Abstract
Background:
Solanum lycocarpum has great importance for food and medicinal traditional use. Recently, it was also evidenced that extracts of S. lycocarpum St. Hill (Solanaceae) and its glycoalkaloids, solamargine (Sg) and solasonine (Sn), are active against flagellated protozoa.
Objective:
The aim was to assess the effects of the extract of S. lycocarpum and its glycoalkaloids, Sn, and Sg, on Giardia lamblia trophozoites.
Materials and Methods:
A crude extract (96%ethanol) (EB) of fruits of S. lycocarpum was prepared and fractionated by partition with 40%ethanol and n-hexane: Ethyl acetate. Glycoalkaloids, Sn, and Sg were recognized in the ethanol fraction (EF) and further isolated by column chromatography. EB, EF, the isolated Sn and Sg and a mixture (1:1) of both glycoalkaloids were tested on cultures of G. lamblia trophozoites and macrophages.
Results:
EB, EF and glycoalkaloids of S. lycocarpum showed activity against Giardia (95.0 < Inhibitory concentration 50 [IC50] ≤120.3 μg/mL). The mixture of glycoalkaloids (1:1) was more active (IC50 = 13.23 μg/mL) than each one individually, suggesting a synergic effect. Moreover, the mixture is nontoxic to macrophage cells.
Conclusion:
Results are optimistic concerning the anti-Giardia potential of the mixture Sn + Sg. Further studies, in vitro and in vivo, will be required to consolidate the usefulness of the mixture of Sn + Sg in view of a new therapeutic strategy for giardiasis.
Keywords: Giardia lamblia, glycoalkaloids, solamargine, Solanum lycocarpum, solasonine
INTRODUCTION
Solanum lycocarpum St. Hill is a plant species of the Solanaceae family, popularly known in Brazil as “lobeira”, “fruta-do-lobo”, “juribebão” or “maça-do-cerrado”. It grows spontaneously in tropical and temperate zones, including the Brazilian Cerrado biome, having great importance for food and medicinal use.[1,2] At Brazil, a traditional remedy (“polvilho-da-lobeira”) is produced from the pulp of the fruit to be used by its alleged hypoglycemic effect.[3]
Biological activities have been intensively investigated, being proved the anti-viral, diuretic, anti-fungi, anti-spasmodic, anti-inflammatory and other pharmacodynamic proprieties.[4,5,6,7] Recently it was also evidenced that extracts of S. lycocarpum and its glycoalkaloids, solamargine (Sg) and solasonine (Sn), are active against flagellated protozoa, Trypanosoma cruzi[8] Leishmania infantum,[7] Leishmania amazonensis,[9] as well as, against helminthes, Strongyloides stercoral is[10] and Schistosoma mansoni.[11] The anti-parasitic effect of S. lycocarpum seems to be also useful in wild nature: Ripen fruits are sought by the maned-wolf (Chrysocyon brachyurus), the largest canid of South America and some authors believe that eaten fruits help in the control of parasitic diseases that affect the maned-wolf and point glycoalkaloids as the active constituents.[12,13]
Solamargine and Sn [Figure 1] are the typical metabolites of S. lycocarpum, however, several other classes of compounds, such as phenolic acids, tannins, flavonoids, steroids, and triterpenes were already recognized.[14,15]
Figure 1.
Structures of glycoalkaloids: Solasonine and solamargine
Considering the indications on the anti-parasitic usefulness of S. lycocarpum extracts and compounds we proposed to investigate their effects on Giardia lamblia, a flagellated protozoon responsible for an intestinal infection (giardiasis) prevalent in many parts of the world and endemic in countries with poor standards of hygiene and sanitation.[16,17] In those countries, common drugs to treat giardiasis (nitroimidazoles, benzimidazoles, quinacrine, paromomycin or furazolidone) are not of easy access[18] and together with increasing resistances, adverse effects, and toxicity, therapeutic alternatives are welcome.
Plants with alleged anti-parasitic activity are excellent starting point to be investigated on G. lamblia parasite.
MATERIALS AND METHODS
Plant material
Fruits of S. lycocarpum St. Hill were collected in Barretos, São Paulo State, Brazil, S 20° 34 × 15.898”/W 48° 34 × 29.989”. A voucher specimen (SPFR 11.308) was deposited at the Herbarium of the Faculty of Philosophy Science and Letters, University of São Paulo, Ribeirão Preto, São Paulo State, Brazil.
Fruits were dried in hot air flow at 60°C and then crushed and reduced to power in a blade grinder.
Extraction
Fruits powder (35 g) of S. lycocarpum was submitted to extraction with 250 mL of 96%ethanol during 4 h at boiling temperature, under reflux, following the procedure described by Almeida and Rocca (1995).[19] After filtration under reduced pressure, remainder powder was re-extracted with 200 mL of 96%ethanol. Extractive solutions were blended, concentrated under reduced pressure to achieve a syrupy consistency and then, dried at room temperature, yielding 8.12 g of dry extract (EB). A thin layer chromatography (TLC) profile of EB was obtained, using 20 cm × 20 cm TLC aluminum plates pre-coated with a 0.25 mm layer of silica gel 60 F254 (Macherey-Nagel GmbH and Co). Plate was activated at 105°C for 30 min prior to use. 15 μL of EB solution (1 mg/mL in 96%ethanol), as well as, 15 μL of solutions of Sn and Sg (1 mg/mL in 96%ethanol) were applied. Reference Sn and Sg (purity 96%) were kindly provided by Professor Adélia Emília de Almeida, Department of Drugs and Medicines, School of Pharmaceutical Sciences, UNESP, Araraquara, São Paulo State - Brazil. Plate was developed with a solution of n-butanol: Acetic acid: Water (6:3:1), then dried and revealed with sulfuric acid 10% (v/v) and heated at 150°C for 5 min. Five bands were observed in the chromatogram at retention factors (Rfs) of 0.38, 0.46, 0.57, 0.67 and 0.78. Sn and Sg bands were revealed at Rfs 0.46 and 0.57, respectively.
Isolation of solamargine and solasonine
0.5 g of EB was solubilized in 10 mL of 40%ethanol and partitioned (v/v) with 3 × 50 mL of n-hexane: Ethyl acetate (9:1). The obtained two fractions, theethanol fraction (EF) and the n-hexane: Ethyl acetate fraction (HF), were concentrated under reduced pressure and monitored by TLC in the condition described above. Sn and Sg [Figure 1] are two major glycoalkaloids found in at least 100 Solanum species.[2,7] In the present study, glycoalkaloids (Sn and Sg) were found in EF and not detected in the HF. 1.0 g of EF was then solubilized in 10 mL of 40%ethanol (v/v) and submitted to open column chromatography, using a 40.0 cm × 5.0 cm glass column filled with a bed of neutral aluminum oxide (VETEC) (63–200 μm).
Elution was performed with 40%ethanol (v/v). Six sub-fractions (F0, F1, F2, F3, F4, and F5), 60 mL each were collected and inspected by TLC. Sn, and Sg were found in sub-fraction F2. Sub-fraction F2 was, then, submitted to preparative TLC, using 20 × 20 cm plates coated with silica gel 60 (0, 75 μm layer thickness) activated at 105°C during 30 min 500 μL of the F2 solution were applied and developed with n-butanol: Glacial acetic acid: water (6:3:1). TLC bands corresponding to Sg and Sn were scraped-off and then extracted with ethanol 96%. After centrifugation, the supernatant was evaporated under reduced pressure, and glycoalcaloids recovered.
Concentrations of Sg and Sn in EB and EF, as well as the purity of the isolated glycoalkaloids were estimated by high-performance liquid chromatography-photodiode array detection analysis, following the method described by Tiossi et al.[20]
Parasites and cultures
The antiparasitic activity assay was performed by growth inhibition assays of trophozoites G. lamblia according to the methodology described by Sousa and Poiares-da-Silva.[21]
Giardia lamblia (WBC6 strain [ATCC 30957] originally from a patient with chronic diarrhea) was obtained from the American Type Culture Collection, Rockville, Md. Trophozoites were maintained in axenic culture at 37°C in 10 mL of Diamond's TYI-S-33 medium, as modified by Keister (1983),[22] in screw-cap cell culture vials. Penicillin G (250 μg/mL) and streptomycin sulfate (250 μg/mL) were added during routine culture. Log-phase cultures (2–3 days) were harvested by cooling culture vials (4°C/15 min) and centrifuged (1500 g/5 min). Trophozoites were washed three times and were then counted in a hemocytometer (Neubauer cell-counter chamber) (5.0 × 104 cells). These cells were used to study the effects of S. lycocarpum samples and glycoalkaloids on G. lamblia trophozoites growth.
Growth inhibition assay
Susceptibility of G. lamblia growth was determined as previously described by Sousa and Poiares-da-Silva (1999).[21] Samples, S. lycocarpum extract (EB), the ethanolic fraction (EF), the isolated Sg, the isolated and Sn and mixture of both glycoalkaloids (Sn + Sg) were diluted in dimethylsulfoxide (DMSO; Sigma Chemical) at 100 mg/mL and then in TYI-S-33 medium in order to get a range of concentrations from 10 to 200 μg/mL. Cultures of log-phase trophozoites (5 × 104) were incubated at 37°C for 48 h as a function of samples concentrations in fresh culture medium using 1.5 mL eppendorf vials. Controls were performed in similar experimental conditions with the DMSO solvent and in the absence of samples. After incubation, vials were cooled at 4°C/15 min and the total number of trophozoites were counted under the light microscope (Nikon Eclipse E100) using a Neubauer cell-counter chamber. The results were expressed as cell number, percentage of control, and the concentration that inhibited the growth at 50% inhibitory concentration 50 (IC50) was determined. All experiments were performed in duplicate and in at least three independent assays.
Cytotoxicity assays
For cytotoxicity assays, late log phase of macrophages cells (J774) were trypsinized and was incubated at 37°C in 24-well tissue culture plates under microaerophilic condition. When the monolayers reached confluence (3–4 days), the medium was removed, and the cells incubated with S. lycocarpum samples for 48 h. The cells viability was evaluated by tetrazolium-dye (MTT) colorimetric method.[23] All experiments were performed in duplicate and in at least three independent assays.
RESULTS AND DISCUSSION
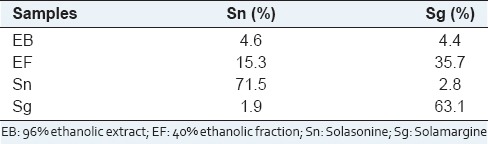
Essayed samples from S. lycocarpum were characterized in what concerns to the concentrations of Sn, and Sg [Table 1]. In the extract EB, Sn, and Sg account, respectively, 4.6% and 4.4% of the composition. In the EF, these glycoalkaloids attain 15.3% and 35.7%, respectively. Isolated Sn, and Sg are, respectively, at 71.5% and 63.1% purity.
Table 1.
Concentration of glycoalkaloids in the samples from Solanum lycocarpum
The biological activity of samples of S. lycocarpum was evaluated on G. lamblia trophozoites and the cytotoxicity on macrophage cells. The antigiardial assays, based on cell growth inhibition of G. lamblia trophozoites, revealed that the samples EB, FE, Sn, Sg, and mixture Sn + Sg (1:1), decreased the number of G. lamblia trophozoites as a function of concentration (not shown). Table 2 summarizes results of anti-Giardia activity and cytotoxicity on macrophages. The cytotoxicity of S. lycocarpum samples was compared with the activity against G. lamblia using the selective index (SI; IC50 (macrophage cells)/IC50 (protozoa)) [Table 2]. A value greater than 1.0 is considered that the sample is more selective for the parasite.[24] According the classification of giardicidal activity established by Amaral et al. (2006),[25] all samples can be considered active (100 <IC50 ≤250.00 μg/mL) or in the case of EF and the mixture as highly actives (IC50 ≤100.00 μg/mL). However, EB as well as Sn were cytotoxic for macrophages showing a low index of selectivity (SI of 0.29 and 0.61, respectively) [Table 2].
Table 2.
Anti-Giardia activity and cytotoxicity on macrophages of samples from Solanum lycocarpum

Surprisingly, although the lower concentrations of glycoalkaloids, the activity of EF (IC50 values of 95.00 μg/mL) is better than those registered for each one of isolated glycoalkaloids (Sn: IC50 = 103.70 μg/mL; Sg: IC50 = 120.30 μg/mL). This finding raised the hypothesis that the activity of fraction EF (and also extract EB) could depend from synergistic effects resulting from the presence of both glycoalkaloids.
In fact, when testing the mixture of Sn and Sg (Sn + Sg) activity raises considerably (IC50 value of 13.20 μg/mL) with a high index of selectivity (SI = 18.9) [Table 2]. Synergistic effects of these glycoalkaloids were already reported concerning the activity on tumor cells,[26] fungi,[5] L. amazonensis,[9] L. infantum,[7] T. cruzi[8] and fibroblast cell line (LLCMK2 cells).[9]
Previously were demonstrated that extracts of S. lycocarpum and its glycoalkaloids, Sg and Sn, are active against T. cruzi (IC50 values of 194.7–15.3 μg/mL),[7,8] L. infantum (IC50 value of 16.2 μg/mL)[7] and L. amazonensis (IC50 values of 238.4–6.6 μM).[9] Comparing these with the present data, we see that S. lycocarpum and its glycoalkaloids shows antigiardial activity that is similar or higher than that described for the other flagellated protozoa.
The report of the literature suggests that steroidal glycoalkaloids containing a chacotriose moiety (rhamnose-glucose-rhamnose) are usually more biologically active than those containing a solatriose moiety (rhamnose-galactose-glucose). Sg, containing chacotriose moiety, was more active on nematodes.[27] T. cruzi[8] and L. amazonensis[9] than Sn that contains solatriose moiety. The cell membranes recognize the rhamnose moieties and absorb the compound as a whole.[9,28] In the present study, Sg was not more active on G. lamblia trophozoites. In this case, it seems that the type of the sugar moiety do not influences how Sg and Sn interacts with a cell membrane of Giardia.
Although having in mind that glycoalkaloids are recognized as potentially toxic, in particular, fetal toxicity and phytoestrogenic effects after long-term consumption,[29,30,31,32] our results are optimistic concerning the usefulness of the mixture of Sn and Sg for development of new therapeutic strategies for treatment of giardiasis.
CONCLUSION
The present study describes for the first time the anti-Giardia activity of Sn and Sg, proving the synergic effects of these compounds. Therefore, we propose further in vitro and in vivo studies to consolidate the usefulness of the mixture of Sn + Sg in view of a new alternative therapeutic strategy for giardiasis.
ACKNOWLEDGMENTS
This work was supported by “Programa Operacional Ciência e Inovação Program 2010 (POCI)/FEDER’ of Foundation for Science and Technology FCT; Ciência Sem Fronteiras Program/CNPq; INCT-if: National Institute of Science and Technology for Pharmaceutical Innovation, Brazil. PADC-FCFar-UNESP-Araraquara, São Paulo state, Brazil.
Footnotes
Source of Support: This work was supported by “Programa Operacional Ciência e Inovação 2010 (POCI)/FEDER’ da Fundação para a Ciência e Tecnologia (FCT). Ciência Sem Fronteiras/CNPq; INCT-if: Instituto Nacional de Ciência e Tecnologia para Inovaçêo Farmacêutica-Brazil,
Conflict of Interest: None declared.
REFERENCES
- 1.Lorenzi H. 3rd ed. Nova Odessa: Plantarum; Plantas Daninhas do Brasil: Terrestres, Aquáticas, Parasitas, e Tóxicas. 2000 Available from: http://www.bdpa.cnptia.embrapa.br/busca?b=ad&id=15175&biblioteca=vazio&busca=autoria:%22LORENZI,%20H.%22&qFacets=autoria:%22LORENZI,%20H.%22&sort=&paginacao=t&paginaAtual=1 . [Google Scholar]
- 2.Pio Corrêa M, pena LA. Dicionário De plantas úteis do Brasil e das exóticas estudadas Rio de Janeiro: Ministério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal, 1984. [Last accessed on 2014 May 15]. Available from: http://wwwworldcatorg/title/dicionario-das-plantas-uteis-do-brasil-e-das-exoticas-cultivadas/oclc/21880710&referer=brief_results .
- 3.Dall’Agnol R, Lino von Poser G. The use of complex polysaccharides in the management of metabolic diseases: The case of Solanum lycocarpum fruits. J Ethnopharmacol. 2000;71:337–41. doi: 10.1016/s0378-8741(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 4.Vieira G, Jr, Ferreira PM, Matos LG, Ferreira EC, Rodovalho W, Ferri PH, et al. Anti-inflammatory effect of Solanum lycocarpum fruits. Phytother Res. 2003;17:892–6. doi: 10.1002/ptr.1247. [DOI] [PubMed] [Google Scholar]
- 5.Fewell AM, Roddick JG, Weissenberg M. Interactions between the glycoalkaloids solasonine and solamargine in relation to inhibition of fungal growth. Phytochemistry. 1994;37:1007–11. doi: 10.1016/s0031-9422(00)89518-7. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian G, Sarathi M, Kumara SR, Hameed AS. Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture. 2007;23:15–9. [Google Scholar]
- 7.Martins GZ. Pharmacognostic Study and Biological Screening of Solanum lycocarpum St Hill (Solanaceae) Pharm D Thesis, Faculty of Pharmaceutical Sciences, UNESP – Estadual Paulista University, Araraquara, Brazil;2013. [Last accessed on 2014 May 15]. Available from: http://baserepositoriounespbr/bitstream/handle/11449/104767/martins_gz_dr_arafcfpdfsequence=1&isAllowed=y .
- 8.Moreira RR, Martins GZ, Magalhães NO, Almeida AE, Pietro RC, Silva FA, et al. In vitro trypanocidal activity of solamargine and extracts from Solanum palinacanthum and Solanum lycocarpum of Brazilian cerrado. An Acad Bras Cienc. 2013;85:903–7. doi: 10.1590/S0001-37652013000300006. [DOI] [PubMed] [Google Scholar]
- 9.Abreu Miranda M, Tiossi RF, da Silva MR, Rodrigues KC, Kuehn CC, Rodrigues Oliveira LG, et al. In vitro leishmanicidal and cytotoxic activities of the glycoalkaloids from Solanum lycocarpum (Solanaceae) fruits. Chem Biodivers. 2013;10:642–8. doi: 10.1002/cbdv.201200063. [DOI] [PubMed] [Google Scholar]
- 10.Miranda MA. Avaliação do potencial antiparasitário do extrato alcaloídico e de alcalóides esteroidais dos frutos de Solanum lycocarpum A St-Hil Pharm D Thesis Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil; 2010. [Last accessed on 2014 May 15]. Available from: http://wwwtesesuspbr/teses/disponiveis/60/60138/tde-04112010-161118/pt-brphp .
- 11.Miranda MA. Potential evaluation of antiparasitic of alkaloid extract and steroidal alkaloids of the fruit of Solanum lycocarpum A St-Hil Pharm D Thesis Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil; 2010. [Last accessed on 2014 May 15]. Available from: http://wwwteses uspbr/teses/disponiveis/60/60138/tde-04112010-161118/pt-brphp .
- 12.Bueno AA, Belentani SC, Motta-Junior JC. Feeding ecology of the maned wolf, Chrysocyon brachyurus (Illiger, 1815) (Mammalia: Canidae), in the Ecological Station of Itirapina, São Paulo state, Brazil. Biota Neotrop. 2002;2:1–8. [Google Scholar]
- 13.Rodrigues FH, Hass A, Lacerda AC, Grando RL, Bagno MA, Bezerra AM, et al. Feeding habits of the maned wolf (Chrysocyon brachyurus) in the Brazilian Cerrado. MastozoologÍa Neotrop. 2007;14:37–51. [Google Scholar]
- 14.Nakamura S, Hongo M, Sugimoto S, Matsuda H, Yoshikawa M. Steroidal saponins and pseudoalkaloid oligoglycoside from Brazilian natural medicine, “fruta do lobo” (fruit of Solanum lycocarpum) Phytochemistry. 2008;69:1565–72. doi: 10.1016/j.phytochem.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Araujo MG, Galeane MC, Castro AD, Salgado HR, Almeida AE, Cunha WR, et al. Pharmacognostical evaluation of fruits of Solanum lycocarpum A St Hill (Solanaceae) Pharmacogn J. 2010;2:248–53. [Google Scholar]
- 16.Black RE, Dykes AC, Sinclair SP, Wells JG. Giardiasis in day-care centers: Evidence of person-to-person transmission. Pediatrics. 1977;60:486–91. [PubMed] [Google Scholar]
- 17.Wolfe MS. Giardiasis. Clin Microbiol Rev. 1992;5:93–100. doi: 10.1128/cmr.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14:114–28. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida AE, Rocca MA. Glicoalcalóides dos frutos do Solanum flaccidum Vell. Rev Ciênc Farm. 1995;16:111–8. [Google Scholar]
- 20.Tiossi RF, Miranda MA, de Sousa JP, Praça FS, Bentley MV, McChesney JD, et al. A validated reverse phase HPLC analytical method for quantitation of glycoalkaloids in Solanum lycocarpum and its extracts. J Anal Methods Chem 2012. 2012 doi: 10.1155/2012/947836. 947836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sousa MC, Poiares-Da-Silva J. A new method for assessing metronidazole susceptibility of Giardia lamblia trophozoites. Antimicrob Agents Chemother. 1999;43:2939–42. doi: 10.1128/aac.43.12.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 23.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 24.Santos AO, Ueda-Nakamura T, Dias Filho BP, Veiga Junior VF, Pinto AC, Nakamura CV. Effect of Brazilian copaiba oils on Leishmania amazonensis. J Ethnopharmacol. 2008;120:204–8. doi: 10.1016/j.jep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Amaral FM, Ribeiro MN, Barbosa-Filho JM, Reis AS, Nascimento FR, Macedo RO. Plants and chemical constituents with giardicidal activity. Braz J Pharmacogn. 2006;16:696–720. [Google Scholar]
- 26.Millward M, Powell A, Daly P, Tyson S, Ferguson R, Carter S. Results of phase I clinical trials of Coramsine in patients with advanced solid tumors. J Clin Oncol. 2006;24(Supp 20):2070. [Google Scholar]
- 27.Udalova ZV, Zinoveva SV, Vasileva IS, Paseshnichenko VA. Correlation between the structure of plant steroids and their effects on phytoparasitic nematodes. Appl Biochem Microbiol. 2004;40:93–7. [Google Scholar]
- 28.Cham BE, Daunter B. Solasodine glycosides. Selective cytotoxicity for cancer cells and inhibition of cytotoxicity by rhamnose in mice with sarcoma 180. Cancer Lett. 1990;55:221–5. doi: 10.1016/0304-3835(90)90122-e. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz A, Pinto E, Haraguchi M, de Oliveira CA, Bernardi MM, de Souza Spinosa H. Phytochemical study of Solanum lycocarpum (St. Hil) unripe fruit and its effects on rat gestation. Phytother Res. 2007;21:1025–8. doi: 10.1002/ptr.2200. [DOI] [PubMed] [Google Scholar]
- 30.Chang CV, Felício AC, Reis JE, Guerra Mde O, Peters VM. Fetal toxicity of Solanum lycocarpum (Solanaceae) in rats. J Ethnopharmacol. 2002;81:265–9. doi: 10.1016/s0378-8741(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 31.Maruo VM, Bernardi MM, Spinosa HS. Toxicological evaluations of long-term consumption of Solanum lycocarpum St. Hill fruits in male and female adult rats. Phytomedicine. 2003;10:48–52. doi: 10.1078/094471103321648656. [DOI] [PubMed] [Google Scholar]
- 32.Maruo VM, Soares MR, Bernardi MM, Spinosa HS. Embryotoxic effects of Solanum lycocarpum St. Hill fruits consumption during preimplantation and organogenesis in rats. Neurotoxicol Teratol. 2003;25:627–31. doi: 10.1016/s0892-0362(03)00038-2. [DOI] [PubMed] [Google Scholar]


