Abstract
The use of anabolic-androgenic steroids (AASs) by professional and recreational athletes is increasing worldwide. The underlying motivations are mainly performance enhancement and body image improvement. AAS abuse and dependence, which are specifically classified and coded by the DSM-5, are not uncommon. AAS-using athletes are frequently present with psychiatric symptoms and disorders, mainly somatoform and eating, but also mood, and schizophrenia-related disorders. Some psychiatric disorders are typical of athletes, like muscle dysmorphia. This raises the issue of whether AAS use causes these disorders in athletes, by determining neuroadaptive changes in the reward neural circuit or by exacerbating stress vulnerability, or rather these are athletes with premorbid abnormal personalities or a history of psychiatric disorders who are attracted to AAS use, prompted by the desire to improve their appearance and control their weights. This may predispose to eating disorders, but AASs also show mood destabilizing effects, with longterm use inducing depression and short-term hypomania; withdrawal/discontinuation may be accompanied by depression. The effects of AASs on anxiety behavior are unclear and studies are inconsistent. AASs are also linked to psychotic behavior. The psychological characteristics that could prompt athletes to use AASs have not been elucidated.
Keywords: Anabolic-androgenic steroids, doping, mood disorders, psychopathology, psychosis
INTRODUCTION
Professional and recreational athletes commonly use anabolic-androgenic steroids (AASs) to enhance performance or improve their physical appearance; the impact of this practice on psychopathology is unknown and so is the presence of psychopathology in those who will make later use of AASs. AASs include testosterone and its numerous synthetic analogs that have been modified to boost their anabolic, rather than their androgenic effects. The higher the anabolic:androgenic ratio, the higher the anabolic effect of a given AAS. Anabolic effects consist in protein synthesis, muscle growth, and erythropoiesis [1-3]. Therefore, they allow athletes to increase muscle size and reduce body fat. AAS users find that their muscles recover faster from intense strain and muscle injury, allowing them to train longer and harder [4]. However, Imanipour et al. [5] have shown AASs to increase serum creatine kinase and muscle damage. AASs produce their anabolic effects through binding to steroid receptors; they activate androgen receptors, thus controlling the transcription of target genes that regulate DNA accumulation required for muscle growth. When AASs bind to skeletal muscle androgen receptor, they cause an increase in muscular mass and strength, since amino acids are used more effectively for protein synthesis [1, 6]. They also reduce glucocorticoid-dependent metabolic breakdown by binding competitively to glucocorticoid receptors [7].
AASs, due to their diverse biological actions, have shown benefit in a variety of conditions, including HIV-related muscle wasting, muscle dystrophies, severe burn injuries, bone marrow failure, hereditary angioedema, and growth retardation in children [8-3]. However, AAS use is associated with various dose-related side-effects. High doses of AASs can lead to serious physical and psychological complications, such as hypertension, atherosclerosis, myocardial hypertrophy and infarction, abnormal blood clotting, hepatotoxicity and hepatic tumors, tendon damage, reduced libido, and psychiatric/behavioral symptoms like aggressiveness and irritability [14-22]. In addition, AASs are related to hypofertility and gynecomastia in men [12, 23] and to virilization in women, with hirsutism, male-pattern baldness, irregular menses, and lower-pitched voice [24].
AAS use to improve performance and acquire more muscular bodies is on the rise worldwide. In the US alone, at least two million individuals use or have used AASs [25, 26] and epidemiologic data suggest that there are millions of other AAS users worldwide, from the UK [27] to Sweden [28], to other European countries [29], to Canada [30], Australia [31], and Brazil [32]. Economic costs vary, with annual per capita expense for AASs ranging from $90 to $6780. Men use AASs significantly more than women, even if the latter are using them increasingly [33]. AAS users frequently have at least one friend or acquaintance that uses or has used AASs. AAS use can also be associated with or predict future consumption of other types of psychoactive substances and might be part of a general pattern of poly-substance use and risk-taking behavior, especially among adolescents [34-37]. In fact, AAS use is no longer limited to athletes, bodybuilders, or weightlifters, but is currently extending to the general population, including young people, probably because of the highly competitive nature of high school and college varsity sports [38-41]. Welder and Melchert [42] reported that in the US over half a million high school students have taken AASs for nonmedical purposes. This raises serious concerns among physicians regarding the numerous adverse effects of these substances.
AASs have been added to the International Olympic Committee (IOC)’s list of banned substances in 1975; their use without physician’s prescription and supervision is illegal in the US and Canada and is termed “doping”. The World Anti-Doping Agency (WADA), which works for the IOC, has published in 2013 a list of prohibited substances, both in- and out-competition [43]; the list includes substances lacking governmental regulatory health authorities’ approval for human therapeutic use (e.g., discontinued drugs or still under preclinical or clinical development, designer drugs, substances approved only for veterinary use). It contains over 100 different AASs, both synthetic and natural. The most common are androstenediol, androstenedione, boldenone, danazol, dehydroepiandrosterone, dihydrotestosterone, methandienone, mesterolone, methenolone, nandrolone, oxandrolone, and tetrahydrogestrinone. These substances are formulated to be administered either orally, or parenterally by intramuscular injections, or subcutaneously by implantable pellets, or transdermally by patches or topical gels. It must be noted that some athletes also use legal “dietary supplements” that are not regulated in both US and Europe. These substances are not subjected to the same testing for safety and effectiveness as all over-the-counter and prescription medications. They are legal substances, sold in the form of pills, which the body converts into testosterone. How efficiently this converted testosterone might work is unknown, as is the extent to which it may obtain desirable effects, and it is suspected that these pills may be as harmful as AASs in other formulations.
The aim of this review is to investigate the relationship between AAS use and psychopathology in athletes and to identify possible prevention and treatment methods. We may suspect that exogenously administered testosterone and its synthetic analogs may alter the developmental trajectory of the brain in adolescents and young adults and its pattern of adaptation to environmental stimuli in middle-aged or older adults. AASs have numerous central nervous system effects, the extent of which varies with several factors, such as the athlete’s background resilience, the duration of AAS use and dose, concurrent organic diseases, and use of other medications, alcohol or illegal substances [44-47].
METHODS
Eligibility Criteria
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement [48]. Retrospective and prospective studies examining the relationship between AAS use and psychopathology in athletes were included. Psychiatric diagnoses had to be based on the Diagnostic and Statistical Manual 5th Edition (DSM-5) criteria [49] or its predecessors or on the International Classification of Diseases 10th revision (ICD-10) [50] criteria or its predecessor. Studies involving animals only or not including athletes were excluded.
Search Criteria and Critical Appraisal
The search strategy differed according to the database explored. For PubMed we used anabolic steroid users AND "Mental Disorders" [MeSH], limiting to humans, as a search strategy after trying several variations that produced an excess of irrelevant papers. The search yielded 151 papers as of July 27, 2014. Subsequent appraisal based on titles and abstracts left 55 papers. The same strategy with no time limits and without using the MeSH function in the other databases yielded 388 papers in ScienceDirect Scopus and four additional relevant papers, 263 papers and one additional with respect to the other two in the INFORMA healthcare database, 2953 papers in Excerpta Medica Database (EMBASE, limited to July 24, 2014) adding further two relevant papers, 5195 papers in PsycINFO (limited to the fourth week of July), adding further three relevant articles, and 18 papers searching for all anabolic steroid users in the Cochrane Central Register of Controlled Trials (CENTRAL), which added no new relevant paper (Fig. 1). Reference lists of all located articles were further searched to detect still unidentified literature, but yielded no new articles. Methodological appraisal of each study was conducted according to PRISMA standards, including bias evaluation.
Fig. (1).
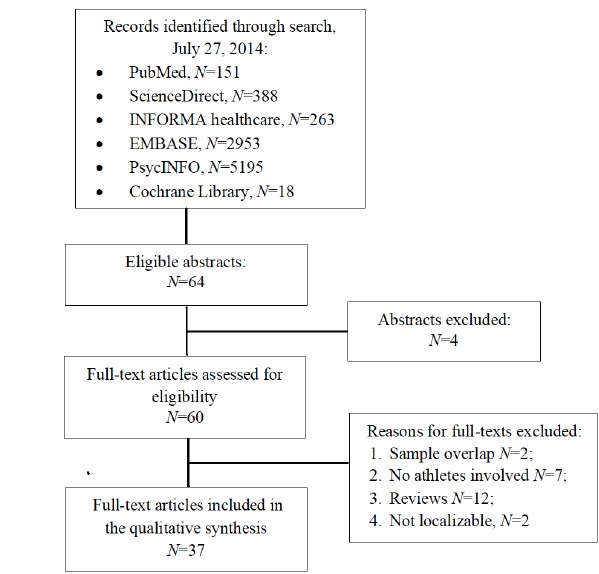
PRISMA flowchart of systematic review inclusion and exclusion.
RESULTS
Search Results and Included Studies
From each electronic database we read all titles and selected those promising to be relevant, which were 64. Subsequently, we read abstracts and downloaded the full text of those actually relevant, which amounted to 60 articles. The reviews were downloaded to further search their reference lists similarly to other papers, but they yielded no other potentially eligible article. Reviews were subsequently excluded. All reasons for exclusion of studies are shown in Fig. 1. The final number of included papers was 37. Sample sizes for AAS users in included studies ranged from 1 (i.e., eight case reports) to 550, with a mean of 93 and a median of 45, indicating skewness towards smaller samples.
Study Characteristics
The following data were extracted from included studies: study source; type of study; total number of participants in the study; number of participants with AAS use/abuse/dependence; number of participants without AAS use (controls); study duration; criteria used for psycho-pathological diagnosis and any rating scale used for psycho-pathological screening; key findings. A data collection form was developed and used to extract data from the included studies. Data items extracted are listed in Table 1.
Table 1.
Data items extracted from the selected studies
| Author(s)/ Year of Publication | Country | Study Type | Participants Sample | Psychopathology Assessment Methods | Key Findings |
|---|---|---|---|---|---|
| Mood disorders (n=12) | |||||
| Pope & Katz (1988) [53] | US | Cross-sectional | 41 bodybuilders and football players current or past AAS users | DSM-III-R criteria | 9 (22%) current AAS users displayed a full affective syndrome and 5 (12%) displayed psychotic symptoms. Another five (12%) developed major depression when they stopped taking AASs |
| Tennant et al. (1988) [66] | US | Case report | One bodybuilder AAS user | Semi-structured interview | The AAS user stated that he was “addicted” to AASs and could not stop to take them without experiencing severe withdrawal symptoms, including depression, disabling fatigue, nausea, and dizziness. A diagnosis of AAS dependence is hypothesized |
| Brower et al. (1989) [63] | US | Case report | One weightlifter AAS-dependent user | DSM-III-R criteria | The AAS-dependent user manifested severe depression and aggression during AAS use |
| Brower et al. (1990) [64] | US | Cross-sectional | 8 weightlifters AAS-dependent user | DSM-III-R criteria | The AAS-dependent users often lamented depressive symptoms |
| Lindström et al. (1990) [65] | Sweden | Cross-sectional | 53 bodybuilders AAS users | Semi-structured interview | 27 (51%) AAS users reported unspecified mood disturbances |
| Perry et al. (1990) [55] | US | Cross-sectional | 20 weightlifters AAS users; 20 weightlifters AAS nonusers (controls) | Standardized Psychological Index; DSM-III-R criteria |
AAS users had significantly more somatic, depressive, anxiety, hostility, and paranoid symptoms when using AASs than when not using them (p<0.005). They also had significantly more depressive, anxiety, and hostility symptoms compared to AAS nonusers (p<0.005). However, no difference in the frequency of major psychiatric disorders was found between the two groups |
| Bahrke et al. (1992) [4] | US | Retrospective, controlled | 12 male weightlifters current AAS users; 14 male weightlifters past AAS users; 24 male weightlifters AAS nonusers (controls) | Semi-structured interview; physical and medical history questionnaire; Profile of Mood States Questionnaire; Buss-Durkee Hostility Inventory | AAS current users, past users, and nonusers showed no significant difference in the Profile of Moods scale and subscale scores |
| Pope & Katz (1994) [54] | US | Cross-sectional | 88 weightlifters AAS users; 68 weightlifters AAS nonusers (controls) | DSM-III-R criteria | 20 (23%) AAS users reported major mood disorders vs. only 4 (6%) of AAS nonusers (p<0.01). Moreover, AAS users displayed mood disorders during AAS exposure significantly more than in the absence of AAS exposure (p<0.001) |
| Malone et al. (1995) [58] | US | Retrospective, controlled | 164 weightlifters and bodybuilders, either current AAS users, or past AAS users, or AAS nonusers | DSM-III-R criteria | Past AAS users had a higher incidence of psychiatric diagnosis than current users and nonusers. About 10% of current AAS users had hypomania. Depression occurred when AAS were stopped in about 10% of weightlifters and bodybuilders. Present psychoactive substance abuse or dependence was relatively low across all user categories. AAS dependence was found in 12.9% of current AAS users and in 15.2% of past AAS users |
| Mood disorders (n=12) | |||||
| Gruber & Pope (2000) [59] | US | Retrospective, controlled | 25 female athletes current or past AAS users; 50 female athletes AAS nonusers (controls) | DSM-IV criteria | 14 (56%) AAS users reported hypomanic symptoms during AAS use and 10 (40%) reported depressive symptoms during AAS withdrawal, but none met full DSM-IV criteria for a hypomanic or major depressive episode. Both AAS users and nonusers frequently reported muscle dysmorphia |
| Perry et al. (2003) [57] | US | Retrospective, controlled | 10 male weightlifters AAS users; 18 male weightlifters AAS nonusers (controls) | Hamilton Depression Rating Scale; Hamilton Anxiety Scale; Mania Rating Scale; Buss-Durkee Hostility Inventory; Point Subtraction Aggression Paradigm; Personality Disorder Questionnaire | AAS users reported more affective – i.e., depressive and manic – symptoms than AAS nonusers |
| Papazisis et al. (2007) [67] | Greece | Case report | One bodybuilder/martial artist AAS user | Semi-structured interview | The AAS user, with a prior history of psychotic depression, was hospitalized for a manic episode and was diagnosed with an AAS-induced mood disorder with manic features |
| Ip et al. (2012) [86] | US | Cross-sectional | 112 male fitness amateurs, bodybuilders and weightlifters AAS-dependent users; 367 male fitness amateurs, bodybuilders and weightlifters AAS-nondependent users (controls) | DSM-IV-TR criteria | AAS-dependent users were more likely to have a diagnosis of a major depressive disorder (15.2% vs. 7.4%, p=0.012) than AAS-nondependent users |
| Lindqvist et al. (2013) [56] | Sweden | Retrospective, controlled | 136 AAS user male former élite athletes in power sports vs. 547 nonuser male former elite athletes in power sports | 30-year follow-back | AAS users sought significantly more often than nonusers professional help for depression (13% vs. 5%; p<0.001), anxiety (13% vs. 6%; p<0.01), melancholy (13% vs. 4%; p<0.001), and worry for mental health (8% vs. 3%; χ2=6.77; p<0.01) |
| Suicide (n=7) | |||||
| Brower et al. (1989) [63] | US | Case report | One weightlifter AAS-dependent user with suicidal ideation | DSM-III-R criteria | The AAS-dependent user manifested severe depression. He had no personal or family history of depression or suicidal tendencies |
| Thiblin et al. (1999) [74] | Sweden | Case series | 8 male athletes AAS-users deceased by suicide | Medico-legal examination; systematic interview with survivors (i.e., family members, friends) |
5 (62.5%) suicides were committed during current AAS use and 2 (25%) following two and six months of AAS withdrawal, respectively. In one case (12.5%) it was unclear whether the suicide was committed during current use or after recent discontinuation |
| Suicide (n=7) | |||||
| Pärssinen et al. (2000) [76] | Finland | Prospective, controlled | 62 male elite weightlifters suspected AAS users; 1094 population controls | 12-year follow-up | A 4.6 times higher mortality rate (95% CI 2.04-10.45; p=0.000) was found in the athletes compared to the general population. The main causes of death were suicide (5%) and myocardial infarction (5%) |
| Thiblin et al. (2000) [75] | Sweden | Retrospective | 25 deceased male athletes current AAS users; 9 deceased male athletes past AAS users | Medico-legal examination | A high proportion (59%) of AAS users died by violent death, i.e., suicide (n=11, 32%) or homicide (n=9, 27%). Suicide was related to impulsive, aggressive behavior characterized by violent rages and mood swings and associated with AAS use |
| Petersson et al. (2006) [77] | Sweden | Retrospective, controlled | 52 deceased athletes AAS users; 68 deceased amphetamine and/or heroin users negative for AAS; 329 deceased AAS and amphetamine and/or heroin nonusers | Medico-legal examination | 44% percent of AAS users died by violent death, i.e., homicide (n=12, 23%) or suicide (n=11, 21%), compared to 7% of amphetamine and/or heroin users. No significant difference between AAS users and AAS and amphetamine and/or heroin nonusers was found, as the proportion of violent death in the latter group was 38% |
| Papazisis et al. (2007) [67] | Greece | Case report | One bodybuilder/martial artist AAS user | Semi-structured interview | The AAS user, with a prior history of psychotic depression, committed suicide after a brief hospitalization for a manic episode, during which he was diagnosed with an AAS-induced mood disorder with manic features |
| Lindqvist et al. (2014) [78] | Sweden | Retrospective, controlled | 181 male elite athletes in power sports suspected AAS users; population controls | 30-year follow-up | No significant increased mortality rate was found in the athletes compared to the general population, but in the age interval of 40-50 years. there was a slightly increased mortality (3.0 vs. 2.2). The main causes of death were cardiovascular disease (n=66, 36%), tumors (n=37, 20%), and suicide (n=21, 11%). A 1.74 times higher mortality rate from suicide (95% CI 1.08-2.66, p=0.025) was noticed in the observed dead athletes when compared to the general population |
| Darke et al. (2014) [79] | Australia | Case series | 24 deceased male bodybuilders, fitness trainers, and bodyguards AAS users | Medico-legal examination | 15 (62.5%) AAS users died from accidental drug toxicity, 4 (16.7%) from suicide by gunshot or hanging, and 3 (12.5%) form homicide |
| Anxiety (n=3) | |||||
| Pope & Katz (1988) [53] | US | Cross-sectional | 41 bodybuilders and football players current or past AAS users | DSM-III-R criteria | 9 (22%) AAS users displayed a full affective syndrome and 5 (12%) displayed psychotic symptoms |
| Ip et al. (2012) [86] | US | Cross-sectional | 112 male fitness amateurs, bodybuilders and weightlifters AAS-dependent users; 367 male fitness amateurs, bodybuilders and weightlifters AAS-nondependent users (controls) | DSM-IV-TR criteria | AAS-dependent users were more likely to report an anxiety disorder diagnosis of (16.1 vs. 8.4%, p=0.02) than AAS-nondependent users |
| Anxiety (n=3) | |||||
| Ip et al. (2014) [60] | US | Cross-sectional | 67 male AAS users and 76 male AAS nonusers recruited from fitness, weightlifting, bodybuilding, and steroid websites |
Semi-structured interview | 8 (12%) AAS users reported an anxiety disorder diagnosis in comparison with only 2 (2.6%) AAS nonusers, showing a significant difference (p=0.046) |
| Somatoform and eating disorders (n=8) | |||||
| Pope and Katz (1994) [54] | US | Cross-sectional | 88 male weightlifters AAS users; 68 male weightlifters AAS nonusers (controls) | DSM-III-R criteria | 16 (18.2%) AAS users reported a history of muscle dysmorphia vs. 0 (0%) of AAS nonusers |
| Blouin & Goldfield (1995) [105] | Canada | Cross-sectional | 139 male bodybuilders, runners, and martial artists | Eating Disorder Inventory; Beck Depression Inventory; Rosenberg Self-Esteem Scale; Anabolic Steroid Questionnaire; Drive for Bulk Scale; three Participation Questionnaires designed for bodybuilding, running, and martial arts |
Bodybuilders reported the greatest use of AASs and the most liberal attitudes towards AAS use. AAS users reported that the main reason for taking them was to improve looks |
| Schwerin et al. (1996) [106] | US | Cross-sectional | 35 male bodybuilders AAS users; 50 male bodybuilders AAS nonusers; 50 athletically active male exercisers AAS nonusers; 50 male nonexercisers AAS nonusers |
Body Dissatisfaction Index; Upper Body Strength Scale; Social Physique Anxiety Scale; Brief-Fear Of Negative Evaluation | AAS-users had significantly lower levels of social physique anxiety, significantly higher upper body strength ratings, and higher – but not reaching statistical significance – body dissatisfaction than AAS nonusers |
| Goldfield et al. (2006) [108] | Canada | Cross-sectional | 27 competitive male bodybuilders; 25 recreational male bodybuilders; 22 men with bulimia nervosa | Semi-structured interview | Competitive bodybuilders reported higher rates of AAS use compared to recreational bodybuilders |
| Kanayama et al. (2003) [107] | US | Cross-sectional | 48 male weightlifters current or past AAS users; 41 weightlifters AAS nonusers (controls) | Rosenberg Self-Esteem Scale; Eating Disorders Inventory; Male Role Attitudes Scale | AAS users showed few differences from AAS nonusers on most measures, but they showed greater symptoms of muscle dysmorphia. AAS experimenters were largely indistinguishable from nonusers, whereas heavy AAS users showed significant differences from nonusers on many measures, including marked symptoms of muscle dysmorphia and stronger endorsement of conventional male roles. An association between both body image concerns and narrow stereotypic views of masculinity and AAS use was found |
| Somatoform and eating disorders (n=8) | |||||
| Cafri et al. (2008) [110] | US | Cross-sectional | 15 male weightlifters with current muscle dysmorphia; 8 male weightlifters with past muscle dysmorphia; 28 male weightlifters with no history of muscle dysmorphia (controls) | Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I); Muscle Appearance Satisfaction Scale; Muscle Dysmorphic Disorder Inventory-Functional Impairment Subscale; Body Dysmorphic Disorder Diagnostic Module; Body Dysmorphic Disorder modification of the Yale-Brown Obsessive-Compulsive Scale; Muscle Dysmorphia Symptom Questionnaire | No significant differences in AAS use, abuse or dependence were found among the three groups |
| Goldfield (2009) [109] | Canada | Cross-sectional | 20 female competitive bodybuilders; 25 female recreational weighttrainers (controls) | Beck Depression Inventory; Eating Disorder Inventory; DSM-III-R Diagnostic Interview Schedule; Bodybuilding Questionnaire; Anabolic Steroid Questionnaire; Drive for Bulk Scale |
8 (40%) competitive bodybuilders reported AAS use compared to 0 (0%) recreational weighttrainers |
| Walker et al. (2009) [111] | US | Cross-sectional | 550 male college athletes | Male Body Checking Questionnaire; Muscle Dysmorphic Disorder Inventory; Beck Depression Inventory-II; Positive and Negative Affect Schedule; Eating Disorder Examination Questionnaire; Questionnaire on Appearance- and Performance-Enhancing Drugs (APEDs) | 471 (85.8%) college athletes reported to be APED (appearance- and performance-enhancing drugs) nonusers, 78 (14.2%) reported to be current or, for the majority, past APED users. Among APED users, 2 (2.9%) took AAS. Body checking was the best predictor of APED use after weight and shape concerns, muscle dysmorphia, depression, and positive and negative affect were included in the logistic regression analysis. Body checking uniquely accounted for the largest amount of variance in the Muscle Dysmorphic Disorder Inventory scores (16%) |
| Behavioral disorders (n=9) | |||||
| Conacher & Workman (1989) [112] | Canada | Case report | One bodybuilder AAS users |
Semi-structured interview; psychiatric and criminal history recollection | The AAS user murdered his wife after three months of taking AASs, which caused significant personality changes, including irritability, quarreling, and sleeplessness |
| Choy et al. (1990) [116] | UK | Prospective, controlled | 3 male strength athletes AAS users; 3 male strength athletes AAS nonusers (controls) | Several months follow-up; Profile of Mood States Questionnaire; Buss-Durkee Hostility Inventory; Rosenweig Picture Frustration Test | AAS users were tested on four occasions: two on-drug periods and two off-drug periods; nonusers were tested in equivalent periods. Self-rated aggression increased significantly in AAS users during their on-drug periods, especially when using multiple AASs |
| Behavioral disorders (n=9) | |||||
| Lefavi et al. (1990) [115] | France | Cross-sectional | 14 male bodybuilders current AAS users; 13 male bodybuilders past AAS users; 18 male bodybuilders AAS nonusers (controls) | Psychological Profile Questionnaire | AAS users had more frequent, more intense, and lengthier episodes of anger, with some of them reporting instances of violence and lack of control. No dose-response or duration-response relationships were found and no significant difference was detected in the ways that AAS users expressed their anger in comparison with nonusers |
| Brower et al. (1991) [117] | US | Cross-sectional | 49 male weightlifters current AAS users | Anonymous, self-administered questionnaire on AAS use; DSM-III-R criteria | 46 (94%) AAS users met at least one DSM-III-R criteria for AAS dependence, 28 (57%) met three or more criteria, consistent with a diagnosis of AAS dependence. AAS-dependent users (n=28, 38%) could be distinguished from nondependent ones (n=21, 28%) by their use of larger doses, more cycles of use, and more aggressive symptoms |
| Bahrke et al. (1992) [4] | US | Retrospective, controlled | 12 male weightlifters current AAS users; 14 male weightlifters past AAS users; 24 male weightlifters AAS nonusers (controls) | Semi-structured interview; physical and medical history questionnaire; Profile of Mood States Questionnaire; Buss-Durkee Hostility Inventory | Current and past AAS users reported the following changes associated with AAS use: increases in aggression and irritability; changes in insomnia, muscle size, muscle strength; faster recovery from workouts and injuries; changes in libido. Current and past users and nonusers showed no significant difference in the Buss-Durkee Hostility Inventory scale and subscale scores |
| Choy & Pope (1994) [119] | UK | Retrospective, controlled | 23 strength athletes AAS users; 14 strength athletes AAS nonusers (controls) | Semi-structured interview; Dyadic Adjustment Scale; Conflict Tactics Scales | AAS users reported significantly more fights, verbal aggression, and violence towards their wives and girlfriends when using AAS than when not using them and in comparison to AAS nonusers |
| Stanley & Ward (1994) [118] | UK | Case report | One bodybuilder AAS abuser | Semi-structured interview | The bodybuilder with AAS abuse manifested psychiatric symptoms and a violent outburst associated with AAS consumption |
| Bond et al. (1995) [120] | UK | Cross-sectional | 16 male strength athletes current AAS users; 16 male strength athletes past AAS users; 14 male strength athletes AAS nonusers (controls) | Aggression Rating Scale; modified Stroop Color Word Conflict Task |
Current AAS users rated themselves more negatively than past users (p<0.10) and took longer than past users and nonusers to name the colors of all word sets of the Stroop test, but there were no significant differences between word sets. Therefore, attentional bias did not differ between groups, but current AAS use produced subtle mood changes and slowed performance compared to past AAS users and nonusers |
| Perry et al. (2003) [57] | US | Cross-sectional | 10 weightlifters AAS users; 18 weightlifters AAS nonusers (controls) | DSM-IV criteria; Mania Rating Scale; Hamilton Depression Rating Scale; Buss-Durkee Hostility Inventory; Point Subtraction Aggression Paradigm; Personality Disorder Questionnaire | AAS users showed increased aggression compared to AAS nonusers, which was related to higher testosterone plasmatic levels (p<0001). However, AAS users were also characterized by higher scores of DSM-IV histrionic (p=0.035), borderline (p=0.016), and antisocial (p=0.000) personality disorders, which may have acted as confounding factors |
| Psychosis (n=3) | |||||
| Annitto et al. (1980) [125] | US | Case report | One male athlete AAS user | Semi-structured interview | The AAS user developed an acute schizophreniform illness during AAS use |
| Pope & Katz (1988) [53] | US | Cross-sectional | 41 bodybuilders and football players current or past AAS users | DSM-III-R criteria | 9 (22%) AAS users displayed a full affective syndrome and 5 (12%) displayed psychotic symptoms |
| Teuber et al. (2003) [126] | Germany | Case report | One male athlete AAS user |
Semi-structured interview | The AAS user, eight weeks after an intramuscular injection of nandrolone, developed anxiety and psychotic symptoms |
Risk of Bias
No evidence of language bias was found, as the search was not limited to English language studies. This reduced the possibility of missing relevant studies. Moreover, no evidence of significant publication bias was found. The Cochrane database search produced no unpublished study, initiating, ongoing, or finished.
AASs and Mood Disorders
Bidirectional relationships between AAS use and mood disorders have been described, with physiological AAS doses affecting minimally mood and even showing beneficial effects (at least with regard to testosterone) in dysthymia and refractory depression [22, 46, 51, 52] and supraphysiological doses being associated with depression, hypomania, or mania. Pope and Katz [53] interviewed bodybuilders and football players using AASs and found that 22% qualified for mood disorders. In a subsequent study, Pope and Katz [54] showed that 23% of athletes who abused AASs met DSM-III criteria for mood disorders, from major depressive disorder to type I and II bipolar disorders. Athletes met these criteria during AAS consumption significantly more than in the absence of it and significantly more than AAS nonusers (6%). The higher the AAS dose, the more severe the psychopathological symptoms. A controlled, retrospective study [55], comparing weightlifters with and without AAS use, found that AAS users lamented significantly more depressive symptoms when they were using AASs than when they were not, and also compared to nonusers. However, no difference in the frequency of major mood disorders was found between the current and past AAS users and the nonusers. The authors concluded that “the organic mood changes associated with AAS abuse usually present as a subsyndromal depressive disorder of insufficient severity to be classified as a psychiatric disorder”. Depressive symptomatology was prominent in a sample of power athletes who were former AAS users [56], implying that AAS use may have long-lasting effects on mood. Subsequently, the same group of investigators [57] compared mood alterations between male weightlifters with and without AAS use. AAS users scored higher on the Hamilton Rating Scale for Depression and on the Modified Mania Rating Scale than nonusers; however, no participant met formal criteria for any mood disorder. A retrospective study by Malone et al. [58] examined weightlifters and bodybuilders who used AASs and found hypomania in approximately 10% of the sample. Depression occurred in another 10% of the sample who discontinued AASs.
A study that recruited exclusively female athletes [59] found a 33% prevalence of current or past AAS use. AAS users reported poly-substance use more frequently than nonusers and showed hypomanic symptoms during AAS use (56%) and depressive symptoms during AAS withdrawal (40%), even if they did not meet full DSM-IV criteria for a hypomanic or a major depressive episode. Ip et al. [60] found that 15% of male AAS-dependent users reported a history of major depressive disorder, compared to only 7% of AAS-nondependent users. In addition, Pope and Katz [53] found AAS users to be at significant risk for major depressive episodes during the first months following AAS discontinuation. Similar results were obtained by Brower [61, 62], who observed that AAS withdrawal usually presented with depressive symptoms, such as apathy, anhedonia, concentration issues, sleep changes, and decreased libido, especially after intense abuse. However, another study [4] failed to find differences on the Profile of Mood States Questionnaire between male weightlifters with AAS use vs. without AAS use; both samples did not differ from the general population.
Brower et al. [63] presented a case report of a 24 year-old weightlifter with AAS dependence and revealed how the uncontrolled pattern of AAS use persisted despite adverse effects, such as severe mood disorders – more specifically, depression –, marital conflict, and deterioration of the individual’s moral values. Subsequently, the same group of investigators [64] found prominent depressive symptoms in eight AAS-using weightlifters who met criteria for DSM-III-R substance dependence. A cross-sectional study of 130 bodybuilders showed how 38% of them had used AASs for a median length of time of two years [65]. Half of the AAS users reported unspecified mood disturbances.
Tennant et al. [66] authored a case report regarding a 23-year-old bodybuilder who admitted to being “addicted” to AASs and not being able to stop taking them without experiencing severe withdrawal symptoms, including low mood, feelings of hopelessness, loss of energy, nausea, and dizziness. Thus, a diagnosis of AAS dependence was hypothesized and the resemblance with opioid dependence was underlined.
Another case report described the effects of long-term AAS use [67]. A 25-year-old man, who had been involved in bodybuilding, martial arts, and illegal boxing matches or other fights and had used AASs since the early adolescence, underwent an involuntary psychiatric hospital admission for a manic episode. He had a prior history of psychotic depression and was diagnosed with substance-related bipolar disorder, attributed to AAS use.
Animal models seem to support the association between AAS abuse and mood disorders. Matrisciano et al. [68] injected subcutaneously 5 mg/kg of body weight nandrolone or stanozolol for four weeks (i.e., a dose considered to the one abused by athletes) in male adult rats; they found that AASs decreased hippocampal and prefronto-cortical brain-derived neurotrophic factor levels and hippocampal low-affinity glucocorticoid receptor expression, while increasing morning trough plasma corticosterone. Intrestingly, major depressive disorder has been variously associated with all these alterations. Furthermore, both nandrolone and stanozolol increased immobility of rats tested on the widely used antidepressant drug screening test, i.e., the forced swimming test. Co-administration of clomipramine, a tricyclic antidepressant drug, prevented all AAS-produced effects. The fact that supraphysiological AAS doses per se may be associated with changes indicating depression in normal rats, prompts us to consider that AAS abuse in humans may produce similar states without needing stress- or other risk factor-exposure. The effects of stanozolol in rats were also tested by Tucci et al. [69], who injected subcutaneously 5 mg/kg/day stanozolol vs. vehicle for four weeks in young male rats. Dopamine increased in the hippocampus and decreased in the prefrontal cortex, serotonin decreased in the prefrontal cortex, nucleus accumbens, striatum, and hippocampus, and norepinephrine decreased in the nucleus accumbens 24 hours after the last injection. All these changes are compatible with the neurochemistry of depression, hence, subchronic use of stanozolol is associated with rat brain biogenic amine changes that in humans could possibly lead to depression. Recently, Zotti et al. [70] injected subchronically 5 mg/kg/day nandrolone for 4 weeks in male rats, showing a depressive, but not anxious profile in animals, accompanied by brain region-dependent dopaminergic, serotonergic, and noradrenergic changes.
AASs and Suicide
Substance abuse in general is a risk factor for suicide [71, 72]. Alink between AAS use and suicide in athletes was firstly suspected by Brower et al. [63], who reviewed case reports and newspaper articles and found that suicidal ideation and completed suicide were not infrequent among AAS users. They described a case of a 24 year-old weightlifter with AAS dependence and severe depression, who developed suicidal ideation after AAS withdrawal [73]. This user had no personal or family history of depression or suicidality.
In a case series of eight young men who committed suicide and were taking (N=5) or had discontinued AASs (N=2) (in one it was not possible to establish whether taking or discontinued), family members had noticed depressive symptoms associated with AAS discontinuation in five cases. After protracted AAS use, four had developed major depressive disorder. Two had manifested hypomanic symptoms before committing suicide. Only one had manifested suicidal ideation before starting AAS use [74]. The authors speculated that long-term AAS use may induce psychopathology, which may contribute to suicidality in predisposed individuals. The same group described a series of 34 violent deaths occurring in AAS users, which were due to suicide (N=11), homicide (N=9), accidents (N=12), or undetermined cause (N=2) [75]. In suicides, forensic history and significant others’ reports highlighted AAS-related impulsive behavior characterized by violent rage, mood swings, and propensity to depression.
Pärssinen et al. [76] studied prospectively for 12 years 62 male elite Finnish weightlifters with suspected AAS use (N=62), comparing them to the general population (N=1094). The athletes showed a 4.6 times higher mortality rate that was mainly due to suicide (5%) and myocardial infarction (5%). Two individuals belonging to the population control group committed suicide, compared to three belonging to the athlete group. Suicide committed in unspecified conditions was described in a case report of AAS-induced mood disorder with manic features occurring in a young bodybuilder/martial artist who was briefly hospitalized for a manic episode manifesting itself with psychomotor agitation, euphoria, irritability, and aggressiveness [67].
An autoptic study [77] compared toxicological findings and death modality in 52 deceased AAS users vs. 68 deceased heroin and/or amphetamine users who were negative for AASs. About 60% of AAS users were taking illicit substances. Compared to heroin/amphetamine related-deaths, AAS-related ones occurred earlier and more frequently by violence, suffered (23% AASs vs. 0% heroin/amphetamine; Fisher’s exact test, p<0.0001) or self-inflicted (21% AASs vs. 7% heroin/amphetamine, χ2, p<0.0001), suggesting that AAS users are highly suicidal and engage frequently in risky behaviors. AAS users’ suicides were caused by voluntary intoxication (N=4), explosion/shooting (N=4), hanging (N=1), car crashing (N=1), or jumping in front of a train (N=1). No cases of defenestration occurred. A recent 30-year follow-back study [78] compared the rates and causes of death in 181 male former élite athletes of power sports “like wrestling, weightlifting, and some track and field disciplines” who were suspected for AAS use, with those of age- and gender-matched healthy controls. Although overall mortality was not increased in the athletes’ sample, individuals in the 20-50 age range in the former athlete group died 45% more often than people in the general population, and those in the 30-50 age range died from suicide 30% more often than the general population. Suicide was committed by 11.6% of the former athletes.
Darke et al. [79] studied 24 cases of abrupt or unnatural death among male AAS users, who were all in their early 30s. Deaths were due to inadvertent drug lethality (62.5%), suicide by gunshot or hanging (16.7%), and homicide (12.5%). In all individuals, except for one, substances other than AASs, mainly psychostimulants, opioids (e.g., heroin, morphine), and benzodiazepines, were detected. This case series study underlines how deaths among AAS users usually concern young male poly-substance users, whose AAS- or poly-substance-related aggressive, disinhibited, and depressive behavior may increase the risk of premature death.
As for the pathophysiological basis of the relationship between AASs and suicidal behavior, conflicting data on plasma testosterone levels in suicide attempters have been published. Low testosterone levels after suicide attempts were detected by two studies [80, 81]; a study of male veterans with posttraumatic stress disorder showed that there was no association between testosterone levels and a history of suicide attempt [82]; a recent study reported no difference in testosterone levels between male suicide attempters and healthy controls [83]. Differently, another recent study of suicide attempters with bipolar disorder [84] found plasma testosterone to correlate with the number of manic episodes and suicide attempts, suggesting that testosterone could represent a pathophysiological link between mood disorders and suicidal behavior.
AASs and Anxiety Disorders
AAS use by adults, but even more by adolescents – at a time when affect-regulating brain areas are still developing and highly hormone/neuromodulator-sensitive – is associated with increased anxiety and altered responses to stress [85]. We already mentioned that 22% of 41 AAS-using bodybuilders and football players met DSM III-R criteria for at least one mood or anxiety disorder [53]. In a survey of 479 athletes recruited from Internet forums of several fitness, bodybuilding, weightlifting, and steroid websites, 16% of male AAS-dependent users had a history of DSM-IV-TR anxiety disorders (generalized anxiety disorder, panic disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or social phobia), compared to only 8% of AAS-nondependent users [86]. A further study by the same research group [60] examined the characteristics of 67 male AAS users and 76 male nonusers, all aged 40 and older, who were recruited through fitness, weightlifting, bodybuilding, and steroid websites. Anxiety disorders were present in more AAS users than nonusers (12.0% vs. 2.6%; p<0.05).
The most recent animal studies, using a variety of experimental paradigms, support that both male and female animals are sensitive to the anxiogenic effects of AASs [87-89], the latter more so [90]. These studies, performed on rodents, used subchronic administrations of either 5 mg/kg nandrolone decanoate or 7.5 mg/kg of a mixture containing testosterone cypionate, nandrolone decanoate, and methandrostenolone. However, studies administering 3.5-5 mg/kg testosterone in rodents found anxiolytic effects [91-93]. These studies used in addition to the elevated plus maze test, the open-field and the Vogel conflict paradigms [94]. Another study that found anxiolytic effects for AASs employed 17-β-hydroxy-1-methyl-5α-androstan-3-one in male rats [95].
Finally, studies using 17α-methyltestosterone in mice found no influence of AASs on anxiety [96-97]. Summarizing, the inconsistency of animal studies focusing on the effects of AASs on anxiety-like behavior reflect species, sex, design, paradigm, and drug heterogeneity.
AASs, Somatoform and Eating Disorders
It is not uncommon for athletes to be affected by somatoform and/or eating disorders. The underlying psychological basis may share several features. Physical appearance and eating patterns are interrelated; lately, there is evidence that young boys and men are becoming as concerned about these aspects as young girls and women. However, women point at thinness, while men strive to increase muscular mass and body size, in line with media-endorsed prototypes. The latter have encountered major changes in the last 50 years and more and more individuals are using AASs simply to look good. The pressure to look good may be one of the main triggers for the above mentioned disorders.
Several studies point to the relationship between body image and eating-related attitudes and disorders. A study compared the psychological characteristics of females with anorexia nervosa and males taking part in bodybuilding competitions, who had both adopted strict standards of body perfection and displayed unhealthy behaviors, such as rigorous food restriction, extreme exercise, and AAS use to pursue their goals [98]. Findings confirmed that anorexic women’s and bodybuilders’ psychological profile was extremely similar. Both groups were significantly more obsessive, perfectionistic, anhedonic, and narcissistic than the general population. Yet, bodybuilders reported positive perceptions of themselves, as opposed to anorexic women. These results may be interpreted in the context of a theoretical model of anorexia nervosa and bodybuilding, which focuses on the role of personality in the onset and maintenance of excessive behaviors.
Another study compared exercise, body shape, eating habits, and weight-related symptomatology in a sample of 15 male gym-users, 21 males with muscle dysmorphia, and 24 males with anorexia nervosa [99]. Muscle dysmorphia, also known as reverse anorexia, or bigorexia, or Adonis complex, is a subtype of body dysmorphic disorder generally affecting men, with its onset in adolescence or early adulthood, characterized by obsessiveness and compulsivity directed towards achieving a lean and muscular physique, even at the expense of health. The study used various questionnaires and a measure of appearance- and performance-enhancing drugs (APED) use. Similarities in the domains of altered body image, disordered eating, and exercise behavior between men with muscle dysmorphia and men with anorexia nervosa were found, however the two groups differed in their pursued goals, which were opposite. Furthermore, significant correlations between muscle dysmorphia and eating disorder measures were observed. These findings provide support for the hypothesis that muscle dysmorphia and anorexia nervosa may have a nosological similarity. A study of male and female university students, dichotomized them into high (HSPA) and low (LSPA) social physique anxiety groups on the basis of their median scores on the Social Physique Anxiety Scale; students also filled out the Eating Attitudes Test and the Physical Activity Assessment Questionnaire [100]. Young men had healthier eating attitudes and greater physical activity levels than women. HSPA participants showed unhealthier eating attitudes and greater physical activity levels than LSPA participants. A group × gender interaction was found for eating attitudes, but not for physical activity. HSPA women scored higher on the Eating Attitudes test than HSPA men and LSPA men and women. Swami et al. [101] compared body size ideals, dissatisfaction with body image, and media influence among 88 female athletes – in particular, 41 track-and-field athletes and 47 martial artists – and 44 female nonathletes. There were no significant between-group differences in ideal body size after controlling for body mass index (BMI). By contrast, track-and-field athletes reported the highest dissatisfaction with body image and internalization of athletic media messages. Participants’ BMI and internalization of athletic media messages predicted dissatisfaction with body image for each sport type and for all sports. These results suggest that women participating in leanness-promoting sports experience greater dissatisfaction with body image than those in other sports or nonathletes.
The media have a relevant role in inducing body dissatisfaction, weight control, and muscle-development. They promote a body stereotype that emphasizes strength and muscularity for men and thinness for women, leading to poorer satisfaction with physical attractiveness and body dimensions and, ultimately, to related disorders [102]. A study investigating the interactions between media use and eating disorders in young adults found that media exposure significantly influenced men’s, but not women’s endorsement of personal thinness and dieting [103]. A study exploring the role of media in triggering weight concerns among preadolescent/early adolescent children, found that boys and girls who strived to resemble same-sex media icons were more likely than their peers to develop preoccupation with weight and become constant dieters [104].
As for the features of the relationship between AAS use and somatoform and/or eating disorders, Pope and Katz [54] showed that 18.2% of 88 male weightlifters who abused AASs reported a history of muscle dysmorphia, compared to none of the 68 male weightlifters who did not use AASs (controls). Blouin and Goldfield [105] examined the relationship between body image disturbances, eating attitudes, and AAS use in 43 male bodybuilders vs. 48 runners and 48 martial artists of the same sex, all recruited from fitness centers. Bodybuilders showed significantly greater body dissatisfaction, with a high tendency to bulk and thinness, and increased inclinations towards bulimia than the other two groups. Additionally, they reported higher perfectionism and ineffectiveness, as well as lower self-esteem. They also consumed more AASs and had freer attitudes towards AAS use. The main reason for taking AASs, according to AAS users, was physical improvement: AAS users reported a stronger drive to put on muscle mass in the form of bulk, more maturity fears, and greater tendencies towards bulimia than AAS nonusers. Thus, male bodybuilders seem to be at risk for body image disturbances and the associated psychopathological characteristics that have been commonly observed in patients with eating disorders. These psycho-pathological characteristics also appear to predict AAS use in this group of men.
One study examined body dissatisfaction, social anxiety, social physique anxiety, and upper body esteem among 135 male athletes and 50 male nonathletes [106]. The athletes were represented by 35 bodybuilders with AAS use, 50 bodybuilders without AAS use, and 50 athletically active exercisers (involved in aerobics, jogging, basketball, or racquetball) without AAS use. Results indicated that the AAS-using bodybuilder group was characterized by lower levels of social physique anxiety, higher upper body strength perception, and a trend towards higher body dissatisfaction than the nonuser groups (AAS-nonusing bodybuilders, athletically active exercisers, and nonexercisers). No difference in terms of social anxiety was found among the four groups.
Kanayama et al. [107] assessed 89 weightlifters, 48 of whom AAS current and past users and 41 AAS nonusers, on measures of self-esteem, attitudes towards male roles, body image, eating attitudes and disorders, and muscle dysmorphia. Current and past AAS users showed no significant differences from nonusers on most measures, although they did show a marginally higher total score on the six subscales of the Eating Disorders Inventory, and, most importantly, they showed more muscle dysmorphia. Furthermore, a distinction was made among AAS users between short-term AAS “experimenters”, i.e., those who reported lifetime use of AASs for 2-5 months, and long-term AAS “heavy users”, i.e., those who reported lifetime AAS use for 6-150 months. The former group was almost indistinguishable from nonusers, but the latter showed significant differences from nonusers on many measures, including stronger endorsement of conventional male roles and marked symptoms of muscle dysmorphia. Thus, both pathology of body image and narrow views of masculinity seam to be frequent among men with long-term AAS use and could contribute to the onset of AAS use disorders. A study by Goldfield et al. [108] compared 27 competitive male bodybuilders, 25 recreational male bodybuilders, and 22 men with bulimia nervosa on a wide variety of eating attitudes and disorders, body image, weight and shape preoccupation, weight loss practices, and AAS use. Competitive bodybuilders reported more body dissatisfaction, bulimia nervosa, binge eating, and AAS use than recreational ones, but less eating-related and general psychopathology than bulimic men. It remains unknown whether men with a history of eating disorders are attracted to competitive bodybuilding or, vice versa, competitive bodybuilding triggers disordered eating and AAS use. Another study by Goldfield and collaborators [109] assessed body image, eating disorders, general psychopathological characteristics, and AAS use in 20 female competitive bodybuilders compared to 25 female recreational weighttrainers (controls), all recruited by posting advertisements in local gymnasia. Competitive bodybuilders had a higher incidence of binge eating, excessive concern with body weight or shape, strict dieting, and extreme exercise for weight control compared to recreational weighttrainers. The rate of AAS use was higher in the former group compared to the latter (40% vs. 0%).
Another study compared psychological status and AAS use in 51 male weightlifters, 15 of whom meeting current criteria for muscle dysmorphia, 8 reporting past muscle dysmorphia, and 28 with no history of muscle dysmorphia (controls), recruited through advertisements placed in gymnasia and nutrition stores [110]. Men with current muscle dysmorphia experienced more aversive symptoms regarding body image, including frequent thoughts about muscularity, appearance dissatisfaction, body checking, bodybuilding dependence, and functional impairment than controls. Men with past muscle dysmorphia were characterized by a higher prevalence of mood and anxiety disorders than controls. No significant difference in AAS use, abuse, or dependence was found among the three groups.
Walker et al. [111] examined the association between body checking, importance of shape and weight, symptoms of muscle dysmorphia, mood disorders, and use of APEDs, such as AASs, in 550 undergraduate males taking part in college sports. In men, body checking correlated with weight and shape concern, symptoms of muscle dysmorphia, depression, negative affect, and APED use. Overall, 85.8% of men were APED nonusers, 14.2% past or current APED user. Among the latter, 2.9% used AASs, 3.6% illegal ergo/thermogenics (i.e., fat burners), 3.8% nonsteroidal anabolics, and 10% over-the-counter ergo/thermogenics. Moreover, 69% used only one type of APED, 19% two types, 10% three types, and only 1% all four types. It is of interest that when interviewed, most men who had used APEDs reported past use, rather than current use. When asked how many years they planned to use AASs or licit and illicit ergo/thermogenics, even if occasionally, 98% reported that they did not plan to use them, the remaining 12% that they planned to use them for a mean of another 3.5 years. More than a quarter of those who had ever used APEDs (28.4%) reported that they would continue to use them even if it was to be proved beyond any doubt that they caused severe health problems. Approximately one-fourth (26.4%) reported that if they could rapidly meet all of their physical training goals through the use of APEDs, they would be willing to decrease their life duration by an average of 4.8 years. Of all the study variables, only body checking predicted APED use and accounted alone for the largest amount of variance in the Muscle Dysmorphic Disorder Inventory scores (16%). This result was confirmed by the significant difference observed on the Muscle Dysmorphic Disorder Inventory scores of APED users and nonusers (p<0.001). Overall, the results of all the described studies support the view that body checking is an important construct in male body image, muscle dysmorphia, and body change strategies and is the best predictor of APED use.
AASs and Behavioral Disorders
AAS use can lead to changes in behavior, such as increased aggression, hostility, and unprovoked rage attacks. It has been shown that AAS users have higher levels of alertness, lower tolerance to frustration or poor performance, and loss of impulse control. The typical sudden and exaggerated aggressive AAS-induced response to minimal provocations has been termed as “roid rage” [54, 112]. A significant incidence of violent crimes and physical partner abuse during AAS use has also been reported. To explain the role of AASs in the control of aggression in males, the “challenge hypothesis” has been formulated. It was initially suggested to account for testosterone-aggression associations in monogamous birds. Testosterone levels were postulated to increase and reach moderate levels at puberty, hence supporting reproductive physiology and behavior. During sexual arousal and challenges to fertile-age males, testosterone levels would rise further. Moreover, this would facilitate competitive behavior, such as aggression. When males are asked to care for offspring, testosterone levels will decrease. The challenge hypothesis was then extended to humans, requiring some modifications: in fact, testosterone levels in men are associated with different behavioral profiles, in relation to life history strategies involving emphasis on either mating or parenting [113]. However, since athletes who take AASs generally train hard, it is also possible that changes attributed to AAS use may partly reflect exercising. A triad may exist between behavioral changes, AAS use, and exercise. Weighttraining, bodybuilding, and other practices should be considered as potential confounding factors in studies designed to examine the behavioral effects of AASs [114].
A case report by Conacher and Workman [112] studied the association between AAS use and violent crime in a 32-year-old amateur bodybuilder who had been convicted of his wife’s murder. He had no psychiatric or criminal history. Three months before the crime, he had started taking AASs. About four weeks before the crime, he had become irritable, quarreling, and sleepless. Thus, AASs may be involved in personality changes and in the increase of violent behavior. Lefavi et al. [115] examined AAS-induced psychological alterations in 45 male bodybuilders, in particular, 13 with current AAS use, 14 with past AAS use, and 18 who never used AASs. Bodybuilders completed a psychological-profile questionnaire. AASs were associated with more frequent and intense episodes of anger, with some AAS users reporting episodes of lack of control and violence. No dose-response or duration-response relationships were found and no significant difference was detected in the ways that current or past AAS users expressed their anger in comparison with nonusers. In a prospective study, six male strength athletes, three of whom were AAS users and three were not, were monitored over several months as they underwent normal training and competitions [116]. They filled out the Profile of Mood States Questionnaire, the Buss-Durkee Hostility Inventory, and the Rosenweig Picture Frustration Test on four occasions: two on-drug periods and two off-drug periods for AAS users and equivalent test periods for AAS nonusers. AAS presence was assessed by gas chromatography and mass spectrometry. While those AASs openly reported by the athletes were confirmed in the on-drug samples and most of the off-drug samples, AAS traces were detectable in some supposed off-drug periods. This could explain why AAS users were always more aggressive compared to nonusers. At any rate, self-rated aggression increased significantly in AAS users during their acknowledged on-drug periods. In particular, multiple AAS use (a practice known as “stacking”) determined severe aggression and hostility; one AAS user admitted to be guilty of attempted murder during a previous on-drug period. Brower et al. [117] investigated addictive patterns of AAS use and related symptomatology in 49 AAS using male weightlifters through an anonymous, self-administered questionnaire. At least one DSM-III-R symptom of dependence was reported by 94% of the sample; three or more symptoms, consistent with a diagnosis of dependence, were reported by 57%. Dependent users could be distinguished from nondependent ones by higher doses, more cycles, more dissatisfaction with body size, and more aggressive symptoms. Doses and dissatisfaction with body size were the best predictors of dependence. Bahrke et al. [4] conducted a study of 50 male weightlifters to assess physiological and psychological features accompanying AAS use. Participants were 12 current AAS users, 14 past AAS users, and 24 nonusers. They underwent physical examination, blood chemistry assessment, and urinalysis, were interviewed with a semi-structured interview concerning their physical training and the effects of any substance use, and completed the Profile of Mood States questionnaire and the Buss-Durkee Hostility Inventory. Current and past AAS users reported, concurrently with AAS use, increased aggression and irritability, insomnia or oversleeping, changes in muscle size and muscle strength, faster recovery from workouts and injuries, and loss of interest in sexual activity. No significant differences in mood disturbances and in hostility scores were found among the three groups and in comparison with normative values in the general population.
AAS abuse by a bodybuilder has been reported to lead to psychiatric symptoms and violent outbursts [118]. Choy and Pope [119] investigated whether athletes’ increased aggression during AAS use could lead to wife battering. For this purpose, 23 strength athletes with AAS use and 14 without AAS use were assessed with the Dyadic Adjustment and Conflict Tactics Scales. Questions focused on relationship with partner, with AAS users invited to respond about their last AAS use cycle and their last AAS-free period and nonusers required to report on their relationship in the preceding three months. AAS users differed from nonusers for an increased number of fights, verbal aggression episodes, and violent episodes against their wives/girlfriends during their on-drug periods, but not during their off-drug periods. Furthermore, these episodes were significantly more frequent in AAS users during the on-drug, compared to the off-drug periods. These findings point to a serious risk of abuse of AAS users’ partners when the user is in an on-AAS period. These results were confirmed by a subsequent study of the same group of investigators. Bond et al. [120] carried out a study on 46 male strength athletes to measure the effects of AASs on attentional bias to aggressive cues. They were 16 current AAS users, 16 former AAS users, and 14 nonusers. Testosterone, nandrolone, and oxymetholone were the most commonly consumed AASs during the last cycle and an average of 2-3 AASs were used during each cycle (“stacking”). Participants completed an Aggression Rating Scale to assess current feelings of anger and hostility and were presented with a modified Stroop Color Word Conflict Task containing sets of neutral (e.g., shelves, broom), verbally aggressive (e.g., hostile, ridicule), and physically aggressive (e.g., attack, violence) words. Current AAS users rated themselves more impatient (p< 0.10) and belligerent (p< 0.10) than past users and took longer than past users and nonusers to name the colors of all word sets, but there were no significant differences between word sets. Therefore, there were no attentional bias differences among and betwixt the three groups, but current AAS use produced subtle mood changes and slowed performance compared to past AAS users and AAS nonusers. These findings draw attention to the fact that AAS users’ partners may be at risk of serious abuse during the on-drug periods. A study by Perry et al. [57] attempted to clarify the supposed relationship between supraphysiological doses of AASs and increased aggression, which is believed to be associated with high plasma testosterone levels. Ten weightlifters with AAS use and 18 without were interviewed using the Modified Mania Rating Scale, the Hamilton Depression Rating Scale, the Buss-Durkee Hostility Inventory, the Point Subtraction Aggression Paradigm, and the Personality Disorder Questionnaire. Higher aggression levels were found in AAS users than in nonusers with both self-rated and clinician-rated measures; aggression levels were associated with higher plasma testosterone concentrations. However, the higher prevalence of DSM-IV cluster B Personality Disorders, such as antisocial, borderline, and histrionic ones, in AAS users than in nonusers, as assessed with the Personality Disorder Questionnaire, could have mediated these differences.
Animal models seem to support the relationship between AASs and behavioral disorders. Steensland et al. [121] examined the effect of the chronic administration of nandrolone on dominant and subordinate male rats in a pair-housed condition and found that during allowed social interactions, dominant nandrolone-pretreated rats had highly aggressive behaviors more often than dominant placebo-treated rats. Furthermore, the probability for highly aggressive behaviors was constantly present for the nandrolone-treated rats throughout the study, whereas it decreased in the placebo-treated group. Nandrolone-treated rats showed less fear in case of potentially threatening events, compared to placebo group. These findings point out to the relatively long-term behavioral alterations due to AAS abuse that have been noted in human beings. A study of male rats tested the hypothesis that behavioral alterations associated with AAS use during the adolescence may be carried over to adulthood even after discontinuing AASs [122]. Prepubertal rats were treated with five weekly intramuscular injections of 5 mg/kg testosterone propionate vs. vehicle for five weeks. The AAS group presented greater aggressiveness in the pubertal phase and higher levels of horizontal and vertical exploration and anxiety-like behavior in the adult phase than the controls, as shown by aggression, elevated plus maze, and open-field tests. A study with pubertal Syrian hamsters [123] found aggression and anxiety during early AAS use to predict behavioral responding during withdrawal. The hamsters received daily subcutaneous injections of a “stack” of testosterone cypionate (2 mg/kg), nortestosterone (2 mg/kg), and dihydrotestosterone undecyclate (1 mg/kg), which are three of the most commonly abused AASs, vs. vehicle for one month. Three weeks after the last AAS/vehicle injection, experimental and control animals were tested for anxiety on the elevated plus maze, the dark/light, and the seed finding tests and then examined for differences in serotonin afferent innervation to selected anxiety-related brain areas. To confirm the absence of an aggressive phenotype, on the twentieth day of AAS withdrawal animals were tested for offensive aggression using the resident-intruder test. The study showed that AAS intake elicits aggression in youngsters and AAS discontinuation elicits anxiety.
AASs and Psychosis
In the seventies, the term “steroid psychosis” described a spectrum of psychoses with no precise presentation; symptoms ranged from affective to schizophrenia-like, to organic brain syndrome-related. The most commonly reported were emotional lability, agitation, trouble focusing, increase in the speed of conversation, sensory flooding, sleep changes, auditory and visual hallucinations, intermittent memory impairment, mutism, and delusions. Premorbid personality, history of previous psychopathological disorders, and history of previous steroid psychosis did not clearly increase an individual's risk of developing a psychotic reaction during any given course of AASs [124]. One of the first papers highlighting the relationship between AASs and psychosis is a case report of an athlete consuming AASs [125]. The authors temporally related the use of these substances to the development of an acute schizophreniform illness. Recommendation was made to consider this “side-effect” in the differential diagnosis of a schizophrenic episode. A study by Pope Katz [53] of 41 body-builders and football players taking AASs estimated the prevalence of psychotic symptoms associated to AAS use to be about 12%. Lastly, a 30-year old male athlete who had taken AASs in the last 1.5 years, eight weeks after having received an intramuscular injection of nandrolone developed anxiety, paranoid ideation and other psychotic symptoms leading to a full-blown psychotic syndrome, for which he had to be admitted to an inpatient psychiatric service [126]. There is dearth of evidence suggesting schizophrenia-like reactions to acute or chronic AAS use, thus the conclusion must be that these reactions may develop on a personal vulnerability basis. An animal study offers some support to this view, as nandrolone pretreatment was found to potentiate amphetamine-mediated aggression in rats [121]. However, it should be mentioned that there have been early attempts to treat schizophrenia with AASs; in particular, it has been claimed that the anabolic steroid Δ1,17-α-methyl-testosterone ameliorated its symptoms [127], but another study found no effect [128].
DISCUSSION
Throughout their lives, athletes generally consume AASs only for few blocks of time, commonly known as “cycles”, that last 8-16 weeks and are separated by substance-free intervals of months or years [35]. However, some individuals go on to abuse AASs to improve their athletic performance and muscular appearance, and in 30% of cases they develop dependence [129]. AAS use becomes abuse when an uncontrolled urge to take AASs arises, even if it proves to be detrimental for health; it becomes dependence when withdrawal symptoms appear upon abrupt discontinuation. Dependence is usually accompanied by tolerance, which fosters the need for dose increase to obtain the same effect. This ensues in habitual, chronic use that renders AASs even more harmful. In AAS dependence, athletes begin to take doses from 10 to 100 times higher than those used in legitimate medical practice (hence the term “supraphysiological”) and often employ a pyramid administration schedule (a practice known as “pyramiding”), progressively increasing doses in a stepwise manner through the first half of a cycle before reducing them symmetrically in the second half, disregarding all safety issues [117]; they take a combination of two or more different AASs (a practice known as “stacking”), sometimes through different routes of administration, although the combination has not been demonstrated to possess any added desirable effect [130]; they consume very frequent or lengthy cycles, often despite adverse effects [117, 131-132]; they use AASs almost continuously for years, reaching an excessive cumulative duration of use [130].
The psychopathological aspects of AAS dependence are still under investigation. One issue in studying AAS dependence has been the illicit nature of these substances, thus the existing studies, mainly observational, have often found difficulties in verifying their exact nature or amounts taken. Furthermore, many AAS users simultaneously consume other APEDs and dietary supplements that may have psychopathological effects which become indistinguishable from those due to AASs in the context of a complex psychiatric presentation. Moreover, there are no universally accepted appropriate criteria to apply to a psychiatric clinical picture with AAS-related mental symptoms. A “sex steroid hormone dependence disorder” was suggested by Yale psychiatrists [133], but has still to gain consensus. Over the years, the existing literature has applied DSM-III, DSM-III-R, DSM-IV, and DSM-IV-TR criteria to assess dependence among populations of AAS users. A problem that has arisen is that the standard DSM-IV-TR substance-dependence criteria are difficult to apply to AASs, since they were mainly projected for intoxicating drugs. Even if AAS suspension causes the classic withdrawal syndrome, mediated by neuroendocrine and cortical neurotransmitter systems, AASs are cumulatively acting drugs that produce little or no acute intoxication, hence they do not deliver an immediate reward upon consumption and do not usually impair daily functions like other intoxicating drugs, such as narcotics, stimulants, and hallucinogens. AAS dependence may involve the opioidergic system. In an attempt to address this problem, a group of researchers [129] suggested a set of diagnostic criteria for AAS dependence, based on the standard substance dependence criteria of DSM-IV-TR, slightly modified and adapted to apply specifically to AASs. The DSM-5 has accepted these suggestions and has recommended, for AAS dependence, the use of the code "other substance use disorder", where the specific substance has to be indicated. This code indicates a mental disorder in which the repeated use of an “other substance” typically continues, despite the individual's knowing that the substance is causing serious problems. The substance cannot be classified within the alcohol, caffeine, cannabis, hallucinogen (phencyclidine and others), inhalant, opioid, sedative, hypnotic, anxiolytic, stimulant, or tobacco categories. DSM-5 diagnostic criteria are shown in Table 2.
Table 2.
DSM-5 diagnostic criteria for AAS dependence [49]. At least two of the following criteria must be met over a 12-month period..
| Criteria |
|---|
| The substance is often taken in larger amounts or over a longer period than was intended |
| There is a persistent desire or unsuccessful efforts to cut down or control use of the substance |
| A great deal of time is spent in activities necessary to obtain the substance, use the substance, or recover from its effects |
| Craving, or a strong desire or urge to use the substance |
| Recurrent use of the substance resulting in a failure to fulfill major role obligations at work, school, or home |
| Continued use of the substance despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of its use |
| Important social, occupational, or recreational activities are given up or reduced because of use of the substance |
| Recurrent use of the substance in situations in which it is physically hazardous |
| Use of the substance is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance |
| Tolerance, as defined by either of the following: a) a need for markedly increased amounts of the substance to achieve intoxication or the desired effect b) a markedly diminished effect with continued use of the same amount of the substance |
| Withdrawal, as manifested by either of the following: a) the characteristic withdrawal syndrome for other (or unknown) substance b) the substance (or a closely related substance) is taken to relieve or avoid withdrawal symptoms |
Evidence of a relationship between AAS use and psychopathology in athletes has been extensively reported in the literature. The first to observe AAS use to enhance athletic performance and its link with psychology has been John Ziegler, the physician for the US men’s weightlifting team, back in the 1950s. He is reported to have stated “What I failed to realize until it was too late was that most of the weightlifters had such obsessive personalities. To them, if two tablets were good, four would be better” [134]. However, this topic has gained public attention and media exposure in recent years, owing to news of AAS abuse by high-profile athletes in professional associations and Olympic sports [13, 15]. The nature of the relationship between AAS use and psychopathology is complex. Individuals who are more vulnerable to the stress associated with improving and maintaining physical functioning, coping with social pressures, and striving for goals, or those with specific “pre-AAS” personality traits, such as antisocial personality disorder and narcissism [35, 135], or “pre-AAS” psychopathological disorders, such as somatoform (e.g., body dysmorphic disorder) and eating disorders (e.g., anorexia or bulimia nervosa), are more prone to develop an AAS dependence. A possible explanation is given by motivation to exercise. Although physical activity presents in different forms, most research designed to increase motivation for it, and adherence to it, pay attention to exercise behavior and not to sports participation. When motivation for physical activity is more extrinsic and focused on appearance and weight control (the so-called “self-objectification”), [136], rather than intrinsic and focused on enjoyment and health improvement, it may facilitate a type of training based on high levels of performance. This may trigger high stress levels and psychopathological symptoms and may herald the onset of AAS use [137-139]. On the other hand, individuals with AAS dependence have been shown to be frequently affected by mood, anxiety, or psychotic disorders, and to present behavioral disturbances some time after AAS use. This may simply reflect the established comorbidity between drug addiction and psychopathological disorders or, alternatively, AASs might cause psychopathological symptoms by determining neuroadaptive changes in the reward neural circuit or by affecting stress vulnerability and neurotrophism that lie in the core of psychopathological disorders [140].
Thus, the bidirectional comorbidity between AAS use and psychopathology is not so much the expression of a linear relationship, but rather of a circular process with a possible neuroendocrine basis (i.e., central nervous system circuitry and the integrated hypothalamic-pituitary-adrenal axis) [141], making it difficult to establish a clear cause-effect relationship. AASs act on the central nervous system in several ways. They can influence neuronal function both directly through the modulation of intracellular receptors and indirectly through influencing the function of ligand-gated ion channels and neurotransmitter receptors [142-145]. Furthermore, they can be converted into estrogen derivatives and activate second messenger systems, they may release endogenous opiate peptides, and they can modulate the expression of γ-aminobutyric acid (GABA) inhibitory receptors [146] and 5-HT1B and 5-HT2 serotonin receptors [147]. These effects involve brain areas associated with depression, stress, anger, and sexual behavior.
The psychopathological complications associated with AAS use, especially manic and mixed symptoms – usually treated with antipsychotic drugs or lithium [148, 149], for which a careful therapeutic drug monitoring is recommended [150] – are a major concern for physicians [151]. As depression and suicide are other possible complications, a medically supervised detoxification may be useful. Addicted AAS users may possibly benefit from a dose reduction through a tapering course of medically prescribed steroids, as abrupt AAS discontinuation may precipitate severe depression and suicide [66, 73]. However, the detoxification of AAS abusers is a poorly studied area, and the prescription of an abused substance to a substance user can be problematic, unless close supervision is provided. Caution in using clonidine for AAS detoxification is recommended, as clonidine itself has been associated with depression. Antidepressants, which have shown promising results during cocaine withdrawal, deserve to receive trials in the treatment of AAS withdrawal.
CONCLUSIONS
AAS use in athletes is associated with mood and anxiety disturbances, as well as reckless behavior, in some predisposed individuals, who are likely to develop various types of psychopathology after long-term exposure to these substances. There is a lack of studies investigating whether the preexistence of psychopathology is likely to induce AAS consumption, but the bulk of available data, combined with animal data, point to the development of specific psycho-pathology, increased aggressiveness, mood destabilization, eating behavior abnormalities, and psychosis after AAS abuse/dependence. The use of AASs in athletes deserves further investigation.
FINANCIAL COMPETING INTERESTS DISCLOSURE
In the past two years, Paolo Girardi has received research support from Lilly, Janssen, and Springer Healthcare, and has participated in Advisory Boards for Lilly, Otsuka, Pfizer, Schering, and Springer Healthcare and received honoraria from Lilly and Springer Healthcare. All other authors of this paper have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
ROLE OF THE FUNDING SOURCE
This study is part of the FIRB project code RBFR12LD0W_002 and has been funded by a grant of the Italian Ministry of Research.
ACKNOWLEDGMENTS
The authors wish to thank Ms Mimma Ariano, Ms Ales Casciaro, Ms Teresa Prioreschi, and Ms Susanna Rospo, Librarians of the Sant’Andrea Hospital, School of Medicine and Psychology, Sapienza University, Rome, for rendering precious bibliographical material accessible, dr. Flavia Napoletano for helping in collecting relevant references, as well as their Secretary Lucilla Martinelli for her assistance during the writing of the manuscript.
REFERENCES
- 1.Lombardo J.A. Anabolic-androgenic steroids. NIDA Res. Monogr. 1990;102:60–73. [PubMed] [Google Scholar]
- 2.Giorgi A., Weatherby R.P., Murphy P.W. Muscular strength, body composition and health responses to the use of testosterone enanthate: a double blind study. J. Sci. Med. Sport. 1999;2(4):341–355. doi: 10.1016/S1440-2440(99)80007-3. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder E.T., Vallejo A.F., Zheng L., Stewart Y., Flores C., Nakao S., Martinez C., Sattler F.R. Six-week improvements in muscle mass and strength during androgen therapy in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60(12):1586–1592. doi: 10.1093/gerona/60.12.1586. [DOI] [PubMed] [Google Scholar]
- 4.Bahrke M.S., Wright J.E., Strauss R.H., Catlin D.H. Psychological moods and subjectively perceived behavioral and somatic changes accompanying anabolic-androgenic steroid use. Am. J. Sports Med. 1992;20(6):717–724. doi: 10.1177/036354659202000613. [DOI] [PubMed] [Google Scholar]
- 5.Imanipour V., Sadeghi M., Mahdi F. Effects of anabolic-androgenic steroids drugs on increase of muscle injuries in bodybuilders who use these drugs. J. Sci. Med. Sport. 2004;7:7–8. doi: 10.1016/S1440-2440(04)80070-7. [DOI] [Google Scholar]
- 6.Mooradian A.D., Morley J.E., Korenman S.G. Biological actions of androgens. Endocr. Rev. 1987;8(1):1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J., Bauman W.A., Huang R., Caplan A.J., Cardozo C. Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manne. Steroids. 2004;69:357–366. doi: 10.1016/j.steroids.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand J.A., Sherins R.J., Alling D.W., Frank M.M. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N. Engl. J. Med. 1976;295(26):1444–1448. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- 9.Ammus S.S. The role of androgens in the treatment of hematologic disorders. Adv. Intern. Med. 1989;34:191–208. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- 10.Joss E.E., Mullis P.E. Delayed puberty in boys. In: Bardin C.W., editor. Current therapy in endocrinology and metabolism. 5th ed. St. Louis: Mosby-Year Book; 1994. pp. 299–300. [PubMed] [Google Scholar]
- 11.Demling R.H., Orgill D.P. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J. Crit. Care. 2000;15(1):12–17. doi: 10.1053/jcrc.2000.0150012. [DOI] [PubMed] [Google Scholar]
- 12.Mottram D.R., George A.J. Anabolic steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2000;14(1):55–69. doi: 10.1053/beem.2000.0053. [DOI] [PubMed] [Google Scholar]
- 13.Knopp W.D., Wang T.W., Bach B.R., Jr Ergogenic drugs in sports. Clin. Sports Med. 1997;16(3):375–392. doi: 10.1016/S0278-5919(05)70031-4. [DOI] [PubMed] [Google Scholar]
- 14.Stannard J.P., Bucknell A.L. Rupture of the triceps tendon associated with steroid injections. Am. J. Sports Med. 1993;21(3):482–485. doi: 10.1177/036354659302100327. [DOI] [PubMed] [Google Scholar]
- 15.Yesalis C.E., Bahrke M.S. Anabolic-androgenic steroids. Current issues. Sports Med. 1995;19(5):326–340. doi: 10.2165/00007256-199519050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Mewis C., Spyridopoulos I., Kuhlkamp V., Seipel L. Manifestation of severe coronary heart disease after anabolic drug abuse. Clin. Cardiol. 1996;19:153–155. doi: 10.1002/clc.4960190216. [DOI] [PubMed] [Google Scholar]
- 17.Winkler U.H. Effects of androgens on haemostasis. Maturitas. 1996;24(3):147–155. doi: 10.1016/S0378-5122(96)82004-4. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan A.J., Kennedy M.C., Casey J.H., Day R.O., Corrigan B., Wodak A.D. Anabolic-androgenic steroids: medical assessment of present, past and potential users. Med. J. Aust. 2000;173(6):323–327. doi: 10.5694/j.1326-5377.2000.tb125667.x. [DOI] [PubMed] [Google Scholar]
- 19.Stimac D., Milić S., Dintinjana R.D., Kovac D., Ristić S. Androgenic/Anabolic steroid-induced toxic hepatitis. J. Clin. Gastroenterol. 2002;35(4):350–352. doi: 10.1097/00004836-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Hartgens F., Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [Review]. [DOI] [PubMed] [Google Scholar]
- 21.Socas L., Zumbado M., Pérez-Luzardo O., Ramos A., Pérez C., Hernández J.R., Boada L.D. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br. J. Sports Med. 2005;39(5):e27. doi: 10.1136/bjsm.2004.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talih F., Fattal O., Malone D., Jr Anabolic steroid abuse: psychiatric and physical costs. Cleve. Clin. J. Med. 2007;74(5):341–344, 346, 349-352. doi: 10.3949/ccjm.74.5.341. [DOI] [PubMed] [Google Scholar]
- 23.Dohle G.R., Smit M., Weber R.F. Androgens and male fertility. World J. Urol. 2003;21(5):341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- 24.Kicman A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) 33rd report on the health status of the US. 2012. 2012.
- 26.Pope H.G., Jr, Kanayama G., Athey A., Ryan E., Hudson J.I., Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am. J. Addict. 2014;23(4):371–377. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker J.S., Graham M.R., Davies B. Steroid and prescription medicine abuse in the health and fitness community: A regional study. Eur. J. Intern. Med. 2006;17(7):479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson S., Baigi A., Marklund B., Fridlund B. The prevalence of the use of androgenic anabolic steroids by adolescents in a county of Sweden. Eur. J. Public Health. 2001;11(2):195–197. doi: 10.1093/eurpub/11.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Rachoń D., Pokrywka L., Suchecka-Rachoń K. Prevalence and risk factors of anabolic-androgenic steroids (AAS) abuse among adolescents and young adults in Poland. Soz. Praventivmed. 2006;51(6):392–398. doi: 10.1007/s00038-006-6018-1. [DOI] [PubMed] [Google Scholar]
- 30.Melia P., Pipe A., Greenberg L. The use of anabolic-androgenic steroids by Canadian students. Clin. J. Sport Med. 1996;6(1):9–14. doi: 10.1097/00042752-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Handelsman D.J., Gupta L. Prevalence and risk factors for anabolic-androgenic steroid abuse in Australian high school students. Int. J. Androl. 1997;20(3):159–164. doi: 10.1046/j.1365-2605.1997.d01-285.x. [DOI] [PubMed] [Google Scholar]
- 32.Galduróz J.C., Noto A.R., Nappo S.A., Carlini E.A. Household survey on drug abuse in Brazil: study involving the 107 major cities of the country--2001. Addict. Behav. 2005;30(3):545–556. doi: 10.1016/j.addbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kutscher E.C., Lund B.C., Perry P.J. Anabolic steroids: a review for the clinician. Sports Med. 2002;32(5):285–296. doi: 10.2165/00007256-200232050-00001. [DOI] [PubMed] [Google Scholar]
- 34.Rogol A.D., Yesalis C.E., III, Yesalis C.E. Anabolic-androgenic steroids and the adolescent. Pediatr. Ann. 1992;21(3):175–, 183, 186-188. doi: 10.3928/0090-4481-19920301-09. [DOI] [PubMed] [Google Scholar]
- 35.Kanayama G., Cohane G.H., Weiss R.D., Pope H.G. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J. Clin. Psychiatry. 2003;64(2):156–160. doi: 10.4088/JCP.v64n0208. [DOI] [PubMed] [Google Scholar]
- 36.Dunn M. Are anabolic-androgenic steroid users polysubstance users? J. Sci. Med. Sport. 2009;12:1–2. doi: 10.1016/j.jsams.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Skarberg K., Nyberg F., Engstrom I. Multisubstance use as a feature of addiction to anabolic-androgenic steroids. Eur. Addict. Res. 2009;15(2):99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- 38.Pallesen S., Jøsendal O., Johnsen B.H., Larsen S., Molde H. Anabolic steroid use in high school students. Subst. Use Misuse. 2006;41(13):1705–1717. doi: 10.1080/10826080601006367. [DOI] [PubMed] [Google Scholar]
- 39.McCabe S.E., Brower K.J., West B.T., Nelson T.F., Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90(2-3):243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanjek B., Rosendahl J., Strauss B., Gabriel H.H. Doping, drugs and drug abuse among adolescents in the State of Thuringia (Germany): prevalence, knowledge and attitudes. Int. J. Sports Med. 2007;28(4):346–353. doi: 10.1055/s-2006-924353. [DOI] [PubMed] [Google Scholar]
- 41.Kokkevi A., Fotiou A., Chileva A., Nociar A., Miller P. Daily exercise and anabolic steroids use in adolescents: a cross-national European study. Subst. Use Misuse. 2008;43:2053–2065. doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- 42.Welder A.A., Melchert R.B. Cardiotoxic effects of cocaine and anabolic-androgenic steroids in the athlete. J. Pharmacol. Toxicol. Methods. 1993;29(2):61–68. doi: 10.1016/1056-8719(93)90052-G. [DOI] [PubMed] [Google Scholar]
- 43.World Anti-Doping Agency (WADA) The 2014 Prohibited List. International Standard. Lausanne (CH): World Anti-Doping Agency2014 at http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/2014/WADA-prohibited-list-2014-EN.pdf . 2014. [September 11, 2013. Accessed on July 25].
- 44.Rubinow D.R., Schmidt P.J. Androgens, brain, and behavior. Am. J. Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt P.J., Rubinow D.R. Neuroregulatory role of gonadal steroids in humans. Psychopharmacol. Bull. 1997;33(2):219–220. [PubMed] [Google Scholar]
- 46.Zarrouf F.A., Artz S., Griffith J., Sirbu C., Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- 47.Ebinger M., Sievers C., Ivan D., Schneider H.J., Stalla G.K. Is there a neuroendocrinological rationale for testosterone as a therapeutic option in depression? J. Psychopharmacol. 2009;23(7):841–853. doi: 10.1177/0269881108092337. [DOI] [PubMed] [Google Scholar]
- 48.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Diagnostic and Statistical Manual of Mental Disorders. Arlington; VA; USA: APA: Fifth Edition (DSM-5TM); 2013. American Psychiatric Association. [Google Scholar]
- 50.World Health Organization. International Classification of Diseases. 10th Revision (ICD-10); World Health Organization/Organisation Mondial de la Santé.1992. [Google Scholar]
- 51.Basaria S., Wahlstrom J.T., Dobs A.S. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001;86(11):5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 52.Amiaz R., Seidman S.N. Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15(3):278–283. doi: 10.1097/MED.0b013e3282fc27eb. [DOI] [PubMed] [Google Scholar]
- 53.Pope H.G., Jr, Katz D.L. Affective and psychotic symptoms associated with anabolic steroid use. Am. J. Psychiatry. 1988;145(4):487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- 54.Pope H.G., Jr, Katz D.L. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch. Gen. Psychiatry. 1994;51(5):375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 55.Perry P.J., Yates W.R., Andersen K.H. Psychiatric symptoms associated with anabolic steroids: a controlled; retrospective study. Ann. Clin. Psychiatry. 1990;2:11–17. doi: 10.3109/10401239009150000. [DOI] [Google Scholar]
- 56.Lindqvist A.S., Moberg T., Eriksson B.O., Ehrnborg C., Rosén T., Fahlke C. A retrospective 30-year follow-up study of former Swedish-elite male athletes in power sports with a past anabolic androgenic steroids use: a focus on mental health. Br. J. Sports Med. 2013;47(15):965–969. doi: 10.1136/bjsports-2012-091340. [DOI] [PubMed] [Google Scholar]
- 57.Perry P.J., Kutscher E.C., Lund B.C., Yates W.R., Holman T.L., Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J. Forensic Sci. 2003;48(3):646–651. [PubMed] [Google Scholar]
- 58.Malone D.A., Jr, Dimeff R.J., Lombardo J.A., Sample R.H. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin. J. Sport Med. 1995;5(1):25–31. doi: 10.1097/00042752-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Gruber A.J., Pope H.G., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother. Psychosom. 2000;69(1):19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 60.Ip E.J., Trinh K., Tenerowicz M.J., Pal J., Lindfelt T.A., Perry P.J. Characteristics and behaviors of older male anabolic steroid users. J. Pharm. Pract. 2014 doi: 10.1177/0897190014527319. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Brower K.J. Anabolic steroids: addictive; psychiatric; and medical consequences. Am. J. Addict. 1992;1:100–114. [Google Scholar]
- 62.Brower K.J. Withdrawal from anabolic steroids. Curr. Ther. Endocrinol. Metab. 1994;5:291–296. [PubMed] [Google Scholar]
- 63.Brower K.J., Blow F.C., Eliopulos G.A., Beresford T.P. Anabolic androgenic steroids and suicide. Am. J. Psychiatry. 1989;146(8):1075. doi: 10.1176/ajp.146.8.1075a. [DOI] [PubMed] [Google Scholar]
- 64.Brower K.J., Eliopulos G.A., Blow F.C., Catlin D.H., Beresford T.P. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am. J. Psychiatry. 1990;147(4):510–512. doi: 10.1176/ajp.147.4.510. [DOI] [PubMed] [Google Scholar]
- 65.Lindström M., Nilsson A.L., Katzman P.L., Janzon L., Dymling J.F. Use of anabolic-androgenic steroids among body builders--frequency and attitudes. J. Intern. Med. 1990;227(6):407–411. doi: 10.1111/j.1365-2796.1990.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 66.Tennant F., Black D.L., Voy R.O. Anabolic steroid dependence with opioid-type features. N. Engl. J. Med. 1988;319(9):578. doi: 10.1056/NEJM198809013190910. [DOI] [PubMed] [Google Scholar]
- 67.Papazisis G., Kouvelas D., Mastrogianni A., Karastergiou A. Anabolic androgenic steroid abuse and mood disorder: a case report. Int. J. Neuropsychopharmacol. 2007;10(2):291–293. doi: 10.1017/S1461145706007243. [DOI] [PubMed] [Google Scholar]
- 68.Matrisciano F., Modafferi A.M., Togna G.I., Barone Y., Pinna G., Nicoletti F., Scaccianoce S. 2010. [DOI] [PubMed]
- 69.Tucci P., Morgese M.G., Colaianna M., Zotti M., Schiavone S., Cuomo V., Trabace L. Neurochemical consequence of steroid abuse: stanozolol-induced monoaminergic changes. Steroids. 2012;77(3):269–275. doi: 10.1016/j.steroids.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Zotti M., Tucci P., Colaianna M., Morgese M.G., Mhillaj E., Schiavone S., Scaccianoce S., Cuomo V., Trabace L. Chronic nandrolone administration induces dysfunction of the reward pathway in rats. Steroids. 2014;79:7–13. doi: 10.1016/j.steroids.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Murphy G.E. Suicide and substance abuse. Arch. Gen. Psychiatry. 1988;45(6):593–594. doi: 10.1001/archpsyc.1988.01800300091013. [DOI] [PubMed] [Google Scholar]
- 72.Sani G., Tondo L., Koukopoulos A., Reginaldi D., Kotzalidis G.D., Koukopoulos A.E., Manfredi G., Mazzarini L., Pacchiarotti I., Simonetti A., Ambrosi E., Angeletti G. Suicide in a large population of former psychiatric inpatients. Psychiatry Clin. Neurosci. 2011;65(1):286–295. doi: 10.1111/j.1440-1819.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 73.Brower K.J., Blow F.C., Beresford T.P., Fuelling C. Anabolic-androgenic steroid dependence. J. Clin. Psychiatry. 1989;50(1):31–33. [PubMed] [Google Scholar]
- 74.Thiblin I., Runeson B., Rajs J. Anabolic androgenic steroids and suicide. Ann. Clin. Psychiatry. 1999;11(4):223–231. doi: 10.3109/10401239909147074. [DOI] [PubMed] [Google Scholar]
- 75.Thiblin I., Lindquist O., Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J. Forensic Sci. 2000;45(1):16–23. [PubMed] [Google Scholar]
- 76.Pärssinen M., Kujala U., Vartiainen E., Sarna S., Seppälä T. Increased premature mortality of competitive powerlifters suspected to have used anabolic agents. Int. J. Sports Med. 2000;21(3):225–227. doi: 10.1055/s-2000-304. [DOI] [PubMed] [Google Scholar]
- 77.Petersson A., Garle M., Holmgren P., Druid H., Krantz P., Thiblin I. Toxicological findings and manner of death in autopsied users of anabolic androgenic steroids. Drug Alcohol Depend. 2006;81(3):241–249. doi: 10.1016/j.drugalcdep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Lindqvist A.S., Moberg T., Ehrnborg C., Eriksson B.O., Fahlke C., Rosén T. Increased mortality rate and suicide in Swedish former elite male athletes in power sports. Scand. J. Med. Sci. Sports. 2014;24(6):1000–1005. doi: 10.1111/sms.12122. [DOI] [PubMed] [Google Scholar]
- 79.Darke S., Torok M., Duflou J. Sudden or unnatural deaths involving anabolic-androgenic steroids. J. Forensic Sci. 2014;59(4):1025–1028. doi: 10.1111/1556-4029.12424. [DOI] [PubMed] [Google Scholar]
- 80.Tripodianakis J., Markianos M., Rouvali O., Istikoglou C. Gonadal axis hormones in psychiatric male patients after a suicide attempt. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257(3):135–139. doi: 10.1007/s00406-006-0686-y. [DOI] [PubMed] [Google Scholar]
- 81.Markianos M., Tripodianakis J., Istikoglou C., Rouvali O., Christopoulos M., Papageorgopoulos P., Seretis A. Suicide attempt by jumping: a study of gonadal axis hormones in male suicide attempters versus men who fell by accident. Psychiatry Res. 2009;170(1):82–85. doi: 10.1016/j.psychres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Butterfield M.I., Stechuchak K.M., Connor K.M., Davidson J.R., Wang C., MacKuen C.L., Pearlstein A.M., Marx C.E. Neuroactive steroids and suicidality in posttraumatic stress disorder. Am. J. Psychiatry. 2005;162:380–382. doi: 10.1176/appi.ajp.162.2.380. [DOI] [PubMed] [Google Scholar]
- 83.Perez-Rodriguez M.M., Lopez-Castroman J., Martinez-Vigo M., Diaz-Sastre C., Ceverino A., Núñez-Beltrán A., Saiz-Ruiz J., de Leon J., Baca-Garcia E. Lack of association between testosterone and suicide attempts. Neuropsychobiology. 2011;63(2):125–130. doi: 10.1159/000318085. [DOI] [PubMed] [Google Scholar]
- 84.Sher L., Grunebaum M.F., Sullivan G.M., Burke A.K., Cooper T.B., Mann J.J., Oquendo M.A. Testosterone levels in suicide attempters with bipolar disorder. J. Psychiatr. Res. 2012;46(10):1267–1271. doi: 10.1016/j.jpsychires.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato S.M., Schulz K.M., Sisk C.L., Wood R.I. Adolescents and androgens, receptors and rewards. Horm. Behav. 2008;53(5):647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ip E.J., Lu D.H., Barnett M.J., Tenerowicz M.J., Vo J.C., Perry P.J. Psychological and physical impact of anabolic-androgenic steroid dependence. Pharmacotherapy. 2012;32(10):910–919. doi: 10.1002/j.1875-9114.2012.01123. [DOI] [PubMed] [Google Scholar]
- 87.Rocha V.M., Calil C.M., Ferreira R., Moura M.J., Marcondes F.K. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10(4):326–331. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- 88.Costine B.A., Oberlander J.G., Davis M.C., Penatti C.A., Porter D.M., Leaton R.N., Henderson L.P. Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse. Psychoneuroendocrinology. 2010;35:1473–1485. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oberlander J.G., Henderson L.P. Corticotropin-releasing factor modulation of forebrain GABAergic transmission has a pivotal role in the expression of anabolic steroid-induced anxiety in the female mouse. 2012. [DOI] [PMC free article] [PubMed]
- 90.Onakomaiya M.M., Porter D.M., Oberlander J.G., Henderson L.P. Sex and exercise interact to alter the expression of anabolic androgenic steroid-induced anxiety-like behaviors in the mouse. Horm. Behav. 2014;66(2):283–297. doi: 10.1016/j.yhbeh.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bitran D., Kellogg C.K., Hilvers R.J. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm. Behav. 1993;27(4):568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 92.Bing O., Heilig M., Kakoulidis P., Sundblad C., Wiklund L., Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. Eur. Neuropsychopharmacol. 1998;8(4):321–323. doi: 10.1016/S0924-977X(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 93.Aikey J.L., Nyby J.G., Anmuth D.M., James P.J. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm. Behav. 2002;42(4):448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 94.Vogel J.R., Beer B., Clody D.E. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacology . 1971;21(1):1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 95.Ågren G., Thiblin I., Tirassa P., Lundeberg T., Stenfors C. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol. Behav. 1999;66(3):503–509. doi: 10.1016/S0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 96.Barreto-Estrada J.L., Barreto J., Fortis-Santiago Y., Rivera-Ramos I., Fortis-Santiago A., Jorge J.C. Modulation of affect after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Behav. Neurosci. 2004;118(5):1071–1079. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- 97.Rojas-Ortiz Y.A., Rundle-González V., Rivera-Ramosa I., Jorge J.C. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice Horm. Behav. 2006. [DOI] [PubMed]
- 98.Davis C., Scott-Robertson L. A psychological comparison of females with anorexia nervosa and competitive male bodybuilders: body shape ideals in the extreme. Eat. Behav. 2000;1(1):33–46. doi: 10.1016/S1471-0153(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 99.Murray S.B., Rieger E., Hildebrandt T., Karlov L., Russell J., Boon E., Dawson R.T., Touyz S.W. A comparison of eating, exercise, shape, and weight related symptomatology in males with muscle dysmorphia and anorexia nervosa. Body Image. 2012;9(2):193–200. doi: 10.1016/j.bodyim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 100.Aşçi F.H., Tüzün M., Koca C. An examination of eating attitudes and physical activity levels of Turkish university students with regard to self-presentational concern. Eat. Behav. 2006;7(4):362–367. doi: 10.1016/j.eatbeh.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Swami V., Steadman L., Tovée M.J. A comparison of body size ideals, body dissatisfaction, and media influence between female track athletes, martial artists, and non-athletes. Psychol. Sport Exerc. 2009;10:609–614. doi: 10.1016/j.psychsport.2009.03.003. [DOI] [Google Scholar]
- 102.Labre M.P. Adolescent boys and the muscular male body ideal. J. Adolesc. Health. 2002;30(4):233–242. doi: 10.1016/S1054-139X(01)00413-X. [DOI] [PubMed] [Google Scholar]
- 103.Harrison K., Cantor J. The relationship between media consumption and eating disorders. J. Commun. 1997;47:40–67. doi: 10.1111/j.1460-2466.1997.tb02692.x. [DOI] [Google Scholar]
- 104.Field A.E., Camargo C.A., Jr, Taylor C.B., Berkey C.S., Roberts S.B., Colditz G.A. Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics. 2001;107(1):54–60. doi: 10.1542/peds.107.1.54. [DOI] [PubMed] [Google Scholar]
- 105.Blouin A.G., Goldfield G.S. Body image and steroid use in male bodybuilders. Int. J. Eat. Disord. 1995;18(2):159–165. doi: 10.1002/1098-108X(199509)18:2<159::AID-EAT2260180208>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 106.Schwerin M.J., Corcoran K.J., Fisher L., Patterson D., Askew W., Olrich T., Shanks S. Social physique anxiety, body esteem, and social anxiety in bodybuilders and self-reported anabolic steroid users. Addict Behav. 1996;21:1–8. doi: 10.1016/0306-4603(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 107.Kanayama G., Pope H.G., Cohane G., Hudson J.I. Risk factors for anabolic-androgenic steroid use among weightlifters: a casecontrol study. Drug Alcohol Depend. 2003;71:77–86. doi: 10.1016/S0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 108.Goldfield G.S., Blouin A.G., Woodside D.B. Body image, binge eating, and bulimia nervosa in male bodybuilders. Can. J. Psychiatry. 2006;51(3):160–168. doi: 10.1177/070674370605100306. [DOI] [PubMed] [Google Scholar]
- 109.Goldfield G.S. Body image, disordered eating and anabolic steroid use in female bodybuilders. Eat. Disord. 2009;17(3):200–210. doi: 10.1080/10640260902848485. [DOI] [PubMed] [Google Scholar]
- 110.Cafri G., Olivardia R., Thompson J.K. Symptom characteristics and psychiatric comorbidity among males with muscle dysmorphia. Compr. Psychiatry. 2008;49:374–379. doi: 10.1016/j.comppsych.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 111.Walker D.C., Anderson D.A., Hildebrandt T. Body checking behaviors in men. Body Image. 2009;6(3):164–170. doi: 10.1016/j.bodyim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conacher G.N., Workman D.G. Violent crime possibly associated with anabolic steroid use. Am. J. Psychiatry. 1989;146(5):679. doi: 10.1176/ajp.146.5.679b. [DOI] [PubMed] [Google Scholar]
- 113.Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Bahrke M.S., Yesalis C.E., III Weight training. A potential confounding factor in examining the psychological and behavioural effects of anabolic-androgenic steroids. Sports Med. 1994;18(5):309–318. doi: 10.2165/00007256-199418050-00003. [DOI] [PubMed] [Google Scholar]
- 115.Lefavi R.G., Reeve T.G., Newland M.C. Relationship between anabolic steroid use and selected psychological parameters in male bodybuilders. J. Sport Behav. 1990;13:157–166. doi: 10.1002/hup.470050407. [DOI] [Google Scholar]
- 116.Choy P.Y.L., Parrott A.C., Cowan D. High-dose anabolic steroids in strength athletes: effects upon hostility and aggression. Hum. Psychopharmacol. Clin. Exp. 1990;5:349–356. [Google Scholar]
- 117.Brower K.J., Blow F.C., Young J.P., Hill E.M. Symptoms and correlates of anabolic-androgenic steroid dependence. 1991;5:349–356. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 118.Stanley A., Ward M. Anabolic steroids--the drugs that give and take away manhood. A case with an unusual physical sign. Med. Sci. Law. 1994;34(1):82–83. doi: 10.1177/002580249403400115. [DOI] [PubMed] [Google Scholar]
- 119.Choi P.Y., Pope H.G., Jr Violence toward women and illicit androgenic-anabolic steroid use. Ann. Clin. Psychiatry. 1994;6(1):21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- 120.Bond A.J., Choi P.Y., Pope H.G., Jr Assessment of attentional bias and mood in users and non-users of anabolic-androgenic steroids. Drug Alcohol Depend. 1995;37(3):241–245. doi: 10.1016/0376-8716(94)01071-R. [DOI] [PubMed] [Google Scholar]
- 121.Steensland P., Blakely G., Nyberg F., Fahlke C., Pohorecky L.A. Anabolic androgenic steroid affects social aggression and fear-related behaviors in male pair-housed rats. Horm. Behav. 2005;48(2):216–224. doi: 10.1016/j.yhbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 122.Olivares E.L., Silveira A.L.B., Fonseca F.V., Silva-Almeida C., Côrtes R.S., Pereira-Junior P.P., Nascimento J.H., Reis L.C. Administration of an anabolic steroid during the adolescent phase changes the behavior, cardiac autonomic balance and fluid intake in male adult rats. Physiol. Behav. 2014;126:15–24. doi: 10.1016/j.physbeh.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 123.Ricci L.A., Morrison T.R., Melloni R.H., Jr Adolescent anabolic/androgenic steroids: Aggression and anxiety during exposure predict behavioral responding during withdrawal in Syrian hamsters (Mesocricetus auratus). Horm. Behav. 2013;64(5):770–780. doi: 10.1016/j.yhbeh.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hall R.C., Popkin M.K., Stickney S.K., Gardner E.R. Presentation of the steroid psychoses. J. Nerv. Ment. Dis. 1979;167(4):229–236. doi: 10.1097/00005053-197904000-00006. [DOI] [PubMed] [Google Scholar]
- 125.Annitto W.J., Layman W.A. Anabolic steroids and acute schizophrenic episode. J. Clin. Psychiatry. 1980;41(4):143–144. [PubMed] [Google Scholar]
- 126.Teuber I., Freiwald D., Volz H.P. Acute paranoid symptoms following intramuscular injection of nandrolone. Psychiatr. Prax. 2003;30(2):73–74. doi: 10.1055/s-2003-39732. [DOI] [PubMed] [Google Scholar]
- 127.Lapinsohn L.I. Anabolic steroids in the treatment of psychiatric states (effect of oxymetholone). Dis. Nerv. Syst. 1962;23:226–230. [PubMed] [Google Scholar]
- 128.Tarlo L., Zachariadis N., Marks V. Anabolic steroids in chronic schizophrenia. Br. J. Psychiatry. 1964;110:287–289. doi: 10.1192/bjp.110.465.287. [DOI] [PubMed] [Google Scholar]
- 129.Kanayama G., Brower K.J., Wood R.I., Hudson J.I., Pope H.G. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009;104:1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gridley D.W., Hanrahan S.J. Anabolic-androgenic steroid use among male gymnasium participants: knowledge and motives. Sports Health. 1994;12:11–14. [Google Scholar]
- 131.Copeland J., Peters R., Dillon P. Anabolic-androgenic steroid use disorders among a sample of Australian competitive and recreational users. Drug Alcohol Depend. 2000;60(1):91–96. doi: 10.1016/S0376-8716(00)80011-3. [DOI] [PubMed] [Google Scholar]
- 132.Perry P.J., Lund B.C., Deninger M.J., Kutscher E.C., Schneider J. Anabolic steroid use in weightlifters and bodybuilders: an internet survey of drug utilization. Clin. J. Sport Med. 2005;15(5):326–330. doi: 10.1097/01.jsm.0000180872.22426.bb. [DOI] [PubMed] [Google Scholar]
- 133.Kashkin K.B., Kleber H.D. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA. 1989;262(22):3166–3170. doi: 10.1001/jama.1989.03430220089036. [DOI] [PubMed] [Google Scholar]
- 134.Bowers L.D., Clark R.V., Shackleton C.H. A half-century of anabolic steroids in sport. Steroids. 2009;74:285–287. doi: 10.1016/j.steroids.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 135.Porcerelli J.H., Sandler B.A. Narcissism and empathy in steroid users. Am. J. Psychiatry. 1995;152(11):1672–1674. doi: 10.1176/ajp.152.11.1672. [DOI] [PubMed] [Google Scholar]
- 136.Noll S.M., Fredrickson B.L. A mediational model linking self-objectification, body shame, and disordered eating. Psychol. Women Quart. 1998;22:623–636. doi: 10.1111/j.1471-6402.1998.tb00181.x. [DOI] [Google Scholar]
- 137.Prichard I., Tiggemann M. Relations among exercise type; self-objectification; and body image in the fitness centre environment. The role of reasons for exercise. Psychol. Sport Exerc. 2008;9:855–866. doi: 10.1016/j.psychsport.2007.10.005. [DOI] [Google Scholar]
- 138.Ehrnborg C., Rosén T. The psychology behind doping in sport. Growth Horm. IGF Res. 2009;19:285–87. doi: 10.1016/j.ghir.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Kilpatrick M., Hebert E., Bartholomew J. College students’ motivation for physical activity: differentiating men’s and women’s motives for sport participation and exercise. J. Am. Coll. Health. 2005;54(2):87–94. doi: 10.3200/JACH.54.2.87-94. [DOI] [PubMed] [Google Scholar]
- 140.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hughes T.K. Potential for the effects of anabolic steroid abuse in the immune and neuroendocrine axis. J. Neuroimmunol. 1998;83:162–167. doi: 10.1016/S0165-5728(97)00233-6. [DOI] [PubMed] [Google Scholar]
- 142.Masonis A.E., McCarthy M.P. Effects of the androgenic/anabolic steroid stanozolol on GABAA receptor function: GABA-stimulated 36Cl- influx and [35S] TBPS binding. J. Pharmacol. Exp. Ther. 1996;279(1):186–193. [PubMed] [Google Scholar]
- 143.Rossbach U.L., Steensland P., Nyberg F., Le Grevès P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. Biochem. Biophys. Res. Commun. 2007;357(4):1028–1033. doi: 10.1016/j.bbrc.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 144.Birgner C., Kindlundh-Högberg A.M., Alsiö J., Lindblom J., Schiöth H.B., Bergström L. The anabolic androgenic steroid nandrolone decanoate affects mRNA expression of dopaminergic but not serotonergic receptors. Brain Res. 2008;1240:221–228. doi: 10.1016/j.brainres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 145.Schwartzer J.J., Melloni R.H., Jr Dopamine activity in the lateral anterior hypothalamus modulates AAS-induced aggression through D2 but not D5 receptors. Behav. Neurosci. 2010;124(5):645–655. doi: 10.1037/a0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McIntyre K.L., Porter D.M., Henderson L.P. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABA(A) receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43(4):634–645. doi: 10.1016/S0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 147.Kindlundh A.M., Lindblom J., Bergström L., Nyberg F. The anabolic-androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience. 2003;119(1):113–120. doi: 10.1016/S0306-4522(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 148.Centorrino F., Fogarty K.V., Sani G., Salvatore P., Cimbolli P., Baldessarini R.J. Antipsychotic drug use: McLean Hospital, 2002. Hum. Psychopharmacol. 2005;20(5):355–358. doi: 10.1002/hup.700. [DOI] [PubMed] [Google Scholar]
- 149.Pacchiarotti I., Nivoli A.M., Mazzarini L., Kotzalidis G.D., Sani G., Koukopoulos A., Scott J., Strejilevich S., Sánchez-Moreno J., Murru A., Valentí M., Girardi P., Vieta E., Colom F. The symptom structure of bipolar acute episodes: in search for the mixing link. J. Affect. Disord. 2013;149(1-3):56–66. doi: 10.1016/j.jad.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 150.Musenga A., Saracino M.A., Sani G., Raggi M.A. Antipsychotic and antiepileptic drugs in bipolar disorder: the importance of therapeutic drug monitoring. Curr. Med. Chem. 2009;16(12):1463–1481. doi: 10.2174/092986709787909604. [DOI] [PubMed] [Google Scholar]
- 151.Peet M., Peters S. Drug-induced mania. Drug Saf. 1995;12(2):146–153. doi: 10.2165/00002018-199512020-00007. [DOI] [PubMed] [Google Scholar]


