chromatography-mass spectroscopy) of the methanol extract was tested with cyclin D1 (Protein Data Bank ID: 2W96) and compared with the reference cyclin D1/Cdk4 inhibitor.
Results:
Methanol extract of wheatgrass effectively reduced the cell viability. The cell cycle analysis showed that the extract treatment caused G1 arrest. The level of cyclin D1 was decreased, whereas p53 level was increased. Molecular docking studies revealed interaction of seven active compounds of the extract with the vital residues (Lys112/Glu141) of cyclin D1.
Conclusion:
These findings indicate that the methanol extract of wheatgrass inhibits human laryngeal cancer cell proliferation via cell cycle G1 arrest and p53 induction. The seven active compounds of the extract were also found to be directly involved in the inhibition of cyclin D1/Cdk4 binding, thus inhibiting the cell proliferation.
Keywords: Clycin D1, docking study, G1 arrest, Methanol extract, p53, wheatgrass
INTRODUCTION
Cancer is a large family of complex diseases, which is life-threatening and partially understood. Multiple forms of cancers are prevailing in the world, out of which head and neck cancer is the sixth most common type. Head and neck cancer include cancer of oral cavity and cancer in the larynx. Human laryngeal cancer (Hep2) cells are laryngeal cancer cells.[1] In past two decades, there has not been much development toward prevention, improvement and 5 years survival of the patient with laryngeal cancer. Due to the limited success of available treatment, alternative strategies are needed for early prognosis and treatment of laryngeal or/and other types of cancer.[1]
Multiple factors are involved in the development of cancer. Thus cancer is known as a multifactorial disease.[2] But uncontrolled growth is a characteristic feature of all types of cancer, which occurs due to alterations in pathways regulating the cell cycle.[3] The integrity and fidelity of the genome are tightly regulated in cell cycle. However, modulation of check points in the G1/S and G2/M phase of cell cycle is associated with malignancy and also contributes to resistance in chemotherapy.[3,4]
Mitogenic signal induces quiescent cells (G0) to undergo proliferation, which follows distinct phases like G1 (pre-DNA synthesis), S (DNA synthesis), G2 (pre-division), and M (cell division).[5] Cell cycle arrest is a very crucial step, which provides an opportunity for DNA to repair and to eliminate the aberrant cell.[2] Deregulation of cell cycle and abnormal proliferation is ubiquitous to all cancer types, and hence inhibition of cell cycle is the potential target for cancer therapy.[5]
Tumor suppressor protein, p53, plays a pivotal role in protecting normal cells from cancer by inducing cell cycle arrest, apoptosis and DNA repair.[6] One of the important targets of p53 to control cell cycle is p21, which is an inhibitor of cyclin dependent kinase Cdk. It induces the cell cycle arrest at G1 phase by inhibiting the activity or by binding to cyclin D1/Cdk4 complex.[7] Thus plays a significant role in controlling cell cycle.
Chemotherapy is crucial for cancer treatment, and various chemotherapeutic drugs are available. Though they are effective, the associated side effects have urged the demand for the development of novel drugs from the natural source. Plant based products are the vital resources for cancer therapy therefore gaining more popularity for the management and prevention of cancer.[2] Phytoconstituents of plant extract act by various mechanisms, including cell cycle arrest, induction of apoptosis and DNA repair system, alteration of the immune system and carcinogen metabolism.[8]
In a continuation of the search for the potent natural anticancer drug, we have selected wheatgrass in our present study. Wheat (Triticum aestivum L.) is a common food item at home. The shoot of wheat is called as wheatgrass. It is known to be a rich source of phenol, flavonoids, vitamins and minerals. Wheatgrass also contains Vitamin A, B1, C and E, β-carotene, ferulic acid, and vanilic acid, many other minerals and trace elements including calcium, iodine, selenium and zinc. Wheatgrass is a common health supplement, consumed in the form of a tablet and fresh juice to boost up the health and vitality. Wheatgrass (juice and powder) have shown the beneficial effect against various degenerative disease including cancer, rheumatoid arthritis, ulcer, thalassemia, etc.[9,10,11]
Until now there has been very little study on the anticarcinogenic effect of wheatgrass. As it contains phenolic compounds and flavonols, and as it is known to possess antioxidant, it can be a potent anticarcinogenic agent. Inhibition or arrest of cell cycle in cancer cells has become a major indicator of anticancer effects of the drug. Hence, the present study was aimed at analyzing the cytotoxic and antiproliferative effect of the methanol extract of wheatgrass against the Hep2 cell line. In our earlier study, we have found the presence of five Polyphenols (identified by high-performance liquid chromatography [HPLC]) [Table 1, B1-B5] and nine bioactive constituents (analyzed by gas chromatography-mass spectroscopy (GCMS) [Table 1, C1-C9])[11] in methanol extract of wheatgrass. In the present study, we have also performed in silico docking to evaluate the interaction of these active components with cyclin D1 (Protein Data Bank ID [PDB]: 2W96) and compared with the known cyclin D1/Cdk4 inhibitor, 2-bromo-12, 13-dihydro-5H-indolo [2, 3-a] pyrrolo[3, 4-c] carbazole-5, 7 (6H)-dione [Table 1a].
Table 1.
Comparison of docking results of CyclinD1/Cdk4 inhibitor and active components (identified by high-performance liquid chromatography/gas chromatography-mass spectroscopy) of methanol extract of wheatgrass to the Cyclin D1 targets protein
MATERIALS AND METHODS
Chemicals
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco's modified eagle medium (DMEM), trypan blue, antibiotics and fetal bovine serum (FBS) were purchased from Sigma–Aldrich, Bangalore, India. Primary antibodies against cyclin D1 (sc-753), p53 (sc-98) and secondary antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). All other reagents and chemicals used were of analytical grade. The Hep2 cell line was purchased from National Centre for Cell Science, Pune, India. Wheatgrass powder was purchased from Eden Park Agro Products Pvt Ltd, under the brand name of “Green Heart”, who is the grower, manufacturer and exporter of wheatgrass.
Sample preparation
Wheatgrass powder was subjected to soxhlet extraction at 50°C using methanol as a solvent for about 24 h. Solvent extract was further evaporated to dryness and stored at <4°C for further studies.
Cell lines and culture conditions
Human laryngeal cancer cells were cultured in T-25 flask maintained in 1 × DMEM supplemented with 10% FBS at 37°C in CO2 incubator in an atmosphere of humidified 5% CO2 and 95% air. DMSO (0.2%) was used to dissolve the methanol extract of wheatgrass to perform various assays.
Cytotoxicity effect
Cell proliferation was measured using the MTT, which colorimetrically measures as a purple formazan compound produced by viable cells.[12] Hep2 cells were collected at a final concentration of 2 × 104 cells/ml in a fresh DMEM with 10% FBS. Aliquots of 100 μl cell suspension were plated in 96-well tissue culture plates. Cells were treated with different concentrations (100–1000 μg/ml) of methanol extract of wheatgrass and incubated for 48 h. After 48 h, 20 μl of a 5 mg/ml MTT solution was added to each well, and the plate was incubated for 4 h, allowing the viable cells to reduce the yellow MTT to dark-blue formazan crystals, which were dissolved in 100 μl of DMSO. The absorbance in individual wells was determined at 570 nm using microplate reader (molecular devices). The cell viability was calculated as percentage of viable cells and then plotted on a graph.
Growth inhibition (%) = (A570 nm of treated cells/A570 nm of controlled cells) ×100
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) was determined by the method of Chenault and Whitesides.[13] Cells were treated for 48 h with different concentrations (100–1000 μg/ml) of methanol extract of wheatgrass. An extra 3 wells were treated for 1 h or until all the cells were lysed with 0.3% Triton X in phosphate buffer saline (PBS). For each sample, 200 μl of 1.22 mM pyruvate in 50 mM phosphate buffer and 4 μl of 12.4 mg/ml NADH dissolved in 50 mM phosphate buffer were added. A volume of 20 μl of supernatant from treated cells was then added and (immediately first reading was taken at 340 nm) then plate was incubated at 37°C for 30 min (and second reading was taken). The LDH concentration was measured at 340 nm and the treated groups were compared with Triton X, which was considered to release 100% LDH.
Activity was expressed in units/mg protein. One unit is defined as μM NADH utilized/min.
Cell cycle analysis by flow cytometry
To determine the effect of wheatgrass on the cell cycle, Hep2 (2 × 106 cells each) cells were treated with the 100 μg/ml concentration of methanol extract of wheatgrass for 48 h and fixed with 70% ethanol. After overnight incubation at 4°C, cells were washed with PBS prior to staining with propidium iodide (PI) (25 μg/ml PI; 0.1% triton X; 50 μg/ml RNase in PBS pH 7.2). The cells were analyzed using BD FACS Canto™ (BD, Singapore) and data were analyzed using the BD FACSDiva software (BD Biosciences, CA).
Western blotting
Western blot analysis was carried out using crude lysate of Hep2 cells, treated with selected concentrations (100 μg/ml and 200 μg/ml) of methanol extract of wheatgrass. Cells were homogenized and lysed in RIPA buffer (150 mM NaCl, 50 M Tris, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 7.4). Protein concentration was determined using Lowry reagent (Sigma-Aldrich) and 40 μg of protein lysate from each group were loaded and were resolved on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels. The proteins were then electro-transferred onto nitrocellulose membrane membrane. After blocking with 5% nonfat milk in Tris buffered saline (TBS, 0.1M, pH 7.4), blots were incubated with primary antibody at 4°C overnight. Protein abundance of β-actin served as a control for protein loading. Membranes were incubated with HRP-conjugated secondary antibody, diluted at an appropriate dilution in 1% BSA, for 2 h at room temperature. After each step, blots were washed thrice with tween (0.2%)-tris-buffer saline. Protein bands were detected by using a chromogenic substrate. Relative band intensity of protein was analyzed by Image J software.
Molecular docking
Ligand preparation
The 14 compounds identified by HPLC/GCMS were used for the docking studies and is listed in Table 1. The cyclin D1/Cdk4 inhibitor (PubChem ID: 5330797) is reported to be a specific inhibitor of cyclin D1-Cdk4 interaction and hence was used as a reference compound in this study. The initial structures were obtained from PubChem database and prepared for docking studies using Schrodinger. The LigPrep 2.5 utility (LigPrep, version 2.5, Schrödinger, LLC, New York, NY, 2011) of Schrodinger was used to (i) generate the tautomers and stereo isomers, and (ii) to perform ligand minimization using the OPLS-2005 force field.
Protein preparation
The crystal structure of cyclin D1 complexed with Cdk4 (PDB ID: 2W96)[14] was selected for docking analysis using Schrodinger. Prior to docking, water molecules beyond 5 Å from the cyclin D1-Cdk4 interface were removed from the crystal structure, and the important water molecules within 5 Å that may involve in interactions were retained. Hydrogens and missing atoms in the crystal structure were added using Maestro interface (Schrödinger Inc., New York). The newly added hydrogens were minimized by restraining the protein conformation followed by a complete minimization. The Polak-Ribiere Conjugate Gradient minimization has been used with OPLS-2005 force field to achieve energy tolerance lesser than 0.05 kcal/mol.
Docking procedure
The 14 compounds along with the reference compound were docked with cyclin D1 at the cyclin D1-Cdk4 interface using Glide 5.7 (Glide, version 5.7, Schrödinger, LLC, New York, NY, 2011). The van der Waals radii of nonpolar atoms were scaled by the default parameters of Receptor-Grid Generation program. The residues Ala153, Ala34, Met113, Trp150, Lys149, Lys33, Ala30, Pro118, Phe108, Ala121, Thr120, Lys112, Glu141, Leu138, Leu142, Asn146 and Glu115 of Cyclin D1 at the cyclin D1-Cdk4 interface were used to define the centroid of the receptor grid. The docking analysis has been performed using the standard precision (SP) algorithm to extract the low energy conformations with a van der Waals scaling factor of 0.80 and a partial charge cut off of 0.15 for ligands. The conformation of ligands with good docking score was used for free energy of binding analysis.
Calculation of free energy of binding
The free energy of binding of protein-ligand complex has been calculated using implicit MM-GBSA approach as implemented in Prime module of Schrodinger (Prime, version 3.0, Schrödinger, LLC, New York, NY, 2011). This method includes, OPLS derived molecular mechanics energies (EMM), a Surface Generalized Born continuum solvation model that governs polar solvation (SGB), a nonpolar solvation term (GNP) explaining the contribution from nonpolar solvent accessible surface area (SASA) and van der Waals interactions (vdW) to obtain the free energy of binding and the respective equation is shown below.
ΔGbind = Gcomplex − (Gprotein + Gligand)
where G = Emm + SGB + GNP
The ligand, receptor and the complex were individually minimized in GBSA continuum solvent and the free energy of binding (ΔGbind) was calculated as the difference between bound and unbound state energies. All the docked conformations were minimized using conjugate gradient algorithm, and the free energy of binding was calculated.
Statistical analysis
The results are presented as mean ± standard deviation of triplicate observations. All the data were analyzed using the SPSS 13-Windows Students version software. Statistical analysis was performed by analysis of variance (ANOVA), followed by Tukey's test. P ≤ 0.05 was considered to be statistically significant.
RESULTS
Cytotoxic effect
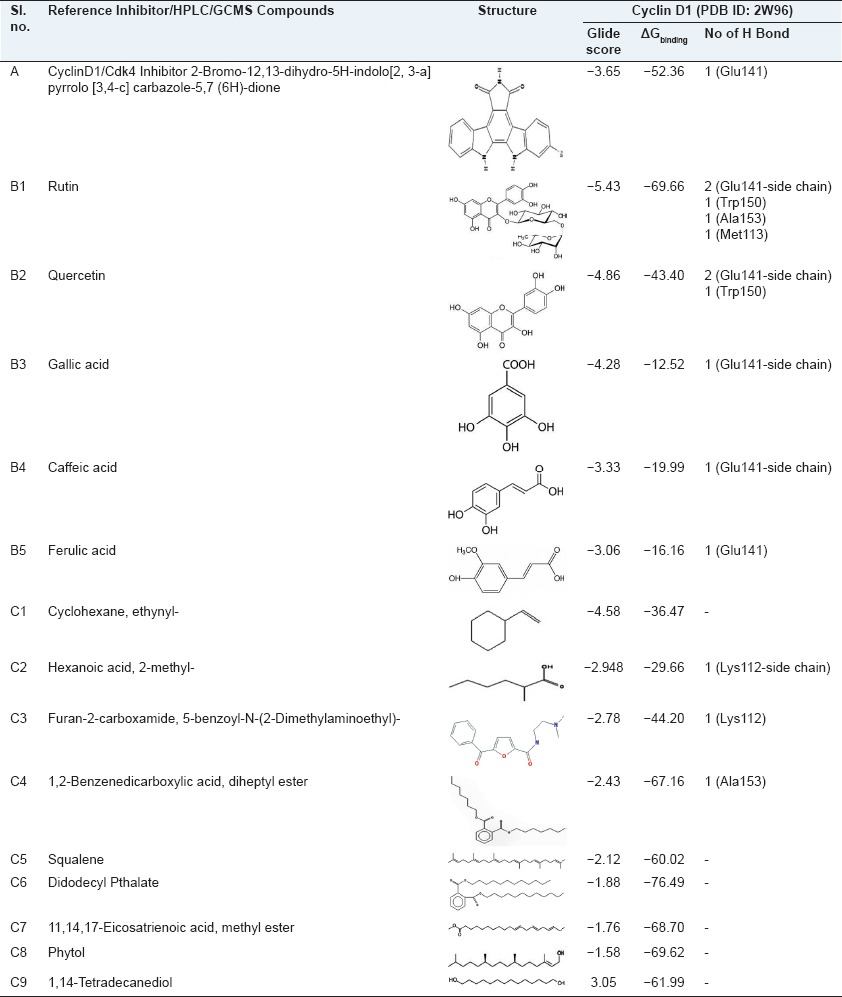
In MTT assay [Figure 1a], extract showed a significant reduction in the proliferation of the Hep2 cell line and the inhibitory concentration (IC50) was found to be 200 μg/ml at 48 h treatment. LDH assay [Figure 1b] showed a significant dose dependent increase in LDH activity in the spent medium of treated cell.
Figure 1.
Cytotoxic effect of methanol extract of wheatgrass by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Lactate Dehydrogenase (LDH) assays at 48 h treatment (a) MTT assay shows growth inhibition (b) LDH assay shows the effect on LDH release of human laryngeal cancer cells (in vitro) by different concentration of methanol extract of wheatgrass at 48 h treatment. Values are means ± standard deviation of triplicate observations from one representative of at least three experiments with similar results
Morphological changes
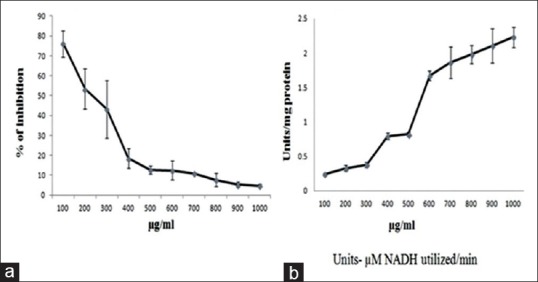
The cytotoxic effect of the drug leads to the morphological changes in the cell structure. Figure 2 clearly shows the change in the morphology (shrinkage of cells and distorted structure) of Hep2 cells when treated with inhibitory concentration of methanol extract of wheatgrass compared with control (nontreated cells).
Figure 2.
Changes in the morphology of Hep2 cells after 48 h treatment with IC50 concentration of methanol extract of wheatgrass
Cell cycle distribution through flow cytometry
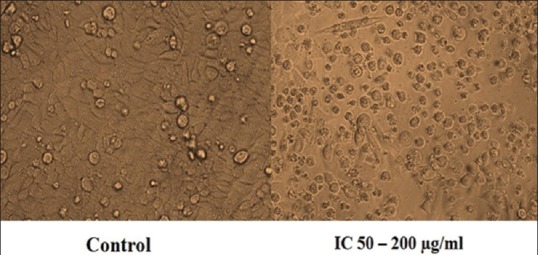
Cell cycle analysis revealed a progressive accumulation of cells in the G1 phase of the cell cycle [Figure 3]. Flow cytometric analysis of the treated cells showed an increase in the percentage of cells in the G1 phase from 44% to 66% and a decrease in the percentage of cells from 27% to 14% in the S phase and 15% to 14% in G2 /M phase.
Figure 3.
Cell cycle distribution through flow cytometry measured with propidium iodide assay. Effect of methanol extract of wheatgrass on cell cycle distribution of human laryngeal cancer cells at 48 h incubation
Cyclin D1 and p53 levels by western blotting
The results showed a significant decrease in the expression of cyclin D1 and increase in the expression of p53 in Hep2 cells treated with 100 μg/ml and 200 μg/ml concentration of methanol extract of wheatgrass for 48 h [Figure 4]
Figure 4.
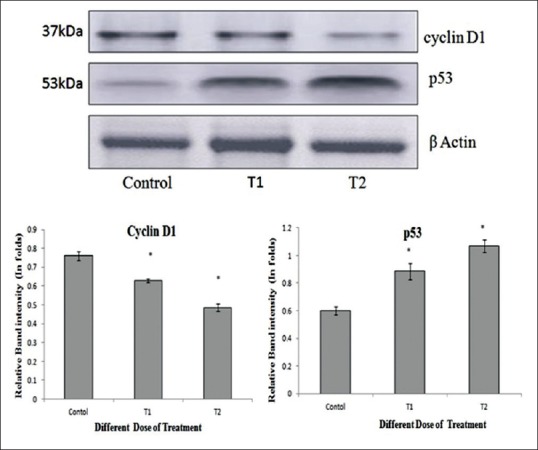
Western blot and densitometry analysis of proteins. Effect of methanol extract of wheatgrass on the levels of cyclin D1 and p53 in human laryngeal cancer cells. The cells were treated with methanol extract of wheatgrass at T1-100 µg/ml and T2-200 µg/ml doses for 48 h. Forty μg protein from each samples (control and treated) were electrophoresed and western blot was carried out using antibody against cyclin D1, p53 and β-actin was detected as a loading control. Values are expressed as mean ± standard deviation of three independent experiments considered at the P ≤ 0.05 levels
Docking study
In silico docking study of all five HPLC identified polyphenols showed H-bond interaction with cyclin D1 [Table 1, B1-B5]. Rutin showed two side chain H-bond interactions with Glu141, other H-bond with Trp150, Ala153, Met113 and one π-π interaction with Trp150. Quercetin showed two side chains H-bond with Glu141, other H-bond with Trp150 and one π-π interaction with Trp150, gallic acid showed one side chain H-bond with Glu141, caffeic acid showed one side chain H-bond with Glu141 and one π-π interaction with Trp150, ferulic acid showed one H-bond with Glu141 and one salt bridge with Lys112. The reference cyclin D1/Cdk4 inhibitor (2-bromo-12, 13-dihydro-5H-indolo[2, 3-a] pyrrolo[3,4-c] carbazole-5, 7 (6H)-dione) was found to form strong H-bond with Glu141 of cyclin D1 [Figure 5a]
Figure 5a.
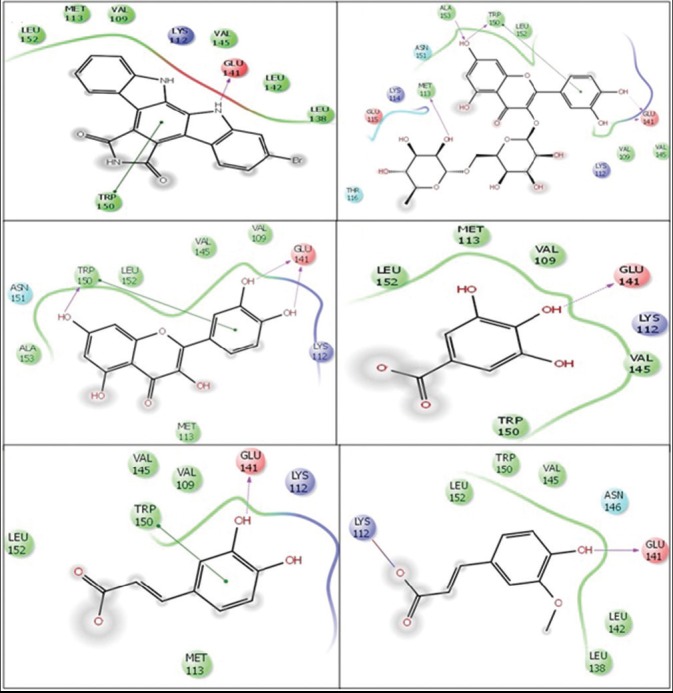
Molecular docking (two-dimensional images) of cyclin D1/Cdk4 inhibitor and polyphenols (identified by high-performance liquid chromatography) of methanol extract of wheatgrass with cyclin D1 (Protein Data Bank ID (PDB ID): 2W96). Docking pose of (a) cyclin D1/Cdk4 inhibitor (2-Bromo-12, 13-dihydro-5H-indolo[2, 3-a] pyrrolo[3, 4-c] carbazole-5, 7 (6H)-dione) (b) Rutin (c) Quercetin (d) Gallic acid (e) Caffeic acid and (f) Ferulic acid. Green lines show π-π interactions and pink lines show H-bond interactions with the arrow head pointing the acceptor atom
In silico docking study of GCMS components of methanol extract of wheatgrass showed the strong H-bond interaction of three out of nine components with cyclin D1 [Table 1, C1–C9]. Hexanoic acid, 2-methyl showed side chain H-bond interaction and furan-2-carboxamide, 5-benzoyl-N-(2-Dimethylaminoethyl) showed H-bond interaction with Lys112 whereas, 1, 2-benzene dicarboxylic acid, diheptyl ester showed H-bond interaction with Ala141. Rest other compounds have not shown any H-bond interaction with the target protein [Figure 5b]
Figure 5b.
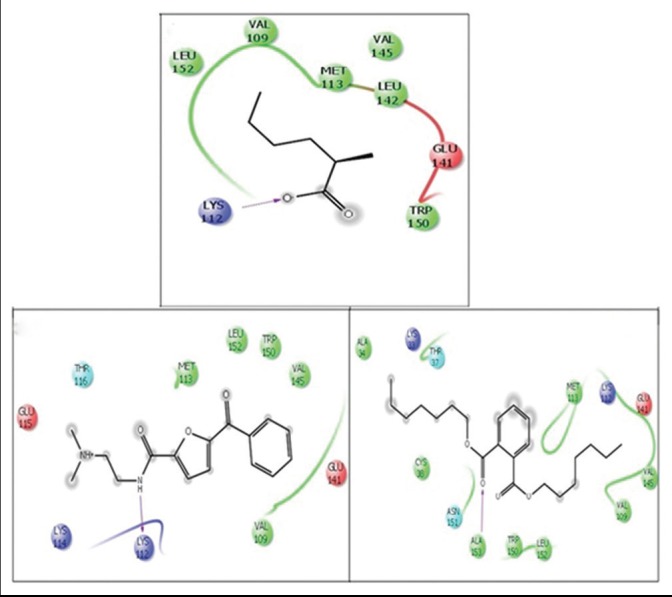
Molecular docking (two-dimensional images) of constituents of methanol extract of wheatgrass (identified by gas chromatography-mass spectroscopy) with cyclin D1 (Protein Data Bank ID (PDB ID) 2W96). Docking pose of (a) 2-methyl-hexanoic acid, (b) furan-2-carboxamide, 5-benzoyl-n-(2-dimethylaminoethyl) and (c) 1, 2-Benzenedicarboxylic acid, diheptyl ester. Pink lines show H-bond interactions with the arrow head pointing the acceptor atom
DISCUSSION
In recent years, plant derived natural agents have become the important focus for managing cancers.[3] Until today, multiple plant extracts have been evaluated for anti-cancerous efficacy and shown to have cytotoxic or cytostatic effects in various cancer cell lines.[15] It is believed that such kind of studies would be helpful to provide scientific evidence to develop effective, a potent drug, which could be useful for chemoprevention/chemotherapy of various cancers without any side-effect.[3] Thus in the present study, we have used the methanol extract of wheatgrass and analyzed its cytotoxicity and antiproliferative effect.
The cytotoxicity effect was assessed by MTT and LDH assay. MTT assay measures the active mitochondria of the metabolically active cells and is indicative of cell viability. On the other side, LDH assay analyses the dead cells. LDH leakage assay is a reliable, fast, and simple method, which is analyzed in the extracellular medium of treated cells. Anticancer drug induces irreversible cell death due to cell membrane damage, which reduces the intracellular LDH and increases the release of LDH into the culture medium.[16] Methanol extract of wheatgrass effectively reduced the cell viability of the cancerous cells in a dose dependent manner and simultaneously increased the release of LDH in the surrounding medium. Figure 2, clearly shows the change in the morphology of Hep2 cells (shrinkage of cells and distorted structure), compared to the control cell, which supports the anticancerous effect of our extract. These results indicate that the methanol extract of wheatgrass has good cytotoxic efficacy against Hep2 cancer cells.
Uncontrolled cell growth plays a significant role in cancer incidence. In this respect, researches on the plant based cell cycle regulators are gaining more importance.[6] Therefore in our study, we have checked the cell cycle distribution of treated cells. The flow cytometric analysis of the DNA content of the treated cells has shown a significant G1 phase arrest. This confirmed that the methanol extract of wheatgrass controls the progression of cells through the cell cycle by arresting cells at G1 phase. Accordingly cells were increased in G1 phase, and decreased in S phase of cell cycle.
Various intrinsic and extrinsic stress signals activates p53, which targets some of the genes that are important for cell cycle arrest or for inducing apoptosis.[1] Cyclin D1 is a downstream effector of p53 activity. Expression of cyclin D1 and p53 both at gene and protein level has a high significant association. Loss of p53 and G1 control are important factors in tumorogenesis, as it directly affects the cell cycle checkpoints.[3] To test the mechanism behind this G1 phase arrest, we analyzed the expression of p53 and cyclin D1 in Hep2 cells. We found a significant decrease in the expression of cyclin D1 and an increase in the expression of p53 at inhibitory concentrations of methanol extract of wheatgrass. From these findings, we can conclude that p53 has a significant role in G1 cell cycle arrest and is induced by methanol extract of wheatgrass. This might be by increasing the expression of p21, which is an inhibitor of Cdk4, thus decreasing the expression of cyclin D1.
Previous studies have shown that cyclin D1 is over expressed, and p53 is deregulated in several of human cancers including laryngeal cancer.[5,17] Due to the significant down regulation of cyclin D1 and up regulation of p53 by methanol extract of wheatgrass, it can be a good candidate for cancers with deregulated expression of these proteins.
In our previous study, phytochemical evaluation of methanol extract of wheatgrass showed considerable amounts of polyphenolic compounds including phenols and flavonoids. HPLC analysis showed the presence of five important polyphenols, rutin, caffeic acid, quercetin, gallic acid, ferulic acid [Table 1, B1-B5] and GC-MS analysis revealed the nine bioactive components [Table 1, C1–C9] in the methanol extract[11] and most of these compounds possess hydroxyl group and/or double bonds. Compounds such as squalene, phytol and 11,14,17-eicosatrienoic acid are previously shown to possess good anticancerous property.[18,19,20] Recent studies have shown that plant based polyphenols directly or indirectly can inhibit cancer cells at different phases of cell cycle.[3] Thus, in the present work, in silico docking study was done to check the interaction of all 14 components of methanol extract of wheatgrass with cyclin D1 (PDB: 2W96) and compared with the cyclin D1/Cdk4 inhibitor (2-bromo-12, 13-dihydro-5H-indolo[2,3-a] pyrrolo[3,4-c] carbazole-5, 7 (6H)-dione).
Cyclin D1/Cdk4 inhibitor is an asymmetrical indolocarbazole compound that acts as a potent and ATP-competitive inhibitor of cyclin D1/Cdk4 at inhibitory concentration of IC50 76 nM. It is shown to inhibit tumor cell growth in HCT-116 and NCI-H460 cells at IC50 <3.0 μM, by inhibiting Rb (retinoblastoma) phosphorylation and inducing G1 cell cycle arrest.[21]
First important step in the activation of any particular Cdk is binding with cognate cyclin phosphorylating the residues within the T-loop of Cdk and then undergoing a conformational change for full activation. The X-ray crystallography study has revealed that the loop conformation of Cdk4 with cyclin D1 is stabilized by two main H-bond interactions. This involves bonds from the loop of Cdk4 to a highly conserved lysine (Lys112) and glutamate (Glu141) on the cyclin D1. Previous studies have shown that the defect or mutation of Lys112 or Glu141 results in inactivation or prevention of binding of cyclin with Cdk4. Mutation of Lys112 disrupts the stabilization of H-bond with the loop and Glu141, which ultimately disrupts the active conformation,[14] thus inhibiting the cell progression from G1 to S phase.
Lys112 and Glu141 are very important residues involved in the strong binding of Cdk4 with cyclin D1. In our docking study, we found that all five Polyphenols (HPLC identified) showed H-bond interaction with Glu141, ferulic acid additionally formed strong salt bridge with Lys112. Whereas the two out of nine active components (identified by GCMS) of methanol extract of wheatgrass showed H-bond interactions; Hexanoic acid, 2-methyl was involved in side chain H-bond interaction and furan-2 carboxamide, 5-benzoyl-N-(2-dimethylaminoethyl) was found to form the strong H-bond to Lys112, electrostatic interaction with Glu141 and hydrophobic interactions with other residues of the active site of cyclin D1. Cyclin D1/Cdk4 Inhibitor (2-bromo-12, 13-dihydro-5H-indolo 2, 3-a] pyrrolo 3, 4-c] carbazole-5, 7 (6H)-dione), was found to form strong H-bond interaction with Glu141 and electrostatic interaction with Lys112 and other hydrophobic interactions [Figures 5a and b]. Glide score of rutin, quercetin and gallic acid was found to be (−5.43, −4.87 and − 4.28 respectively) higher than cyclin D1/Cdk4 inhibitor (−3.65), whereas the glide score of caffeic acid and ferulic acid was − 3.33 and − 3.06 respectively, which is very close to the reference compound. Hexanoic acid, 2-methyl showed glide score of − 2.95, furan-2 carboxamide, 5-benzoyl-N-(2-dimethylaminoethyl) showed glide score of-2.78. Also, the free energy of binding of these compounds are found to be higher than or similar to the reference compound [Table 1].
Interaction of these active component of methanol extract of wheatgrass with important residue of cyclin D1 proves that they are involved in inhibiting the cyclin D1/Cdk4 interaction thus preventing the change in conformation of Cdk to the active form, which is required for the cell progression from G1 to S phase of cell cycle, thus arresting the cells at G1 phase.
CONCLUSION
This is the first report which shows that the methanol extract of wheatgrass induced the cytotoxic effect by cell cycle arrest at G1 phase in association with increased p53 and decreased cyclin D1 expression in Hep2 cells. Moreover, seven active compounds of wheatgrass were also found to interact with important residue (Lys112 and Glu141) of cyclin D1 and inhibit the cyclin D1/Cdk4 interaction, thus halting cell progression at G1 phase. Hence, the active components of wheatgrass can be effective candidates for the treatment of cancer. Further research on elucidating their molecular mechanisms is in progress.
ACKNOWLEDGMENTS
The authors acknowledge the support provided by University Grant Commission for providing the Junior Research Fellowship (reference no.F.17-115/98 (SA-I) dated 01.03.2012) to Garima Shakya. The authors thank DST-FIST, UGC-SAP, DBT-IPLS for providing the infra structural facility.
Footnotes
Source of Support: The authors acknowledge the support provided by University Grant Commission for providing the Junior Research Fellowship (reference no.F.17-115/98 (SA-I) dated 01.03.2012) to Garima Shakya
Conflict of Interest: None declared.
REFERENCES
- 1.Jiao J, Qin Z, Li S, Liu H, Lu Z. Potential role of Notch1 signaling pathway in laryngeal squamous cell carcinoma cell line Hep-2 involving proliferation inhibition, cell cycle arrest, cell apoptosis, and cell migration. Oncol Rep. 2009;22:815–23. doi: 10.3892/or_00000504. [DOI] [PubMed] [Google Scholar]
- 2.Armania N, Yazan LS, Ismail IS, Foo JB, Tor YS, Ishak N, et al. Dillenia Suffruticosa extract inhibits proliferation of human breast cancer cell lines (MCF-7 and MDA-MB-231) via induction of G2/M arrest and apoptosis. Molecules. 2013;18:13320–39. doi: 10.3390/molecules181113320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezaei PF, Fouladdel S, Ghaffari SM, Amin G, Azizi E. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. DARU J Pharm Sci. 2012;20:101. doi: 10.1186/2008-2231-20-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson MA, Schwartz GK. Development of cell-cycle inhibitors for cancer therapy. Curr Oncol. 2009;16:36–43. doi: 10.3747/co.v16i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyur LF, Lee SH, Chang ST, Lo CP, Kuo YH, Wang SY. Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways. Phytomedicine. 2010;18:16–24. doi: 10.1016/j.phymed.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS One. 2012;7:e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krifa M, Alhosin M, Muller CD, Gies JP, Chekir-Ghedira L, Ghedira K, et al. Limoniastrum guyonianum aqueous gall extract induces apoptosis in human cervical cancer cells involving p16 INK4A re-expression related to UHRF1 and DNMT1 down-regulation. J Exp Clin Cancer Res. 2013;32:30. doi: 10.1186/1756-9966-32-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Verma P, Pandey BR. Therapeutic potential of organic Triticum aestivum Linn.(Wheat Grass) in prevention and treatment of chronic diseases: An overview. Int J Pharm Sci Drug Res. 2012;4:10–4. [Google Scholar]
- 10.Garima S, Charan G, Sankar P, Rukkumani R. Protective role of wheatgrass on oxidative stress in streptozotocin induced type 2 diabetic rats. Int J Pharm Pharm Sci. 2012;4:415–23. [Google Scholar]
- 11.Garima S, Sankar P, Muddasarul H, Varalakshmi D, Rukkumani R. GCMS analysis, in vitro antioxidant and cytotoxic studies of wheatgrass extract. Am J Phytomed Clin Ther. 2014;2:877–93. [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Chenault HK, Whitesides GM. Lactate dehydrogenase-catalyzed regeneration of NAD from NADH for use in enzyme-catalyzed synthesis. Bioorg Chem. 1989;17:400–9. [Google Scholar]
- 14.Day PJ, Cleasby A, Tickle IJ, O’Reilly M, Coyle JE, Holding FP, et al. Crystal structure of human CDK4 in complex with a D-type cyclin. Proc Natl Acad Sci U S A. 2009;106:4166–70. doi: 10.1073/pnas.0809645106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arung ET, Wicaksono BD, Handoko YA, Kusuma IW, Yulia D, Sandra F. Anticancer properties of diethylether extract of wood from Sukun (Artocarpus altilis) in human breast cancer (T47D) cells. Trop J Pharm Res. 2009;8:317–24. [Google Scholar]
- 16.Rambabu V, Subab S, Manikandan P, Vijayakumara S. Cytotoxic and apoptotic nature of migrastatin, a secondary metabolite from streptomyces evaluated on HepG2 cell line. Int J Pharm Pharm Sci. 2014;6:333–8. [Google Scholar]
- 17.Wu Z, Wu L, Li L, Tashiro S, Onodera S, Ikejima T. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J Pharmacol Sci. 2004;94:166–76. doi: 10.1254/jphs.94.166. [DOI] [PubMed] [Google Scholar]
- 18.Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–90. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- 19.Malek SN, Wahab NA, Yaacob H, Shin SK, Lai HS, Serm LG, et al. Cytotoxic activity of pereskia bleo (Cactaceae) against selected human cell lines. Int J Cancer Res. 2008;4:20–7. [Google Scholar]
- 20.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–49. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 21.Zhu G, Conner SE, Zhou X, Shih C, Li T, Anderson BD, et al. Synthesis, structure-activity relationship, and biological studies of indolocarbazoles as potent cyclin D1-CDK4 inhibitors. J Med Chem. 2003;46:2027–30. doi: 10.1021/jm0256169. [DOI] [PubMed] [Google Scholar]


