Abstract
Background:
Recently, many efforts have been made to discover new products of natural origin which can limit the xenobiotic-induced hepatic injury. Carbon tetrachloride (CCl4) is a highly toxic chemical that is widely used to study hepatotoxicity in animal models.
Objective:
The present study was conducted to investigate the curative and protective effects of Schinus terbenthifolius ethanolic extract against CCl4 -induced acute hepatotoxicity in rats.
Materials and Methods:
S. terbenthifolius extract was orally administered in a dose of 350 mg dried extract/kg b.wt. before and after intoxication with CCl4 for curative and protective experiments, respectively. A group of hepatotoxicity indicative enzymes, oxidant-antioxidant capacity, DNA oxidation, and apoptosis markers were measured.
Results:
CCl4 increased liver enzyme leakage, oxidative stress, hepatic apoptosis, DNA oxidation, and inflammatory markers. Administration of S. terebinthifolius, either before or after CCl4 intoxication, significantly decreased elevated serum liver enzymes and reinstated the antioxidant capacity. Interestingly, S. terebinthifolius extract inhibited hepatocyte apoptosis as revealed by approximately 20 times down-regulation in caspase-3 expression when compared to CCl4 untreated group. On the other hand, there was neither protective nor curative effect of S. terebinthifolius against DNA damage caused by CCl4.
Conclusion:
The present study suggests that S. terebinthifolius extract could be a substantially promising hepatoprotective agent against CCl4 toxic effects and may be against other hepatotoxic chemical or drugs.
Keywords: Antioxidant, apoptosis, hepatoprotective, hepatotoxicity, Schinus terebinthifolius
INTRODUCTION
Liver is the most important organ responsible for xenobiotic metabolisms, acting as sensitive target site for substance modulating biotransformation.[1] During aerobic metabolic reactions, considerable amounts of reactive oxygen species (ROS) such as superoxide anion (O2−) and hydrogen peroxide (H2O2) are produced,[2] which enter a variety of chain reactions and generate free radicals such as •OH. These hydrogen species assault polyunsaturated fatty acids and thereby initiate the process of lipid peroxidation causing degradation and inactivation of various important biomolecules.[3] This situation resulting in many complications such as; oxidative stress, antioxidant alteration, decreasing of the intracellular level of glutathione (GSH), reduction of catalase (CAT) activity which leading to tissue injury and carcinogenesis.[4,5]
Many xenobiotics (drugs and environmental chemicals) are capable of causing some degree of liver injury.[6] As lifestyle changes, hepatic injuries have become one of the most common diseases in the world attributed to great morbidity and mortality especially in developing countries.[7] Therefore, many efforts have been made recently for discovering new products of natural origin, which can limit the xenobiotic-induced hepatic injury.
Carbon tetrachloride (CCl4) is widely used to study hepatotoxicity in animal models by starting lipid peroxidation.[8] CCl4 is biotransformed in the liver forming reactive metabolic trichloromethyl radicals (ˉ CCl3) which reacts with excess O2 producing reactive free radicals. These free radicals begin peroxidation of membrane polyunsaturated fatty acids and covalently bind microsomal lipids and proteins producing lipid peroxides leading to cellular disorders and pathological alterations.[9,10]
Herbs have been an important source of natural materials used for treatment and/or prevention of several illnesses.[11] S. terebinthifolius, also named Brazilian pepper, aroeira-vermelha, pink pepper, mastic tree or christmas-berry, belongs to the Anacardiaceae family of plants.[12] Anacardiaceae family comprises 800 species in 82 genera. The genus Schinus, which is named after the Greek name of the mastic tree (Schinos), is used for decoration, tanning agent and as spice. Among the 25 species, Schinus terbenthifolius was growing in Egypt several decades ago.[13] S. terebinthifolius poses antifungal, healing, analgesic, anti-inflammatory, antioxidant, anti-allergic, and insecticidal activities.[12,14,15,16] Marzouk et al.[17] reported that aqueous methanolic extract of the leaves of Schinus molle showed moderate to strong radical scavenging properties on lipid peroxidation, hydroxyl radical, and O2− generations. Meanwhile, the possibility of S. terebinthifolius to protect the liver from drugs or toxins was not studied yet. Therefore, this study was carried out to examine the possible hepatoprotective effect of S. terbenthifolius against CCl4 induced liver toxicity in rats.
MATERIALS AND METHODS
Animals
A total of 42 adult male Wistar rats with initial body weights of 206 ± 12 g and 8 weeks age were obtained from the Animal House Colony of the National Research Center, Dokki, Cairo, Egypt. Rats were allowed to acclimatize to their new environment for 2 weeks prior to the start of the experiment. The rats were housed in stainless steel cages under proper environmental conditions on a 12 h light-dark cycle. Rats were received a commercial pellet diet and water ad libitum.
Ethics statement
The laboratory animal ethical committee of Veterinary Medicine Faculty has approved this study; rats were maintained and received human care in accordance with the guiding principles for the Care and Use of Animals. The experimental design was approved by the Scientific Ethical Committee, Faculty of Veterinary Medicine, Suez Canal University (Protocol 014/2013). All the efforts were taken to minimize the animal suffering and to decrease the number of animals used.
Chemicals and reagents
Kits of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), γ glutamyl transferase (GGT), lactate dehydrogenase (LDH), GSH S-transferase reduced GSH, malondialdehyde (MDA), superoxide dismutase (SOD), CAT, glutathione peroxidase (GPx), and glutathione S-transferase (GST) were purchased from Biodiagnostics Co. (Cairo, Egypt). C-reactive protein (CRP) and α1 -fetoprotein (AFP) levels were analyzed using diagnostic kits (Roche Diagnostics, Cairo, Egypt). Immunohistochemical kits and antibodies were obtained from abcam® (Cambridge, MA, USA). 8-hydroxydeoxyguanosine (8-OHdG) was measured by the Oxi Select™ oxidative DNA damage ELISA kit (Cell Biolabs, Inc., USA). CCl4 and all other chemicals of analytical grade were purchased from Sigma Chemical Co. (St Louis, Mo, USA).
Plant collection and extract preparation
The aerial parts of S. terebinthifolius were collected from Ismailia, Egypt. The leaves were washed by fresh water to remove the contaminants, and then dried in hot air oven at 40°C. The dried leaves were grounded into powder with a laboratory electrical mill, de-fattened by n-hexane and then extracted in 70% ethanol at 4°C for 24 h. The extract was filtered and evaporated till dryness under reduced pressure using a rotatory evaporator (rotavapor RE111, BÜCHI). The obtained dried extract (each 1 kg dry leaves yield 126 g dry ethanolic extract) was stored in dry cold place until used in the experiment.[18] Ethanol: Deionized water (70:30) was selected as an organic polar solvent to be able to extract the vacuolar components (such as total phenolic compounds including flavonoids exist in glycosides form) of S. terebinthifolius leaves.[19]
Phytochemical constituents detection and quantification
Total flavonoid contents (TFC) and total phenolic contents TPC were measured in Schinus terebinthifolius extract following the method described by Park et al.[20] and Kim et al.[21] respectively. The TFC and the TPC were expressed as mg of rutin equivalents (RE) and mg of gallic acid equivalents per gram dried extract, respectively. Thin layer chromatography (TLC) was performed for detection and semi-quantification of other phytochemicals such as alkaloids, cardiac glycosides, saponins, tannins, terpenoids, quinones, and essential oils. Silica gel 60 F254 (0.25 mm thickness) plates (Merck Co, Darmstadt, Germany) were used as a stationary phase. Four mobile phases were used for separations; n-hexane: Ethyl acetate (8:2), chloroform: Methanol: Water (8:2:0.2), ethyl acetate: Chloroform: Methanol: Water (12:8:8:2) and chloroform: Methanol: Water (65:35:10). The spots have been detected under UV at 254 and 366 nm and sprayed with specific reagents to visualize some major phytochemicals. These reagents were Dragendorff reagent for alkaloids, Kedde reagent for cardiac glycosides, potassium hydroxide reagent for anthraquinones (red), anthones (yellow), and coumarins group (blue) at UV–366 nm. Also, anisaldehyde reagent and vanillin-sulfuric reagent were used for detection of terpenoids, steroids, and essential oils. Saponins and tannins were detected using forth and gelatin-salt block tests respectively.[22]
Acute oral toxicity
Median lethal dose (LD50) of S. terebinthifolius ethanolic extract was determined using 12 male Wistar rats. Graduated doses between 0.5 and 5.0 g dried extract/kg b.wt. were reconstituted in 1 mL saline and administered orally using the stomach tube. Rats were observed for clinical signs of toxicity and mortality for 24 h. LD50 for the extract was determined according to the modified up-and-down technique[23] to reduce the number of animals used. LD50 value was calculated using AOT425 statistical program (Version 1.0, Westat, NW, Washington DC, USA) developed by Westat for US EPA.
Experimental design
Preventive study
Fifteen rats were divided into three groups (five animals of each). Control group was injected interapretonially (i.p.) with 1 ml physiological saline instead of CCl4. CCl4 intoxicated group was given a single i.p. dose of 1 ml CCl4/kg b.wt. according to Manoj and Aqued.[24] Preventive group was orally administered 350 mg S. terebinthifolius dried extract/kg b.wt dissolved in 1 ml saline for 7 consecutive days prior to CCl4 injection.
Treatment study
Control and CCl4 groups were treated same as in preventive study. Treatment group was orally administered 350 mg S. terebinthifolius dried extract/kg b.wt. reconstituted in 1 ml saline for 14 consecutive days after CCl4 injection. Number of animals used for treatment study was 15 rats.
Dose of S. terebinthifolius was decided according to preliminary pilot study aimed to determine the least effective dose. This preliminary study revealed a good protective effect at a dose of 300 mg dried extract/kg b.wt and a moderate curative effect at a dose of 400 mg extract/Kg b.w.
Blood and liver sampling
After 72 h (in preventive study) and after 2 weeks (in treatment study) of CCl4 administration, all animals were lightly anesthetized using diethyl ether. Blood samples were collected from postorbital plexus and divided into two parts, first part was collected in heparinized tubes and used for the determination of selected hematological parameters, while the second part was allowed to clot at room temperature then centrifuged at 1200 × g gravitational force (G) for 10 min to separate the serum which was used for biochemical estimation of liver integrity enzymes, antioxidants and oxidative stress biomarkers, and inflammatory markers. Rats then were sacrificed by diethyl ether anesthesia and the liver was removed from each rat and washed several times with normal saline. Part of the liver was taken for biochemical estimations of antioxidant and oxidative stress biomarkers and the remaining tissue was kept in 10% formalin buffer and used for immunohistochemical examination.
Processing of the liver
Liver sample was washed twice with saline solution, crushed and homogenized in ice-cold 0.01 M sodium-potassium phosphate buffer (pH 7.4). The homogenate was centrifuged at 860 × g for 20 min at 4°C, and the resultant supernatant was frozen at −20°C and used for hepatic oxidative stress markers.
Analysis of 8-Hydroxydeoxyguanosine in DNA
A commercially available ELISA kit (Cell biolabs, inc) was used to evaluate the levels of 8-OH-dG in DNA. The assays were performed following the instructions of the manufacturer.[25] Briefly, DNA was extracted then incubated at 95°C for 5 min and rapidly chilling on ice. Digest DNA sample to nucleosides by incubating the denatured DNA with 5–20 units of nuclease P1 for 2 h at 37°C in 20 mM sodium acetate (pH 5.2), then treated with 5–10 units of alkaline phosphatase for 1 h at 37°C in 100 mM Tris buffer (pH 7.5). The reaction mixture was centrifuged for 5 min at 6000 × g, and the supernatant was used for the 8-OHdG ELISA assay. Briefly, 50 μL of unknown sample or 8-OHdG standard was incubated at room temperature for 10 min. 50 μL of the diluted anti-8-OHdG antibody was added and incubated at room temperature for 1 h. Washed 3 times, then 100 μL of the diluted secondary antibody-enzyme conjugate was added and incubate at room temperature for 1 h before re-washing. 100 μL of substrate solution was added to each well and incubate at room temperature for 20 min. Enzyme reaction was stopped by adding 100 μL of stop solution and absorbance was measured using a spectrophotometer at 450 nm (Shimadzu, model UV-3000, Kyoto-Japan).
Activated caspase-3 immunohistochemistry
Formalin liver samples were embedded in paraffin. The paraffin blocks were cut into 5–7 μm thickness. Deparaffinization and antigen recovery were carried out. Endogenous peroxidase activity was blocked by incubation for 5 min with 3% H2O2. Sections were incubated with the primary antibody for caspase-3 overnight at 4°C. Detection kit was used for visualization of the antibody reaction to detect and counting the number of apoptotic cells. The visualization performed according to the manufacturer instructions (Sigma, St. Louis, MO). Revelation done using 3’, 3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO). Sections washed in distilled water, and counter stained with Mayer's Hematoxylin for 5 min then examined using Olympus CX 21 microscope under 400 × powers.
Statistical analysis
The results were expressed as mean ± standard deviation for five animals in each group. One-way ANOVA was used followed by Holm-Sidak Multiple comparison test. Pearson's Correlation test was done for correlating parametric variables. Statistical significance was accepted at P ≤ 0.05.
RESULTS
Phytochemical constituents detection and quantification
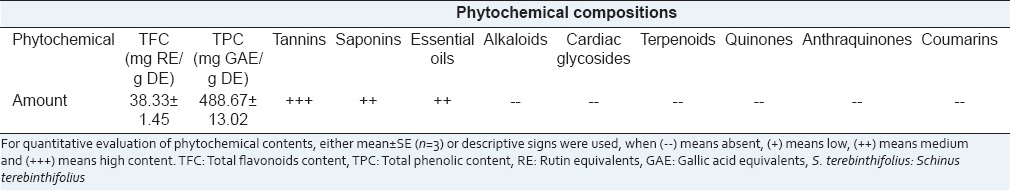
The quantitative and semi-quantitative phytochemical compositions of S. terebinthifolius ethanolic extract are shown in Table 1. TPC was found to be the major constitute, it represented about half of the total ethanolic extract dry weight. From the TPC, the TFC constituted about 8% with a concentration of 38.33 ± 1.45 mg REs/g dried extract. Tannins, saponins, and essential oils were also detected in medium to high concentrations in the extract while no alkaloids, cardiac glycosides, terpenoids, quinones, anthraquinones or coumarins could be detected in the extract.
Table 1.
Quantitative and semi-quantitative phytochemical compositions of S. terebinthifolius ethanolic extract
Acute oral toxicity
Oral administration of S. terebinthifolius ethanolic extract up to 5 g/kg b.wt. did not show any toxic signs or deaths within 24 h from extract administration. Moreover, no signs of delayed toxicity were perceived when rats were observed for further 14 days.
Serum hepatic parameters
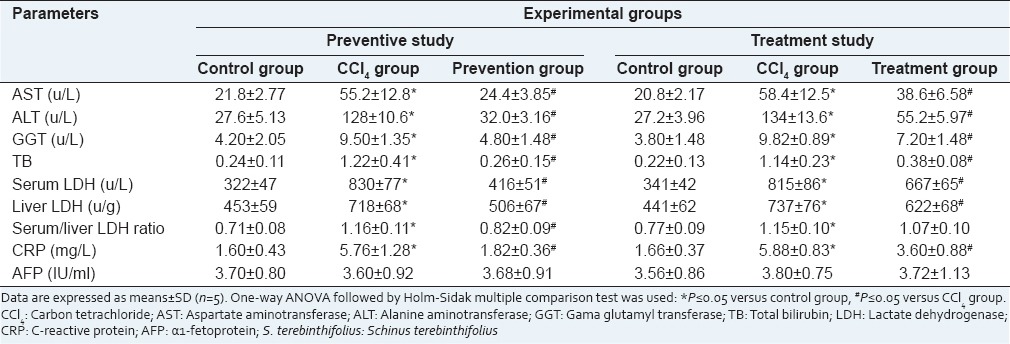
The effects of S. terebinthifolius on serum transaminases, bilirubin, and LDH, are summarized in Table 2. There were significant increases (P ≤ 0.05) in liver enzymes leakage (AST, ALT, GGT and LDH) as well as blood bilirubin concentrations in CCl4 intoxicated rats when compared to control groups in both preventive and treatment experiments. Administration of S. terebinthifolius, either for 1 week before or for 2 weeks after CCl4 injection, significantly (P ≤ 0.05) decreased liver enzymes leakage and restored the altered bilirubin to approximately the control levels.
Table 2.
Liver serum parameters and inflammatory markers in control rats, rats intoxicated with CCl4 and rats received S. terebinthifolius either before CCl4 (prevention group) or after CCl4 (treatment group)
Inflammatory markers
While there were significant increases in CRP levels in all CCl4 intoxicated groups, AFP was almost unchanged in comparison with the control group [Table 2]. Using of S. terebinthifolius either as preventive or as a treatment in rats intoxicated with CCl4 significantly decreased the CRP levels when compared to CCl4 groups.
Antioxidant activities and oxidative DNA damage
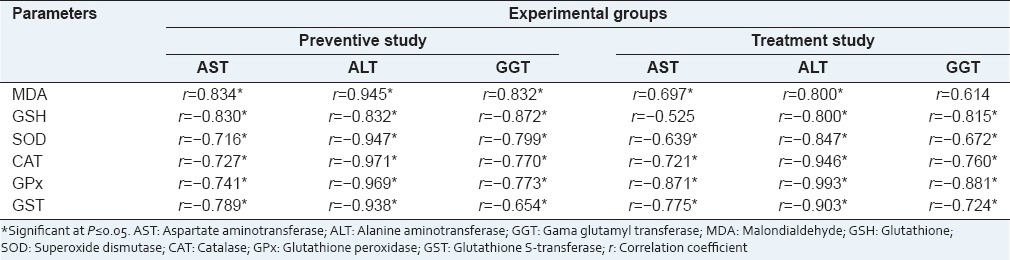
Significant decreases in the erythrocyte and hepatic GSH in addition to hepatic SOD, CAT, GPx and GST were observed in CCl4 intoxicated rats when compared with normal control rats. Oral administration of S. terebinthifolius extract in both preventive and treatment groups reinstated the GSH levels in comparison with CCl4 group [Table 3]. The rats received CCl4 alone showed elevated lipid peroxidation demonstrated by high serum and liver MDA levels as compared to the control rats. Once more, administration of S. terebinthifolius extract before or after CCl4 showed significant decreases in serum MDA levels as compared to those in CCl4 intoxicated groups. Unlike in the preventive group, liver MDA level in the treatment group was still higher than that in the control group. The oxidative DNA damage marker (8-OHdG) was significantly elevated in the rats intoxicated with CCl4 in comparison with that in control rats. There was neither protective nor curative effect of S. terebinthifolius against DNA damage caused by CCl4 [Table 3]. When data were analyzed for correlations using Pearson's correlation analysis, our results showed negative correlations between liver enzymes (AST, ALT, and GGT) and the hepatic antioxidant parameters GSH, SOD, CAT, GPx, and GST. Meanwhile, only MDA showed positive correlations with these liver enzymes [Table 4].
Table 3.
Oxidant and antioxidant parameters in control rats, rats intoxicated with CCl4 and rats received S. terebinthifolius either before CCl4 (prevention group) or after CCl4 (treatment group)
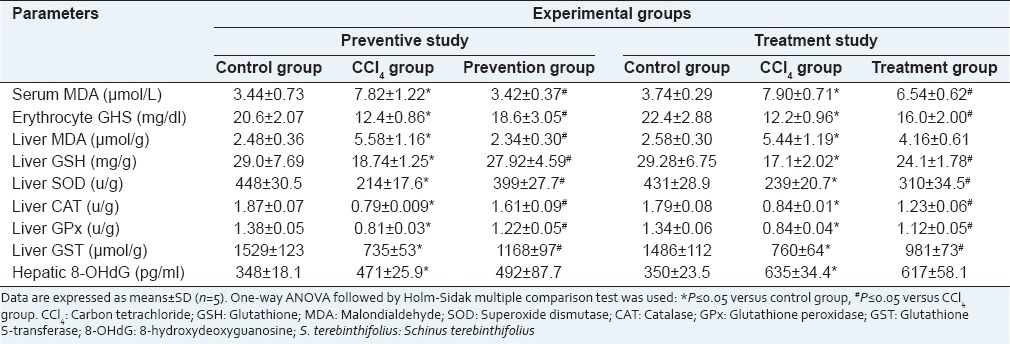
Table 4.
Pearson's correlation coefficients among the serum liver enzymes and the oxidant-antioxidant markers in the prevention and treatment studies
Blood parameters
There was a significant decrease in blood platelets while there was a significant increase in WBCs count in rats treated with CCl4 compared to control rats. Although pre-treatment with S. terebinthifolius restored these values to approximately normal values, administration of S. terebinthifolius after CCl4 significantly decreased the WBCs but not platelets counts when compared to CCl4 group [Table 5]. When the correlation coefficient (r) was calculated using Person's correlation analysis, our data showed significant negative correlations between blood platelets count and TB (r = −0.824 and-0.442 in prevention and treatment studies, respectively), whereas there were significant positive correlations between WBCs count and CRP levels in prevention and treatment studies (r = 0.889 and 0.778, respectively) using Pearson's correlation.
Table 5.
Blood parameters in control rats, rats intoxicated with CCl4 and rats received S. terebinthifolius either before (prevention group) or after (treatment group) CCl4 intoxication
Immunohistochemical findings
The expression levels of cleaved caspase-3 in the livers of CCl4 intoxicated rats were relatively high (16.8 ± 1.48 cells/HPF in prevention study and 22.6 ± 3.36 cells/HPF in treatment study). Interestingly, these levels were significantly decreased when CCl4 intoxicated rats were administered S. terebithifolius extract (1.2 ± 0.45 cells/HPF in prevention study and 0.4 ± 0.55 cell/HPF in treatment study) as detected by immunohistochemistry [Figure 1].
Figure 1.
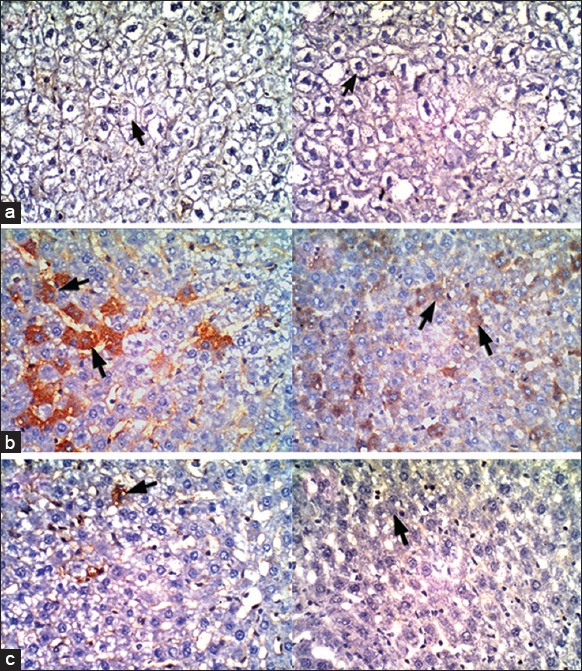
Immunohistochemical staining of the liver sections (×400) stained with caspase 3 antibodies for apoptotic cells and hematoxylin counterstaining of the nuclei and non-apoptotic cells. (a) control groups, (b) CCl4 groups and (c) rats received ethanolic extract of Schinus terebinthifolius either before [left side pictures] or after [right side pictures] CCl4-intoxications. Arrowheads in figures (b) point out caspase-3 positive apoptotic cells
DISCUSSION
In this study, the TPC was found to be the major constitute of S. terebithifolius ethanolic extract. Besides, the TFC was found to be 38.33 ± 1.45 mg (REs) per gram of dried extract. These two constituents are good indicators for the antioxidant activity of plant extracts. Many researchers reported a significantly high correlation between the high antioxidant activity of plant extracts and its high TPC and TFC.[26]
Median lethal dose was estimated to evaluate the acute toxicity of S. terebithifolius ethanolic extract aiming to use one-twentieth of LD50 in our experiment. Our result revealed neither toxic signs nor deaths up to a dose of 5 g dried extract/kg b.wt. Substances of oral LD50 < 5000 mg/kg are considered practically nontoxic.[27] Accordingly, the dose of S. terebithifolius in this study was decided based on its effective not on its toxicity.
Carbon tetrachloride is one of the most frequently used hepatotoxins to study liver diseases in experimental animal models; it has been reported to show many hepatic disorders similar to that perceived in viral hepatitis or chemically-induced liver injury.[28,39,30,31] In liver, CCl4 transformed into trichloromethyl radicals those react with excess O2 resulting in the production of reactive free radicals and stimulation of lipid peroxidation followed by cellular damage, inflammation, and pathological changes.[10,32,33] Administration of CCl4 significantly increased the leakage of liver ALT, AST, GGT, and LDH enzymes into the serum; it also increased blood TB. ALT, AST, GGT, and LDH are sensitive indicators for the hepatic function and their excessive leakage into the blood is usually associated with impaired hepatocellular function and designates the disruption of integrity of hepatic cell membranes.[34,35,36,37] In the present study, CCl4 administration increased lipid peroxidation via elevation of serum and hepatic MDA levels and decreasing GSH antioxidant capacity in erythrocytes and liver as well as decreasing the activities of hepatic SOD, CAT, GPx, and GST [Table 3]. These effects are implicated in hepatic oxidative stress and toxicity. This pointed out that CCl4 -mediated hepatic injury occurred as a result of excessive production of ROS, which attack various biological molecules including lipids and causing lipid peroxidation. These results are parallel with those obtained by many other researchers.[8,38,39] CRP is an acute-phase protein that produced by hepatocytes and it is a sensitive marker for inflammation, infection, and tissue damage.[40] The results in this study revealed that CCl4 administration induced liver inflammation as there were significant increases in CRP levels coupled with an increase in WBCs count. Administration of S. terebinthifolius pre and post CCl4 intoxication significantly reduced the elevated liver enzymes, CRP, and WBCs count; these findings designate the protection of the hepatocytes integrity or renewal of injured hepatocytes.[34] In addition, there were elevations in antioxidant enzymes and reduction of the lipid peroxidation in blood and livers of groups those received S. terebinthifolius compared to those of CCl4 -intoxicated groups. These findings verify the antioxidant effects of S. terebinthifolius and explain its restorative effect that could be attributed to its ability to minimize the production of free radicals and/or to enhance the scavenger activities. The antioxidant activity of S. terebinthifolius was confirmed by other researchers.[14,16,17]
8-OHdG is a specific marker for DNA oxidation.[41] The current study showed significant elevation in 8-OHdG in CCl4 -intoxicated groups when compared to the control groups. Such elevation in 8-OHdG levels can be explained by the oxidative alteration of macromolecules and consequently genomic unsteadiness caused by the formation of excessive ROS.[42] In our study, S. terebinthifolius failed to protect hepatocyte DNA from oxidative damage or reverse the damage. This could be attributed to the pharmacokinetic properties of the antioxidant substances (mainly the flavonoids) inside the liver cells. When the CCl4 is a non-polar compound and can easily pass both cellular and nuclear membranes causing damage to DNA, it seemed that the flavonoids in S. terebinthifolius extract become more polar and less fat soluble inside the liver calls through binding with one or more intracellular compounds such as proteins (which its concentrations are 8 times higher than extracellular concentrations). Specially that the polarity of flavonoids depends to a large degree on whether they occur bound to one or more polar moiety (such as sugar), which renders them highly polar, or whether they occur in the free form in which case they are much less polar.[19]
As a general, the present data showed a significant positive correlation between liver function enzymes and the oxidant-antioxidant markers, which confirms the antioxidant mechanism of S. terebinthifolius as a hepatoprotective agent.
Apoptosis of hepatocytes is mainly initiated by activation of the caspase family of cysteine proteases, which is a major form of cell death.[43] Our findings indicated that a substantial number of hepatocytes undergo apoptosis after CCl4 administration; this finding was in consistent with those obtained by other researchers.[44,45,46] Our results also revealed that S. terebinthifolius intensely inhibited hepatocyte apoptosis by down-regulating the expression of caspase-3 compared with CCl4 group. Besides the decreased LDH leakage, which could be a beneficial marker for the integrity of the outer cell membrane, that result confirmed the protective and therapeutic effects of S. terebinthifolius against CCl4 -induced hepatotoxicity.
In summary, the present study affirmed a beneficial ability of S. terebinthifolius ethanolic extract to reduce CCl4 -induced liver damage. The markers of protection include significantly decreased serum hepatic enzyme, increased antioxidant capacity, and decreased hepatocyte apoptosis when compared with the CCl4 treatment group. This hepatoprotective effect might be attributed to its antioxidant and free radical scavenging effects. Such findings put up S. terebinthifolius as a promising hepatoprotective agent against CCl4 toxic effects and may be against other hepatotoxic chemicals or drugs, both in animal and human.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ahmed M. Abdel-Azeem (Botany and Microbiology Department, Faculty of Science, Suez Canal University, Egypt) for identification and authentication of S. terebinthifolius and supervising its collection and preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Madkour FF, Khalil WF, Dessouki AA. Protective effect of ethanol extract of Sargassum Dentifolium (Phaeophyceae) in carbon tetrachloride induced hepatitis in rats. Int J Pharm Pharm Sci. 2012;4:637–41. [Google Scholar]
- 2.Gupta M, Mazumder UK, Thamilselven V, Manikandan L, Senthilkumar GP. Potential hepatoprotective effect and antioxidant role of ethanol extract of Oldenlandia umbellate in carbon tetrachloride induced hepatotoxicity in Wistar rats. Iran J Pharmacol Ther. 2007;6:5–9. [Google Scholar]
- 3.Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmacol Res. 2004;49:481–6. doi: 10.1016/j.phrs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JM. 3rd ed. New York: Oxford University Press; 1999. Free Radicals in Biology and Medicine; pp. 617–783. [Google Scholar]
- 5.Stal P, Olson J. Ubiquinone: Oxidative stress, and liver carcinogenesis. In: Kagan VE, Quinn DJ, editors. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; 2000. pp. 317–29. [Google Scholar]
- 6.Sturgill MG, Lambert GH. Xenobiotic-induced hepatotoxicity: Mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem. 1997;43:1512–26. [PubMed] [Google Scholar]
- 7.Lau DT, Membreno FE. Antiviral therapy for treatment-naïve hepatitis B virus patients. Gastroenterol Clin North Am. 2004;33:581–99, ix. doi: 10.1016/j.gtc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Tirkey N, Pilkhwal S, Kuhad A, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2. doi: 10.1186/1471-2210-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraganahalli BD, Chinampudur VC, Dethe S, Mundkinajeddu D, Pandre MK, Balachandran J, et al. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn Mag. 2012;8:116–23. doi: 10.4103/0973-1296.96553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazima B, Manoharan V, Prabu SM. Cadmium toxicity: Oxidative stress and organ dysfunction. Res Rev J Toxicol. 2014;4:1–18. [Google Scholar]
- 11.Feroz Z, Khan RA, Amber F, Mahayrookh A. Hepatoprotective effect of herbal drug on CCl (4) induced liver damage. Pak J Pharm Sci. 2013;26:99–103. [PubMed] [Google Scholar]
- 12.Affonso CR, Fernandes RM, Oliveira JM, Martins MC, Lima SG, Sousa Júnior GR, et al. Effects of the essential oil from fruits of Schinus terebinthifolius Raddi (Anacardiaceae) on reproductive functions in male rats. J Braz Chem Soc. 2012;23:180–5. [Google Scholar]
- 13.Glen HF. Johannesburg, South Africa: Jacana; 2002. Cultivated Plants of Southern Africa. Botanical Names, Common Names, Origins, Literature; p. 404. [Google Scholar]
- 14.Chowdhury AR, Tripathi S. Essential oil from leaves of S. terebinthifolius Raddi. Indian Perfumer. 2001;4:257–9. [Google Scholar]
- 15.Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz M, Jr, Tien OS, Kakinami SH, et al. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002;73:69–91. doi: 10.1016/s0367-326x(01)00362-8. [DOI] [PubMed] [Google Scholar]
- 16.Cavalher-Machado SC, Rosas EC, Brito Fde A, Heringe AP, de Oliveira RR, Kaplan MA, et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int Immunopharmacol. 2008;8:1552–60. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Marzouk MS, Moharram FA, Haggag EG, Ibrahim MT, Badary OA. Antioxidant flavonol glycosides from Schinus molle. Phytother Res. 2006;20:200–5. doi: 10.1002/ptr.1834. [DOI] [PubMed] [Google Scholar]
- 18.Jirayus W, Kanok-Orn I, Korakod I. Antioxidant activity and cytotoxicity of six selected, regional, Thai vegetables. Am Eurasian J Toxicol Sci. 2012;4:108–17. [Google Scholar]
- 19.Bohm BA. Vol. 2. Amsterdam: Harwood Academic Publishers; 1998. Introduction to flavonoids. Chemistry and Biochemistry of Organic Natural Products; pp. 176–7. [Google Scholar]
- 20.Park YS, Jung ST, Kang SG, Heo BK, Arancibia-Avila P, Toledo F, et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–8. [Google Scholar]
- 21.Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–6. [Google Scholar]
- 22.Harborne JB. London, UK: Chapman and Hall; 1998. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis; p. 302. [Google Scholar]
- 23.Bruce RD. A confirmatory study of the up-and-down method for acute oral toxicity testing. Fundam Appl Toxicol. 1987;8:97–100. doi: 10.1016/0272-0590(87)90104-7. [DOI] [PubMed] [Google Scholar]
- 24.Manoj B, Aqueed K. Protective effect of Law sona alba L. against CCl4 induced hepatic damage in albino rats. Indian J Exp Biol. 2003;4:85–7. [PubMed] [Google Scholar]
- 25.Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy-2’- deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007;109:574–80. doi: 10.1002/cncr.22417. [DOI] [PubMed] [Google Scholar]
- 26.Sarepoua E, Tangwongchai R, Suriharn B, Lertrat K. Relationships between phytochemicals and antioxidant activity in corn silk. Int Food Res J. 2013;20:2073–9. [Google Scholar]
- 27.Hodge HC, Sterner JH. Combined tabulation of toxicity classes. In: Spector WS, editor. Handbook of Toxicology. Vol. 1. Philadelphia: W. B. Saunders Company; 1956. p. 37. [Google Scholar]
- 28.Sannigrahi S, Mazumder UK, Pal D, Mishra SL. Hepatoprotective potential of methanol extract of Clerodendrum infortunatum Linn. against CCl4 induced hepatotoxicity in rats. Pharmacogn Mag. 2009;5(S1):394–9. [Google Scholar]
- 29.Williams AT, Burk RF. Carbon tetrachloride hepatotoxicity: An example of free radical-mediated injury. Semin Liver Dis. 1990;10:279–84. doi: 10.1055/s-2008-1040483. [DOI] [PubMed] [Google Scholar]
- 30.Johnston DE, Kroening C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Pharmacol Toxicol. 1998;83:231–9. doi: 10.1111/j.1600-0773.1998.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 31.Shenoy KA, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginkgo Biloba against tetrachloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33:260–6. [Google Scholar]
- 32.Basu S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–27. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 34.Patrick-Iwuanyanwu KC, Wegwu MO, Ayalogu EO. Prevention of CCl4-induced liver damage by ginger, garlic and vitamin E. Pak J Biol Sci. 2007;10:617–21. doi: 10.3923/pjbs.2007.617.621. [DOI] [PubMed] [Google Scholar]
- 35.Firdous SM, Sravanthi K, Debnath R, Neeraja K. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of fruits against carbon tetrachloride induced hepatic injury rats. Int J Pharm Pharm Sci. 2012;4:354–9. [Google Scholar]
- 36.Bhakta T, Mukherjee PK, Mukherjee K, Banerjee S, Mandal SC, Maity TK, et al. Evaluation of hepatoprotective activity of Cassia fistula leaf extract. J Ethnopharmacol. 1999;66:277–82. doi: 10.1016/s0378-8741(98)00220-7. [DOI] [PubMed] [Google Scholar]
- 37.Wafay H, El-Saeed G, El-Toukhy S, Youness E, Ellaithy N, Agaibi M, et al. Potential effect of garlic oil and Silymarin on carbon tetrachloride-induced liver injury. Aust J Basic Appl Sci. 2012;6:409–14. [Google Scholar]
- 38.Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med. 2012;12:178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Fartosi KG, Majid A, Auda AM, Hussein MH. The Role of Camel's Milk against some oxidant-antioxidant markers of male rats treated with CCl4. Int J Res Pharm Biomed Sci. 2012;3:385–9. [Google Scholar]
- 40.Husain MT, Kim HD. C-Reactive protein and erythrocyte sedimentation rate in orthopaedics. Univ Pa Orthop J. 2002;15:13–6. [Google Scholar]
- 41.Subash P, Gurumurthy P, Sarasabharathi A, Cherian KM. Urinary 8-OHdG: A marker of oxidative stress to DNA and total antioxidant status in essential hypertension with South Indian population. Indian J Clin Biochem. 2010;25:127–32. doi: 10.1007/s12291-010-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 43.Inoue S, Browne G, Melino G, Cohen GM. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009;16:1053–61. doi: 10.1038/cdd.2009.29. [DOI] [PubMed] [Google Scholar]
- 44.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515–25. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee TY, Chang HH, Wang GJ, Chiu JH, Yang YY, Lin HC. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J Pharm Pharmacol. 2006;58:659–65. doi: 10.1211/jpp.58.5.0011. [DOI] [PubMed] [Google Scholar]
- 46.Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, et al. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 2012;32:522–9. doi: 10.1016/j.nutres.2012.06.007. [DOI] [PubMed] [Google Scholar]


