Abstract
Background:
Fabaceae family members are known to possess preventive and therapeutic potentials against various types of cancers.
Objective:
The aim of this study was to investigate the cytotoxic and apoptotic effects of hydroalcoholic extracts from the aerial parts and roots of an endemic Ebenus species; Ebenus boissieri Barbey in human lung cancer cell line.
Materials and Methods:
After treatment with hydroalcoholic extracts cytotoxic activities of both extracts were measured by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay, whereas caspase-3 activity, tumor necrosis factor-a lpha (TNF-α) and interferon gamma (IFN-γ) releasewere measured by enzyme linked immunosorbent assay.
Results:
According to in vitro assay results, the increase in all caspases activity suggested that extracts induce cells to undergo apoptosis. Especially, induction in caspase-3 activity was the most remarkable result of this study. Both aerial part and root extracts induced apoptosis by increasing caspase-3 activity, TNF-α and IFN-γ release. When compared to their relative controls, the concentrations of both TNF-α and IFN-γ in extract-treated groups were significantly and dose dependently exalted.
Conclusion:
Taken together, our results indicate that the hydroalcoholic extracts of E. boissieri can be considered as a source of new anti-apoptotic and therefore anti-carcinogenic agent.
Keywords: Apoptosis, caspase, Ebenus boissieri, interferon-gamma, lung cancer, tumor necrosis factor-alpha
INTRODUCTION
Cancer is known as a leading cause of death worldwide and has accounted for 7.6 million deaths (around 13% of all deaths) in 2008. Cancer-related deaths are predicted to increase to over 11 million in 2030.[1] Lung cancer has become the most leading cause of cancer-related death due to the high incidence, rapid progression, and poor prognosis.[2] It is the major cancer killer and a health care problem worldwide with an overall 5-years survival rate of < 15%.[2,3] Currently, chemotheraphy has been widely used for treatment of lung cancer. However, serious side-effects have been reported for some anti-tumor chemotherapeutics. Thus, exploration of new chemicals for treatment of lung cancer is necessary.[2]
Apoptosis or programmed cell death is an irreversible event initiated by various extra cellular stimuli and is also an important phenomenon in cancer chemotherapy.[4] Apoptosis has extrinsic and intrinsic signaling pathways. The intrinsic pathway, triggered by intracellular stresses or by deprivation of growth factors, requires disruption of the mitochondrial membrane and release of cytochrome c, the latter functions with apoptosis protease activating factor (Apaf-1) to induce activation of caspase-9, thereby initiating the apoptotic caspase cascade.[4,5]
Cancer chemotherapeutic drugs are obtained either from natural products or total/semi-synthesis. Over 60% of the drugs in clinical trials for anticancer activity were isolated from natural sources[6] such as vinblastine from Catharanthus roseus (Apocynaceae), taxol from Taxus brevifolia Nutt. (Taxaceae).[7] Apart from a direct antitumoral effect, anticancer agents may contribute to tumor destruction indirectly by stimulating cell-mediated immune responses; altering the amount of ınterferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and ınterleukin-2 (IL-2).[5]
Plants and plant products are known to be effective and versatile chemopreventive agents against various types of cancers.[8] Since traditional background of Anatolian, medicine shows an extensive use of plants as useful pharmaceuticals, Turkey is therefore considered as a promising region for discovery of new plant products. The genus Ebenus L. belongs to the family of Fabaceae (subfamily Papilionoideae) and is an Eastern Mediterranean and Irano-Turanian element. Ebenus was revised by A. Huber-Morath in the Flora of Turkey.[9] Due to their close resemblance to Astragalus species they are often called with similar vernacular names by the inhabitants. Experimental (in vitro and in vivo) and clinical investigations on Astragalus membranaceus roots, the components of traditional Chinese medicine, have revealed that the extracts to possess significant effects against various types of cancers.[10] In the present study, therefore, we aimed to investigate the possible in vitro cytotoxic, antiproliferative and immunomodulatory effects of hydroalcoholic extracts of Ebenus boissieri in A549 human lung cancer cell line. To the best of our knowledge, this is thefirst report indicating any pharmacological properties of E. boissieri.
MATERIALS AND METHODS
Plant materials
Ebenus boissieri Barbey is a 50 − 60 cm long perennial herb. It has yellow flowers. This plant prefers stony hillsides and steppe from 1200 to 2000 m above the sea level. It is endemic to Turkey, where it only grows in Antalya (Turkey).
The roots and flowering aerial parts of E. boissieri were collected from the locality C3 Antalya, Korkuteli district (36° 56’ 51’’N, 030° 09’ 41’’E), stony hillsides and steppe about 1290 m above sea level at the middle of June 2008. A voucher specimen is deposited at AKDU (Herbarium of the Biology Department of Akdeniz University) as Gokturk 7201.
Preparation of plant extracts
Either dried roots or aerial parts of E. boissieri were powdered and individually macerated in 80% ethanol for 2 days at room temperature. The extracts were then filtered and evaporated to dryness under reduced pressure to yield root extract (20.2% w/w) and aerial part extract (32.7% w/w).
Cell lines and culture conditions
Human lung adenocarcinoma epithelial cell line A549 and human embryo kidney 293T cells were kindly provided by Prof. Dr. OZES (Akdeniz University, Faculty of Medicine, Antalya, Turkey). A549 cells were cultured in Dulbecco's Modified Eagle's Medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 10 μg/mL gentamicin and 5% sodium pyruvate. 293T cells were maintained in RPMI 1640 supplemented with 10% FBS, 100 mg/L streptomycin, and 100 mU/L penicillin. The cells were incubated in 5% CO2 with 95% humidity at 37°C.
Cell proliferation assay
Cell proliferation was assessed using a CellTiter 96 aqueous nonradioactive cell proliferation assay (Promega, Madison, WI, USA), which is based on the cleavage of 3 -(4, 5 dimethylthiazol -2 -yl) -5 -(3 -carboxymethoxy -phenyl) -2 -(4 -sulfonyl) -2H -tetrazolium into a soluble, yellow formasan salt. The cells were seeded at 1 × 104 cells/well in 200 μL complete medium onto 96 -well plates and allowed to attach for 24 h. After the cells had reached 80–90% confluency, the medium was removed, washed with phosphate -buffered saline (PBS). Subsequently, cells were treated with various concentrations (0.01–1000 μg/mL) of hydroalcoholic aerial and root extracts prepared in 1% FBS containing complete medium. As positive control, the cells were treated with a cytotoxic drug, doxorubicin -HCl. Each treatment was performed in eight well replicates. The cells were grown at 37°C for time course (24 h, 48 h and 72 h). The medium was gently aspirated to terminate the experiment and 180 μL serum free complete medium and 20 μL of 3 -(4, 5 -dimethylthiazolyl -2) -2, 5 -diphenyltetrazolium (MTT) were added to each well for 4 h. The absorbance at 490 nm was measured in a microplate reader (Thermo Labsystem Multiscan Spectrum, Thermolabsystem, Chantilly, VA, USA), using wells without cells as background. The sample readings calculated by subtracting the average of background absorbances. All experiments were performed at least four times. The half -maximal inhibitory concentration (IC50) of each extract was derived by a nonlinear regression model (curve -fit) based on sigmoidal dose response curve (variable slope) and computed using Graph -Pad Prism, version 4.00 (Graph -Pad Software, San Diego, CA). The growth inhibition was determined using: Growth inhibitory activity (%) = (1 − [OD value of sample/OD value of control group]) ×100%.
Cell viability
The live/dead viability/cytotoxicity kit for Mammalian cells (Invitrogen, Eugene, OR, USA) was used to determine cell viability. Briefly, the cells were seeded at 1 × 104 cells/well in 200 μL complete medium on to 96-well plates and allowed to attach for 24 h. The same protocol described above was used for treatment of the cells with the extracts. Cells were dually stained with two probes (calcein-AM and EthD-1) to simultaneously determinate live and dead cells in a sample. Fluorescent intensity was measured on a LS55 luminescence plate reader (PerkinElmer Inc., Waltham, MA, USA) at 485–530 nm excitation and 630–645 nm emission wavelengths. The experimental data was pooled and used for statistical comparisons using Student's t-test.
Trypan blue exclusion was used to verify results. Cells were plated on the 12-well plates at a cell density of 5 × 104/well, in 1 mL of complete medium and allowed to attach in a CO2 incubator for 24 h. Cells were treated with the extracts and at the end of each experiment, the cells were collected by trypsinization and cell viability was assessed by a hemocytometer using standart trypan blue (0.4% in PBS) exclusion staining. The percentage of viable cells (%) was calculated as; ([the number of live plus dead cells – the number of dead cells]/the number of live plus dead cells) ×100%.[5]
Multi-caspase assay
ApoTarget caspase colorimetric protease assay sample kit (KHZ1001, Invitrogen Corp., Camarillo, CA, USA) was used to determine the activity of caspase-2, 3, 6, 8 and 9. Treated and untreated cells (5 × 105 cells/well in 6-well plate) were collected and transferred into sterile test tube and lysed using the cell lysis buffer supplied. Samples (50 μL) of the lysate were aliquoted into wells of a standard black 96-well microplate, to which 50 μL of reaction buffer containing 10 mM dithiothreitol were then added to the sample wells. The selective substrates used; VDVAD-pNA, DEVD-pNA, VEID-pNA, IETD-pNA and LEHD-pNA substrates for caspase-2, caspase-3, caspase-6, caspase-8 and caspase-9, respectively were added to the appropriate wells (5 μL of 4 mM; final concentration 200 μM) and the plate was then incubated at 37°C for 4 h. The absorbance at 405 nm was measured in a microplate reader (Thermo Labsystem Multiscan Spectrum, Thermolabsystem, Chantilly, VA, USA). To determine the change in caspase activity, the absorbance of treated samples compared with untreated control group.
Assay for tumor necrosis factor-alpha
The concentration of TNF-α in the supernatants of the cells was determined by enzyme linked immunosorbent assay (ELISA) as described by the manufacturer (KHC3011, Invitrogen Corp., Camarillo, CA, USA). Human TNF-α was used as standard, and serial dilutions (1000–15.6 pg/mL) were used to establish the standard curve. Average results from four independent experiments were compared to nontreated and treated cells by measuring the optical density at 450 nm.
Estimation of interferon gamma
Release of IFN γ was assayed by a sandwich ELISA Kit (KHC4021, Invitrogen Corp., Camarillo, CA, USA). The detection of IFN-γ in cell culture supernatant was performed according to the manufacturer instructions. Human IFN-γ was diluted (1000–6 pg/mL) and used as a standard to establish the standard curve.
Assay for ınterleukin-2
Release of IL-2 was assayed by sandwich ELISA Kit (KHR0021, Invitrogen Corp., Camarillo, CA, USA). The detection of IL-2 concentrations in cell culture supernatant was performed according to the manufacturer instructions. Recombinant human IL-2 was diluted (8000–1 pg/mL) and used to establish the standard curve.
Western blot
Cell homogenates were studied by Standard Western blot techniques. Briefly, 25 μg of homogenate was separated on a 10% acrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Hybond-P, Amersham Pharmacia Biotech, Piscataway, NJ) with a semidry transfer apparatus. The membranes were blocked with Tris Buffered Saline-5% milk and then probed with anti caspase-3, anti TNF-α, anti-IFN-γ and anti- IL-2 (sc-2710281, sc-1350, sc-59993 and sc-48545; 1:200, 1:100, 1:100 and 1:100, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The primary antibody was detected with horseradish peroxidase-conjugated anti-mouse secondary antibody (sc-2005, diluted 1:5000, 1:2000, 1:1000 and 1:2000 for caspase-3, TNF-α, IFN-γ and IL-2, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were washed, and blots were developed using an enhanced chemiluminescence Western blotting detection kit(ECL Plus kit, Amersham Biosciences RPN2132, USA) and exposed to X-ray film (Sigma C4729-1EA, Sigma Z363006-50) for 2–30 s. Molecular weight standards (See Blue® Plus2, LC 5925, Life Technologies, USA) were used to determine molecular weights of the visualized bands.
Statistical analysis
All values are expressed as the mean ± standard error of mean data were analyzed using One-way analysis of variance, followed by Dunnett's multiple comparisons test for comparison of group means to control or by the Student's t-test. (Graph Pad InStat., USA). A P < 0.05 was considered statistically significant.
RESULTS
In vitro cytotoxicity against lung and 293T cells
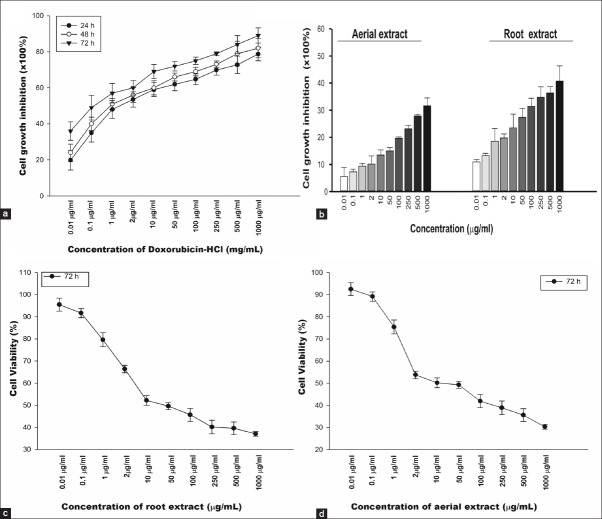
In order to compare antiproliferative effects of various concentrations of aerial and root extracts (0.01–1000 μg/mL) of E. boissieri and doxorubicin-HCl (0.01–1000 μg/mL) on normal cells, in vitro growth inhibition ratio of A549 and 293T cells were determined. As shown in Figure 1a, doxorubicin-HCl had significantly inhibited cell growth in the dose and time-dependent manner. The inhibition ratio of doxorubicin-HCl on proliferation of 293T cells enhanced with the concentration and time increase (P < 0.001). The IC50 of doxorubicin-HCl was approximately 1.43 ± 0.49, 0.9 ± 0.15 and 0.1 ± 0.02 μg/mL for 24, 48 and 72 h, respectively. Surprisingly, the inhibition ratio of aerial or root extracts (0.01–1000 μg/mL) on 293T cells at was no more than 25%, significantly below that of doxorubicin (P < 0.05) [Figure 1b], indicating that these extracts had no direct cytotoxicity to noncancerous cells (P > 0.05). Therefore, we concluded that, the effect of both aerial and root extracts on proliferation of A549 cells worth to be studied in detail.
Figure 1.
The in vitro inhibition of cell growth. Dose-response analysis of 293T cells to doxorubicin-HCl. 293T cells were incubated with increasing concentrations (0.01–1000 μg/mL) of doxorubicin-HCl for 24, 48 and 72 h (A) and hydroalcoholic aerial and root extracts for 72 h (B). Cytotoxic effects of hydroalcoholic root (C) and aerial (D) extracts against A549 lung cancer cell line for 72 h. Cell proliferation was determined by MTT assay. The graphs are plotted as percent inhibition when compared with control cells. Results are representative of four independent experiments with eight replicates and are presented as mean ± SEM
We examined the antiproliferative effects of hydroalcoholic extracts of aerial and root parts (0.01–1000 μg/mL) on A549 and 293T cell growth and determined the appropriate dose for these cell lines. Unfortunately, up to 20 μg/mL, none of the tested doses was found to effectively inhibit cell proliferation or induce cell death. A549 cell viability was reduced in dose dependent manner for 72 h with an IC50 of approximate 35.8 μg/mL for root [Figure 1c] and 12.75 μg/mL for aerial [Figure 1d] extracts. These findings indicated that both aerial and root extracts significantly inhibited proliferation of lung cancer cells in dose-and time-dependent manner. Based on the viability trend obtained; 20 μg/mL aerial and 40 μg/mL root extracts were chosen for subsequent experiments
According to the MTT test results, at the end of 72 h, 20 μg/mL aerial and 40 μg/mL root extracts caused 85% and 65% inhibition in growth of A549 cells, respectively [Figure 2]. As can be seen from the percentage of the growth inhibition rates, aerial extract was found to be more effective on A549 cells than root extract.
Figure 2.
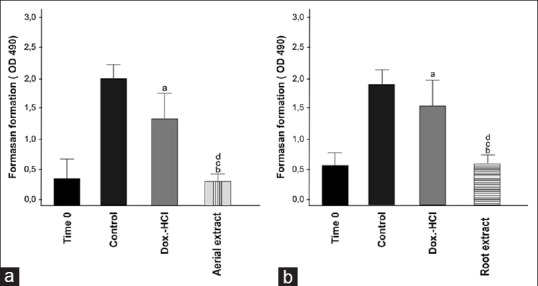
The effect of hydroalcoholic aerial and root extracts on cell growth of A549. Cells were treated with 20 μg/mL aerial extract (A) and 40 μg/ml root extract (B) or doxorubicin-HCl. Cell growth was determined after 72 h using MTS solution. Time 0 demonstrates the number of cells before treatment. Viability of the cells was measured by MTT assay. Each bar represents time dependent changes in viability. Results are representative of four independent experiments with eight replicates and are presented as mean ± SEM. (a and b) significantly different from the control group; P < 0.01and P < 0.001 respectively (c and d) significantly different from doxorubicin-HCl treated group in same incubation time P < 0.05 and P < 0.01.
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay was carried out with dually seeded cells in the same plate. The experiments were terminated in one set at the end of 24, 48 and 72 h following the application of the extracts while the others did not. Viability of the cells in the second group, which were still incubated with the extracts, was examined under an inverted microscope day by day and growth rate of these cells was also determined 1 week after extract treatment to find out whether extract-induced cell death was a late event. Interestingly, the viable cell number for A549 cell line decreased even after a week, suggesting that the impact of both aerial and root extracts was still ongoing. In terms of evaluating the success of antiproliferative effects of this plant, this is a quite important result of this study. We should mention that no decrease in the viability of cells treated with low-doses of aerial or root extracts was observed (data not shown). The results obviously demonstrate that hydroalcoholic aerial and root extracts of E. boissieri inhibit the growth of lung cancer cells (P < 0.001). In contrast, neither aerial nor root extract of this plant inhibit the proliferation of 293T cells for doses up to 250 μg/mL (P > 0.05). Given the fact that no cytotoxic effects were observed against normal cells, our data clearly indicates that E. boissieri exert a selective antiproliferative activity against lung cancer cells
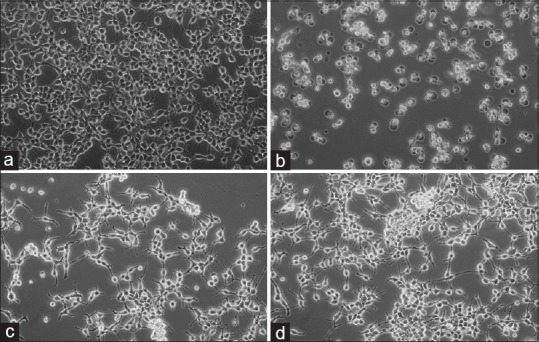
To clarify the effects of hydroalcoholic aerial and root extracts of E. boissieri in normal (293T) and tumor cell (A549) growth, cells were plated on 12-well plates (50,000 cells/well) and 36 h after plating, treated with 20 μg/mL aerial and 40 μg/mL root extracts (in medium supplemented with 1% FBS, with eight repeats of each treatment). The number of dead cells was determined 72 h after the treatment using the trypan blue exclusion assay. The percent decrease in cell survival was calculated for each independent experiment, and the average of five experiments is shown in Table 1. As shown in Figure 3, the number of live 293T cells did not change in either aerial or root extract treated group. The number of live A549 cells decreased in both extracts treated group [Figure 4]. The total cell number was also changed by this treatment, which indicates that extracts not only induce cell death, but also inhibit cell proliferation. Comparing the microscopic images cell death was observable with the abundance of seemingly condensed apoptotic cells and cell fragments in aerial extract-treated group as well as in the group treated with root extract. Using live/dead viability assay as a marker of both viable and nonviable cells, similar results were obtained [Table 2].
Table 1.
Cummulative results of tryphan blue exclusion testa
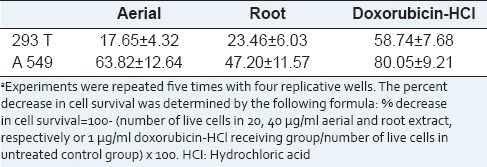
Figure 3.
293T cells were seeded in 12-well plates (50,000 cells/well) and the number of live and dead cells was determined by the tryphan blue exclusion test and the cells were examined at the end of 72 h. Appearances of the cells untreated (A: Control) and treated (B: Doxorubicin-HCl, C: Aerial extract, D: Root extract) were photographed under a contrast phase microscope (Olympus-IX71)
Figure 4.
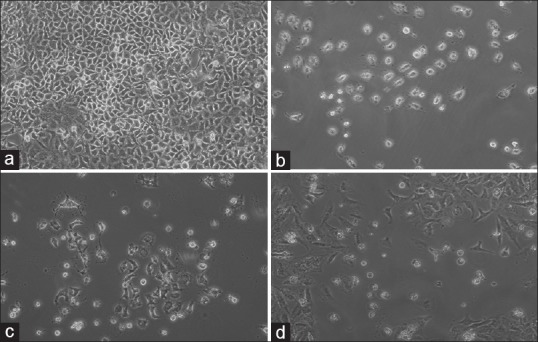
A549 cells were seeded in 12-well plates (50,000 cells/well) and the number of live and dead cells was determined by the tryphan blue exclusion test. Appearances of the cells untreated (A: Control) and treated (B: Doxorubicin-HCl, C: Aerial extract, D: Root extract) were photographed under a contrast phase microscope (Olympus-IX71) at the end of 72 h
Table 2.
Cummulative results of live-dead cell viability assaya

Effects of aerial and root extracts on expression of apoptosis-related proteins
There was a time-dependent increase in all caspase activity, suggesting that extracts induce cells to undergo apoptosis. The effect of both aerial and root extracts on the activity of the initiator caspase-2, 8 and 9 as well as the executioner caspase-3 and 6 are shown in Figure 5a and b. According to the assay results, the activities of all tested caspases were higher after aerial extract (20 μg/mL) than that of root extract (40 μg/mL) treatment for 72 h in A549 cells. The increase in all caspase activity suggests that extracts induce cells to undergo apoptosis (P < 0.01).
Figure 5.
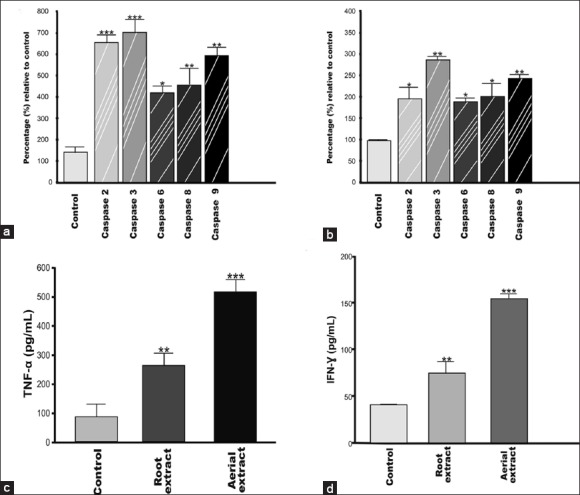
Effects of aerial and root extracts on expression of apoptosis-related proteins. Both aerial (A) and root (B) extracts induced all caspases activation in lung cancer cells. 5 μmol/mL of reversible aldehyde inhibitors, were used to confirm that the observed fluorescence signal in cell population is due to the activity of caspase-like proteases. TNF-α (C) and IFN-γ (D) protein expression is significantly induced in the cells. The expression of TNF-α and IFN-γ in A549 cells following treatment for 72 h with aqueous aerial or root extracts was determined by ELISA. Each data value indicates the mean ± SEM of four independent experiments. ** P < 0.01 and *** P < 0.001, significantly different as compared to the control group; Student's t-test
Since cytokines play a prominent role in the development of both inflammatory and apoptotic responses, we investigated the effects of aerial and root extracts of E. boissieri on TNF-α, IFN-γ and IL-2 expressions by an enzyme-linked immunosorbent assay according to the indication of the manufacturer. Both 20 μg/ml of aerial and 40 μg/ml of root extracts allowed the production of anticancer cytokines if any, in A549 cell culture supernatants. No significant differences (P > 0.05) in IL-2 levels were found between both aerial and root extract treated and untreated A549 cells while the levels of TNF-α and IFN-γ showed considerable increase (P < 0.001 and P < 0.01, respectively) in A549 culture supernatants treated with either aerial or root extract in comparison with the control group (untreated cells) [Figure 5c and d]. Similarly, western blot analysis clearly shows that incubation of A549 cells resulted in increased expression of TNF-α and IFN-γ at protein levels whereas IL-2 expressions did not change [Figure 6].
Figure 6.
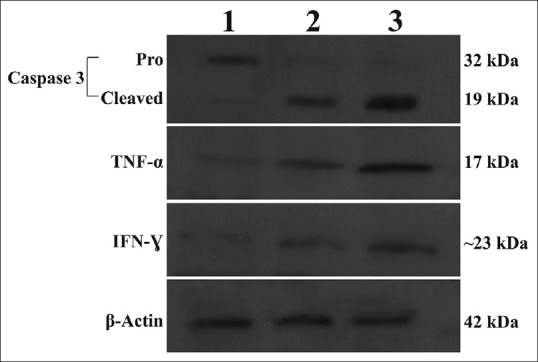
Lysates from A549 cells were incubated with 20 μg/mL aerial (Lane 2) or 40 μg/mL root (Lane 3) extracts were analyzed by Western blotting for caspase-3, TNF-α and IFN-γ protein expression levels. Aerial- or root-treated cells show cleaved (activated) caspase-3, whereas untreated (Lane 1) cells have no cleaved caspase-3. β-actin was used as internal control
DISCUSSION
Cancer is one of the major causes of death worldwide and especially, the lung cancer has become the leading cause of cancer-related death due to its high incidence, rapid progression, and poor prognosis.[2,11] Currently, conventional treatments such as chemotherapy and radiotherapy have been widely used for treatment of lung cancer. However, anti-tumor drugs used in these treatments have serious side effects.[12] Therefore, extensive attentions have been paid to develop plant-derived new drugs for the treatment of lung cancer.[2]
Recently, a number of studies have reported both in vitro and in vivo beneficial effects of herbal medicines in cancer treatments.[13,14,15,16] The members of Fabaceae family have been shown to inhibit different types of cancer cells.[17,18,19,20,21,22,23] Ebenus species also belongs to Fabaceae family. However, there is no report of Ebenus sp. displaying an antitumoreffect and having apoptosis inducing activity on cancer cells.
Programmed cell death or apoptosis plays the vital role in many physiological and developmental stages as well as tissue homeostasis to control the cell number.[24] The induction of cell apoptosis and targeting apoptotic pathways are attractive approaches for the treatment of cancer.[25] Screening of medicinal plants and plant derived products as potential inducers of apoptosis have become the major strategy in anticancer drug research. The present study was focused on the ability of the E. boissieri aerial and root extracts for the first time in influencing the process of apoptosis and their anti-proliferative activity in lung adenocarcinoma A549 cells and normal human embryo kidney 293T cells. It is well known that 293 T cells are used in in vitro experiments as a more closely resemble of noncancerous cells. We found that both aerial and root extracts significantly inhibited the proliferation of lung cancer cells while 293T cells were almost insensitive after all incubation periods. The anti-proliferative effect was evaluated by MTT reduction assay. Many herbals and phytochemicals have been reported for their cytoprotective effect using MTT assay.[26] The criterion of cytotoxic activity for the extracts having potential anticancer activity as established by the American National Cancer Institute is an IC50 < 30 μg/mL in the preliminary assay.[27] Since the IC50 concentration of the aerial extract in A549 cell line was 12.75 μg/mL therefore it is potent as anticancer therapeutic agent.
Some cytotoxic agents cause apoptosis via activating the mitochondrial pathway. Cytochrome c is an important element of this pathway. During apoptotic process, cytochrome c is released from mitochondria to the cytosol. There it forms a complex with Apaf-1, which subsequently activates caspase-9. Finally, this results in activation of caspase-3, which is essential for typical characteristics of apoptosis such as degradation of cellular proteins, cell shrinkage, DNA fragmentation, loss of plasma membrane potential and membrane blebbing.[28,29,30,31] Caspases have potential as therapeutic target due to their crucial role in apoptosis. For A549 cells, exposure to aerial and root extracts of E. boissieri led to induction of activity of all the caspases studied (2, 3, 6, 8 and 9), while significant increase was observed in the activity of caspase-3. Moreover, lysates of treated cells were subjected to Western blot analysis and the results showed that the protein expression of the activepro-caspase-3 was significantly decreased due to transform to active form, associated with the disappearance of inactive pro-caspase-3. To confirm whether induction of apoptosis is actually due to caspase-3 activation, studies using specific inhibitor of caspase-3 were conducted and revealed asignificant reduction in the induction of extract-mediated apoptosis given the fact that the ability of extracts of E. boissieri to induce apoptosis in A549 cells. According to these results the extracts of E. boissieri will be a promising candidate for lung cancer treatment.
Alterations in cytokine production have been observed in different human diseases as in neoplasia,[32] rheumatoid arthritis,[33] diabetes,[34] asthma and allergy[35,36] and cardiovascular diseases.[37] TNF and certain interleukins have been included in carcinogenesis.[38] TNF-α has a synergistic effect with IFN-γ and both of them have been shown to induce apoptosis by activating of the caspase cascade in many cancer cell types.[39,40] We examined the potential effects of aerial and root extracts on TNF-α and IFN-γ concentrations on account of their roles in the activation of apoptosis. The data presented here demonstrate for the first time that E. boissieri aerial parts extract exhibited a high anti-proliferative activity and were a potent inducer for the immunoregulatory cytokines TNF-α and IFN-γ in A549 lung cancer cells. The activation of TNF-α expression by the aerial parts extract in lung cancer cells most likely occurs with the involvement of increased expression of IFN-γ that directly binds to caspases. However, we did not detect any significant amount secreted of IL-2 protein after treatment A549 cells by either aerial or root extracts for 72 h. Our results showed both aerial and root extract appear to be nontoxic to normal 293T cells when compared to their effects on lung cancer cells and hence, we did not record any induction in secretion of TNF-α, IFN-γ and IL-2 in 293T cells.
CONCLUSION
The potential anticancer activity of E. boissieri extracts against lung cancer cells was investigated in this in vitro experimental study, for the first time. Taken together, the results in this study revealed that hydroalcoholic extracts of E. boissieri induce apoptosis in lung cancer cells by altering the amount of caspase-3, TNF-α and IFN-γ. Though the active components and precise mode of action of this plant are not yet ascertained, its potential anti-tumor and immunomodulatory activity, along with the selectivity against cancer cells observed in vitro, suggest that hydroalcoholic extracts from the aerial parts and roots of this plant are worth additional studies. Further chemical and pharmacological investigations to identify the active constituents of this plant and also to screen their mechanisms of action are recommended for anticancer drug discovery.
ACKNOWLEDGMENTS
This work was supported by The Scientific Research Project Coordination Unit of Akdeniz University (Project no: 2012.06.0115.032) and Akdeniz University, Science Faculty, Department of Biology. We are grateful to Akdeniz University, Medicine Faculty, Department of Histology and Embryology for the permission and technical support in the use of the contrast phase microscope (Olympus-IX71).
Footnotes
Source of Support: This work was supported by The Scientific Research Project Coordination Unit of Akdeniz University (Project no: 2012.06.0115.032) and Akdeniz University, Science Faculty, Department of Biology
Conflict of Interest: None declared.
REFERENCES
- 1.Switzerland: World Health Organization; 2010. World Health Organization. WHO Library Cataloguing-in-Publication Data World Health Statistics 2010. 1. Health Status Indicators. 2. World Health. 3. Health Services. Statistics. 4. Mortality. 5. Morbidity. 6. Life Expectancy. 7. Demography. 8. Statistics. I. WHO Press; Geneva 27. NLM classification: WA 900.1. [Google Scholar]
- 2.Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, et al. Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol. 2013;59:118–28. doi: 10.1016/j.fct.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Vaz JA, Almeida GM, Ferreira IC, Martins A. Clitocybe alexandri extract induces cell cycle arrest and apoptosis in a lung cancer cell line: Identification of phenolic acids with cytotoxic potential. Food Chem. 2012;132:482–6. doi: 10.1016/j.foodchem.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Ao M, Wu J, Yu L. TNFa and Fas/FasL pathways are involved in 9-Methoxycamptothecin-induced apoptosis in cancer cells with oxidative stress and G2/M cell cycle arrest. Food Chem Toxicol. 2013;55:396–410. doi: 10.1016/j.fct.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Aydemir EA, Oz ES, Göktürk RS, Ozkan G, Fiskin K. Glycyrrhiza flavescens subsp. antalyensis exerts antiproliferative effects on melanoma cells via altering TNF-a and IFN-a levels. Food Chem Toxicol. 2011;49:820–8. doi: 10.1016/j.fct.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 7.de Mesquita ML, de Paula JE, Pessoa C, de Moraes MO, Costa-Lotufo LV, Grougnet R, et al. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J Ethnopharmacol. 2009;123:439–45. doi: 10.1016/j.jep.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Esakkirajan M, Prabhu NM, Arulvasu C, Beulaja M, Manikandan R, Thiagarajan R, et al. Anti-proliferative effect of a compound isolated from Cassia auriculata against human colon cancer cell line HCT 15. Spectrochim Acta A Mol Biomol Spectrosc. 2014;120:462–6. doi: 10.1016/j.saa.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 9.Huber-Morath A, Ebenus L. In: Flora of Turkey and the East Aegean Island. Davis PH, editor. Vol. 3. London: Edinburgh University Press; 1970. pp. 590–6. [Google Scholar]
- 10.Ren S, Zhang H, Mu Y, Sun M, Liu P. Pharmacological effects of Astragaloside IV: A literature review. J Tradit Chin Med. 2013;33:413–6. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 11.Assaf AM, Haddadin RN, Aldouri NA, Alabbassi R, Mashallah S, Mohammad M, et al. Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medicine of Jordan. J Ethnopharmacol. 2013;145:728–36. doi: 10.1016/j.jep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Force T, Kerkelä R. Cardiotoxicity of the new cancer therapeutics – Mechanisms of, and approaches to, the problem. Drug Discov Today. 2008;13:778–84. doi: 10.1016/j.drudis.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Chen YL, Lin SZ, Chang JY, Cheng YL, Tsai NM, Chen SP, et al. In vitro and in vivo studies of a novel potential anticancer agent of isochaihulactone on human lung cancer A549 cells. Biochem Pharmacol. 2006;72:308–19. doi: 10.1016/j.bcp.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yuan S, Wang J, Lin P, Liu G, Lu Y, et al. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol Appl Pharmacol. 2006;215:168–78. doi: 10.1016/j.taap.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–76. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 17.Chung WT, Lee SH, Kim JD, Sung NS, Hwang B, Lee SY, et al. Effect of the extracts from Glycyrrhiza uralensis Fisch on the growth characteristics of human cell lines: Anti-tumor and immune activation activities. Cytotechnology. 2001;37:55–64. doi: 10.1023/A:1016111713056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinjo J, Nagao S, Tanaka T, Nonaka GI, Okabe H. Antiproliferative constituents in the plant 8. Seeds of Rhynchosia volubilis. Biol Pharm Bull. 2001;24:1443–5. doi: 10.1248/bpb.24.1443. [DOI] [PubMed] [Google Scholar]
- 19.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, et al. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: Induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–47. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Kang SC, Lee CM, Choi H, Lee JH, Oh JS, Kwak JH, et al. Evaluation of oriental medicinal herbs for estrogenic and antiproliferative activities. Phytother Res. 2006;20:1017–9. doi: 10.1002/ptr.1987. [DOI] [PubMed] [Google Scholar]
- 21.Regasini LO, Lopes AA, Silva DH, Furlan M, Yough MC, Maria DA, et al. Antiproliferative effect of Pterogyne nitens on melanoma cells. J Basic Pharm Sci. 2007;28:335–40. [Google Scholar]
- 22.Kim SC, Park SJ, Lee JR, Seo JC, Yang CH, Byun SH. Cytoprotective activity of Glycyrrhizae radix extract against arsenite-induced cytotoxicity. Evid Based Complement Alternat Med. 2008;5:165–71. doi: 10.1093/ecam/nem014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu XQ, Xue CC, Zhou ZW, Li CG, Du YM, Liang J, et al. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice) Life Sci. 2008;82:68–78. doi: 10.1016/j.lfs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Arepalli SK, Sridhar V, Venkateswara Rao J, Kavin Kennady P, Venkateswarlu Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: Induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apoptosis. 2009;14:729–40. doi: 10.1007/s10495-009-0332-z. [DOI] [PubMed] [Google Scholar]
- 25.Li ZB, Wang JY, Jiang B, Zhang XL, An LJ, Bao YM. Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomedicine. 2007;14:846–52. doi: 10.1016/j.phymed.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–5. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Suffness M, Pezutta JM. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in Plant Biochemistry: Assays for Bioactivity. London: Academic Press; 1990. pp. 71–153. [Google Scholar]
- 28.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 29.Mehmet H. Caspases find a new place to hide. Nature. 2000;403:29–30. doi: 10.1038/47377. [DOI] [PubMed] [Google Scholar]
- 30.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–23. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 31.Ramasamy S, Abdul Wahab N, Zainal Abidin N, Manickam S. Effect of extracts from Phyllanthus watsonii Airy Shaw on cell apoptosis in cultured human breast cancer MCF-7 cells. Exp Toxicol Pathol. 2013;65:341–9. doi: 10.1016/j.etp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheim JJ, Murphy WJ, Chertox O, Schirrmacher V, Wang JM. Prospects for cytokine and chemokine biotherapy. Clin Cancer Res. 1997;3:2682–6. [PubMed] [Google Scholar]
- 33.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 34.Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, et al. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011;137:1197–206. doi: 10.1016/j.jep.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Pease JE. Asthma, allergy and chemokines. Curr Drug Targets. 2006;7:3–12. doi: 10.2174/138945006775270204. [DOI] [PubMed] [Google Scholar]
- 36.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 37.Jiang F, Dusting GJ. Natural phenolic compounds as cardiovascular therapeutics: Potential role of their antiinflammatory effects. Curr Vasc Pharmacol. 2003;1:135–56. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–86. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Guan YQ, Li Z, Liu JM. Death signal transduction induced by co-immobilized TNF-a plus IFN-Y and the development of polymeric anti-cancer drugs. Biomaterials. 2010;31:9074–85. doi: 10.1016/j.biomaterials.2010.08.044. [DOI] [PubMed] [Google Scholar]