Abstract
Background:
Kalpaamruthaa (KA) is a formulatory herbal preparation has beneficial antioxidant, anti-apoptotic and anti-inflammatory properties against cardiovascular damage (CVD).
Objective:
The present study was undertaken to investigate the protective role of KA in type II diabetes mellitus-induced CVD through the modulation of protease-activated receptor-1 (PAR1).
Materials and Methods:
CVD was developed in 8 weeks after type II diabetes mellitus induction with high fat diet (2 weeks) and low dose of streptozotocin (2 × 35 mg/kg b.w. i.p. in 24 h interval). CVD-induced rats treated with KA (200 mg/kg b.w. in 0.5 ml of olive oil) orally for 4 weeks.
Results:
KA increased the activities of enzymatic antioxidants and the levels of non-enzymatic antioxidants in pancreas of CVD-induced rats. KA effectively reduced the lipid peroxides and carbonyl content in the pancreas of CVD-induced rats. KA reduced cellular damage by ameliorating the activities of marker enzymes in plasma, heart and liver. The protective nature of KA was further evidenced by histological observation in pancreas. Further, KA reduced CVD by decreasing the expression of PAR1 in heart.
Conclusion:
This study exhibits the defending role of KA in type II diabetes mellitus-induced CVD through altering PAR1.
Keywords: Cardiovascular damage, Kalpaamruthaa, oxidative stress, protease-activated receptor-1, type II diabetes mellitus
INTRODUCTION
Hyperglycemia in diabetes produces multiple biochemical consequence including oxidative stress and plays a vital role in the development of cardiovascular damage (CVD).[1] Chronic hyperglycemia persuades cellular oxidative stress, resulting in the production of oxygen free radicals, a response that plays a major task in the etiology of diabetic complications.[2] Hyperglycemia induces increased superoxide anion production via the activation of multiple pathways including NAD (P) H oxidase, cyclooxygenase, uncoupled nitric oxide synthase, glucose autoxidation, polyol pathway and formation of advanced glycation end products.[3,4,5,6,7] Reactive oxygen species (ROS) can induce cell death via lipid peroxidation (LPO), alteration of cellular proteins and initiation of diverse stress-signaling pathways. Oxidative stress is currently suggested as a major factor underlying the tissue damage in diabetes mellitus and diabetic complications.[8] The increased production of ROS may destruct pancreas and progress β-cell dysfunction in diabetic condition.[9] Protease-activated receptor-1 (PAR1) is a G protein-coupled receptor, is expressed in cardiomyocytes and cardiac fibroblasts and is activated by the coagulation protease thrombin, as well as other proteases. The expression of PAR1 is found to be localized in the endothelial cells of normal arteries, and also it is observed in the smooth muscle cells and macrophages of the vessel wall in atherosclerotic lesions.[10] A common signaling pathway of PAR1 is the activation of phospholipase C, resulting in the formation of inositol triphosphate and diacylglycerol, followed by calcium (Ca2+) mobilization and activation of protein kinase C. In our previous study, we have reported that Kalpaamruthaa (KA) modulates CVD by altering protein kinase C/Akt signaling.[11] Hence in this study, we intend to investigate the role of KA on PAR1, a upstream molecule to PKC. Further, oxidative stress is suggested to be involved in the upregulation of PAR1 expression, whereas the scavengers of free radicals and inhibitors of NAD (P) H oxidase, have been shown to inhibit the upregulation of PAR1.[12] PARs are important modulators of responses that are the hallmark of inflammation. It is well established that PARs contribute to inflammation through cell and tissue specific induction of inflammatory cytokines and chemokines.[13] PAR activation also induces vascular smooth muscle cells proliferation.[14] PARs have recently garnered significant attention from the medical and pharmaceutical communities as potential therapeutic targets in the treatment of CVD.[15] Targeting PAR1 with the drug that has antioxidant property provides better result in CVD associated with insulin resistance.
Kalpaamruthaa is a herbal preparation, consisting of Semecarpus anacardium (SA) Linn. nut milk extract, Emblica officinalis dried powder of fruit and honey in proposed ratio. SA Linn. nut shells contain biflavones A, C, A1, A2, tetrahydrorobustaflavone B (tetrahydromentoflavone), jeediflavone, semecarpuflavone, and galluflavone.[16,17,18,19] Emblica officinalis has emblicanin A, emblicanin B, punigluconin, pedunculagin, rutin and gallic acid, which were established by high-performance thin layer chromatography.[20] KA has been reported for its potent antioxidant, anti-inflammatory, anti-arthritic, anticancer and analgesic, antipyretic and non-ulcerogenic properties.[20,21,22,23,24] Further, in our previous study, KA has been reported for its potent antioxidant, anti-apoptotic and anti-inflammatory properties.[25,26] KA effectively improved the endothelial damage, and metabolic alterations in diabetes-induced CVD.[27,28] The goal of this study was to evaluate the protective role of KA in CVD through the modulation of PAR1 expression.
MATERIALS AND METHODS
Chemicals
Streptozotocin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). SA has been prepared according to Formulary of Siddha Medicine (1972).[29] To this, a fresh dried powder of EO fruit and honey were added. All the other chemicals and reagents used in this study were of analytical grade.
Animals
The experiments were conducted according to ethical norms approved by the Ministry of Social Justices and Empowerment, Government of India and Institutional Animal Ethics Committee Guidelines (Approval No. 01/03/2010). Male Sprague-Dawley rats (160–180 g) were obtained from the Central Animal House, Institute of Basic Medical Sciences, Chennai, India. The animals were acclimatized to the laboratory conditions for a period of 2 weeks. The animals were housed under standard conditions of temperature 25°C ± 1°C, relative humidity 60% ± 5%, 12 ± 1 h light/dark cycles. Animals were given a standard rat feed (Hindustan Lever Ltd., Bangalore) and water ad libitum. The efforts were taken carefully to minimize the animal suffering.
Experimental design
The animals were divided into five groups with six animals in each group. Group I: Control rats received olive oil (1 ml) during the treatment period. Group II: CVD-induced rats. [Insulin resistance was developed by the administration of high-fat diet (84.3% standard laboratory chow, 10% yolk powder, 5% lard, 0.5% bile salt and 0.2% cholesterol) for 2 weeks[30] and then diabetes was induced by streptozotocin administration, 2 × 35 mg/kg body weight intraperitoneally (dissolved in 0.5 ml of 0.1 M citrate buffer, pH 4.5) in 24 h interval, CVD developed in 8 weeks as we reported earlier[25,31] Group III: CVD-induced rats (as group II rats), then treated with KA (200 mg/kg b.w. in 0.5 ml of olive oil) orally for 4 weeks. Group IV: CVD-induced rats (as group II rats), then treated with SA (200 mg/kg b.w. in 0.5 ml of olive oil) orally for 4 weeks. Group V: KA control rats received (200 mg/kg b.w. in 0.5 ml of olive oil) orally during the treatment period.
At the end of the experimental period, animals were sacrificed by decapitation. Blood was collected in a heparinized tube, and plasma was separated by centrifugation at 1000 × g for 10 min. Pancreas, heart and liver were carefully dissected, rinsed in ice-cold physiological saline, homogenized with 0.1 M Tris-HCl buffer (pH 7.4) at 4°C and centrifuged at 800 × g for 10 min at 4°C. The resultant supernatants were used to measure biochemical parameters.
Biochemical assays
The activities of enzymatic antioxidants and the levels of non-enzymatic antioxidants were studied in pancreas. Superoxide dismutase (SOD) was measured by the method of Marklund and Marklund.[32] Catalase (CAT) was estimated by Sinha's method.[33] Glutathione peroxidase (GPx) was assayed according to the method of Rotruck et al.[34] Glutathione reductase (GR) was measured by Staal et al.[35] Reduced glutathione (GSH) was assessed by the method described by Moron et al.[36] Vitamin C was measured by the method of Omaye et al.[37] Vitamin E was assayed according to the method of Desai.[38] LPO was measured by the method of Ohkawa et al. and protein carbonyl levels were also estimated according to the method of Levine et al. in pancreas.[39,40]
Lactate dehydrogenase (LDH) was assayed by the method of King.[41] Creatine kinase (CK) activity was determined by the method of Okinaka et al.[42] Glutamate oxaloacetate transaminase/aspartate aminotransferase (GOT/AST) and glutamate pyruvate transaminase/alanine aminotransferase (GPT/ALT) were assayed by the method of King.[43] alkaline phosphatase (ALP) activity was assayed by the method of King.[44] γ-Glutamyl transferase (γ-GT) was assayed by the method of Rosalki and Rau.[45]
Immunohistochemical expression of protease-activated receptor-1
Paraffin embedded myocardial sections (3 μm) were rehydrated in xylene and then in graded ethanol solutions. The sections were blocked with 5% bovine serum albumin in Tris buffered saline, pH 7.4, for 2 h. The sections were then incubated with primary antibody (PAR1) at 4°C overnight (Santa Cruz Biotechnology, Inc., USA). After washing the slides thrice, the sections were incubated with secondary antibody for 2 h at room temperature. Sections were then washed and incubated for 5–10 min in 0.02% diaminobenzidine solution containing 0.01% hydrogen peroxide. Counter staining was performed using hematoxylin, and the slides were visualized under the microscope (Nikon Eclipse 400).
Histopathological examination
The pancreas was fixed in 10% buffered formalin solution and embedded in paraffin. Three μm thick sections were placed on slides and stained with hematoxylin and eosin. The slides were then evaluated under the light microscope (Nikon Eclipse 400).
Statistical methods
Statistical package for social sciences (SPSS.10) software (IBM, Chicago, IL, US) was used for One-way analysis of variance, followed by the Tukey's test for multiple comparisons. All the values are expressed as mean ± standard deviation for six rats in each group.
RESULTS
The levels of LPO and carbonyl in pancreas of control and experimental groups of rats are shown in Figure 1. The levels of LPO and protein carbonyls were increased in CVD-induced rats as compared to control (P < 0.01). KA treatment (P < 0.01) decreased these levels in CVD-induced rats toward near normal than the reduction seen in SA treatment as compared to CVD-induced rats. No difference between control and drug alone was observed.
Figure 1.
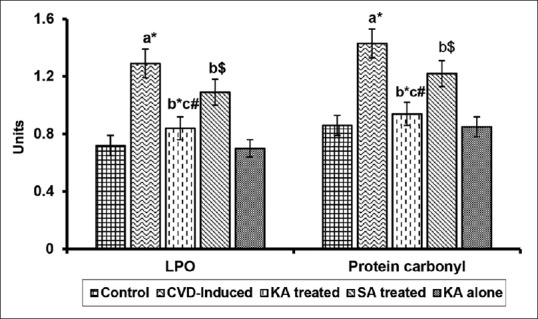
Effect of Kalpaamruthaa on lipid peroxidation (LPO) and protein carbonyl content in pancreas of control and experimental groups of rats. Values are represented as mean ± standard deviation for six rats in each group. Values are given statistically significant at P < 0.01 denoted by *; P < 0.05 denoted by #; P < 0.1 denoted by $. aVersus control, bversus cardiovascular damage-induction, c versus Semecarpus anacardium treatment. Unit: LPO-n mole malondialdehyde (MDA)/mg of protein, Protein carbonyl-n mole/mg of protein
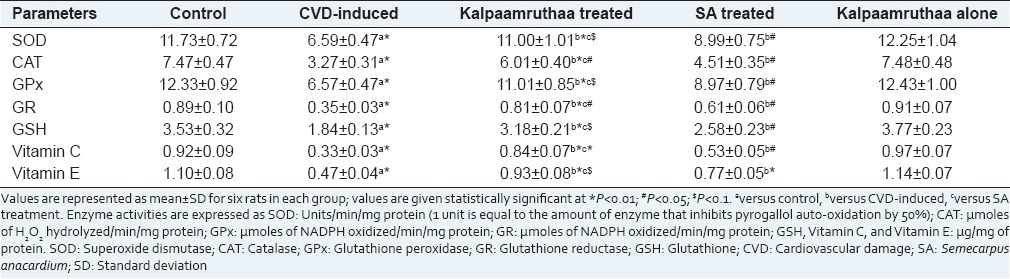
Table 1 represents the effect of KA on the activities/levels of antioxidants in pancreas of control and experimental groups of rats. The activities of SOD, CAT, GPx and GR, and the levels of GSH, Vitamin C, and Vitamin E were decreased in CVD-induced rats as compared to their control counter parts (P < 0.01). Treated groups (KA and SA) showed a significant increase in their activities. Protection rendered by KA (P < 0.01) was found to be effectively higher than SA treatment as compared to CVD-induced rats. No significant difference was observed between control and KA control groups.
Table 1.
Effect of Kalpaamruthaa on pancreatic antioxidants in control and experimental groups of rats
Marker enzymes such as LDH, CK, GOT/AST, GPT/ALT, ALP and γ-GT in plasma, heart and liver of control and experimental groups are summarized in Tables 2 and 3, respectively. CVD-induced rats exhibited increase in these enzyme activities in plasma (P < 0.01) with concordant decrease in heart and liver (P < 0.01) as compared to their control counter parts, whereas treatment with KA and SA ameliorated these activities nearer to normal state. KA imparts better modulatory effect on marker enzymes than the sole SA as compared to CVD-induced rats (P < 0.01). These findings clearly testify the protective effect of KA against CVD.
Table 2.
Effect of Kalpaamruthaa on marker enzymes in the plasma of control and experimental group of rat
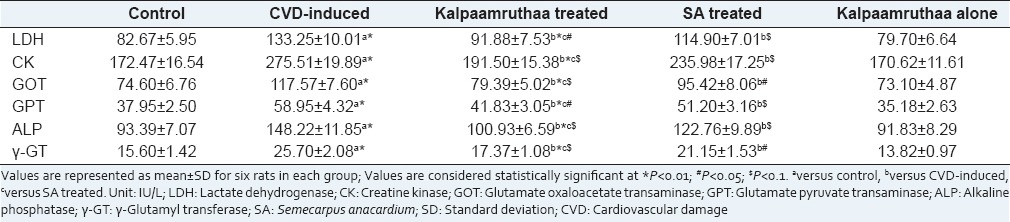
Table 3.
Effect of Kalpaamruthaa on marker enzymes in heart and liver of control and experimental group of rats
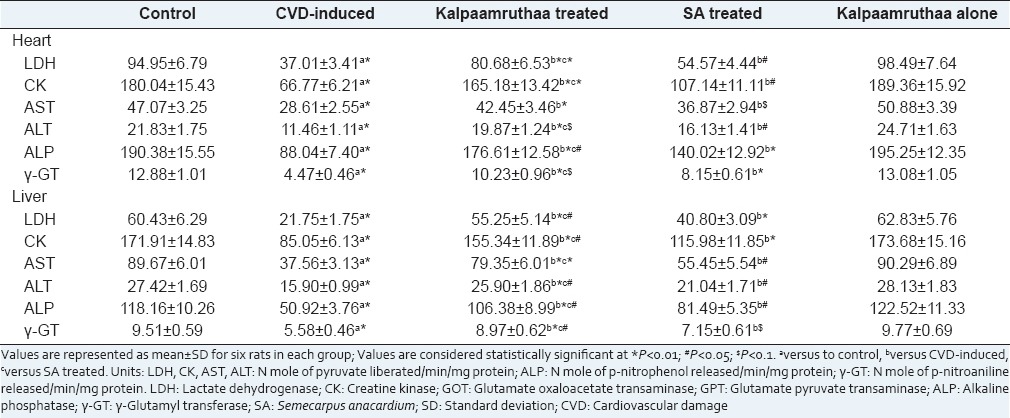
Figure 2 shows the histological changes of the pancreas in control and experimental groups of rats. Control rats and KA control rats showed intact, big and round clusters of islet cells surrounded by exocrine acini. CVD-induced rats had dilated acini gaps, shrinkage of islet and reduced pancreatic islet area. These pathological findings were altered with normal islet cells, improved islet area and reduced acini gaps in KA and SA treated group of rats. Significant degree modulation was observed in KA treated group.
Figure 2.
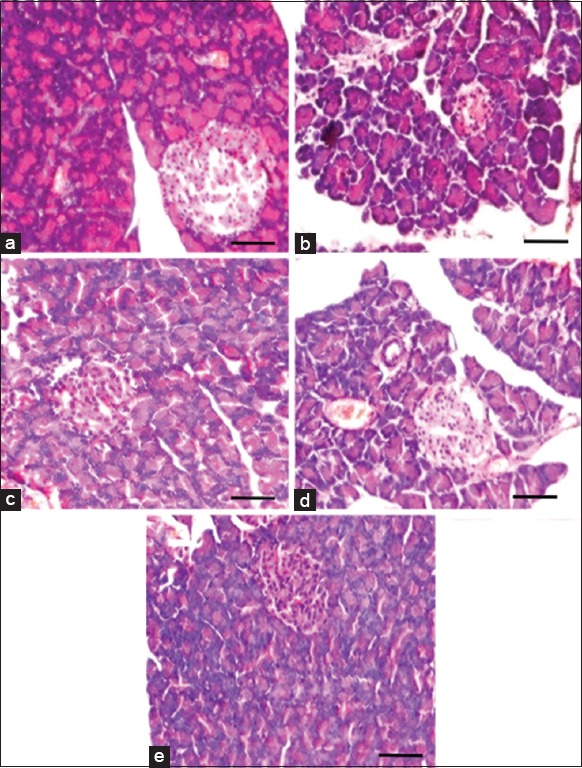
Histological alterations of pancreas in control and experimental groups of rats. Control rat pancreas (a) had intact, big and round clusters of islet cells surrounded by exocrine acini. Cardiovascular damage-induced (b) rats had dilated acini gaps, shrinked islet and reduced islet area. Kalpaamruthaa treated (c) rats had markedly reduced dilation of acini and high degree of islet area than Semecarpus anacardium treated (d) rats. Drug alone group of rats (e) had histological pattern similar that of control rats. Sections were visualized under light microscope at a magnification of × 200 (Scale bar - 100 µm)
The molecular mechanisms involved in the protective action of KA were studied through PAR1 in heart of control and experimental group of rats. Immunohistochemical expression of PAR1 in myocardium is presented in Figure 3. CVD-induced rats had a high degree of stained PAR1 cells (P < 0.01) as compared to control rats. Treatment with KA reduced the PAR1 expression upon treatment with KA (P < 0.01) as compared with CVD-induced rats. KA administered rats and control rats had minimal number of stained positive cells.
Figure 3.
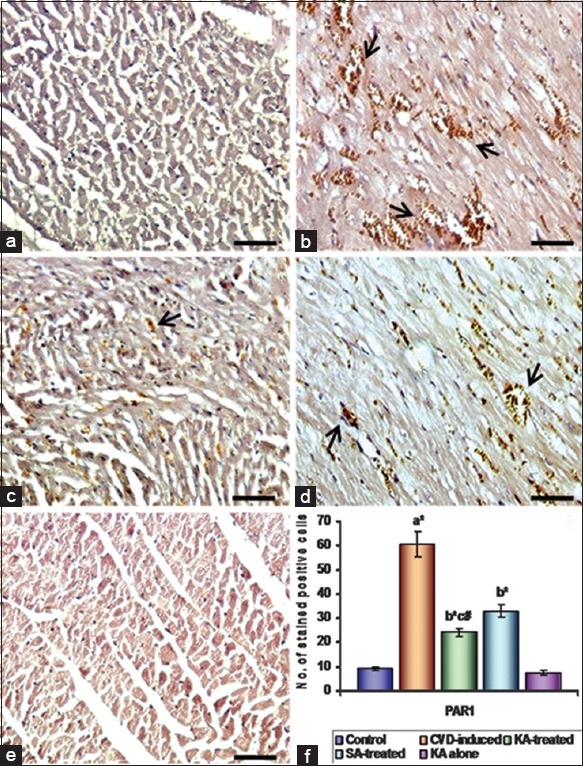
Effect of Kalpaamruthaa (KA) on protease-activated receptor-1 (PAR1) expression in heart of control and experimental group of rats. Immunohistochemical analysis of PAR1 in heart of control and experimental group of rats. (a) Control; (b) cardiovascular damage (CVD)-induced; (c) KA-treated; (d) Semecarpus anacardium (SA)-treated; (e) KA alone. Arrows indicate stained positive cells. (f) Quantitative analysis of PAR1 expression. The number of stained (positive) cells per × 200 field was averaged across 15 fields for each rat. Values are given statistically significant at P < 0.01 denoted by *; P < 0.05 denoted by #.a versus control, b versus CVD-induced, c versus SA treated (scale bar - 100 µm)
DISCUSSION
Cellular antioxidants are defending against oxidative stress in CVD to protect tissue from damage. This has led to an increase in the demand for natural products with constitutive anti-oxidative property and minimal side effects.[46,47] In the present study, we found out KA effectively protect against CVD through its antioxidant property and by modulating PAR1 expression.
Oxidative stress in diabetes coexisted with a decline in the antioxidant capacity, which could increase the deleterious effects of free radicals.[48] In CVD, the development of oxidative stress is not only by oxygen free-radical generation but also due to non-enzymatic protein glycosylation, auto-oxidation of glucose, impaired glutathione metabolism, alteration in antioxidants and LPO formation. Increased LPO under diabetic condition can be due to increased oxidative stress in the cell as a result of depletion of antioxidants protective systems.[49] CVD-induced rats showed an increase in LPO and protein carbonyl level, which are the evidence of intensified free radical production. These free radicals may react with polyunsaturated fatty acids in the cell membrane leading to peroxidation. KA modulates pancreatic damage with balanced free radicals/antioxidants status by reducing LPO and carbonyl content in our study, suggesting that KA could have a high antioxidant capacity to scavenge free radicals generated by ROS and prevent radical damage. This is in correlation with the previous report that KA reduced the oxidative tissue damage in other experimental condition.[23] From the results obtain, KA augmented the enzymatic antioxidants (SOD, CAT, GPx, and GR) and non-enzymatic antioxidants (GSH, Vitamin C and Vitamin E) in the pancreas of CVD-induced condition. Our results are in agreement with the previous report that KA protects tissue from oxidative damage by enhancing antioxidant status.[25]
The marker enzymes namely, LDH; CK; GOT/AST; GPT/ALT; ALP and γ-GT serve as sensitive indices to assess the severity of CVD.[50] When myocardial cells are damaged due to the deficiency of oxygen supply, the cell membrane becomes permeable or may rupture and results in leakage of enzymes ensuing in increased activity of these enzymes in plasma with concomitant decrease in tissue.[51] The activity of CK is an important marker for myocardial damage. LDH is a tetrameric enzyme recognized as a marker with potential use in assessing early stage of myocardial damage in diabetes.[52] γ-Glutamyl transpeptidase has a key task in amino acid transport across membranes and catalyzes the initial step in the breakdown of glutathione. Furthermore, increase in γ-GT activity in plasma is an indicator of impairment in liver function. Measurement of enzymatic activities of aminotransferases (AST and ALT) and ALP is of clinical and toxicological importance as changes in their activities are indicative of tissue damage by toxicants or in disease conditions.[53] The onset of cardiac dysfunction was confirmed with altered activities of these enzymes in CVD-induced rats. Treatment with KA modulated the activities of these enzymes comparable to the control condition. This is an indication of the protective action of KA in reversing cardiac damage. These results are in agreement with the previous report that KA was able to modulate the marker enzymes activities in other experimental system.[54]
The assessment of histological observation of pancreatic section in CVD-induced rats had showed dilated acini gaps, shrinkage of islet and reduction of pancreatic islet area. This result is in agreement with the previous report.[55] KA validates its protective effect by increasing the islet with intact pancreas. This result further substantiates the claim that KA has a protective nature on pancreatic tissue.
Protease-activated receptors have been linked to the regulation of a broad range of cellular functions in cardiovascular systems.[15] PARs are critical for normal homeostasis and contribute to the pathogenesis of vascular disorders characterized by chronic inflammation. Activation of PAR1 mediates responses involved in contractility, inflammation, proliferation and repair.[10] Thus, interference with PAR1 appears to be a promising strategy to treat CVD. It is capable of activating various signaling molecules including protein kinase C and contributes to CVD.[56] KA protects against diabetes-induced CVD by altering protein kinase C/Akt signaling.[11] PAR1 expression was studied in this study to identify the upstream signaling cascade to PKC. In this study, CVD-induced rats had elevated expression of PAR1 in heart. This might be due to increased oxidative stress elicited by hyperglycemia.[12] Treatment with KA reduces the PAR1 expression in CVD-induced rats through it antioxidant and normoglycemic properties.[25,27]
CONCLUSION
This study showed that KA exhibited antioxidant activity, and able to reduce pancreatic oxidative damage. Further, the results of this study suggested that KA is a possible regulator of PAR1 expression and have therapeutic applications in diabetes-induced CVD. These findings provide a significant molecular basis for explaining how KA defends CVD developed in type II diabetes mellitus.
ACKNOWLEDGMENT
We thank Jawaharlal Nehru Memorial Fund, New Delhi, India for financial assistance in the form of Jawaharlal Nehru Memorial Fund Scholarship for Doctoral studies (2011–2012) awarded to R. Latha.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mezzetti A, Cipollone F, Cuccurullo F. Oxidative stress and cardiovascular complications in diabetes: Isoprostanes as new markers on an old paradigm. Cardiovasc Res. 2000;47:475–88. doi: 10.1016/s0008-6363(00)00118-8. [DOI] [PubMed] [Google Scholar]
- 2.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–8. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Soro-Paavonen A, Zhang WZ, Venardos K, Coughlan MT, Harris E, Tong DC, et al. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens. 2010;28:780–8. doi: 10.1097/HJH.0b013e328335043e. [DOI] [PubMed] [Google Scholar]
- 5.Shahab A. Why does diabetes mellitus increase the risk of cardiovascular disease? Acta Med Indones. 2006;38:33–41. [PubMed] [Google Scholar]
- 6.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, et al. Superoxide production by NAD (P) H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–62. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 9.Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–76. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- 11.Latha R, Shanthi P, Sachdanandam P. Kalpaamruthaa modulates oxidative stress in cardiovascular complication associated with type 2 diabetes mellitus through PKC-ß/Akt signaling. Can J Physiol Pharmacol. 2013;91:901–12. doi: 10.1139/cjpp-2012-0443. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KT, Frye SR, Eskin SG, Patterson C, Runge MS, McIntire LV. Cyclic strain increases protease-activated receptor-1 expression in vascular smooth muscle cells. Hypertension. 2001;38:1038–43. doi: 10.1161/hy1101.092840. [DOI] [PubMed] [Google Scholar]
- 13.Cocks TM, Moffatt JD. Protease-activated receptors: Sentries for inflammation? Trends Pharmacol Sci. 2000;21:103–8. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- 14.Hirano K, Kanaide H. Role of protease-activated receptors in the vascular system. J Atheroscler Thromb. 2003;10:211–25. doi: 10.5551/jat.10.211. [DOI] [PubMed] [Google Scholar]
- 15.Shah R. Protease-activated receptors in cardiovascular health and diseases. Am Heart J. 2009;157:253–62. doi: 10.1016/j.ahj.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Gil RR, Lin LZ, Cordell GA, Kumar MR, Ramesh M, Reddy BM, et al. Anacardoside from the seeds of Semecarpus anacardium. Phytochemistry. 1995;39:405–7. doi: 10.1016/0031-9422(94)00842-h. [DOI] [PubMed] [Google Scholar]
- 17.Murthy SS. Jeediflavanone – A bioflavanoid from Semecarpus anacardium. Phytochemistry. 1985;24:1065–9. [Google Scholar]
- 18.Murthy SS. A bioflavanoid from Semecarpus anacardium. Phytochemistry. 1983;22:1518–20. [Google Scholar]
- 19.Murthy SS. Naturally occurring biflavanoid derivatives part VII galluflavanone a new flavanoid from Semecarpus anacardium Linn. Indian J Chem. 1985;24:398–402. [Google Scholar]
- 20.Ghosal S, Tripathy VK, Chouhan S. Active constituents of Emblica officinalis: Part I-the chemistry and anti-oxidant effect of two new hydrolysable tannins, Emblicanin A and B. Ind J Chem. 1996;35:941–8. [Google Scholar]
- 21.Umarani M, Shanthi P, Sachdanandam P. Protective effect of Kalpaamruthaa in combating the oxidative stress posed by aflatoxin B1-induced hepatocellular carcinoma with special reference to flavonoid structure-activity relationship. Liver Int. 2008;28:200–13. doi: 10.1111/j.1478-3231.2007.01615.x. [DOI] [PubMed] [Google Scholar]
- 22.Mythilypriya R, Shanthi P, Sachdanandam P. Synergistic effect of Kalpaamruthaa on antiarthritic and antiinflammatory properties – Its mechanism of action. Inflammation. 2008;31:391–8. doi: 10.1007/s10753-008-9090-2. [DOI] [PubMed] [Google Scholar]
- 23.Veena K, Shanthi P, Sachdanandam P. Therapeutic efficacy of Kalpaamruthaa on reactive oxygen/nitrogen species levels and antioxidative system in mammary carcinoma bearing rats. Mol Cell Biochem. 2007;294:127–35. doi: 10.1007/s11010-006-9252-1. [DOI] [PubMed] [Google Scholar]
- 24.Mythilypriya R, Shanthi P, Sachdanandam P. Analgesic, antipyretic and Ulcerogenic properties of an indigenous formulation – Kalpaamruthaa. Phytother Res. 2007;21:574–8. doi: 10.1002/ptr.2116. [DOI] [PubMed] [Google Scholar]
- 25.Latha R, Shanthi P, Sachdanandam P. Antioxidant and anti-apoptotic properties of Kalpaamruthaa in type 2 diabetes mellitus induced cardiovascular complications. Biomed Prev Nutr. 2012;2:210–4. [Google Scholar]
- 26.Raja L, Palanivelu S, Panchanatham S. Anti-inflammatory property of Kalpaamruthaa on myocardium in type 2 diabetes mellitus induced cardiovascular complication. Immunopharmacol Immunotoxicol. 2013;35:119–25. doi: 10.3109/08923973.2012.712138. [DOI] [PubMed] [Google Scholar]
- 27.Latha R, Shanthi P, Sachdanandam P. Kalpaamruthaa ameliorates myocardial and aortic damage in cardiovascular complications associated with type 2 diabetes mellitus. Can J Physiol Pharmacol. 2013;91:116–23. doi: 10.1139/cjpp-2012-0292. [DOI] [PubMed] [Google Scholar]
- 28.Latha R, Shanthi P, Sachdanandam P. Kalpaamruthaa ameliorates mitochondrial and metabolic alterations in diabetes mellitus induced cardiovascular damage. J Diet Suppl. 2014;11:305–19. doi: 10.3109/19390211.2014.887599. [DOI] [PubMed] [Google Scholar]
- 29.Narayanasami V, Uthamaroyan C. S. 2nd ed. India: Indian Medicine Practitioners Co-operative Pharmacy and Stores, Madras; 1972. Formulary of Siddha Medicine. [Google Scholar]
- 30.Xie W, Xing D, Sun H, Wang W, Ding Y, Du L. The effects of Ananas comosus L. leaves on diabetic-dyslipidemic rats induced by alloxan and a high-fat/high-cholesterol diet. Am J Chin Med. 2005;33:95–105. doi: 10.1142/S0192415X05002692. [DOI] [PubMed] [Google Scholar]
- 31.Marsh SA, Dell’italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am J Physiol Heart Circ Physiol. 2009;296:H282–92. doi: 10.1152/ajpheart.00421.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 33.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 34.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 35.Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta. 1969;185:39–48. doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- 36.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 37.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 38.Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–47. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 41.King J. Lactate dehydrogenase. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand; 1965a. p. 83. [Google Scholar]
- 42.Okinaka S, Kumagai H, Ebashi S, Sugita H, Momoi H, Toyokura Y, et al. Serum creatine phosphokinase. Activity in progressive muscular dystrophy and neuromuscular diseases. Arch Neurol. 1961;4:520–5. doi: 10.1001/archneur.1961.00450110050006. [DOI] [PubMed] [Google Scholar]
- 43.King J. The transferases alanine and aspartate tranaminases. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand Company Limited; 1965b. pp. 121–38. [Google Scholar]
- 44.King J. The phosphohydrolases – Acid and alkaline phosphatases. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand Company Limited; 1965c. pp. 191–208. [Google Scholar]
- 45.Rosalki SB, Rau D. Serum -glutamyl transpeptidase activity in alcoholism. Clin Chim Acta. 1972;39:41–7. doi: 10.1016/0009-8981(72)90297-5. [DOI] [PubMed] [Google Scholar]
- 46.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–73. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13:1203–18. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 48.Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol. 2006;38:794–803. doi: 10.1016/j.biocel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–68. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 50.Motawi TM, Sadik NA, Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: An experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010;48:2326–36. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 51.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Patel BM, Agarwal SS, Bhadada SV. Perindopril protects against streptozotocin-induced hyperglycemic myocardial damage/alterations. Hum Exp Toxicol. 2012;31:1132–43. doi: 10.1177/0960327112446817. [DOI] [PubMed] [Google Scholar]
- 53.Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology. 2007;230:178–88. doi: 10.1016/j.tox.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 54.Mythilypriya R, Shanthi P, Sachdanandam P. Salubrious effect of Kalpaamruthaa, a modified indigenous preparation in adjuvant-induced arthritis in rats – A biochemical approach. Chem Biol Interact. 2008;173:148–58. doi: 10.1016/j.cbi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Daisy P, Balasubramanian K, Rajalakshmi M, Eliza J, Selvaraj J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine. 2010;17:28–36. doi: 10.1016/j.phymed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]


