Abstract
Background:
Vatica diospyroides type LS is a known source of valuable compounds for cancer treatment, however, in contrast little is known about therapeutic efficacy of type SS.
Objective:
This study focused on in vitro cytotoxicity of these fruit extracts, and the cell death mode they induce in breast cancer cells.
Materials and Methods:
Acetone extracts of fruit were tested for cytotoxicity against MCF-7 and MDA-MB-231 cell lines. The apoptosis and necrosis of these cells were quantified by fluorescence activated cell sorter (FACS) and western blot analyses.
Results:
After 72 h of treatment, the 50% growth inhibition concentrations (IC50) levels were 16.21 ± 0.13 µg/mL against MCF-7 and 30.0 ± 4.30 µg/mL against MDA-MB-231, indicating high and moderate cytotoxicity, respectively. From the FACS results, we estimate that the cotyledon extract at half IC50 produced 11.7% dead MCF-7 cells via apoptosis, whereas another concentrations both apoptosis and necrosis modes co-existed in a dose-dependent manner. In MDA-MB-231 cell line, only the apoptosis was induced by the pericarp extract in a dose-dependent manner. With the extracts at half IC50 concentration, in both cells, the expression of p21 decreased while that of Bax increased within 12–48 h of dosing, confirming apoptosis induced by time-dependent responses. Apoptosis dependent on p53 was found in MCF-7, whereas the mutant p53 of MDA-MB-231 cells was expressed.
Conclusion:
The results indicate that fruit extracts of V. diospyroides have cytotoxic effects against MCF-7 and MDA-MB-231 cells via apoptosis pathway in a dose-dependent manner. This suggests that the extracts could provide active ingredients for the development, targeting breast cancer therapy.
Keywords: Apoptosis, breast cancer, flow cytometry, fruit, Vatica diospyroides type SS
INTRODUCTION
Vatica diospyroides Symington is a critically endangered species in Dipterocarpaceae,[1] and is the key source of ingredients in a blood tonic used in Thai traditional ethnopharmacology. These natural compounds have been explored for cancer treatment in a modern drug discovery program.[2] Among the many cancer types, breast cancer, colon cancer, and oral cancer have been treated using V. diospyroides stem compounds.[3] The important compounds found in the stem are resveratrol derivatives, vaticanol series, found not only in this plant, but also in other related genera in this family.[3,4,5,6] Breast cancer is currently the leading cancer type as cause of death, for women in middle-income and high-income countries.[7]
Only the stems of plants in this family seem to provide the resveratrol compounds. Aside from the stem, the most valuable organs reported as new sources of chemopreventives inhibiting breast cancer in vitro are fruit and root of V. diospyroides type LS. Their extracts have been reported as highly cytotoxic against MCF-7 and MDA-MB-468 cell lines.[8,9] A new subtype (SS) of the thinly distributed V. diospyroides, with purple fruit and pink in the flower, has been described recently.[10] Its fruit have not previously been studied for their therapeutic compounds or extracts. Our hypothesis was that this fruit might show potential for breast cancer treatment, similar to the fruit of type LS. The current study was designed to detect and distinguish between cytotoxic, apoptotic, and necrotic effects of SS fruit extracts, in breast cancer cell lines. As we report below, the nonvolatiles from crude acetone extracts of V. diospyroides type SS fruit were highly effective in inhibiting human breast cancer cell lines in vitro, and significantly induced apoptosis via p53, p21 and Bax protein related mechanisms.
MATERIALS AND METHODS
Plant materials and preparation of the two extracts
Fruit of V. diospyroides type SS was collected from a 10-year-old tree at the Nong Thung Thong nonhunting area, Kiansa, Suratthani. The specimens (Collector number T. Srisawat 002) were authenticated by Dr. Charun Maknoi, and deposited at the Queen Sirikit Botanic Garden, Maerim, Chiangmai, Thailand. The fruit pericarp and cotyledon were separated, and each cut to small pieces and completely air-dried in shadow. Dried pieces of each type of samples were separately extracted with acetone ((CH3)2 CO) for 5 days in agitated condition. The solvent extract was then transferred to a new container, filtered through a cotton fabric, and evaporated at room temperature using a rotor evaporator (Heidolph Rotary Evaporator, D-91126, Germany) under reduced pressure, and the dry gum of nonvolatile compounds was stored in cool and dark conditions.
4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cell lines MCF-7 (HTB-22™) and MDA-MB-231 (HTB-26™) were purchased from the American Type Culture Collection, USA. MCF-7 and MDA-MB-231 were maintained in RPMI1640 and high glucose Dulbecco's Modified Eagle's Medium (DMEM), respectively, supplemented with 10% fetal bovine serum and 100 units/mL penicillin-streptomycin, in a humidified atmosphere with 5% CO2 at 37°C. Cells were seeded in 96-well plates at the density of 2 × 104 cells, in 100 µL of medium per well. Crude dry extracts were diluted to 5–80 µg/mL concentrations in a medium. Each MCF-7 or MDA-MB-231 culture was incubated for 72 h with the cotyledon or pericarp extract included in a medium, for which the dry extracts were dissolved in dimethylsulfoxide (DMSO). Each cell line was only tested with a matched extract, not with both extracts. This matching was based on preliminary scoping experiments, where only these matches showed efficacy: MCF-7 with cotyledon extract and MDA-MB-231 with pericarp extract. For the control experiments, DMSO at 0.04% was used as a solvent control whereas negative control (medium) and positive control (doxorubicin) were also used as treatments. The treated cells were washed with phosphate buffer saline (PBS) prior to determinations of cell viability by 4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the method described previously.[8] By measuring the optical density and using a calibration curve, the growth inhibition was determined for each well. The 50% growth inhibition concentrations (IC50 dose level) of crude extracts were calculated from fitted response curves. The cytotoxic activities of the extracts against the cancer cell lines MCF-7 and MDA-MB-231 were determined according to the US. National Cancer Institute and Geran et al.[11] and labeled for activity levels as follows: IC50 ≤20 µg/mL = highly active, IC50 21–200 µg/mL = moderately active, IC50 201-500 µg/mL = weakly active, and IC50 >501 µg/mL = inactive. The whole experiment was replicated three times.
AnnexinV-FITC/propidium iodide binding and flow cytometric analysis
AnnexinV-FITC/propidium iodide (PI) binding was determined with a kit following its manufacturer's protocol (BD Pharmingen™, USA). Cells were seeded at the density of 3 × 105 for MDA-MB-231 and 4 × 105 for MCF-7 in 12-well plates. Cell harvesting was carried out by adding 200 µL of trypsin to detach the cells and centrifugation. After centrifugation, the harvested cells were resuspended in 1x binding buffer (0.1 M Hepes, 0.1 M NaOH pH 7.4, 1.4 M NaCl, 25 mM CaCl2) at a concentration of 1 × 106 cells/mL. Five microliters of AnnexinV-FITC was added, then adding 5 µL of PI into the suspensions. The suspensions were vortexed gently before incubating for 30 min at room temperature, in darkness. Finally, before analysis by flow cytometry, 400 µL of ×1 binding buffer was added into each tube and then incubated at 4°C for 30 min in darkness. The flow cytometer was a fluorescence activated cell sorter (FACS) Calibur (Becton Dickinson Biosciences [BDB], San Jose, CA) with CellQuest software (BDB), equipped with a 488 nm argon ion laser. Five thousand nuclei were acquired with FACS per sample. The data were analyzed by WinMDI version 2.9 software (Scripps Institute, La Jolla, CA).
Western blotting
The MCF-7 and the MDA-MB-231 cells were treated with the extract at half IC50 (0.5 IC50) concentration for up to 48 h, then released by trypsinizing the cells and collected by centrifugation. The cells were washed once in cold PBS, and lysed cells in radioimmunoprecipitation assay buffer (Peirce Biotechnology, IL, USA). Protein concentration was then measured using Bradford protein assay (Biorad, CA, USA). Fifty micrograms of each protein sample were separated in 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and electrophoretically transferred to a nitrocellulose membrane. The membranes were blocked in 5% low-fat dry milk in Tris Buffered Saline with Tween® 20 (TBS-T) (5% low-fat dry milk, 0.1% Tween-20 in Tris-buffered saline) for 1 h at room temperature. After that, each membrane was incubated overnight with primary antibodies against p21WAF1/Cip1 (1:1000), p53 (1:1000), Bax (1:1000) and β-actin (1:1000) used as an internal control. All the antibodies were purchased from Cell Signaling, MA, USA. The membranes were washed 3 times (5 min/wash) with 1% low-fat dry milk in TBS-T, and incubated with ECL anti-rabbit IgG horseradish peroxidase (GE health care) diluted 1:5000 in 1% low fat dry milk in TBS-T, for 1 h at room temperature. After secondary washing with TBS-T 3 times (10 min/wash), the signals were detected using a chemiluminescent detection system (Pierce, IL, USA) and exposed to film.
Statistical analysis
The cytotoxicity experiments were replicated three times, and the results are expressed as mean ± standard deviation Student's t-test was used for comparing means.
RESULTS
Cytotoxicity determination by 4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The breast cancer cell inhibition is shown in Figure 1. Cytotoxic effects, of the cotyledon and pericarp extracts of V. diospyroides type SS fruit, against MCF-7 and MDA-MB-231 were quantified by MTT assay. After 72 h treatments with various concentrations of the extracts, the IC50 dose levels were estimated from fitted response curves. Each curve describes how the percentage of surviving cells depends on the dose level, and the level giving 50% inhibition is the IC50 dose level. The inhibition observed was dose dependent, with a typical sigmoidal shape of dose response, corroborating validity of the observations. The IC50 of the cotyledon extract against MCF-7 was 16.21 ± 0.13 µg/mL, indicating highly cytotoxic activity. On the other hand, the pericarp extract had moderate activity at 30.0 ± 4.30 µg/mL, against MDA-MB-231 cell line. Based on these determinations, half IC50, IC50 and 2-fold IC50 dose levels were used in the cell death mode analysis to be discussed next.
Figure 1.
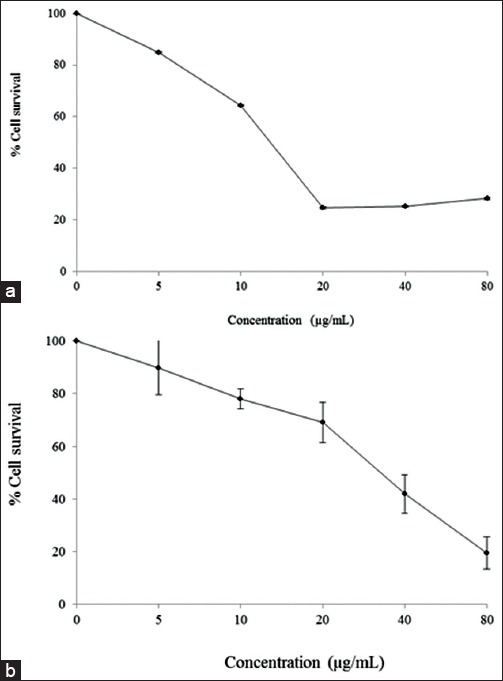
The inhibition responses of MCF-7 (a) and MDA-MB-231 (b) cell lines to 72 h treatments with cotyledon and pericarp extracts of Vatica diospyroides Symington type SS fruit, respectively, at various concentrations (5–80 µg/mL)
Cell death mode analysis by AnnexinV-FITC/propidium iodide staining
Prior to determining the modes of cell death by using FACS, after removing the medium on harvesting cells, the cells were trypsinized by adding 200 µL of trypsin and incubating at 37°C for 3 min. The cells in suspension were then collected by centrifugation and washed twice with ice-cold PBS. In Figure 2, the results of apoptotic pathway assessments are shown separately for untreated and treated MCF-7 and MDA-MB-231 cells, with various concentrations of cotyledon and pericarp extracts, respectively. After a 24-h treatment, the apoptotic pathways of MCF-7 and MDA-MB-231 were different. Treated MCF-7 cells cluster in the plot in the shape of number 7, and the path of apoptosis is indicated with yellow arrows. The plots indicate that treated MDA-MB-231 cells were in an initial stage of apoptosis rather than in mid or late stages. Treatment with cotyledon extract at half IC50 divided the MCF-7 cells to viable, early, late and apoptotic cell death populations (51.2, 15.5, 21.7 and 11.7%) [Figure 2]. The quadrants within which these populations were counted are separated by straight lines in the figure. Interestingly, at IC50 dose level the populations of viable and early apoptosis stage cells were comparatively higher at 54.8 and 18.2%, respectively, whereas the populations of late apoptosis stage and nonviable cells were reduced to 20.5 and 6.4%, indicating inhibition of apoptosis mode despite increased dose. Simultaneously, a track connecting viable cells directly to nonviable cells, indicated by a red arrow, shows initiation of necrosis mode. This track to necrotic cells was also induced at 2-fold IC50, which gave a high population of nonspecific dead cell (12.9%). The population of viable cells increased to 58.1%, while the populations of cells in early and late apoptosis stages decreased to 11.0% and 18.0%. Therefore, delayed apoptosis, and induced necrosis of MCF-7 cells were first observed at IC50, in a dose-dependent manner. On the other hand, the treated MDA-MB-231 cells were consistently in the apoptosis mode, in a dose-dependent manner.
Figure 2.
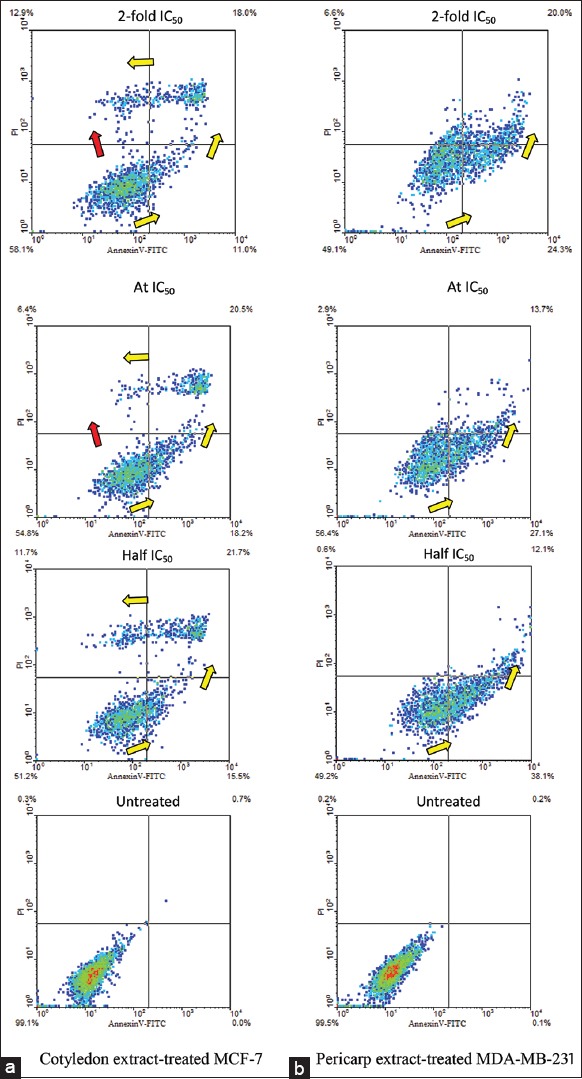
Scatter plots indicative of apoptotic patterns for (a) MCF-7 cells untreated and treated with cotyledon extract, and (b) MDA-MB-231 cells untreated and treated with pericarp extract of Vatica diospyroides type SS fruit. Treatment dose levels were half 50% growth inhibition concentrations (IC50), IC50 and 2-fold IC50, for 24 h. The cells were stained with AnnexinV-FITC/PI and analyzed by flow cytometry. Viable, early apoptotic, late apoptotic, and nonviable cells are positioned in the lower left, lower right, upper right and upper left segments of the diagram. Yellow arrows indicate apoptotic progression of cells, whereas red arrow shows necrotic transition. Five thousand events were counted in each assay
Using the concentration of pericarp extract at half IC50, IC50 and 2-fold IC50, the population of cells in early apoptosis stage declined from 38.1 via 27.1% to 24.3%, with increasing dose level. Correspondingly, the populations of late apoptosis stage and of nonviable cells increased significantly (12.1 via 13.7–20.0%, and 0.6 via 2.9–6.6%, respectively). The fact that the 6.6% population of nonviable MDA-MB-231 cells was about half of the 12.9% for MCF-7 suggests, that the cotyledon extract at IC50 induced both apoptosis and necrosis in MCF-7 cells while only the apoptosis mode in MDA-MB-231 cells was induced by the pericarp extract. Overall, the observations above varied consistently in a dose-dependent manner.
Cell death mode analysis by protein-based assay
To determine the roles of p21, p53 and Bax proteins in the apoptosis of MCF-7 and MDA-MB-231 cells induced by the extracts of V. diospyroides type SS fruit, we studied the effects at half IC50 extract concentrations on apoptosis induction by means of apoptosis-related protein expression as observed by western blots. Western blot analysis revealed that there was no detectable p53 protein expressed in the treated MCF-7 cells, whereas p53 was observed in the treated MDA-MB-231 cells [Figure 3]. On the other hand, continuous changes in the expression levels of p21 and Bax were detected in both cell lines, in a consistently time-dependent manner, indicating activation downstream of p53. There was up-regulation of p21waf1⁄Cip1 at 12 h and Bax at 24–48 h. The expression of p21waf1⁄Cip1 protein could induce cell cycle arrest and allow DNA repair to occur. As long as the cells are not able to repair their damaged DNA, the expression of Bax protein will be stimulated leading eventually to apoptosis. Simultaneously, apoptosis in both of the treated cell lines was also induced by the Bax protein, a proapoptotic protein that had a consistently time-dependent response (12–48 h) [Figure 3].
Figure 3.
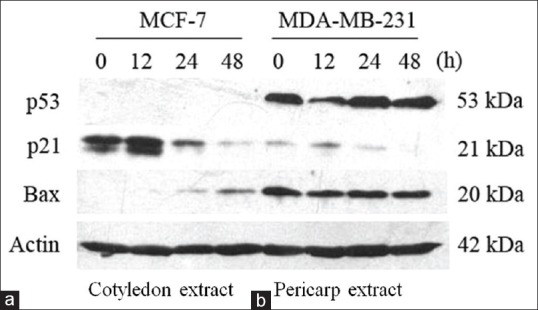
Effect of Vatica diospyroides type SS fruit on expression of the tumor suppressor protein p53 and downstream products affected by it, namely the proapoptotic protein Bax and the antiapoptotic protein p21. (a) MCF-7 cells were treated with the extract of fruit cotyledon, whereas (b) MDA-MB-231 cells were treated with the extract of fruit pericarp, both at concentration of half 50% growth inhibition concentrations. The cellular proteins were separated on SDS-polyacrylamide gels and subsequently transferred to membranes. The protein levels were determined by western blots with appropriate antibodies directed against each protein
DISCUSSION
Several recent in vitro studies on human breast cancer treatment have explored compounds extracted from various medicinal plant species. Most of these investigate cell growth inhibition as an indication of cytotoxicity and may include other molecular-biochemical-cytological assays for apoptosis or necrosis observations.[12] In the present study, we first preliminarily determined the antiproliferative actions of V. diospyroides type SS fruit extracts, on MCF-7 and MDA-MB-231 breast cancer cell lines. The evaluation of cytotoxic effects revealed that cotyledon and pericarp extracts exhibited dose-dependent activity, with high activity against MCF-7 and moderate activity against MDA-MB-231. The cotyledon extract inhibited MCF-7 growth by 50% at 24 h while 50% inhibition of MDA-MB-231 cell growth with pericarp extract took about 48 h (data not shown). The antiproliferative activity of these fruit extracts may be associated with the presence of some secondary metabolites. For example, terpenoids and saponins, which are present in the extracts of V. diospyroides type LS fruit that are antiproliferative against MDA-MB-468 cell line,[8] are likely also extracted from the cotyledon of type SS fruit. The antioxidant activity of terpenoids is well established.[13,14] Interestingly, these compounds inhibit cancer cell proliferation and metastasis by several mechanisms and are currently studied with hopes for breakthrough insights in some anticancer discovery programs.[15] Aside from terpenoids, the saponins are another well-known group of phytochemicals that also exhibit antioxidant and antitumor activities, and are currently tested in several laboratories.[16,17,18] On using cell viability measurements, many phytochemicals might be interfering with MTT tetrazolium reduction, and specifically resveratrol and various antioxidants rapidly form dark blue formazan in the absence of live cells.[19,20,21] Since V. diospyroides is a major source of resveratrol,[3] it is necessary to wash the treated cells with 1X PBS, in order to avoid such interference with MTT determinations. In future studies, the experimental design could include tests of such interference, but clearly washing cannot eliminate the disturbances to measurements from intracellular compounds – the disturbance effects will remain difficult to resolve from true treatment effects on undisturbed determinations, because treatments necessarily will have intracellular effects. Further, other assays related to growth and proliferation might be used in combination with viability assays, but the interpretation from multiple indications can become disputable and partly subjective, as there is no single correct way to aggregate such disparate observations. However, the leap from in vitro cancer studies to clinical treatments is long and risky, and perhaps imperfections in early in vitro screening should be tolerated while acknowledging them and looking for improved methods.
In the apoptosis study, we discriminated between apoptosis and necrosis based on the density traces in diagrams from flow cytometry. These diagrams represent four main states, namely viable cells, early and late apoptosis stages, and nonviable cells. The trace from a cell population that includes transitions from viable cells to early apoptosis stage, on to late apoptosis, and finally to nonviable cells, is called an apoptotic pathway in the context of these diagrams. The staining fluorochromes (AnnexinV-FITC/PI binding) relate to cytological apoptosis, because in a viable cell phosphatidylserine (PS) is located on the inner surface of plasma membrane; it cannot conjugate AnnexinV or PI (both negative). In a cell undergoing apoptosis, with the breakdown of plasma membrane the phospholipids (PS) are translocated from inner to the outer leaflet of the membrane, and thus the PS are available on the outside. This induces AnnexinV-FITC binding with the PS (AnnexinV-positive but PI-negative), for cells in early apoptosis stage. When the plasma membrane has been lost, the PI can pass through to the inside and intercalates with DNA, so both AnnexinV-FITC and PI are conjugated (both positive). These dye-conjugating cells are in the late apoptosis stage. In the final stage of apoptosis, the cells get segmented into membrane-bound apoptotic bodies with intense intercalation of PI with DNA (AnnexinV-negative but PI-positive), and hence the signals in the diagram are in the nonviable cell quarter.[22,23] The AnnexinV-FITC/PI binding assay therefore enables detecting and distinguishing early and late apoptosis stages and apoptotic cell death related to the cell cycle.[22] Meanwhile, the diagram trace directly from viable cells to nonviable cells might be identified as the nonapoptotic pathway. Necrosis, in nonapoptotic cells, describes accidental cell death considered to be an uncontrolled process. The plasma membrane first swells and consequently ruptures, with rapid loss of membrane integrity.[24] This is in contrast with the maintained membrane integrity during apoptosis until the plasma membrane is lost. We thus suggest that the necrotic cells could not be intensely conjugated by AnnexinV-FITC, but they allowed PI to pass through and to intercalate with DNA inside. In the flow cytometry diagram, therefore, necrosis was indicated by a direct trace from the viable to the nonviable cell population.
In the present study, the effects of cotyledon and pericarp extracts at half IC50, IC50 and 2-fold IC50 on apoptotic or necrotic cell death modes of MCF-7 and MDA-MB-231 cells were determined after a 24-h treatment. In MCF-7, the apoptotic cell death population increased to about 12.9% at 2-fold IC50 dose level. Increased populations of viable and dead cells and decreased populations of early and late apoptotic cells were observed when the dose level increased. It is interesting to note that, at IC50 and 2-fold IC50, the population of viable cells is higher than at half of IC50, indicating an effect against apoptosis. Moreover, some viable cells could directly convert to dead cells. As discussed above, these findings indicate that there might be necrosis caused by acute toxicity at high dose levels.[25,26,27] The necrotic and apoptotic deaths in combination gave the highest final cell death, correlating with in vitro antiproliferation. A low concentration of chemopreventive may increase intracellular oxidative stress,[26] shifting the Bax-Bcl-2 ratio to favor apoptosis while high dose levels would not do this. Thus, at IC50 or higher dose levels, the death of MCF-7 cells arose from both apoptosis and necrosis modes. A comparison between half of IC50, IC50, and 2-fold IC50 dose levels of pericarp extract, the populations of late apoptosis stage and dead MDA-MB-231 cells increased significantly (12.1 via 13.7-20.0%, and 0.6 via 2.9-6.6%) whereas the population of viable cells was persistent (49-56%). In this case, the extract had no acute toxicity, and viable cells were not directly converted to dead cells. Hence, necrotic cell death was not induced, instead apoptotic cell death only was triggered. These results are coherent with the IC50 levels of the pericarp extract.
Our findings resemble those found in the work of Balijepalli et al. (2010),[23] who found a higher apoptosis rate in MCF-7 cells than in MDA-MB-231 cells. The MCF-7 is an estrogen receptor-positive (ER+) cancer cell line, and these share substantial global similarities in the structures of their transcriptomes. This cell line is a good model for identifying molecular events of some ER+ human breast cancers.[28] The MDA-MB-231 (estrogen receptor-negative; ER−) cell line is more aggressive and unresponsive to anti-estrogens. A chemopreventive for ER− cancer cell lines needs to have a stronger toxicity and more side-effects than chemopreventives of ER+ cell lines.[29] The high rate of apoptosis in MCF-7 was probably due to lack of estrogen stimulation in our test conditions.[23] The flow cytometry results revealed that MCF-7 is able to respond quicker than MDA-MB-231 to treatment, by means of vigorously shifting from viable, early or late apoptotic stages to nonviable cells. Apoptosis and necrosis were clearly indicated in the cells treated with the fruit extracts in vitro. These results confirm that MCF-7 model allows the study of competing mechanisms in response to chemotherapy, representing estrogen-dependent human breast cancer cells.[30]
Apoptosis induction in cancer cells is more critical than induction of necrosis for the efficacy of a chemopreventive agent.[31] Gorczyca (1999) suggested that distinguishing between apoptotic and necrotic cells by using flow cytometry is not based on cell morphology, and cannot be correlated with morphological characterizations.[22] Therefore, in many laboratories, mostly fluorochromes and other indicators such as acridine orange and Hoechst 33342 have been recently used with fluorescence microscopic observations, as markers for discrimination between viable and apoptotic dead cells.[32,33] Furthermore, molecular-based methods such as flow cytometry, gel electrophoresis and western blot have been commonly used for apoptosis quantification by (1) comparing the sub G0/G1 histogram,[27] (2) detecting the smear band of fragmented DNA, and (3) determining the expression of various tumor-related proteins,[31] respectively. In the present study, the western blots confirmed that apoptosis in both cell lines could be induced by V. diospyroides type SS fruit. Furthermore, the p53 protein was completely absent from the treated MCF-7 cells in at all treatment times, whereas its expression was found consistently in the treated MDA-MB-231 cells. Normally, under stress conditions, the p53 is naturally unstable and can be rapidly degraded by the cellular proteasome,[34] namely by the mouse double minute 2 (MDM2) protein. Therefore, the half-life of p53 may be as short as minutes to an hour.[35] In contrast, the MDA-MB-231 had increasingly p53 associated apoptosis within 12–48 h. In more than 50% of human cancers p53 is mutated, and this is also the case with MDA-MB-231 cells.[36] Unfortunately, the mutant-p53 may resist degradation by MDM2,[34] and thus the p53 band in a western blot may show significant expression. The p53-transcription targets include p21 and Bax, and we confirmed in MCF-7 the induction of p21 early at 12 h (DNA damage repair), while Bax was stimulated in the late 24–48 h stage (apoptosis). This evidence suggested that the induction of p21 and apoptosis by the extracts of V. diospyroides type SS cotyledon is through a p53-dependent pathway. When the MDA-MB-231 cells, with p53 dysfunction, were treated with extracts of V. diospyroides type SS fruit, the effects on cell growth arrest and induction of apoptosis were p53-independent. Apoptosis might occur by an alternative pathway, but further studied would be needed to clarify this. Our results reveal that the p53 protein was not essential for the responses of MCF-7 and MDA-MB-231 cells treated with V. diospyroides type SS fruit extract, similar to observations by Lam et al. (2012),[37] whereas the time-dependent induction of p21 and Bax proteins was significant in both cell lines. In this analysis, the diagrams from flow cytometry were successfully combined with protein expression patterns to discriminate between various stages of apoptosis as well as necrosis. The flow cytometric characterizations are considered accurate, easy, reliable, and more rapid than other current options.
CONCLUSION
Extracts of V. diospyroides type SS fruit had cytotoxic effects on MCF-7 and MDA-MB-231 breast cancer cell lines; cotyledon extract doses at IC50 or above produced mixed MCF-7 cell apoptosis and necrosis, whereas a dose at half IC50 only induced apoptosis. All concentrations of the pericarp extract induced MDA-MB-231 cell death, only via apoptosis. Modern molecular immunology enabled the identification and counting of necrotic and apoptotic cells, to determine the respective populations as well as the cell deaths. The extracts studied may contribute to cancer therapies or prevention, particularly if in vivo studies were pursued, as well as contribute to drug development in case the active ingredients are identified in future studies.
ACKNOWLEDGMENT
This work was financially supported by Prince of Songkla University, Hat Yai campus. The authors thank Associate Professor Dr. Seppo Karrila for his edits, comments, and suggestions.
Footnotes
Source of Support: Financially Supported by Prince of Songkla University, Hat Yai campus
Conflict of Interest: None declared.
REFERENCES
- 1.Ashton P. Vatica diospyroides. IUCN 2013, IUCN Red List of Threatened Species. Version 1. 2013. [Last cited on 2013 Aug 05]. Available from: http://www. iucnredlist.org/details/33482/0 .
- 2.Kinghorn AD, Cui B, Ito A, Chung HS, Seo EK, Long L, et al. Fractionation of plants to discover substances to combat cancer. In: Cutler SJ, Cutler HG, editors. Biologically Active Natural Products: Pharmaceuticals. Florida United State of America: CRC Press LLC; 2000. pp. 16–23. [Google Scholar]
- 3.Seo EK, Chai H, Constant HL, Santisuk T, Reutrakul V, Beecher CW, et al. Resveratrol tetramers from Vatica diospyroides. J Org Chem. 1999;64:6976–83. [Google Scholar]
- 4.Ito T, Akao Y, Yi H, Ohguchi K, Matsumoto K, Tanaka T, et al. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis. 2003;24:1489–97. doi: 10.1093/carcin/bgg105. [DOI] [PubMed] [Google Scholar]
- 5.Atun S, Aznam N, Arianingrum R, Takaya Y, Masatake N. Resveratrol derivatives from stem bark of Hopea and their biological activity test. J Phys Sci. 2008;19:7–21. [Google Scholar]
- 6.Zain WZ, Ahmat N, Norizan NH, Nazri NA. The evaluation of antioxidant, antibacterial and structural identification activity of trimer resveratrol from Malaysia's dipterocaparceae. Aust J Basic Appl Sci. 2011;5:926–9. [Google Scholar]
- 7.World Health Organization. Breast cancer: Prevention and control. [Last cited on 2013 Jan 05]. Available from: http://www.who.int/cancer/detection/breastcancer/en/index1.html .
- 8.Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K. Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides Symington type LS. Trop J Pharm Res. 2013;12:71–6. [Google Scholar]
- 9.Srisawat T, Chumkaew P, Maichum W, Sukpondma Y, Graidist P, Kanokwiroon K. In vitro cytotoxic activity of Vatica diospyroides Symington type LS root extract on breast cancer cell lines MCF-7 and MDA-MB-468. J Med Sci. 2013;13:130–5. [Google Scholar]
- 10.Srisawat T, Kanjanasopa D, Ieamkheng S, Cheur-srisakul R. RAPD technique identifies subtypes of Vatica diospyroides Symington, a critically endangered medicinal and fragrant plant in the Dipterocarpaceae. J Plant Sci. 2013;8:57–64. [Google Scholar]
- 11.Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep III. 1972;3:1–103. [Google Scholar]
- 12.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, et al. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari S, Joshi R, Patro BS, Ghanty TK, Chintalwar GJ, Sharma A, et al. Antioxidant activity of bakuchiol: Experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem Res Toxicol. 2003;16:1062–9. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]
- 14.González-Burgos E, Gómez-Serranillos MP. Terpene compounds in nature: A review of their potential antioxidant activity. Curr Med Chem. 2012;19:5319–41. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 15.Huang M, Lu JJ, Huang MQ, Bao JL, Chen XP, Wang YT. Terpenoids: Natural products for cancer therapy. Expert Opin Investig Drugs. 2012;21:1801–18. doi: 10.1517/13543784.2012.727395. [DOI] [PubMed] [Google Scholar]
- 16.Luo H, Huang J, Liao WG, Huang QY, Gao YQ. The antioxidant effects of garlic saponins protect PC12 cells from hypoxia-induced damage. Br J Nutr. 2011;105:1164–72. doi: 10.1017/S0007114510004939. [DOI] [PubMed] [Google Scholar]
- 17.Guajardo-Flores D, Serna-Saldívar SO, Gutiérrez-Uribe JA. Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.) Food Chem. 2013;141:1497–503. doi: 10.1016/j.foodchem.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Xu F, Huang H, Gu Z, Wang L, Tan W, et al. Evaluation on anti-inflammatory, analgesic, antitumor, and antioxidant potential of total saponins from Nigella glandulifera seeds. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/827230. 827230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhard D, Schwaiger W, Crazzolara R, Tinhofer I, Kofler R, Csordas A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003;195:193–9. doi: 10.1016/s0304-3835(03)00157-5. [DOI] [PubMed] [Google Scholar]
- 20.Bruggisser R, von Daeniken K, Jundt G, Schaffner W, Tullberg-Reinert H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002;68:445–8. doi: 10.1055/s-2002-32073. [DOI] [PubMed] [Google Scholar]
- 21.Talorete TP, Bouaziz M, Sayadi S, Isoda H. Influence of medium type and serum on MTT reduction by flavonoids in the absence of cells. Cytotechnology. 2006;52:189–98. doi: 10.1007/s10616-007-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorczyca W. Cytometric analyses to distinguish death processes. Endocr Relat Cancer. 1999;6:17–9. doi: 10.1677/erc.0.0060017. [DOI] [PubMed] [Google Scholar]
- 23.Balijepalli MK, Tandra S, Pichika MR. Antiproliferative activity and induction of apoptosis in estrogen receptor-positive and negative human breast carcinoma cell lines by Gmelina asiatica roots. Pharmacognosy Res. 2010;2:113–9. doi: 10.4103/0974-8490.62949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golstein P, Kroemer G. Cell death by necrosis: Towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Granchi D, Cenni E, Ciapetti G, Savarino L, Stea S, Gamberini S, et al. Cell death induced by metal ions: Necrosis or apoptosis? J Mater Sci Mater Med. 1998;9:31–7. doi: 10.1023/a:1008878527233. [DOI] [PubMed] [Google Scholar]
- 26.Hsuuw YD, Chan WH. Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann N Y Acad Sci. 2007;1095:428–40. doi: 10.1196/annals.1397.046. [DOI] [PubMed] [Google Scholar]
- 27.Yu SF, Chen TM, Chen YH. Apoptosis and necrosis are involved in the toxicity of Sauropus androgynus in an in vitro study. J Formos Med Assoc. 2007;106:537–47. doi: 10.1016/S0929-6646(07)60004-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Wang A, Liu MC, Zwart A, Lee RY, Gallagher A, et al. Estrogen receptor alpha positive breast tumors and breast cancer cell lines share similarities in their transcriptome data structures. Int J Oncol. 2006;29:1581–9. [PubMed] [Google Scholar]
- 29.Guo M, Wang M, Deng H, Zhang X, Wang ZY. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-a36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur J Pharmacol. 2013;714:56–64. doi: 10.1016/j.ejphar.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Perry RR, Kang Y, Greaves B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Ann Surg Oncol. 1995;2:238–45. doi: 10.1007/BF02307030. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, Han M, Sun RH, Wang JJ, Zhang YP, Zhang DQ, et al. ABL-N-induced apoptosis in human breast cancer cells is partially mediated by c-Jun NH2-terminal kinase activation. Breast Cancer Res. 2010;12:R9. doi: 10.1186/bcr2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajudin TJ, Mat N, Siti-Aishah AB, Yusran AA, Alwi A, Ali AM. Cytotoxicity, antiproliferative effects, and apoptosis induction of methanolic extract of Cynometra cauliflora Linn. Whole fruit on human promyelocytic leukemia HL-60 cells. Evid Based Complement Alternat Med 2013. 2012 doi: 10.1155/2012/127373. 127373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maliyakkal N, Udupa N, Pai KS, Rangarajan A. Cytotoxic and apoptotic activities of extracts of Withania somnifera and Tinospora cordifolia in human breast cancer cells. Int J Appl Res Nat Prod. 2013;6:1–10. [Google Scholar]
- 34.Tsvetkov P, Reuven N, Shaul Y. Ubiquitin-independent p53 proteasomal degradation. Cell Death Differ. 2010;17:103–8. doi: 10.1038/cdd.2009.67. [DOI] [PubMed] [Google Scholar]
- 35.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–8. [PubMed] [Google Scholar]
- 36.ATCC. ATCC cell lines by gene mutation. [Last cited on 2014 Jun 09]. Available from: http://www.atcc.org/~/media/PDFs/Culture%20Guides/Cell_Lines_by_Gene_Mutation.ashx .
- 37.Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS One. 2012;7:e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]


