Abstract
Background:
Long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) increases risk of having a range of gastrointestinal problems. Therefore, new anti-inflammatory, analgesic, antipyretic drugs having lesser side effects are being searched all overthe world as alternatives to NSAIDs.
Aims:
To evaluate the anti-inflammatory, analgesic and antipyretic profile of Ocimum sanctum root extracts.
Materials and Methods:
Anti-inflammatory profile of hexane (STH), chloroform (STC), ethyl acetate (STE), butanol (STB) and water (STW) extracts of OS was carried out by using carrageenan induced paw edema. STE a most active extract was further validated in dose dependent manner for anti-inflammatory, analgesic and antipyretic activity as well as oral toxicity profile in small laboratory animals. Identification of bioactives flux and chemical signature of most active fraction STE was developed by using the high-performance liquid chromatography fingerprinting.
Results:
An ethyl acetate fraction (STE) exhibit most potent anti-inflammatory activity followed by STB, STW, STC and STH. Dose response study of STE showed anti-inflammatory, analgesic and anti-pyretic potential in dose-dependent manner without any toxic effect at dose 2000 mg/kg. Chemical fingerprint revealed the presence of flavanoids.
Conclusions:
The present research revealed that STE possess anti-inflammatory, analgesic and anti-pyretic properties. However, future research is advocated to evaluate the pharmacological properties of isolated bioactive compounds.
Keywords: Analgesic, anti-inflammatory, anti-pyretic, Ocimum sanctum
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most widely used medicine for the treatment of diseases and disorders linked with inflammation, pain and fever.[1] Long-term administration of NSAID may cause severe complications including gastric ulcer, renal damage, bronchospasm and cardiac abnormalities due to their nonselective inhibition of cyclooxygenases (COX) enzymes.[2,3] Therefore, new anti-inflammatory, analgesic drugs having lesser effects are being searched all overthe world as alternatives to NSAIDs.[4] Medicinal and aromatic plants are being used for 1000's of years in traditional system of medicine for the cure of many ailments. Natural molecules are much safer than synthetic molecules. Medicinal plants derived natural compounds such as flavonoids, steroids, lignans, polyphenols, coumarins, terpenes and alkaloids are scientifically proved to relieve inflammation, pain and fever.[5,6,7]
Ocimum sanctum (OS) Linn. (Lamiaceae) commonly known as “Tulsi” has been used for thousands of years in Indian System of Medicine for its diverse healing properties. Extracts of this plant, particularly of leaves, have been traditionally used for various ailments in the traditional Ayurvedic and Unani system of medicine.[8] Scientific investigations have found out that OS possesses various pharmacological activities such as anti-oxidant,[9] anti-inflammatory, analgesic, antipyretic,[10] immunomodulatory,[11] hepatoprotective,[12] and neuroprotective[13] effects have been reported using animal models. Pharmacological activities of OS could be attributed due to the presence of the phytoconstituents such as eugenol, methyl eugenol, carvacrol, sesquiterpine, apigenin, luteolin, and ursolic acid.[14] Eugenol is a phenolic compound which is a major constituent of OS.[15] Roots of OS are waste biomass after the harvesting of herb. Hence, suitable methods should be adopted to minimize the pollution of environment by converting waste materials to value added products. OS roots extracts possess anti-plasmodial,[16] anti-depressant[17] activities. The present study was carried out to determine the anti-inflammatory, analgesic and antipyretic profile of OS root extracts using small laboratory animals. Attempt was also made to develop chemical signature and to identify the bioactives flux using the high-performance liquid chromatography (HPLC) fingerprint.
MATERIALS AND METHODS
Plant materials
Roots of OS were collected from the experimental fields of CSIR - Central Institute of Medicinal and Aromatic Plants, Lucknow, India. The plant material was authenticated by Botany and Pharmacognosy Department of the institute and a voucher specimen of the material has been deposited in the herbarium unit under voucher number no. (CIMAP-12598).
Preparation of extracts
Fresh roots of OS was washed and dried under shade at room temperature. The roots were segregated and grounded in a pulverizer into a coarse powder. The powdered material (500 g) was extracted exhaustively with methanol in a soxhlet apparatus. The methanol extract was concentrated under vacuum to get a dark brown viscous mass (28.57 g, 5.8%). The extract was re-dissolved in water (STW) and was then successively partitioned with hexane (STH), chloroform (STC), ethyl acetate (STE) and butanol (STB). All the fractions were concentrated under controlled conditions in vacuum to obtain the different extracts. The extracts were air dried at room temperature and stored at 4°C till further use.
Chemicals
Diclofenac sodium, indomethacin, paracetamol, carrageenan, and yeast Brewer's were purchased from Sigma-Aldrich (St. Louis, USA). Acetic acid was purchased from S. D fine chemicals (Mumbai, Maharashtra, India), and Formalin was purchased from Qualigens fine Chemicals (Mumbai, Maharashtra, India).
In vivo study
Animals
Charles foster rats (180–200 g) and Swiss albino mice (18–22 g) were used for study. All animals were procured from institute animal house and acclimatized to experimental room before experiment. The animals were feed with pellet animal feed. However, all the animals were fasted overnight before experiment and ad libitum drinking water was provided under standard environmental conditions of 22°C ± 3°C, 12:12 dark-to-light cycle. Animal experiments were carried out as per the approved protocol by the institutional animal ethics committee followed by the committee for the purpose of control and supervision of experimental animals (CPCSEA), Government of India (Registration no: 400/01/AB/CPCSEA).
Anti-inflammatory activity
Carrageenan induced paw edema
Carrageenan induced paw edema model in rats was used to determine anti-inflammatory activity of the plant extracts. Animals were divided into seven groups comprise five animals in each group and treated orally. Group 1: Control treated with vehicle (distilled water), Group 2–6 treated with STH, STC, STE, STB, STW at 300 mg/kg and Group 7 as a standard control treated with Indomethacin (15 mg/kg). STE, a most active fraction was further studied at 0.5 log interval dose at dose 30, 100 and 300 mg/kg. After 60 min of the oral administration of test extracts, each rat was injected with freshly prepared 0.1 ml of 1% (w/v) suspension of carrageenan in physiological saline (0.9% saline solution) into subplantar region of the right hind paw of rat.[18] The paw volume was measured immediately before injection and after 1, 2, 3, 4 h than after 5 h. The difference in footpad thickness was measured by using plethysmometer (IITC, Life Scientific Instruments, Woodland Hills, CA, USA). The ability of anti-inflammatory drug to suppress paw inflammation was expressed as a percentage of inhibition of paw edema and this percentage can be calculated according to the following equation:
Percentage of inhibition (%) = (X − Y)/X × 100
Where,
X = Mean increase in paw volume of rats in the control group,
Y = Mean increase in paw volume of rats in the drug treated group.
Analgesic activity (thermal and chemical induced pain models)
Hot plate analgesic test
Mice were divided into five groups (n = 5). Each experimental group was treated orally with vehicle (distilled water), STE in dose of 30, 100, 300 mg/kg and standard (diclofenac sodium 10 mg/kg). The increased latency time of all animals towards thermal heat was recorded. 30, 60, 90 and 120 min after the administration of test and standard drug, the animals in all the five groups were individually exposed to the hot plate (UGO Basile S. R. L, Biological Research Apparatus, Italy) maintained at 55°C ± 1°C. The time taken in seconds for fore paw licking or jumping was taken as reaction time. A cut off period of 30 s is observed to avoid damage to the paws.[19]
Tail immersion test
Mice used in this test were divided into five groups (n = 5). Mice were placed into individual restraining cages leaving the tail hanging out freely. The animals were allowed to adapt to the cages for 5 min before testing. The lower 5 cm portion of the tail was marked. This part of the tail is immersed in a water bath freshly filled water of exactly 50°C ± 1°C. The reaction time was determined 30, 60, 90, and 120 min after oral administration of vehicle (distilled water), STE in dose of 30, 100, 300 mg/kg and standard (diclofenac sodium 10 mg/kg).[20]
Acetic acid induced writhing test
The peripheral and central antinociceptive activity of OS was evaluated by using the acetic acid-induced writhing test. All animals were divided in to five groups (n = 5). Each experimental group was treated orally with vehicle (distilled water), plant extract (STE in dose of 30, 100, 300 mg/kg), and standard (diclofenac sodium 10 mg/kg). After 30 min of treatment, writhing was induced by intra-peritoneal injection of 1% acetic acid in saline. The number of abdominal constrictions (writhes) and hind limb stretching were counted after 5 min of acetic acid injection for the period of 10 min.[21]
Formalin induced paw licking test
Mice were divided into five groups of five animals each. The chosen mice were treated orally with vehicle (distilled water), plant extract (STE in dose of 30, 100, 300 mg/kg), and standard (diclofenac sodium 10 mg/kg). After 60 min each mouse was injected 20 μl of 5% formalin in saline, (sub-plantar region). These mice were individually placed in a transparent observation chamber. The spent of paw licking was estimated and used as an index to measure the analgesic effect during the 0–5 min period (first phase, neurogenic) and the 15–25 min (second phase, inflammatory) period after formalin injection.[22]
Anti-pyretic activity
Brewer's yeast induced pyrexia
Brewer's yeast (20%) in saline was used to induced pyrexia. Rectal temperature of the rats was measured by using a thermometer. Pyrexia was induced by injection of 10 ml/kg of Brewer's yeast suspension sub-cutaneously in the back below the nape of the neck. The site of injection is massaged in order to spread the suspension beneath the skin. Immediately after yeast administration, food is withdrawn. After 18 h of pyrexia induction, the rise in rectal temperature was measured. Animals which shows rise in temperature more than 0.7°C were selected for study. Each experimental group was treated orally with vehicle (distilled water), plant extract (STE in dose of 30, 100, 300 mg/kg), and the standard drug (Paracetamol at dose of 150 mg/kg). Rectal temperatures were recorded 1, 2, 3, 4, and 5 h after dosage administration.[23]
Acute oral toxicity
For Safety evaluation of STE acute toxicity study was carried out. Animals were divided into two groups (5 mice/group). Animals of first group were treated orally with vehicle (distilled water), animals of group second treated with STE (2000 mg/kg p.o). Animals were observed during the first 12 h for any alteration in the symptoms of mobility, (depression, convulsion, salivation) and for mortality. After 7th day of experiment, the serum was separated from the blood of the animals for measurement of haematological (red blood cells (RBCs), white blood cells (WBCs) and haemoglobin) and serum biochemical (serum glutamic oxaloacetic transaminase [SGOT], serum glutamic pyruvic transaminase [SGPT], alkaline phosphatase (ALKP), creatinine cholestrol, triglyceride) parameters.[24]
High-performance liquid chromatography fingerprint analysis
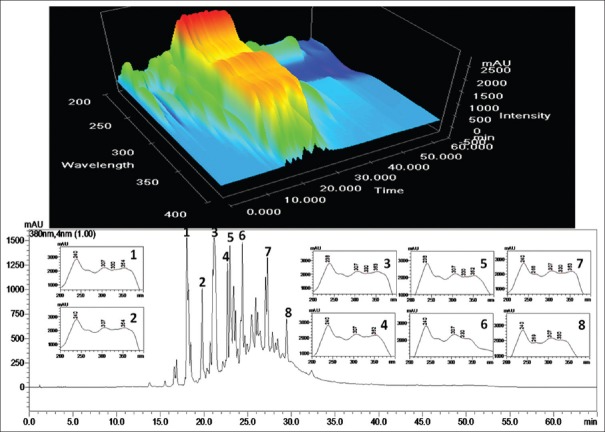
One hundred milligram of the STE was re-dissolved in methanol under ultrasonic exposure (30.0 cm × 25.0 cm × 12.5 cm, 34 ± 3 kHz, piezoelectric transducer sandwich-type six transducer, 250 W, Oscar Micro clean-109, Mumbai, India). The un-dissolved material was separated by centrifugation (5500 × g for 3 min). Prior to HPLC analysis the sample were filtered through 0.45 μm nylon filter. HPLC analyses were performed using a Shimadzu system consisting solvent delivery pumps (LC-20AD), rheodyne manual injector, column oven (CTO-20A), and a multi wavelength photodiode array detector (SPDM20A) with a Waters Symmetry C18 column (250 mm × 4.6 mm; 5 μm) was used for equipment control, data acquisition, and processing of the chromatographic information was done using software LC-MS Solution 3.21. The mobile phase consisted of 0.5% acetic acid in water (solvent A) and 0.5% acetic acid acetonitrile (solvent B). Before use samples and mobile phase were filtered through a 0.45 m membrane nylon filter. The gradient elution program was: 0–10 min, linear gradient from 5% to 15% B; 15–45 min, linear gradient from 15% to 85% B; 45–50 min, linear gradient from 85% to 95% B; 50–55 min, linear gradient from 95% to 15% B; 55–60 min, linear gradient from 15% to 5% B and stop at 65 min. The flow rate was 1.0 ml/min and the sample injection volume was 20 μL. Ultraviolet (UV) spectra were acquired between 200 nm and 600 nm to monitor any possible co-elution in plant sample solution. Therefore, considering maximum chromatographic signal response for fingerprint, wavelength 380 nm was selected [Figure 1].
Figure 1.
A representative high-performance liquid chromatography chromatogram of ethyl acetate, a ethyl acetate extract of Ocimum sanctum roots
Statistical analysis
The statistical significance was assessed using one-way analysis of variance followed by Bonferroni's test. The values are expressed as mean ± standard error of mean and P < 0.05 was considered significant.
RESULTS
Anti-inflammatory activity
Carrageenan induced paw edema
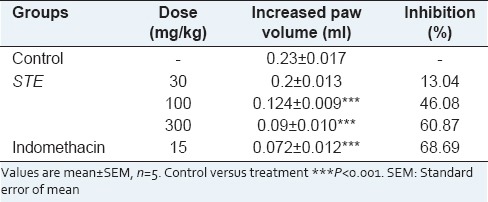
The paw edema of rats increased progressively and reached its maximum after 3 h of carrageenan injection. The results of the ant-inflammatory effects of OS roots extracts on carrageenan induced paw edema are presented in Table 1. Among the all extracts, STE showed most potent anti-inflammatory activity followed by STB, STW, STC and STH [Table 1]. STE, a most active fraction exhibited 13.04%, 46.08%, 60.87% inhibition of edema in animals treated with 30, 100 and 300 mg/kg, respectively [Table 2].
Table 1.
Anti-inflammatory activity of Ocimum sanctum root extracts against carrageenan induced paw edema
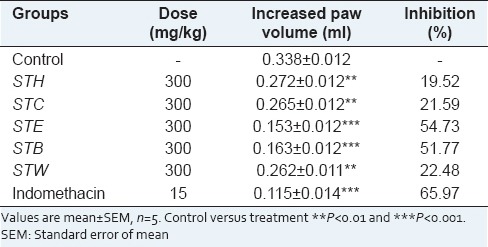
Table 2.
Dose dependent anti-inflammatory response of STE on carrageenan induced paw edema
Analgesic activity
Hot plate analgesic test
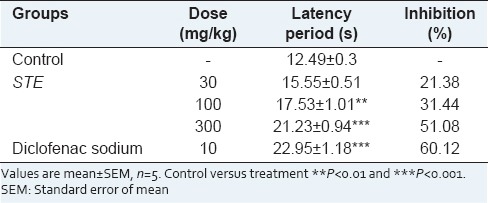
Diclofenac sodium, a standard NSAIDs showed a significant analgesic effect (70.58% protection) against thermally induced pain model, whereas STE showed 31.44 and 51.08% protection at dose rate of 100 and 300 mg/kg, respectively [Table 3].
Table 3.
Analgesic effect of STE on hot plate test
Tail immersion test
As shown in Table 4, STE at 100 and 300 mg/kg significantly reduced the painful sensation 22.90 and 31.05% respectively with respect to control. Diclofenac sodium exhibited 38.18% reduction in painful sensation. Significant anti-nociceptive activity was not observed in animals treated with STE at 30 mg/kg.
Table 4.
Analgesic effect of STE on tail immersion test

Acetic acid induced writhing test
The mean number of writhing caused by acetic acid administration were found to be high in control. Diclofenac sodium (10 mg/kg), a standard NSAIDs inhibited 71.98% writhing response in comparison to control group. Oral administration of STE 30 mg/kg did not show any significant activity, whereas STE at 100,300 mg/kg resulted in a significant reduction of acetic acid induced writhing (33.81, 53.14%) compared to control group in mice [Table 5].
Table 5.
Analgesic effect of STE on acetic-acid induced writhing

Formalin test
In the formalin test, STE shows significant analgesic activity (22.19, 51.77%) in terms of time spent of paw licking responses at 100 and 300 mg/kg respectively compared to control group. Whereas, diclofenac sodium (10 mg/kg) showed 76.81% analgesic activity [Table 6].
Table 6.
Analgesic effect of STE on formalin test

Anti-pyretic activity
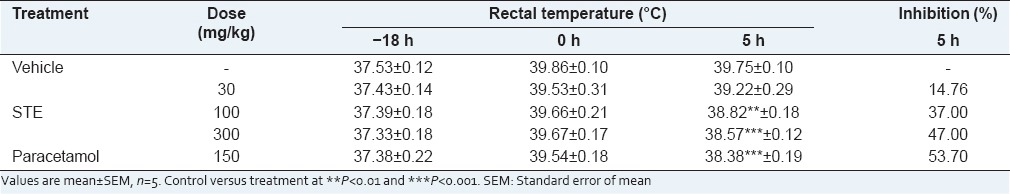
STE started to produce significant reduction of temperature after 1 h that was 29.51 and 38.03% at 100 and 300 mg/kg respectively. While the maximum antipyretic effect of paracetamol (150 mg/kg) and STE (100 and 300 mg/kg) was 53.70%, 37.0%, 47.0% respectively after 5 h. STE (30 mg/kg) did not shown any significant reduction [Table 7].
Table 7.
Effect of STE on Brewer's yeast induced pyrexia
Acute oral toxicity study
Haematological parameter (RBCs, WBCs, Haemoglobin) was found normal as well as the serum biochemistry did not exhibit any significant changes in SGOT, SGPT, ALKP, creatinine cholesterol, triglyceride, when compared to the vehicle control. Similarly no sign of mobility and mortality was found during whole experimental period [Table 8].
Table 8.
Effect of STE on acute toxicity at 2000 mg/kg as a single oral dose in Swiss albino mice

High-performance liquid chromatography fingerprint analysis
Eight major peaks of Reversed-phase (RP)-HPLC chromatograms of STE was tentatively characterized as flavonoids (both glycone and aglycones) based on the UV spectra matching. Each of the flavonoid peaks was well resolved from the neighboring peaks and displays excellent peak symmetry and separation efficiency [Figure 1]. The presence of an UV active chromophore due to the phenyl ring provides great support to flavonoid identification and analysis. The typical feature not only makes flavonoids easy to detect but also provides important structural information that can distinguish the oxidation pattern and type of polyphenol. All flavonoid aglycones contain at least two aromatic rings and consequently, efficiently absorb UV light. The flavonoids in methanolic solutions typically yield two UV absorption peaks: The first maximum, which is found in the 240–285 nm range (Band I), is due to the A-ring benzoyl system - and the second maximum, which is in the 300–400 nm range (Band II), to the substitution pattern and conjugation of the B-ring cinnamoyl system. Band A lies in the 310–350 nm and 350–385 nm range for flavones and flavonols respectively. While, Band B, remains same in the 250–290 nm range for both of all flavones and flavonols subgroup of flavonoid. In flavanones and dihydroflavonols, band A is often reduced to little more than a shoulder at 300–330 nm and Band B, in the 277–295 nm range, is the main peak. Consequently, these two subgroups cannot be distinguished by simple UV-Visible analysis. Flavanols show λmax at nonspecific wavelengths between 270 nm and 290 nm, at which many phenolics absorb, thus not allowing their selective detection. UV spectral data of major eight peaks [Figure 1] showed two absorptions at λmax the range of 238–240 nm of Band II and another at the range of 307–354 nm of Band I indicated that these compounds tentatively belongs to 3-substituted flavonol or flavones. UV-Visible spectra of each peak used as an indicative tool for the characterization of C-ring, whereas the MS spectra provided additional significant information.
DISCUSSION
Despite considerable progress in the treatment of diseases and disorders using modern therapeutic agents, search for newer drugs continues because the existing synthetic drugs have several limitations. Long-term administration of NSAIDs for the treatment of inflammation, pain and fever may cause severe complications includes gastric ulcer, renal damage, bronchospasm and cardiac abnormalities.[2] Therefore, new anti-inflammatory, analgesic drugs having lesser effects are being searched all over the world as alternatives to NSAIDs.[4] Medicinal and Aromatic Plants are recognized for their ability to produce a wealth of secondary metabolites and are being used in human health from several generations. The majority of plant-derived leads showed potent anti-inflammatory, analgesic and antipyretic activity.[25] Roots of OS are waste biomass after the harvesting of herb. Hence, suitable methods should be adopted to minimize the pollution of environment by converting waste materials to value added products. The present study was carried out to determine the anti-inflammatory, analgesic and antipyretic profile of OS root extracts using small laboratory animals.
Carrageenan induced inflammation in rat paw is a suitable model for evaluating anti-inflammatory agents acting by inhibiting the mediators of acute inflammation.[26] OS root extracts coded as STE, STB, STW, STC, STH exhibited significant reduction in paw edema volume. STE showed paw edema inhibition in dose dependent manner. Anti-inflammatory activity of STE might be attributed due to the inhibition of release of pro-inflammatory mediators of acute inflammation such as histamine and prostaglandin.[27] STE, a most active extract was further explored for analgesic and antipyretic activity in small animal model.
Analgesic profile of STE was studied using chemical and thermal induced pain model. These include the acetic acid induced writhing test and formalin induced paw licking test as chemical-induced pain model, whereas hot plate latency test and tail immersion test as thermal-induced pain model. STE exhibited significant inhibition of acetic acid induced writing and formalin induced paw licking in dose-dependent manner. Intra-peritoneal injection of acetic acid caused writhing by increasing the level of prostaglandins in peritoneal fluids.[28] Prostaglandins activate peripheral nociceptors to induce abdominal constrictions.[29] Hence, it is necessary to inhibit the COX enzyme for reducing the writhing. The paw licking induced by subcutaneous injection of formalin as a peripheral noxious stimulus involving two phases of biphasic nociceptive responses. The first phase (neurogenic pain) is occur by direct chemical stimulation of nociceptive afferent fibers (predominantly C fibers), which can be inhibit by opiates like morphine.[30] The second phase (inflammatory phase) is due to release of inflammatory mediators, such as prostaglandins, serotonin, histamine and bradykinin in peripheral tissues.[31] Acetic acid-induced writhing test does not confirm whether STE acts centrally or peripherally. To find out the centrally acting analgesic role of STE, hot plate latency and tail immersion test was performed. Latency period in hot plate and tail immersion test was significantly increased in STE administered mice as compared to the vehicle administered control mice in dose dependent manner. This finding showed that STE also have centrally acting analgesic role. Thermal induced pain model produced response by different grades of centrally acting noxious stimuli. The tail immersion shows spinal reflexes to nociceptive stimuli and the hot plate produced analgesic activity supraspinally.[32]
STE reduce the Brewer's yeast induced rectal temperature and exhibit antipyretic activity in a dose dependent manner. Subcutaneous injection of Brewer's yeast causes pyrexia by enhances the production of prostaglandin which ultimately increase the body temperature. This production can be prevented by inhibiting the cyclo-oxygenase enzyme.[33]
Acute toxicity study showed that a single oral administration of STE (2000 mg/kg) did not produce any mortality, behavioural changes (gait, posture, fur, depression, panting) as well as no significant changes were recorded in serum biochemical (SGOT, SGPT, ALKP, creatinine Cholestrol, Triglyceride) and haematology parameters (RBCs, WBCs and Haemoglobin) in the mice as compared to the control group.[34,35]
It is believed that the therapeutic effects of any herbal product are because of collective response of multiple chemical constituents. Therefore, it is necessary to define as many phytochemicals as possible in order to recognize and explain the bioactivity. It is essential to develop a highly structured and well defined chemical profile or fingerprint of the active fraction. RP-HPLC coupled with diode array detection provides an efficient method for rapid identification of chemical profiles of the compounds with chromophoric groups like, flavonoids. Preliminary phytochemical screening of the STE shows the presence of flavonoids. Flavonoids act as an anti-inflammatory agent by inhibiting the chemical mediators of the inflammatory response in the same way as the NSAIDs, that is, by inhibiting the enzymes that cause the synthesis of prostaglandins.[36]
CONCLUSION
The present study revealed that ethyl acetate extract of OS roots (STE) exhibits the anti-inflammatory, analgesic and anti-pyretic activity. Further investigations are needed in order to isolate the active ingredients in the ethyl acetate extract of OS roots that is responsible for pharmacological activities and to elucidate the mechanism of action of these active ingredients.
ACKNOWLEDGMENT
The authors are highly grateful to the Director CSIR-CIMAP for Providing necessary facilities and financial support for this work under the project CSIR Network project (BSC 0203).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Burke A, Smyth E, Fitzgerald GA. Analgesic-antipyretic agents: Pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilmans the Pharmacological Basis of Therapeutic. New York: McGraw Hill; 2006. [Google Scholar]
- 2.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 3.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–22. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 4.Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87:199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 5.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009;112:587–94. [Google Scholar]
- 6.Singh M, Hamid AA, Maurya AK, Prakash O, Khan F, Kumar A, et al. Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol. 2014;143:323–33. doi: 10.1016/j.jsbmb.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Bawankule DU, Trivedi P, Pal A, Shanker K, Singh M, Sharma P, et al. Protective mechanism of lignans from Phyllanthus amarus against galactosamine/lipopolysaccharide-induced hepatitis: An in-vivo and in-silico studies. Curr Top Med Chem. 2014;14:1045–55. doi: 10.2174/1568026614666140324130047. [DOI] [PubMed] [Google Scholar]
- 8.Warrier PK. New Delhi (India): CBS Publication; 1995. Indian Medicinal Plants; p. 168. [Google Scholar]
- 9.Bawankule DU, Pal A, Gupta S, Yadav S, Misra A, Rastogi S, et al. Protective effect of Ocimum sanctum on ethanol-induced oxidative stress in swiss albino mice brain. Toxicol Int. 2008;15:121–5. [Google Scholar]
- 10.Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum: An experimental study evaluating its anti-inflammatory, analgesic and antipyretic activity in animals. J Ethnopharmacol. 1987;21:153–63. doi: 10.1016/0378-8741(87)90125-5. [DOI] [PubMed] [Google Scholar]
- 11.Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol. 2002;80:15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 12.Akilavalli N, Radhika J, Brindha P. Hepatoprotective activity of Ocimum sanctum Linn. Against lead induced toxicity in albino rats. Asian J Pharm Clin Res. 2011;4:84–9. [Google Scholar]
- 13.Yanpallewar SU, Rai S, Kumar M, Acharya SB. Evaluation of antioxidant and neuroprotective effect of Ocimum sanctum on transient cerebral ischemia and long-term cerebral hypoperfusion. Pharmacol Biochem Behav. 2004;79:155–64. doi: 10.1016/j.pbb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. As a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 15.Singh AA, Bajaj VK, Sekhawat PS, Singh K. Phytochemical estimation and antimicrobial activity of aqueous and methanolic extract of Ocimum sanctum L. J Nat Plant Prod Plant Resour. 2013;3:51–8. [Google Scholar]
- 16.Venkatesalu V, Gopalan N, Pillai CR, Singh V, Chandrasekaran M, Senthilkumar A, et al. In vitro anti-plasmodial activity of some traditionally used medicinal plants against Plasmodium falciparum. Parasitol Res. 2012;111:497–501. doi: 10.1007/s00436-012-2834-9. [DOI] [PubMed] [Google Scholar]
- 17.Maity TK, Mandal SC, Saha BP, Pal M. Effect of Ocimum sanctum roots extract on swimming performance in mice. Phytother Res. 2000;14:120–1. doi: 10.1002/(sici)1099-1573(200003)14:2<120::aid-ptr557>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 19.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 20.Wang YX, Gao D, Pettus M, Phillips C, Bowersox SS. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain. 2000;84:271–81. doi: 10.1016/s0304-3959(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 21.Khan H, Saeed M, Gilani AU, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J Ethnopharmacol. 2010;127:521–7. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Santos AR, Calixto JB. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides. 1997;31:381–9. doi: 10.1016/s0143-4179(97)90075-5. [DOI] [PubMed] [Google Scholar]
- 23.Adams SS, Hebborn P, Nicholson JS. Some aspects of the pharmacology of ibufenac, a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1968;20:305–12. doi: 10.1111/j.2042-7158.1968.tb09744.x. [DOI] [PubMed] [Google Scholar]
- 24.Chanda D, Shanker K, Pal A, Luqman S, Bawankule DU, Mani D, et al. Safety evaluation of Trikatu, a generic Ayurvedic medicine in Charles Foster rats. J Toxicol Sci. 2009;34:99–108. doi: 10.2131/jts.34.99. [DOI] [PubMed] [Google Scholar]
- 25.Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. Seed oil in experimental animal models. Food Chem Toxicol. 2010;48:61–4. doi: 10.1016/j.fct.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Owoyele VB, Oloriegbe YY, Balogun EA, Soladoye AO. Analgesic and anti-inflammatory properties of Nelsonia canescens leaf extract. J Ethnopharmacol. 2005;99:153–6. doi: 10.1016/j.jep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Lin C, Lin W. Anti-inflammatory and hepatoprotective effects of Ventilago leiocarpa. Phytother Res. 1995;9:11–5. [Google Scholar]
- 28.Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 29.Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther. 1998;285:1031–8. [PubMed] [Google Scholar]
- 30.do Amaral JF, Silva MI, Neto MR, Neto PF, Moura BA, de Melo CT, et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull. 2007;30:1217–20. doi: 10.1248/bpb.30.1217. [DOI] [PubMed] [Google Scholar]
- 31.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 32.Jinsmaa Y, Okada Y, Tsuda Y, Shiotani K, Sasaki Y, Ambo A, et al. Novel 2’, 6’- dimethyl-L-tyrosine-containing pyrazinone opioid mimetic mu-agonists with potent antinociceptive activity in mice. J Pharmacol Exp Ther. 2004;309:432–8. doi: 10.1124/jpet.103.060061. [DOI] [PubMed] [Google Scholar]
- 33.Zhu ZZ, Ma KJ, Ran X, Zhang H, Zheng CJ, Han T, et al. Analgesic, anti-inflammatory and antipyretic activities of the petroleum ether fraction from the ethanol extract of Desmodium podocarpum. J Ethnopharmacol. 2011;133:1126–31. doi: 10.1016/j.jep.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Joshua Allan J, Damodaran A, Deshmukh NS, Goudar KS, Amit A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague – Dawley rats. Food Chem Toxicol. 2007;45:1928–37. doi: 10.1016/j.fct.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Mohanty S, Srivastava P, Maurya AK, Cheema HS, Shanker K, Dhawan S, et al. Antimalarial and safety evaluation of Pluchea lanceolata (DC.) Oliv. and Hiern: in-vitro and in-vivo study. J Ethnopharmacol. 2013;149:797–802. doi: 10.1016/j.jep.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Berknow R. 16th ed. New Jersey: Merck Research Laboratories Rathway; 1992. The Merck Manual of Diagnosis and Therapy; pp. 1407–20. [Google Scholar]