Abstract
Background:
1-nitropyrene (1-NPy) is one of the most abundant nitro-polycyclic aromatic hydrocarbons particularly in diesel exhausts. It is a mutagenic and carcinogenic pollutant very widespread in the environment. So the discovery of antimutagenic agents is essential. Harpagophytum procumbens (HP) is traditionally used as anti-inflammatory and analgesic particularly against painful osteoarthritis. Harpagoside (HS), its major iridoid glycoside, is considered as the main active component.
Objective:
The aim of the present study was to evaluate the antimutagenic activity of HS and HP extracts against mutagenic activity of 1-NPy.
Materials and Methods:
The antimutagenic activity was investigated using the in vitro cytokinesis-block micronucleus assay in cultured human lymphocytes. Cells were exposed to HS or HP extracts before (pretreatment), during (co-treatment), and after (posttreatment) treatment with 1-NPy.
Results:
Results showed that HS significantly reduced the mutagenicity of 1-NPy in pretreatment and particularly in co-treatment, whereas all HP extracts significantly reduced the genotoxicity in the three protocols.
Conclusion:
These results suggested that HS was strongly involved in antimutagenic activity of HP extracts in co-treatment, but other components in HP extracts participated in this activity in pre- and post-treatment.
Keywords: 1-nitropyrene, anti-inflammatory, antimutagenic effect, cytokinesis-block micronucleus assay, Harpagophytum procumbens, harpagoside
INTRODUCTION
Human exposure to environmental mutagenic/carcinogenic pollution is still a matter of concern. Pollution of air, water and soil is estimated to account for 1–4% of all cancers.[1] Among the large number of worrying pollutants, nitro-polycyclic aromatic hydrocarbons compounds (nitro-PAHs) have been related to the development of lung, skin and bladder cancers.[2,3] Several studies have shown that 1-nitropyrene (1-NPy) is one of the most abundant nitro-PAH particularly in diesel exhausts.[4] The International Agency for Research on Cancer has assessed that1-NPy is possibly carcinogenic to humans (Group 2B).[3] 1-NPy was found to be mutagenic in mutagenicity test using bacteria and mammalian cells.[5,6,7,8] Many genes related to pro-inflammatory response were induced by 1-NPy, suggesting that 1-NPy plays a pivotal role in the pathogenesis of inflammatory diseases caused by ambient air pollutants such as diesel exhausted particles and urbanair particulates.[9,10,11]1-NPy is present in the human environment, and its elimination seems to be impossible, so studies carried out to avoid its deleterious effects are parts of the general policy of environmental carcinogenesis prevention. An interesting way is the identification of natural agents that could significantly reduce mutagenic effects and/or increase the resistance of the human organism. The use of antimutagenic plants to inhibit DNA and/or chromosomal damage that initiate carcinogenesis appears to be a feasible strategy in the prevention of cancer.[12,13] Various medicinal and dietary plants containing cancer preventive and antimutagenic molecules have been described.[14,15,16,17,18] Plant extracts are interesting in the search of antigenotoxic or antimutagenic activity because they contain several compounds associated with complementary or synergistic activity.
Among these compounds, iridoids represent an important group of natural constituents. These secondary metabolites are cyclopentano[c] pyran monoterpenoids and are usually found as glycosides. They are present in a large number of medicinal plants belonging to various genera particularly Plantago, Cornus, Scrophularia, Gentiana and Harpagophytum. A large number of iridoids, described as the active compounds of various plants used in traditional medicine, display a broad range of biological and pharmacological activities such as choleretic,[19] hepatoprotective,[20] hemodynamic,[21] antimicrobial,[22] antitumoral[23] and anti-inflammatory activities.[24,25]
Harpagophytum procumbens (HP) D.C. (Pedaliaceae) commonly called Devil's claw, is an herbaceous plant growing in the south of Africa, mainly in the Kalahari Desert and in the Namibian steppes. HP is traditionally used as anti-inflammatory in treating pain and inflammation in arthritis and rheumatism. The herbal drug consists the secondary roots that contain iridoids as active compounds. The powdered herbal drug or the aqueous and hydroethanolic extracts are generally used in phytotherapy. The iridoid glycoside fraction contains mainly harpagoside (HS), procumbide, harpagide and 8-0-p-coumaroylharpagide. HS [Figure 1], the main iridoid glycoside of HP, has been extensively investigated with respect to its pharmacological properties including anti-inflammatory and analgesic activities.[26,27,28] The precise mechanisms by which HP may reduce inflammation remain to be elucidated, but HS is designated as a marker in this indication.[29]
Figure 1.
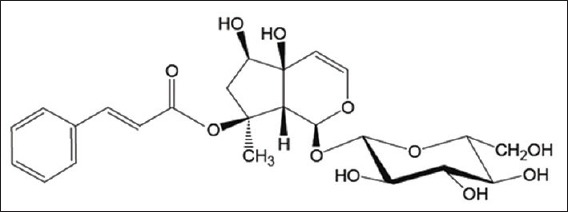
Chemical structure of harpagoside
The in vitro cytokinesis-block micronucleus (CBMN) assay has emerged as one of the preferred methods for assessing chromosomal damage because they enable to reliably measure both aneugenic (chromosome loss) and clastogenic (chromosome breakage) events (Organisation for Economic Co-operation and Development [OECD] guideline 478).[30] Micronuclei (MN) may contain acentric chromosomal fragments formed by unrepaired double-strand breaks, or lagged chromosomes that have failed to segregate into a daughter macronucleus during mitosis. At telophase, a nuclear envelope forms around the lagging chromosomes and fragments forming a small nucleus, named MN. The rate of micronucleated cells is, therefore, a biomarker of chromosome damage. Although CBMN assay is usually used to screen for environmental mutagens, it can be used to identify the antimutagenic potential of natural extracts or chemicals on eukaryotic cells. Three different experimental protocols were used to investigate the mechanism of antimutagenicity: (a) A pretreatment protocol to show the ability of compounds to prevent mutagen-induced DNA damage; (b) a co-treatment protocol in which direct chemical interactions between natural compounds and extracts and mutagen could occur either outside or inside the cells; (c) a posttreatment protocol in which a protective effect could be seen only if the damage to the cells is not converted to chromosome breakage or loss at the end of the mutagen exposure period.
In the present study, we evaluated HS and HP extracts for their ability to decrease 1-NPy induced chromosomal damage in cultured human lymphocytes using the in vitro CBMN assay. In order to evaluate the HS contribution in the chromosome damage modulation, HS and HP extracts containing the same HS concentration were tested. Cells were exposed to HS or HP extracts before (pretreatment), during (co-treatment), and after treatment (posttreatment) with 1-NPy in order to determine the possible mechanism of antimutagenicity.
MATERIALS AND METHODS
Chemicals
Harpagoside (HS – purity 99%) was purchased from Extrasynthèse (Genay, France). 1-nitropyrene (1-NPy – purity 99%), mitomycin C (MMC – purity 98%), chlorophyllin (Chl) sodium copper salt (Chl – purity 99%), dimethyl sulfoxide (DMSO), phytohemagglutinin (PHA), L-ergothioneine (ERT) and cytochalasin B were from Sigma-Aldrich (Saint Quentin Fallavier, France). Heparin was purchased from VWR (Fontenay-sous-Bois, France).
Plant material and extraction
Dried secondary roots of HP were purchased from Cailleau Herboristerie, batch 19995 (Chemillé, France). Roots were protected from light and humidity and were ground to a fine powder before use. HP powder (20 g) was extracted successively with ethanol and water/ethanol (50:50, v/v) by percolation for 12 h at room temperature. The extracts were evaporated to dryness under vacuum. For hydroalcoholic solution, after evaporation of ethanol, the aqueous solution was then freeze-dried. The dried extracts were dissolved in DMSO before antimutagenicity evaluation. Aqueous extract of HP was prepared by boiling 20 g of powdered roots in 300 mL of distilled water for 15 min. The decoction was passed through a layer of cotton, and it was then freeze-dried. This extract was dissolved in water before antimutagenicity evaluation.
High-performance liquid chromatography analysis
Analyses were performed using an Agilent 1100 Series apparatus (degasser G1379A, quaternary pump G1311A, autosampler G1313A, a photodiode array detector G1315B). The system was piloted by ChemStation computer software. The chromatographic separation was achieved using a Symmetry C18 column 250 × 4.6 mm, 5 μm (Waters, MA, USA) protected by a Symmetry C18 (20 × 3.9 mm, 5 μm) guard column. The mobile phase was a mixture of methanol and water (57:43, v/v) under isocratic conditions. The flow rate of the mobile phase was 1.0 mL/min and the injection volume was 20 μL. All separations were performed at room temperature. Quantification was carried out at a single wavelength of 278 nm. High-performance liquid chromatography (HPLC) analysis was performed as described in the monograph “harpagophyton (racine d’)” of the European Pharmacopeia.[31] A standard solution of HS was prepared at a concentration of 0.2 mg/mL in the mobile phase and filtered through a syringe filter (0.45 μm, Millipore) before HPLC analysis. The extract solutions were prepared at a concentration of 10.0 mg/mL in the mobile phase and filtered through a syringe filter (0.45 μm, Millipore) before HPLC analysis.
Cytokinesis-block micronucleus assay
Lymphocyte cultures
Antimutagenic and mutagenic properties of HS and HP extracts were investigated in whole blood lymphocyte cultures, which may facilitate metabolic activation or detoxification of the tested compound.[32] Blood was obtained by venipuncture from 33 healthy nonsmoking male and female donors aged 20 to 55, after informed consent, and collected in heparinized tubes. A 0.35 mL amount of blood was cultured in 4.6 mL of X-vivo serum-free medium (Lonza, Levallois-Perret, France) supplemented with 1% PHA and 1% heparin and incubated in 5% CO2 at 37°C for 72 h. All treatments were performed in duplicate in three independent experiments.
Mutagenic assay
Harpagoside and HP extracts werefirst evaluated for mutagenicity. Sterile DMSO and MMC (5 μg/mL) were used as negative and positive controls respectively. HS, HP extracts and controls were added to culture 24 h after PHA stimulation. HS was dissolved in water and then serially diluted in order to prepare solutions with HS concentration of 0.001, 0.01, 0.1 and 1.0 μg/mL. The highest non-mutagenic concentrations of HS will be selected to evaluate its protective potential against 1-NPy induced DNA damage. HP extracts concentrations used to evaluate their mutagenicity were defined by their HS content so that the HS concentration in each extract was the highest non-mutagenic concentrations of HS.
Antimutagenic assay
Lymphocyte cultures were treated with mutagen, 1-NPy (1 μg/mL) 24 h after PHA stimulation. Antimutagenic activity of Chl, ERT, HP extract and HS was evaluated. Three treatment schedules were used to assess the capability of compounds to inhibit the mutagenicity of 1-NPy: (a) Pretreatment protocol: Lymphocytes were first treated with compounds for 5 h, washed with supplemented X-vivo medium, then treated with 1-NPy for 5 h. (b) Posttreatment protocol: Lymphocytes were first treated with 1-NPy for 5 h, washed with supplemented X-Vivo medium, then posttreated with compounds for 5 h. (c) Co-treatment protocol: Cells were co-treated with 1-NPy and either Chl or HS or HP extracts for 5 h. Vehicles used for Chl, ERT, HS (sterile water EP Grade), HP extracts (sterile water EP Grade or DMSO) and 1-NPy (DMSO) were added in the culture medium of the negative controls, so that controls and tests medium have the same volume. 1-NPy (1 μg/mL) alone was used for the positive control to mutagenic activity, and 1-NPy treated with Chl (35 μg/ml) was used as positive control for antimutagenic activity.
Cell harvest and slide scoring
Cytochalasin B was added to the cultures 43 h after PHA stimulation at a final concentration of 6 μg/mL, and lymphocyte cultures were harvested after 72 h. Lymphocytes were collected, then treated with 5 mL of 0.075 M KCl for 1 min. Cells were fixed immediately using a 3:1 methanol/acetic acid solution. This fixation step was repeated twice after 20 min of storage at 4°C. Cells were spread on precleaned slides, air-dried, and staining was achieved with 5% Giemsa for 10 min. Stained slides were examined under a light microscope. For each culture, the binucleated micronucleated cell (BMNC) rates were expressed as the numbers of micronucleated cells per 1000 binucleated lymphocytes. MN was scored according to standard criteria.[33]
Evaluation of protective capacity against 1-nitropyrene
The reduction of BMNC rate was determined as follows: Reduction (%) = ([BMNC rate in A – BMNC rate in B]/[BMNC rate in A – BMNC rate in C]) ×100, where A is the positive control, B is the group of cells treated with mutagen and HP extract or HS, and C is the negative control.
Evaluation of cytotoxicity
Cytotoxicity was evaluated by determination of cytostasis calculated from the cytokinesis block proliferation index (CBPI).
Cytokinesis block proliferation index indicates the number of cell cycles per cell during the period of exposure to cytochalasin B, was determined by scoring 500 cells per treatment. It was calculated as follows: CBPI = (M1 + 2 [M2] +3 [M3])/500, where M1, M2 and M3 are the number of cells with 1, 2, 3 or more nuclei, respectively.
Cytostasis was calculated as follows: Cytostasis (%) = 100 – 100 ([CBPIB – 1]/[CPBIC – 1]), where B is the group of cells treated with mutagen and HP extract or HS and C is the negative control. A cytotoxicity is defined by a cytostasis of 55 ± 5% or more (OECD guidelines).
Statistical analysis
The BMNC rate was compared to control and treatments using Mann–Whitney U-test. This test is a nonparametric statistical test for assessing whether one of the two samples of independent observations tends to have larger values than the other. U-test can be applied for samples, which don’t follow the normal distribution. P < 0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
1-nitropyrene is a mutagenic and carcinogenic pollutant very widespread in the environment. It is well known that 1-NPy induces several genes involved in the pro-inflammatory response and that an inflammatory pathway seems one of the environmental mutagenic mechanisms.[9,10,11] For this reason, it was relevant to test a natural anti-inflammatory compound for its antimutagenicity against the mutagenic/carcinogenic pollutant 1-NPy. HP is a medicinal plant containing HS and used for its anti-inflammatory properties.
The mutagenicity of HP extracts and HS has not been evaluated yet. In the current study, the mutagenicity of HP extracts and HS were evaluated using CBMN assay. The results of the mutagenicity testing are presented in Table 1. According to OECD 487, the criterion for a positive mutagenic response is a statistically increase in the BMNC rates compared to negative controls, without cytostasis greater than 55% ± 5%. Studies on HP anti-inflammatory activity showed that extracts with HS concentrations ranging from 0.8 μg/ml to 20 μg/ml, inhibited COX-2 and PEG2 activities and interleukine production in whole blood[26] and in human monocytes.[34] Mutagenic activity was determined at these active concentrations. HS (0.01, 0.1, 1 and 10 μg/mL) did not increase the BMNC rate and was not cytotoxic [Table 1]. Results indicated that HS was neither clastogenic nor aneugenic in the range of the tested concentrations. The selected HS concentration to be used in antimutagenic assay was, therefore, the highest tested concentration, 10 μg/mL. For each HP extract, the single tested concentration was defined in order to obtain 10 μg/mL of HS in the culture medium. The content of HS in HP extracts was determined by HPLC analysis. The chromatograms of the three extracts are presented in Figure 2. For each extract, HS is the main compound and 8-O-p-coumaroylharpagide is a minor iridoid identified by comparison with a reference isolated in the laboratory. The ethanolic extract showed the highest HS content (10.80%, w/w) and hydroethanolic extract the lowest HS content (1.46%, w/w), the HS content for aqueous extract is 1.88% (w/w). So, the concentrations were 532 μg/mL, 93 μg/mL and 685 μg/mL for aqueous, ethanolic and hydro-ethanolic extracts, respectively. At these concentrations, the three HP extracts did not increase the BMNC rate in lymphocyte culture, and no cytotoxic effect was observed [Table 1].
Table 1.
Effects of HS and HP extracts on the BMNC rate and cytostasis, in human peripheral lymphocytes cultured in vitro
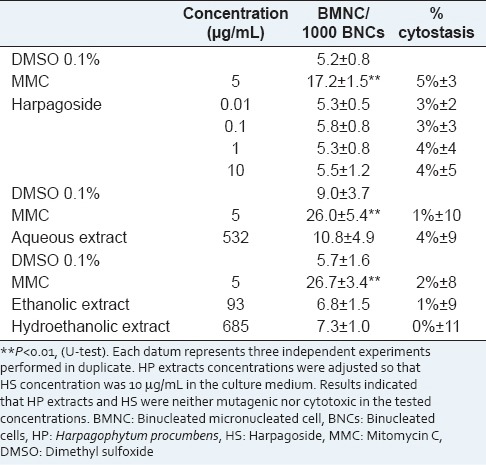
Figure 2.
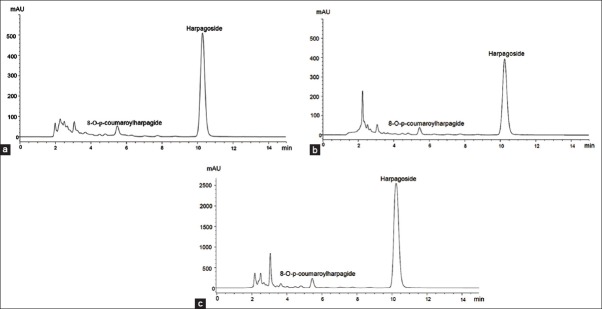
High-performance liquid chromatography chromatographic profiles of Harpagophytum procumbens extracts. (a) Aqueous extract, (b) hydroethanolic extract, (c) ethanolic extract
Results of the antimutagenicity test are presented in Tables 2 and 3. The criterion for a positive antimutagenic response was a statistically significant decrease in the BMNC rate compared to 1-NPy induced BMNC rate, without cytostasis greater than 55% ± 5%. In each treatment, 1-NPy induced a significant increase in BMNC rates compared to vehicle controls and Chl caused a significantdecrease in 1-NPy induced BMNC rates. Chl is a water-soluble derivative of chlorophyll that possesses anticarcinogenic and antimutagenic properties.[35,36] In all protocols, 1-NPy alone or in combination with Chl or HS or HP extracts did not change cytostasis. Antimutagenic activity was evaluated using (i) Chl at 35 μg/mL (ii) the highest HS concentration tested (10 μg/mL) and (iii) aqueous, ethanolic and hydroethanolic HP extracts.
Table 2.
Effects of HS (10 μg/mL) and ERT on the 1-NPy (1 μg/mL) induced BMNC rate and cytostasis, in human peripheral lymphocytes cultured in vitro
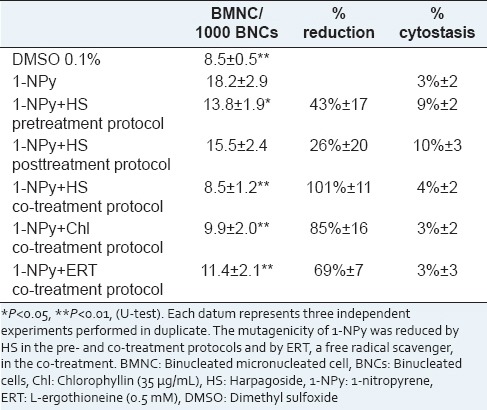
Table 3.
Effects of aqueous, hydroethanolic and ethanolic HP extracts, in pre, co. and posttreatment protocol, on the 1-NPy induced BMNC rate and cytostasis, in human peripheral lymphocytes cultured in vitro
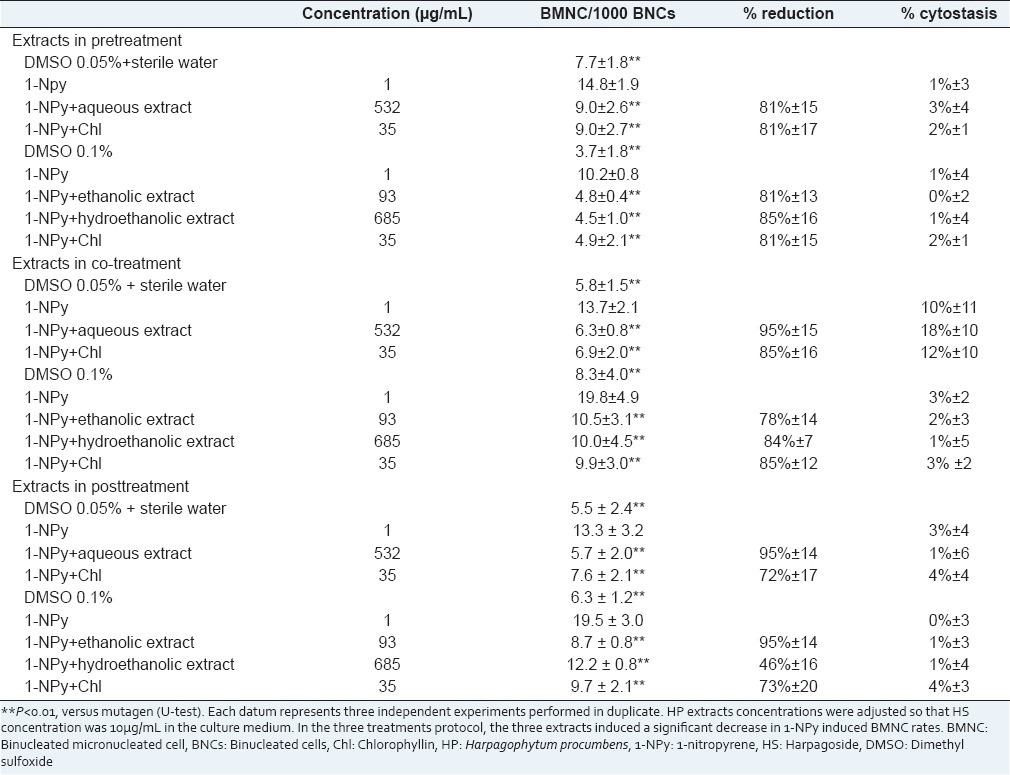
In Table 2, the antimutagenicity assay performed using HS showed a significant decrease in BMNC rates in both pre- and co-treatment protocols but not in the posttreatment. The clastogenicity and/or aneugenicity of 1-NPy was moderately (43%) and strongly (101%) reduced by HS in the pre- and co-treatment protocols, respectively.
The effects of HP extracts, in pre-, co- and post-treatment protocols, on the 1-NPy induced BMNC rates and cytostasis are shown in Tables 3. In pre-treatment protocol, the three extracts induced a significant decrease in 1-NPy induced BMNC rates (P < 0.01). Decreases in BMNC rates were much closed, ranging from 81% to 85%. The protective effects of the three HP extracts toward 1-NPy were higher than the protective effect observed using pure HS. This observation supports the hypothesis that compounds other than HS can prevent chromosome damage are present in the three HP extracts. In co-treatment protocol, the extracts induced a significant decrease in 1-NPy induced BMNC rates (P < 0.01). The BMNC rates were reduced by 95%, 84% and 78% using aqueous, hydroethanolic and ethanolic extracts respectively. The strong protective effect of the three HP extracts toward 1-NPy induced chromosome damage can mainly be attributed to HS. Indeed, decreases in BMNC rates are considerable (>78%) using HP extracts and HS. In posttreatment protocol, similar effects were observed. The three extracts induced a significant decrease in 1-NPy induced BMNC rates: 95%, 95% and 46% for aqueous, ethanolic and hydroethanolic extracts respectively. A strong protective effect of the aqueous and ethanolic extracts toward chromosome damage was observed whereas HS has no protective effect. The hydroethanolic extract was moderately protective toward 1-NPyinduced chromosome damage. These results suggest that other components in HP extracts participated in this activity in posttreatment.
The 1-NPy genotoxicity partly acts through the production of reactive oxygen species (ROS) and partly through adduct formation. To ensure the generation of ROS in our experimental model after 1-NPy treatments, a supporting experiment has been performed. Lymphocyte cultures were treated with 1-NPy and co-treated with ERT (0.5 mM), a well-known free radical scavenger.[37] Results are shown in Table 2. ERT significantly reduced the 1-NPy induced BMNC rates (69%). The ROS production seems to be the major pathway of 1-NPy mutagenesis in our experimental model. Several studies showed that HP exhibits antioxidant capacity by the radical scavenging effect, but HS did not contribute significantly to this activity.[28,38,39,40,41] HP extracts contain, in addition to iridoids, phenylpropanoid glycosides (acteoside, isoacteoside), and flavonoids (kaempferol, luteolin), all well-known for their antioxidant activities.[25,41,42,43] These compounds could explain the strong increase in antimutagenic effect of HP extracts in pretreatment. Antimutagenic properties of flavonoids have been reported against mutagenicity induced by tert-butyl hydroperoxide, a ROS generator[44] and against mutagenicity induced by many other mutagens.[43] In addition, phenolic compounds possess a wide range of biological activities, which contribute to their inhibitory effects on carcinogenesis. Extensive research has been conducted in vitro or in vivo on antioxidant and anticancer activities of phenolic compounds from medicinal and dietary plants, such as curcumin, gingerol and capsaicin.[17,45] Several studies have shown antimutagenic activities of plant extracts against 1-NPy in Salmonella typhimurium, probably due to their polyphenolic composition.[46,47,48,49,50] However, an anti-adduct mechanism cannot be eliminated. Additional experiments are necessary to determine the precise mechanism of antimutagenicity of HP extract. Interestingly, studies on HP/HS anti-inflammatory activities are in accordance with our results on HP/HS antimutagenic activities. Indeed, studies suggested that the anti-inflammatory activity of the HP extracts are higher than that of pure HS.[28,41]
Lymphocytes were exposed to HS or HP extracts before (pretreatment), during (co-treatment), and after (posttreatment) treatment with 1-NPy in order to collect much information as possible on the mechanism of antimutagenicity. Inhibition of a chromosome damaging effect can result from (i) a direct interaction between the mutagen and the antimutagen, and this interaction could occur outside and/or inside the cells, (ii) a HS or HP extract induced cellular effect that could enhance a protective mechanism. In pre- and post-treatment protocol, mutagen and antimutagen were not present simultaneously in the culture, suggesting that the antimutagenic effect was consecutive to a HS or HP extract induced cellular effect. In contrast, in co-treatment protocol, both protective cellular effect and direct interaction between mutagen and antimutagen could occur. A classification of possible mechanisms of mutagenesis and carcinogenesis inhibitors has been proposed.[12] The mutagenicity of 1-NPy was reduced in pretreatment (43% ± 17%) and more intensely in the co-treatment (101% ± 11%). The decrease in BMNC rate observed in pretreatment protocol suggests an increase in protective cellular mechanisms mentioned previously, such as stimulation of expression of antioxidant enzymes. The BMNC rate decrease observed in the co-treatment protocol is greater than in the pretreatment, indicating an additional protective mechanism, as a direct interaction between HS and 1-NPy. HP extracts decrease 1-NPy induced BMNC rates in the three treatment protocols [Table 3]. The BMNC rate decrease observed in the co-treatment protocol for HS and HP extract is similar suggesting that antimutagenic activity in co-treatment can be attributed to HS. The BMNC rate decrease is greater for HP extract than HS in pre- and post-treatment, indicating compounds other than HS are probably involved in the cellular effect.
CONCLUSION
Harpagoside and HP extracts are antimutagenic toward the environmental mutagen/carcinogen 1-NPy. This is the first report of antimutagenic properties of HS and HP. The 1-NPy induced chromosomal damage protection was greater using HP extracts than HS, probably due to the presence of natural antioxidants in the extracts. These results underline the interest of aqueous or hydroethanolic extracts of HP usually used in phytotherapy. Further investigations are necessary to deepen the knowledge of the relationships between anti-inflammatory and antimutagenic effects. This correlation could justify the evaluation of other anti-inflammatory plants and natural molecules for their antimutagenic properties.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stewart BW, Kleihues P. In: World Cancer Report. Vol. 57. Lyon: IARC Press; 2003. The causes of cancer; pp. 21–81. [Google Scholar]
- 2.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–72. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 3.In: IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Vol. 46. Lyon: IARC Press; 1989. IARC International Agency for Research on Cancer. Diesel and gasoline engine exhausts and some nitroarenes. [PMC free article] [PubMed] [Google Scholar]
- 4.Bamford HA, Bezabeh DZ, Schantz S, Wise SA, Baker JE. Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere. 2003;50:575–87. doi: 10.1016/s0045-6535(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefoy A, Chiron S, Botta A. Environmental nitration processes enhance the mutagenic potency of aromatic compounds. Environ Toxicol. 2012;27:321–31. doi: 10.1002/tox.20644. [DOI] [PubMed] [Google Scholar]
- 6.Scheepers PT, Martens MH, Velders DD, Fijneman P, van Kerkhoven M, Noordhoek J, et al. 1-Nitropyrene as a marker for the mutagenicity of diesel exhaust-derived particulate matter in workplace atmospheres. Environ Mol Mutagen. 1995;25:134–47. doi: 10.1002/em.2850250207. [DOI] [PubMed] [Google Scholar]
- 7.Silvers KJ, Eddy EP, McCoy EC, Rosenkranz HS, Howard PC. Pathways for the mutagenesis of 1-nitropyrene and dinitropyrenes in the human hepatoma cell line HepG2. Environ Health Perspect. 1994;102(Suppl 6):195–200. doi: 10.1289/ehp.94102s6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt DL, Utzat CD, Hilario P, Basu AK. Mutagenicity of the 1-nitropyrene-DNA adduct N-(deoxyguanosin-8-yl)-1-aminopyrene in mammalian cells. Chem Res Toxicol. 2007;20:1658–64. doi: 10.1021/tx700131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ovrevik J, Låg M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology. 2009;259:46–53. doi: 10.1016/j.tox.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Park EJ, Park K. Induction of pro-inflammatory signals by 1-nitropyrene in cultured BEAS-2B cells. Toxicol Lett. 2009;184:126–33. doi: 10.1016/j.toxlet.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Pei XH, Nakanishi Y, Inoue H, Takayama K, Bai F, Hara N. Polycyclic aromatic hydrocarbons induce IL-8 expression through nuclear factor kappaB activation in A549 cell line. Cytokine. 2002;19:236–41. [PubMed] [Google Scholar]
- 12.De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–27. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 14.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 15.Endringer DC, Valadares YM, Campana PR, Campos JJ, Guimarães KG, Pezzuto JM, et al. Evaluation of Brazilian plants on cancer chemoprevention targets in vitro. Phytother Res. 2010;24:928–33. doi: 10.1002/ptr.3050. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson LR. Antimutagens as cancer chemopreventive agents in the diet. Mutat Res. 1994;307:395–410. doi: 10.1016/0027-5107(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 17.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 18.Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals: Potential molecular targets, biomarkers and animal models. Acta Pharmacol Sin. 2007;28:1409–21. doi: 10.1111/j.1745-7254.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyagoshi M, Amagaya S, Ogihara Y. Choleretic actions of iridoid compounds. J Pharmacobiodyn. 1988;11:186–90. doi: 10.1248/bpb1978.11.186. [DOI] [PubMed] [Google Scholar]
- 20.Chang IM, Ryu JC, Park YC, Yun HS, Yang KH. Protective activities of aucubin against carbon tetrachloride-induced liver damage in mice. Drug Chem Toxicol. 1983;6:443–53. doi: 10.3109/01480548309014166. [DOI] [PubMed] [Google Scholar]
- 21.Circosta C, Occhiuto F, Ragusa S, Trovato A, Tumino G, Briguglio F, et al. A drug used in traditional medicine: Harpagophytum procumbens DC. II. Cardiovascular activity. J Ethnopharmacol. 1984;11:259–74. doi: 10.1016/0378-8741(84)90072-2. [DOI] [PubMed] [Google Scholar]
- 22.Sticher O, Plant mono-, di. sesquiterpenenoids with pharmacological and therapeutical activity. In: Wagner H, Wolff P, editors. New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. Berlin: Springer Verlag; 1977. pp. 137–76. [Google Scholar]
- 23.Ishiguro K, Yamaki M, Takagi S, Ikeda Y, Kawakami K, Ito K, et al. Studies on iridoid-related compounds. V. Antitumor activity of iridoid dervs. periodate oxidation products. J Pharmacobiodyn. 1988;11:131–6. doi: 10.1248/bpb1978.11.131. [DOI] [PubMed] [Google Scholar]
- 24.Recio MC, Giner RM, Máñez S, Ríos JL. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994;60:232–4. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- 25.Viljoen A, Mncwangi N, Vermaak I. Anti-inflammatory iridoids of botanical origin. Curr Med Chem. 2012;19:2104–27. doi: 10.2174/092986712800229005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anauate MC, Torres LM, de Mello SB. Effect of isolated fractions of Harpagophytum procumbens D. C. (devil's claw) on COX-, COX-activity and nitric oxide production on whole-blood assay. Phytother Res. 2010;24:1365–9. doi: 10.1002/ptr.3124. [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Murata K, Naruto S, Matsuda H. Inhibitory effects of devil's claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J Nat Med. 2010;64:219–22. doi: 10.1007/s11418-010-0395-8. [DOI] [PubMed] [Google Scholar]
- 28.Kaszkin M, Beck KF, Koch E, Erdelmeier C, Kusch S, Pfeilschifter J, et al. Downregulation of iNOS expression in rat mesangial cells by special extracts of Harpagophytum procumbens derives from harpagoside-dependent and independent effects. Phytomedicine. 2004;11:585–95. doi: 10.1016/j.phymed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Grant L, McBean DE, Fyfe L, Warnock AM. A review of the biological and potential therapeutic actions of Harpagophytum procumbens. Phytother Res. 2007;21:199–209. doi: 10.1002/ptr.2029. [DOI] [PubMed] [Google Scholar]
- 30.In: Guideline for Testing of Chemicals. Paris: OECD; 2010. OECD. No. 487: In Vitro Mammalian Cell Micronucleus Test (MNvit) [Google Scholar]
- 31.In: European Pharmacopoeia. 7th ed. Strasbourg, France: Council of Europe; 2011. Council of Europe. Harpagophyton (racine d’) monograph 1095. [Google Scholar]
- 32.Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis. 2011;26:177–84. doi: 10.1093/mutage/geq068. [DOI] [PubMed] [Google Scholar]
- 33.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 34.Fiebich BL, Heinrich M, Hiller KO, Kammerer N. Inhibition of TNF-alpha synthesis in LPS-stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine. 2001;8:28–30. doi: 10.1078/0944-7113-00002. [DOI] [PubMed] [Google Scholar]
- 35.Grossi MR, Berni A, Pepe G, Filippi S, Mosesso P, Shivnani AA, et al. A comparative study of the anticlastogenic effects of chlorophyllin on N-methyl-N’- nitro-N-nitrosoguanidine (MNNG) or 7, 12-dimethylbenz (a) anthracene (DMBA) induced micronuclei in mammalian cells in vitro and in vivo. Toxicol Lett. 2012;214:235–42. doi: 10.1016/j.toxlet.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Dashwood R, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1: A review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat Res. 1998;399:245–53. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 37.Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 2012;1822:784–93. doi: 10.1016/j.bbadis.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Betancor-Fernández A, Pérez-Gálvez A, Sies H, Stahl W. Screening pharmaceutical preparations containing extracts of turmeric rhizome, artichoke leaf, devil's claw root and garlic or salmon oil for antioxidant capacity. J Pharm Pharmacol. 2003;55:981–6. doi: 10.1211/0022357021468. [DOI] [PubMed] [Google Scholar]
- 39.Georgiev MI, Alipieva K, Orhan IE. Cholinesterases inhibitory and antioxidant activities of Harpagophytum procumbens from in vitro systems. Phytother Res. 2012;26:313–6. doi: 10.1002/ptr.3555. [DOI] [PubMed] [Google Scholar]
- 40.Grant L, McBean DE, Fyfe L, Warnock AM. The inhibition of free radical generation by preparations of Harpagophytum procumbens in vitro. Phytother Res. 2009;23:104–10. doi: 10.1002/ptr.2570. [DOI] [PubMed] [Google Scholar]
- 41.Mncwangi N, Chen W, Vermaak I, Viljoen AM, Gericke N. Devil's Claw-a review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J Ethnopharmacol. 2012;143:755–71. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Korkina LG. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell Mol Biol (Noisy-le-grand) 2007;53:15–25. [PubMed] [Google Scholar]
- 43.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 44.Edenharder R, Grünhage D. Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat Res. 2003;540:1–18. doi: 10.1016/s1383-5718(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 45.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem Toxicol. 2002;40:1091–7. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 46.Edenharder R, Tang X. Inhibition of the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-nitropyrene by flavonoids, coumarins, quinones and other phenolic compounds. Food Chem Toxicol. 1997;35:357–72. doi: 10.1016/s0278-6915(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 47.Jeng SN, Shih MK, Kao CM, Liu TZ, Chen SC. Antimutagenicity of ethanol extracts of bee glue against environmental mutagens. Food Chem Toxicol. 2000;38:893–7. doi: 10.1016/s0278-6915(00)00081-8. [DOI] [PubMed] [Google Scholar]
- 48.Kuo ML, Lee KC, Lin JK. Genotoxicities of nitropyrenes and their modulation by apigenin, tannic acid, ellagic acid and indole-3-carbinol in the Salmonella and CHO systems. Mutat Res. 1992;270:87–95. doi: 10.1016/0027-5107(92)90119-m. [DOI] [PubMed] [Google Scholar]
- 49.Martínez CJ, Loarca-Piña G, Ortíz GD. Antimutagenic activity of phenolic compounds, oligosaccharides and quinolizidinic alkaloids from Lupinus campestris seeds. Food Addit Contam. 2003;20:940–8. doi: 10.1080/02652030310001605998. [DOI] [PubMed] [Google Scholar]
- 50.Olvera-García V, Castaño-Tostado E, Rezendiz-Lopez RI, Reynoso-Camacho R, González de Mejía E, Elizondo G, et al. Hibiscus sabdariffa L. extracts inhibit the mutagenicity in microsuspension assay and the proliferation of HeLa cells. J Food Sci. 2008;73:T75–81. doi: 10.1111/j.1750-3841.2008.00781.x. [DOI] [PubMed] [Google Scholar]


