Abstract
Anabolic-androgenic steroids (AAS) are synthetic substances derived from testosterone that are largely employed due to their trophic effect on muscle tissue of athletes at all levels. Since a great number of organs and systems are a target of AAS, their adverse effects are primarily on the following systems: reproductive, hepatic, musculoskeletal, endocrine, renal, immunological, infectious, cardiovascular, cerebrovascular, and hematological. Neuropsychiatric and behavioral effects as a result of AAS abuse are well known and described in the literature. Mounting evidence exists suggesting that in addition to psychiatric and behavioral effects, non-medical use of AAS carries neurodegenerative potential. Although, the nature of this association remains largely unexplored, recent animal studies have shown the recurrence of this AAS effect, ranging from neurotrophin unbalance to increased neuronal susceptibility to apoptotic stimuli.
Experimental and animal studies strongly suggest that apoptotic mechanisms are at least in part involved in AAS-induced neurotoxicity. Furthermore, a great body of evidence is emerging suggesting that increased susceptibility to cellular oxidative stress could play a pivotal role in the pathogenesis of many neurodegenerative disorders and cognitive impairment. As in other drug-evoked encephalopathies, the key mechanisms involved in AAS – induced neuropathology could represent a target for future neuroprotective strategies. Progress in the understanding of these mechanisms will provide important insights into the complex pathophysiology of AAS-induced neurodegeneration, and will pave the way for forthcoming studies. Supplementary to abandoning the drug abuse that represents the first step in reducing the possibility of irreversible brain damage in AAS abusers, neuroprotective strategies have to be developed and implemented in future.
Keywords: Androgen-anabolic steroids, apoptosis, biochemical mechanisms, excitotoxic neuronal death, neurotrophin unbalance, neuroprotective strategies, neurotoxicity, oxidative-stress
INTRODUCTION
Anabolic-androgenic steroids (AAS) are synthetic substances derived from testosterone (Fig. 1) that are employed for their trophic effect on muscle tissue, with a net result in increased muscle mass and strength. These effects, in conjunction with the neurostimulatory ones, may explain the large prevalence of AAS among athletes at all levels [1-7]. Athletes, and namely bodybuilders and weightlifters, are the main users of these substances [1, 6]. Individuals who desire a lean appearance and muscular appearance are also implicated in this [2, 3, 7, 8-18].
Fig. (1).
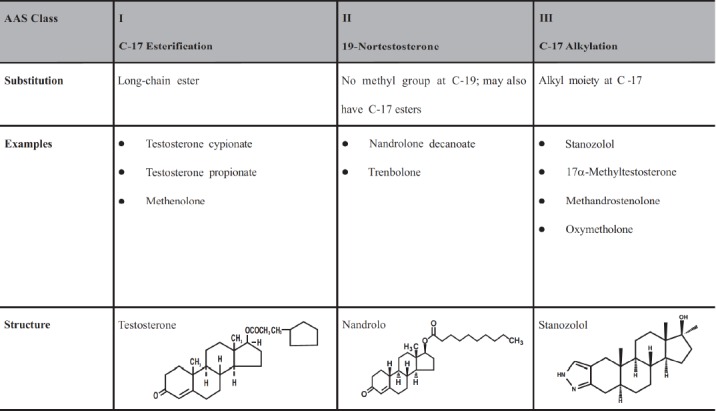
The three major classes of anabolic androgen steroids (modified from Oberlander, J.G.; Henderson, L.P, 2012, cited sub 51).
Although the use of anabolic steroids for cosmetic purposes is incorrectly thought to be relatively harmless, contrarily, anabolic steroids are harmful to health [4, 19-21]. In fact, their consumption can trigger a series of adverse side effects on the body. It is generally believed that side effects of AAS could develop only as a result of long-term use [22]. However, acute adverse effects have also been described, primarily consisting of headaches, fluid retention, gastrointestinal irritation, diarrhoea, abdominal pain, jaundice, menstrual abnormalities, and hypertension. The chronic effects of AAS abuse aside from the neuro-psychiatric and behavioral effects include a wide range of somatic consequences. Many organs and systems are targets of AAS action; consequently, AAS may exert negative effects on reproductive, hepatic, musculoskeletal, endocrine, renal, immunologic, cardiovascular, cerebrovascular, and hematological systems [5, 23-27].
Neuropsychiatric and behavioral effects of AAS abuse are well known and described in the literature [9, 12, 28-52]. In rodents, long-term administration of certain AAS induces behavioral and neurochemical changes [44, 53-58], which may resemble similar behavioral modifications observed in AAS abusers.
However, the neurodegenerative effects of long term AAS abuse are part of not yet evident phenomenon in which the negative effects of these drugs remain clinically silent during the young age. Most of the world’s illicit AAS users are still under the age of 50, too early for clear symptomatic manifestations (cognitive or motor deficits) of possible neurotoxic effects. [59]. Recently, Kanayama et al. [59] reported that visio-spatial memory of long-term AAS users was significantly worse than in AAS non-users. Moreover the same Authors reported that visio-spatial performance showed a significant negative correlation with total lifetime AAS dosing [59]. These observations are in line with the experimental data reported by Pierretti et al. [60] who demonstrated that rats administered with supraphysiologic AAS doses showed spatial memory deficits.
There is growing evidence that non-medical use of AAS has a neurodegenerative potential. Although the nature of this effect is still largely not clarified, recent animal studies have shown the recurrence of neurotoxic effects of AAS, ranging from neurotrophin unbalance to increased neuronal susceptibility to apoptotic stimuli [61].
The current paper aims to investigate the neurotoxicity related to AAS abuse and the underlying hypothesized mechanisms.
AAS NEUROACTIVITY
For a long time, steroid hormones have been demonstrated to control sexual differentiation of the brain, reproduction, behavior, memory, etc. [62-64].
A mass of studies demonstrated that nervous system is a target for two different pools of steroids: steroid hormones produced in the peripheral glands, and neurosteroids originating directly from the nervous system [65].
Steroids are biologically active at classical androgen receptors (ARs), albeit with significantly different activities [66-69].
Once bound by their ligands, ARs act as nuclear transcription factors eliciting the expression of genes under control of steroid-response elements (SRE), within a time course of hours following AAS binding to ARs. This pathway is supposed to be at the basis of medium- and long-term effects of steroid hormones (such as the regulation of hypophyseal hormones secretion, or sexual differentiation of brain circuits). However, the well known short-term effects of steroids led to hypothesize the existence of other receptors (the so-called non-classical steroid receptors) located within the membrane. The following receptors also act in the same manner: γ-Aminobutyric acid (GABA) type A and B (GABA-A receptor, GABA-B receptor), serotonin type 3 (5-HT3), N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate receptor, and an atypical intracellular receptor like the sigma 1 [70-73].
As widely demonstrated in the literature, aggression, anxiety, and reproductive behaviors involving GABAergic transmission are mainly affected by AAS use in both human abusers and animal models [44, 74].
The central region of the medial preoptic area (mPOA), the medial preoptic nucleus (MPN), is characterized by a dense presence of GABAergic neurons [75, 76] and GABA A receptors [56, 77], as well as high levels of ARs, estrogen receptors (ERs), and aromatase, consistent with the high steroid-affinity of this region [56].
In an experimental animal model, it was demonstrated that remarkable modifications in the levels of selective GABA A receptor subunit mRNAs, depending upon the dose of AAS, and on the age and the sex of the animals [53] were induced by chronic administration of AAS.
AAS can also be aromatized to estrogens and interface with both estrogen receptor alpha and beta (ERα, ERβ) [68, 78-84]. Moreover, AAS can also act indirectly altering signaling via ER by their ability to allosterically inhibit aromatase [78, 85], through interactions with a non-AR/ER microsomal binding site [86] as well as allosteric modulation of ion channels [87-89]. Furthermore, the AAS induced decrease of endogenous neurosteroids biosynthesis, may explain the non-receptor-mediated effects on both aggression and anxiety [78, 85, 90-91].
Conclusively, the classical AAS mechanisms of action imply steroid binding to the ARs. However, the kaleidoscopic mechanisms of AAS activity are further complicated by the fact that some steroids involve non-classical and non-genomic mechanism responses [92-94] (Fig. 2).
Fig. (2).
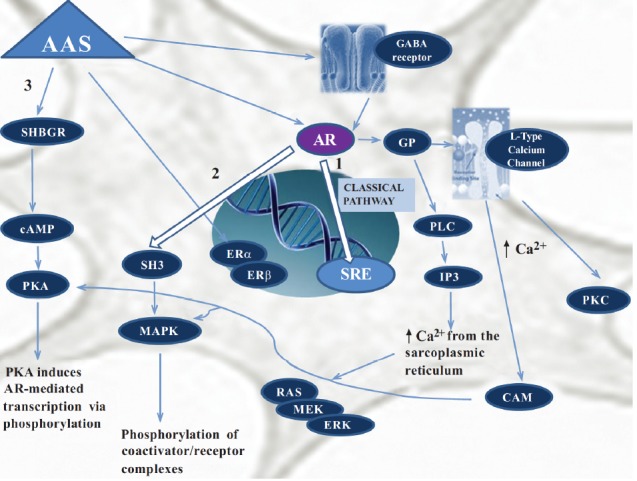
The different mechanisms of AAS neurotoxicity. In the classical pathway (1), the androgen freely passes through the membrane bilayer and binds cytoplasmic Androgen Receptor (AR); after translocation to the nucleus bound AR binds to Steroid-Response Elements (SRE), stimulating gene transcription. Bound AR also interacts with Src Homology Domain 3 (SH3) of the tyrosine kinase c-Src to activate the Mitogen-Activated Protein Kinase (MAPK) pathway and induces gene transcription via phosphorylation of coactivator/receptor complexes (2). The androgen binds to Steroid Hormone Binding Globulin (SHBG) activating the SHBG Receptor (SHBGR) and leading to an increase in Protein Kinase A (PKA) activity. PKA may influence AR-mediated transcription via alteration of phosphorylation status of AR and AR coregulators (3). AAS can be also metabolized to estrogens, interacting not only with AR but also with Estrogen Receptors (ERα and ERβ) to regulate gene transcription. These interaction can result in change in GABAA receptor subunit gene expression. AAS also induce directly changes in GABAergic signaling altering gene expression. On the right the non-genomic androgen action via changes in intracellular ion concentrations and membrane fluidity is represented. Androgen interacts with a membrane associated AR leading to the activation of Ltype calcium channels through some type of inhibitory g-protein (GP). This increase in intracellular calcium activates Protein Kinase C (PKC), and activates via calmodulin (CAM) PKA and MAPK pathway. The modulation of GP may also activate Phospholipase C (PLC) resulting in increases in inositol 1,4,5-thriphosphate (IP3) and consequently in release of intracellular calcium stores from the sarcoplasmic reticulum and in activation of the RAS/MEK/ERK pathway (MEK=MAPK/ERK kinase, ERK=extracellular-signal regulated kinase).
There is a growing body of evidence suggesting that non- genomic effects of AAS act in concert with genomic effects. Rapid, non - genomic effects of AAS differ from genomic ones in: 1) faster onset (seconds to minutes), 2) insensitivity to inhibition of RNA and protein synthesis, 3) effects produced by steroids unable to access the nucleus (either covalently linked to membrane impermeable macro-molecules or in cells lacking a nucleus), and 4) not usually blocked by classical antagonists due to different steroidal specificity from classical cognate nuclear receptors [95]. As for other steroids, non-genomic AAS effects include a broad spectrum of intracellular pathways such as the activation of membrane bound receptors, triggering of downstream pathways involving protein kinases and phosphatases, mobilization of intracellular Ca2+, and SRE-independent changes in transcription [95]. Androgens can bind to receptors in or around the plasma membrane, activate cell-signaling pathways, and regulate responses on a time scale of seconds or minutes [93]. This effect has been demonstrated in several kinds of cells, including osteoblasts, platelets, skeletal muscle cells, cardiac myocytes and neurons.
A rapid (seconds to minutes) change in [Ca2+]i is the main non-genomic effect of androgen exposure [96-103].
The versatility of Ca2+ as a second messenger is implicated in a variety of pathophysiological processes, such as cell proliferation, apoptosis, necrosis, motility, and gene expression [93, 103, 104]. Neurons seem to be particularly sensitive to Ca2+ oscillations [102] because Ca2+ controls pivotal neural processes including synaptic plasticity [105], exocytosis [106], gene expression [107, 108], bioenergetics [109], autophagy [110], and apoptosis [111].
Moreover, androgen induced [Ca2+]i rise may regulate ARs activation since increased [Ca2+]i levels stimulate the binding of androgens to ARs [112]. In addition, androgens can activate calcium dependent kinase pathways, such as extracellular signal-regulated kinase (ERK) or Src, which could phosphorylate the ARs and enhance its activity [113, 114]. The increase in [Ca2+]i following treatment with Ca2+ ionophores or inhibitors of Ca2+-ATPase have also been found to reduce ARs expression [115]. Conclusively, Ca2+ could act as a pivotal link between non-genomic and genomic AAS signaling [103].
AAS NEUROTOXICITY
At physiological levels, androgens influence neuronal differentiation, neuroprotection, neuronal survival, and development [116-118], likely through the classic, slow AR pathway.
Estrada et al. demonstrated that following the administration of physiological doses of testosterone, rapid intracellular Ca2+ increases in neuroblastoma cells are evoked, leading to neurite outgrowth [119], which is essential in neuronal differentiation [120].
Neurobehavioral changes like hyperexcitability and supra-aggressive nature observed following the administration of large doses of androgen could represent the clinical expression of neuronal damage resulting from exposure to high concentrations of AAS [9, 12, 28-52].
The mechanisms of these deleterious neuropathological effects of AAS have not yet been completely elucidated, and are still largely unexplored; however evidence has shown the recurrence of neurodegenerative and neurotoxic potential of these compounds, ranging from neurotrophin unbalance to increased neuronal susceptibility to apoptotic stimuli [61].
The expression ‘apoptosis’ was coined by Kerr et al. [121] to describe peculiar morphological aspects of cell death. In apoptosis, cells appear rounded – up, pseudopods are retracted, cellular volume is reduced (pyknosis), chromatin is condensed, and nuclei are fragmented (karyorrhexis). Other typical findings of apoptosis are little or no ultrastructural modifications of cytoplasmic organelles, plasma membrane blebbing, and engulfment by resident phagocytes (in vivo) [122] (Fig. 3).
Fig. (3).
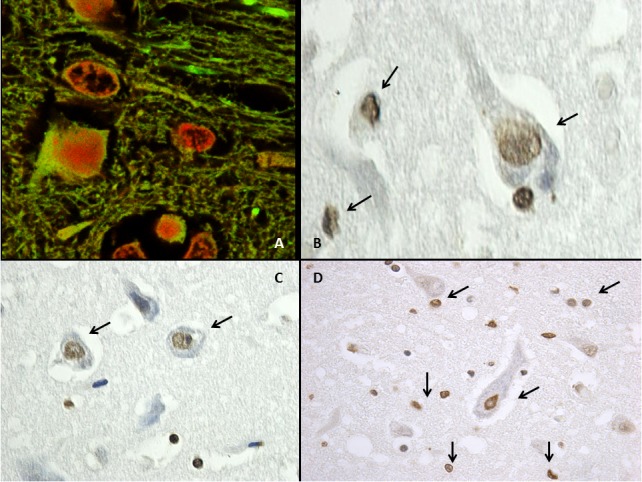
Mouse brain treated with nandrolone decanoate (A) Confocal laser scanning microscope. Increase of apoptosis (Tunel assay) with intense positive reaction (red). (B-C-D) TUNEL assay revealed neuronal and glial over-expression of apoptotic nuclei (arrows) with a brownish positive reaction.
The mechanisms leading to apoptotic death are very complex. Extrinsic apoptosis is a caspase – dependent cell death subroutine elicited by extracellular stress signals that are sensed and propagated by specific transmembrane receptors. It can be initiated by the binding of lethal ligands, such as FAS/CD95 ligand (FASL/CD95L), tumor necrosis factor α (TNFα) and TNF superfamily member 10 (TNFSF10, also known as TNF-related apoptosis inducing ligand, TRAIL), to various death receptors (i.e., FAS/CD95, TNFα receptor 1 (TNFR1), and TRAIL receptor (TRAILR) 1–2, respectively [123].
The apoptotic death can be triggered by many different intracellular stress conditions, including DNA damage, oxidative stress, cytosolic Ca2þ overload, mild excitotoxicity (related to glutamate receptor overstimulation in the nervous system), accumulation of unfolded proteins in the endoplasmic reticulum (ER), and many others (the so-called “intrinsic apoptosis”) [123]. The initiating stimuli and the signaling cascade involved in intrinsic apoptosis are strictly linked to a mitochondrion-centered control mechanism.
It is well known that AAS can exert apoptotic stimuli in different tissue and organs, therefore representing a pivotal mechanism in AAS – induced toxicity [124-135]. Growing evidence is emerging that apoptotic mechanisms are at least in part, also involved in AAS induced neurotoxicity.
Estrada et al. [136] firstly showed that the treatment of neuroblastoma cells with elevated concentrations of testosterone induces a decrease in cell viability by activation of an apoptotic cell death program, as demonstrated by increased numbers of annexin V-positive cells, DNA fragmentation, and caspase activation. The hypothesis put forward was that elevated testosterone alters InsP3R (Inositol trisphosphate receptor) type 1-mediated intracellular Ca2+ signaling, and that the prolonged Ca2+ signals lead to apoptosis [136].
Orlando et al. [137] studied the effect of some AAS (testosterone, nandrolone, stanozolol, and gestrinone) on excitotoxic neuronal death induced by N-methyl-d-aspartate (NMDA) in primary cultures of mouse cortical cells. The term “excitotoxicity” was coined by John Olney [138, 139] to describe a specific neuronal death pathway induced by an excessive stimulation of glutamate receptors, resulting in excessive Ca2+ influx through a receptor’s associated ion channels [140-142]. The Authors demonstrated that only very high concentrations of testosterone were able to amplify neuronal excitotoxicity; on the contrary lower testosterone concentrations seemed to be protective. Testosterone was inactive at intermediate concentrations. However, the presence of aromatase inhibitors made even low concentrations of testosterone neurotoxic, therefore suggesting that aromatization of testosterone into 17β-estradiol could counter – balance its intrinsic toxicity. Contrary to testosterone, nortestosterone, stanozolol, and gestrinone-amplified NMDA toxicity at nanomolar concentrations was insensitive to aromatase inhibitors, but was abrogated by the androgen receptor antagonist, flutamide. None of the AASs was toxic in the absence of NMDA. These results led to the hypothesis that AAS increase neuronal vulnerability to excitotoxic insults and may therefore induce neuronal death observed in acute or chronic neurological diseases [137].
Other groups have experimentally demonstrated an apoptotic effect of high dosages of AAS. Cunningham et al. [143] showed that physiologically relevant doses of androgens induce neurotoxicity in dopaminergic neurons (N 27 cells). In this experimental model, androgens enter the cell, bind to the classical intracellular ARs, and induce oxidative stress leading to mitochondrial dysfunction. The release of cytochrome c from the mitochondria activates the apoptotic caspase cascade, promoting PKCδ (Protein kinase C-δ) activation. It has been hypothesized that androgens may induce apoptosis specifically in dopamine neurons since testosterone-induced apoptosis was not observed in gonadotropin-releasing hormone (GnRH) neurons [143]. One group [144] demonstrated additionally that nandrolone and methandrostenolone potentiated the apoptotic trigger induced by beta-amyloid, widely involved in the pathogenesis of Alzheimer’s disease. The same Authors also hypothesized that AAS abuse might support the onset and the progression of neurodegenerative diseases. Tugyan et al. [145], in an animal model (rats) demonstrated a net increase in apoptosis and a significant decrease in neuronal count in the parietal cortex, prefrontal cortex and hippocampal regions of the brain induced by nandrolone decanoate. In their elegant experiment on neuron – like cells (undifferentiated pheochromocytoma 12 cells), Basile et al. [146] demonstrated that the treatment with steroid hormones (androsterone, nandrolone, methandienone and 17-α- methyltestosterone) induced cell death through apoptotic pathways. The Authors detected the appearance of the cleaved and hence active form of caspase 3, along with the cleaved form of poly adenosine diphosphate [ADP]-ribose polymerase, (PARP), therefore suggesting that apoptosis might be a generalized response to high concentrations of steroids. The obtained results demonstrated that AAS-induced apoptosis in neuron-like differentiated pheocromocytoma cell line PC12 [146]. Finally, as it was noted that the effects on cells following AAS treatment were delayed, the Authors speculated that these hormones might exert their effects by acting on AR-mediated genomic pathway [146] and might therefore alter gene transcription [147].
The complexity of the mechanisms of AAS induced neurotoxicity implicates oxidative stress since apoptosis itself can be induced by oxidative stress [148] (Fig. 4).
Fig. (4).
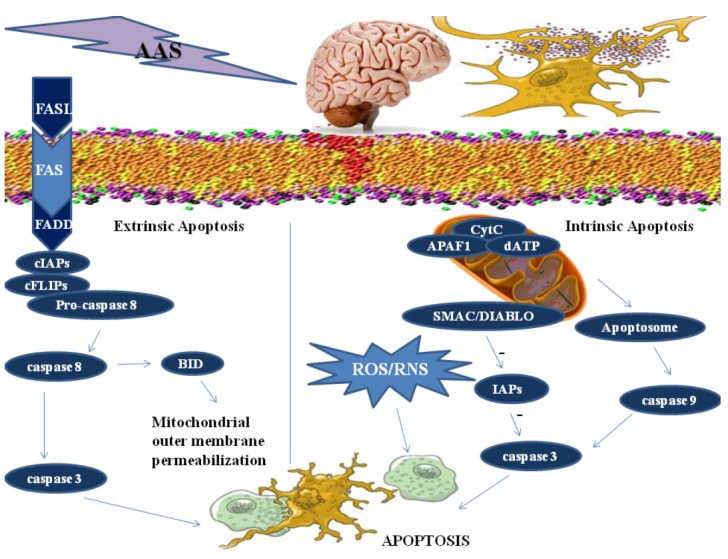
A schematic illustration of the complex mechanisms leading to neuronal death. Extrinsic apoptosis is a caspase – dependent cell death subroutine that is initiated by the binding of lethal ligands, such as FAS/CD95 ligand (FASL/CD95L) to death receptor FAS/CD95; the complex recruit FAS-associated protein with a death domain (FADD), cellular inhibitor of apoptosis proteins (cIAPs), c-FLIPs and procaspase 8. This supramolecular platform controls the activation of caspase-8, that can directly trigger the caspase cascade by mediating the proteolytic maturation of caspase-3, or stimulate mitochondrial outer membrane permeabilization by cleaving the BH3 interacting-domain (BID). In the intrinsic apoptosis the multiple intracellular stress conditions lead to a mitochondrion-centered control mechanisms. When lethal signals prevail, mitochondrial outer membrane permeabilization occurs and leads to mitochondrial transmembrane potential dissipation and arrest of mitochondrial ATP synthesis. The respiratory chain gets uncoupled, leading to reactive oxygen species (ROS) and reactive nitrogen species (RNS) production. Cytochrome C (CytC), together with the cytoplasmic adaptor protein APAF1 and dATP, create the apoptosome, that triggers the caspase 9-caspase 3 proteolytic cascade. Direct IAP-binding protein with low pl (DIABLO, also known as second mitochondria-derived activator of caspases, SMAC) induces caspase activation
A redox system imbalance with an excess of reactive oxygen species (ROS) and reactive nitrogen species (RNS) can contribute to neuronal cell injury and death [149-153] and has been associated with apoptosis [123, 154-157]. The redox system may play different roles in apoptosis. Protein oxidation may essentially influence the gene expression necessary for the signals leading to apoptosis. Caspase activation, DNA binding of several transcription factors, and cytoskeletal alterations in cells undergoing apoptosis may directly or indirectly be affected by oxidative events [148].
In experimental models of neurodegenerative diseases, excessive generation of ROS such as superoxide anion (02-), and RNS such as nitric oxide (NO) can contribute to neuronal cell injury and death [141, 153]. NO derived from iNOS also play a significant role in the pathogenesis of several neurodegenerative diseases such as Alzehimer’s Disease (AD), Parkinson’s Disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and HIV-associated neurocognitive disorder, through the activation of glial cells (astrocytes and microglia) induced by a plethora of neuroinflammatory and neurodegenerative stimuli [158, 159]. Activated glial cells result in expression of iNOS and production of high levels of NO [158, 159]. Oxidative stress has been shown to initiate the apoptotic pathway also in animal models of PD [160-163].
A great body of evidence is emerging that increased susceptibility to cellular oxidative stress could play a pivotal role in the pathogenesis of many neurodegenerative disorders and cognitive impairment [164-169]. These findings are strongly supported by experimental data that confirm the potential neurotoxicity of oxidative stress [170-174].
Our group has pointed out the role of oxidative stress in the mechanisms of AAS-induced toxicity in various organs, such as liver, cardiovascular system and kidney [125, 175-177].
Other Authors focused on the potential dangerous link between androgens and oxidative stress in neurotoxicity. Holmes et al. investigated the effects of androgens under conditions of oxidative stress to determine whether androgens play a neuroprotective or neurotoxic role in dopamine neuronal function. It was discovered that androgens themselves increased mitochondrial function via a calcium-dependent mechanism. Androgen pre-treatment protected cells from oxidative stress-induced cell death. However, treatment with androgens after the oxidative insult increased cell death, and these effects were in part mediated by calcium influx into the mitochondria, and the negative effects of androgens were not blocked by either androgen or estrogen receptor antagonists. A membrane-associated androgen receptor was supposed to be implicated. The results of this study suggested that androgens are neuroprotective when oxidative stress levels are minimal, but when oxidative stress levels are elevated, androgens exacerbate oxidative stress damage [178]. Similar results were reported by Cunningham et al. [179] who demonstrated that testosterone appears to have negative consequences on brain function under conditions of elevated oxidative stress in the Caucasian race. The same group [180] demonstrated that in a preexisting oxidative stress environment, androgens can further exacerbate oxidative stress damage.
Taken together, all these studies indicate a potential role for androgens in oxidative stress – mediated neurodegeneration.
Clinical observations further support this hypothesis. Compared to women, men have a higher incidence of post ischemic stroke Parkinsonism, a neurodegenerative condition in which oxidative stress is strongly implicated in the progression of cell death [181, 182]. Moreover, at the same age, men have a greater incidence of neurodegenerative diseases such as PD than women [183-185].
However, some studies suggest a neuroprotective role for androgens [186-188]. More recently, Nguyen et al. hypothesized that androgen protection could be specific to apoptosis. The results of this study demonstrated that testosterone attenuated neuronal death induced by apoptotic pathways [189]. A different effect was demonstrated for the oxidative stress – induced neuronal injury: direct anti-oxidant mechanism may be not involved in neuroprotection induced by androgens since micromolar concentrations of testosterone have no protective effects against cell death induced by the oxidative stressors [189]. However, other Authors previously reported conflicting results as they showed that in neuroblastoma cells, neuroprotective effects of testosterone could deal with the cellular antioxidant defense system [190, 191]. Other studies support a neuro-protective role for androgens, in which androgens can protect against oxidative stress damage [192, 193]. A possible mechanism for androgen-induced neuroprotection is preconditioning because androgens themselves can moderately increase oxidative stress and apoptosis [143]. These results suggest that the level of oxidative stress determines whether androgens play a positive or negative role in neuronal function [178].
The scenario of the potential neurotoxicity by AAS is further complicated by other mechanisms that have been focused by several authors.
A biochemical basis for the AAS – induced neuropsychiatric sequelae has been hypothesized from the observation that in rats administered with high doses of AAS, levels of brain-derived neurotrophic factor (BDNF) in the hippocampus (HIPP) and prefrontal cortex (PFC) are reduced by stanozolol. It also decreased the expression of low-affinity glucocorticoid receptors in the HIPP, and increased morning trough basal plasma corticosterone levels [194], and decrements of BDNF production occurring in the context of a maladaptive response to stress which may contribute to the reduced volume of the hippocampus and prefrontal cortex observed in depressive disease [195]. In rats, chronic administration of AAS induced modifications in the HPA (hypothalamic-pituitary-adrenal) axis and BDNF levels which are coherent with the current hypothesized pathophysiology of depression [194]. Tucci et al. [196] tested this suggestive hypothesis and examined the possible biochemical changes in different brain areas of the stanozolol-treated animals. The results reported by the authors showed that 5-HT levels decreased in all brain areas following stanozolol treatment. Moreover, the steroid differently affects dopaminergic system in the PFC and HIPP of rats whereas no significant changes were observed in the STR (striatum) or NAC (nucleus accumbens). In particular, in rats chronically administered with stanozolol reduced dopamine levels in PFC are observed, so reinforcing the view that chronic treatment with stanozolol seems to reproduce the neurochemical background of depression [196].
Finally, a neurotrophin unbalance has been proposed as an AAS neurotoxic mechanism involving the nerve growth factor (NGF), a member of the neurotrophin family that promotes the differentiation, growth, and survival of specific neuronal populations during development and in the adulthood [60, 61]. In experimental setting it was demonstrated that adult rats chronically injected with high doses of testosterone or nandrolone showed an increase in NGF levels in the hippocampus and septum [197]. In their animal model, Pieretti et al. found that the expression of NGF and its receptors changed with a region – specific pattern following AAS treatment. NGF levels were reduced in the basal forebrain of rats treated with nandrolone or stanozolol, suggesting that the retrograde transport of NGF from the hippocampus to the basal forebrain was impaired by AAS, This observation reinforced the hypothesis that supraphysiological doses of AAS induce neurotrophin unbalance and related behavioral disturbances [60].
Taken together, all these studies strongly support the evidence of deleterious responses of brain tissue when exposed to high doses of AAS.
Although the mechanisms of AAS effects on the brain are still unclear, some key points may be drawn.
Androgens can exert neurotoxic effects both via direct mechanisms including apoptosis and oxidative stress, and by intensifying neuronal excitotoxicity, a manner of neuronal death that is strongly involved in the pathophysiology of acute and chronic neurodegenerative disorders, even at low concentrations [137]. On the other hand, neuroprotective effects of androgens have been reported in the literature and seem to be related to the level of oxidative stress [178]. In addition, the data provide evidence that suggests a different mechanism for the role of AAS in neurodegeneration and cognitive impairment related to neurotrophin unbalance and biochemical mechanisms.
CONCLUSIONS
A wide plethora of AAS side effects which mainly affect the cardiovascular system, the liver, the kidney, the musculoskeletal, and the endocrine systems have been described in the literature. In recent years, it has become more and more evident that AAS abuse may be detrimental to brain function [198].
Clinical studies demonstrated that in both adults and adolescents, AAS abuse is associated with disorders such as high level of anxiety, irritability, poor impulse control, mood fluctuation. In addition neurodegenerative potential effects of AAS abuse have been strongly suggested. However, great difficulties exist in studying this particular kind of AAS – induced toxicity. First of all, a general limitation of human studies is due to the fact that information about the modality and doses of AAS use/abuse are often self reported and not objectively assessed [23]. Furthermore, the habit of polydrug abuse makes hardly possible to distinguish the toxic effects of AAS from those caused by other drugs [199]. Human studies mimicking the real entity of self underground administration of AAS activity are infeasible since it would be unethical to administer high doses of AAS over prolonged periods of time to assess the risks to health [19]. Finally, the susceptibility of the individuals themselves is partly dependent on genetic factors, a well known key factor in developing adverse events drug – related [200].
In this context, animal models and experimental in vitro studies allowing the detection of the pathological response resulting from anabolic compound administration are well consolidated approaches to detect the negative effects of AAS abuse. Assessment of how AAS exert their neurotoxic effects is a critical question that needs to be further addressed to fully understand the underlying neurobiological signaling mechanisms associated with AAS abuse, and provide new insights for therapeutic intervention. The global evidence emerging from the studies existing on the issue of AAS-induced neurodegeneration allow the affirmation that apoptosis and oxidative stress are strongly involved, and represent the major pathway for AAS neurotoxicity. It is well known that oxidative and nitrosative stresses play a key role in normal physiology and imbalances are attributed to pathology and disease. Redox signaling through oxidation and reduction reactions is a pivotal key in numerous cell signaling cascades, including those with opposing cellular consequences, proliferation, and apoptosis [201, 202]. Our current understanding of the features and the molecular targets of ROS/RNS that regulate cell-signaling pathways is limited, especially in the substance abuse field [203].
By further understanding the components of the cellular dysregulatory events induced by AAS abuse, there should be opportunities to intervene in drug – evoked pathologies, through specific therapeutic platforms. In the context of a complex and multi-faceted therapeutic approach [203, 204], the possibility of targeting redox pathways and reducing excitotoxicity and apoptosis may represent future promising therapeutic neuroprotective strategies [205, 206] (Fig. 5).
Fig. (5).
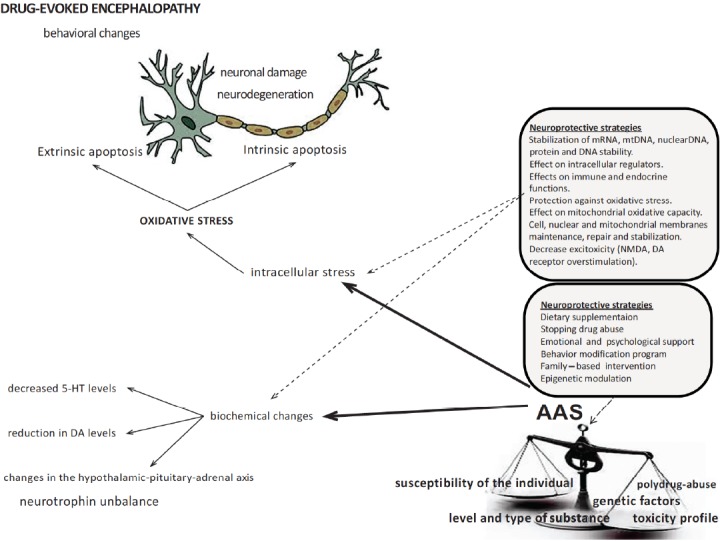
The multifaceted therapeutic approach to AAS abuse evoked neuropathy focuses on “traditional” and new treatment strategies. The first strategy would be to stop the subject from the abuse. Restore normal metabolism and correct nutritional deficiencies is a further fundamental step because drug abuse may cause nutritional insufficiencies. Psychological and family – based support also play and effective role. Another potential strategy is the potential of epigenetic modulation in reducing the risk of developing the drug abuse-evoked encephalopathy. Finally, new therapeutic strategies imply targeting the genomic, metabolic, and mitochondrial dysfunction resulting in increased excitotoxicity, reduced energy production, and lowered antioxidant potential.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We are indebted to Amos Tambo Bsc (Hons) for technical assistance.
References
- 1.Di Luigi L., Romanelli F., Lenzi A. Androgenic-anabolic steroids abuse in males. J. Endocrinol. Invest. 2005;28(3) Suppl:81–84. [PubMed] [Google Scholar]
- 2.Evans N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sports Med. 2004;32(2):534–542. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- 3.Foster Z.J., Housner J.A. Anabolic-androgenic steroids and testosterone precursors: ergogenic aids and sport. Curr. Sports Med. Rep. 2004;3(4):234–241. doi: 10.1249/00149619-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Maravelias C., Dona A., Stefanidou M., Spiliopoulou C. Adverse effects of anabolic steroids in athletes. A constant threat. Toxicol. Lett. 2005;158(3):167–175. doi: 10.1016/j.toxlet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Shahidi N.T. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001;23(9):1355–1390. doi: 10.1016/S0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 6.Sjöqvist F., Garle M., Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371(9627):1872–1882. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 7.Woerdeman J., de Hon O., Levi M., de Ronde W.P. [Anabolic androgenic steroids in amateur sports in the Netherlands] Ned. Tijdschr. Geneeskd. 2010;154:A2004. [PubMed] [Google Scholar]
- 8.González B., Hernando R., Manso R. Anabolic steroid and gender-dependent modulation of cytosolic HSP70s in fast- and slow-twitch skeletal muscle. J. Steroid Biochem. Mol. Biol. 2000;74(1-2):63–71. doi: 10.1016/S0960-0760(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 9.Hall R.C., Hall R.C., Chapman M.J. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46(4):285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- 10.Nasr J., Ahmad J. Severe cholestasis and renal failure associated with the use of the designer steroid Superdrol (methasteron): a case report and literature review. Dig. Dis. Sci. 2009;54(5):1144–1146. doi: 10.1007/s10620-008-0457-x. [DOI] [PubMed] [Google Scholar]
- 11.Wade N. Anabolic steroids: doctors denounce them but athletes aren’t listening. Science. 1972;176(4042):1399–1403. doi: 10.1126/science.176.4042.1399. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama G., Hudson J.I., Pope H.G., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98(1-2):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pope H.G., Brower K.J. Ninth Edition. Philadelphia : Lippincott Williams & Wilkins:; 2009. Anabolic-Androgenic Steroid-Related Disorders. In: Comprehensive Textbook of Psychiatry; pp. 1419–1431. [Google Scholar]
- 14.Hildebrandt T., Alfano L., Langenbucher J.W. Body image disturbance in 1000 male appearance and performance enhancing drug users. J. Psychiatr. Res. 2010;44(13):841–846. doi: 10.1016/j.jpsychires.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley W.E., Yesalis C.E., III, Friedl K.E., Anderson W.A., Streit A.L., Wright J.E. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260(23):3441–3445. doi: 10.1001/jama.1988.03410230059028. [DOI] [PubMed] [Google Scholar]
- 16.Kanayama G., Pope H.G., Jr, Hudson J.I. “Body image” drugs: a growing psychosomatic problem. Psychother. Psychosom. 2001;70(2):61–65. doi: 10.1159/000056228. [DOI] [PubMed] [Google Scholar]
- 17.Parkinson A.B., Evans N.A. Anabolic androgenic steroids: a survey of 500 users. Med. Sci. Sports Exerc. 2006;38(4):644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama G., Hudson J.I., Pope H.G. Features of men with anabolic-androgenic steroid dependence: A comparison with nondependent AAS users and with AAS nonusers. Drug Alcohol Depend. 2009;102(1-3):130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartgens F., Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 20.O’Malley P.A. The drive to win and never grow old: the risks of anabolic steroid abuse, an update for the clinical nurse specialist. Clin. Nurse Spec. 2013;27(3):117–120. doi: 10.1097/NUR.0b013e31828c83db. [DOI] [PubMed] [Google Scholar]
- 21.Kersey R.D., Elliot D.L., Goldberg L., Kanayama G., Leone J.E., Pavlovich M., Pope H.G., Jr, National Athletic Trainers’ Association National Athletic Trainers’ Association position statement: anabolic-androgenic steroids. J. Athl. Train. 2012;47(5):567–588. doi: 10.4085/1062-6050-47.5.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiblin I., Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam. Clin. Pharmacol. 2005;19(1):27–44. doi: 10.1111/j.1472-8206.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 23.Turillazzi E., Perilli G., Di Paolo M., Neri M., Riezzo I., Fineschi V. Side effects of AAS abuse: an overview. Mini Rev. Med. Chem. 2011;11(5):374–389. doi: 10.2174/138955711795445925. [DOI] [PubMed] [Google Scholar]
- 24.van Amsterdam J., Opperhuizen A., Hartgens F. Adverse health effects of anabolic-androgenic steroids. Regul. Toxicol. Pharmacol. 2010;57(1):117–123. doi: 10.1016/j.yrtph.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Brower K.J. Anabolic steroid abuse and dependence. Curr. Psychiatry Rep. 2002;4(5):377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- 26.Pope H.G., Brower K.J. Anabolic-androgenic steroids. In: The American Psychiatric Publishing Textbook of substance abuse treatment. In: Galanter M., Kleber HD, editors. Thirth Edition. Washington, D.C: American Psychiatric Publishing; 2004. pp. 257–264. [Google Scholar]
- 27.Basaria S. Androgen abuse in athletes: detection and consequences. J. Clin. Endocrinol. Metab. 2010;95(4):1533–1543. doi: 10.1210/jc.2009-1579. [DOI] [PubMed] [Google Scholar]
- 28.Daly R.C., Su T.P., Schmidt P.J., Pagliaro M., Pickar D., Rubinow D.R. Neuroendocrine and behavioral effects of high-dose anabolic steroid administration in male normal volunteers. Psychoneuroendocrinology. 2003;28(3):317–331. doi: 10.1016/S0306-4530(02)00025-2. [DOI] [PubMed] [Google Scholar]
- 29.Caplan J.P., Epstein L.A., Quinn D.K., Stevens J.R., Stern T.A. Neuropsychiatric effects of prescription drug abuse. Neuropsychol. Rev. 2007;17(3):363–380. doi: 10.1007/s11065-007-9037-7. [DOI] [PubMed] [Google Scholar]
- 30.Pope H.G., Katz D. Psychiatric effects of exogenous anabolicandrogenic steroids. In: Wolkowitz O.M., Rothschild A.J., editors. Psychoneuroendocrinology: The Scientific Basis of Clinical Practice. Washington, D.C.: American Psychiatric Press; 2003. pp. 331–358. [Google Scholar]
- 31.Pope H.G., Jr, Kouri E.M., Hudson J.I. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch. Gen. Psychiatry. 2000;57(2):133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 32.Kanayama G., Hudson J.I., Pope H.G., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98(1-2):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pope H.G., Jr, Katz D.L. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch. Gen. Psychiatry. 1994;51(5):375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 34.Gruber A.J., Pope H.G., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother. Psychosom. 2000;69(1):19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 35.Choi P.Y., Pope H.G., Jr Violence toward women and illicit androgenic-anabolic steroid use. Ann. Clin. Psychiatry. 1994;6(1):21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- 36.Perry P.J., Kutscher E.C., Lund B.C., Yates W.R., Holman T.L., Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J. Forensic Sci. 2003;48(3):646–651. [PubMed] [Google Scholar]
- 37.Petersson A., Garle M., Granath F., Thiblin I. Morbidity and mortality in patients testing positively for the presence of anabolic androgenic steroids in connection with receiving medical care. A controlled retrospective cohort study. Drug Alcohol Depend. 2006;81(3):215–220. doi: 10.1016/j.drugalcdep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Petersson A., Garle M., Holmgren P., Druid H., Krantz P., Thiblin I. Toxicological findings and manner of death in autopsied users of anabolic androgenic steroids. Drug Alcohol Depend. 2006;81(3):241–249. doi: 10.1016/j.drugalcdep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Thiblin I., Kristiansson M., Rajs J. Anabolic androgenic steroids and behavioral patterns. among violent offenders. J. Forensic Sci. 1997;8(2):299–310. [Google Scholar]
- 40.Thiblin I., Pärlklo T. Anabolic androgenic steroids and violence. Acta Psychiatr. Scand. Suppl. 2002;106(412):125–128. doi: 10.1034/j.1600-0447.106.s412.27.x. [DOI] [PubMed] [Google Scholar]
- 41.Klötz F., Garle M., Granath F., Thiblin I. Criminality among individuals testing positive for the presence of anabolic androgenic steroids. Arch. Gen. Psychiatry. 2006;63(11):1274–1279. doi: 10.1001/archpsyc.63.11.1274. [DOI] [PubMed] [Google Scholar]
- 42.Yates W.R., Perry P.J., MacIndoe J., Holman T., Ellingrod V. Psychosexual effects of three doses of testosterone cycling in normal men. Biol. Psychiatry. 1999;45(3):254–260. doi: 10.1016/S0006-3223(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 43.Su T.P., Pagliaro M., Schmidt P.J., Pickar D., Wolkowitz O., Rubinow D.R. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269(21):2760–2764. doi: 10.1001/jama.1993.03500210060032. [DOI] [PubMed] [Google Scholar]
- 44.Clark A.S., Henderson L.P. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci. Biobehav. Rev. 2003;27(5):413–436. doi: 10.1016/S0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 45.Pope H.G., Jr, Katz D.L. Affective and psychotic symptoms associated with anabolic steroid use. Am. J. Psychiatry. 1988;145(4):487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- 46.Perry P.J., Yates W.R., Andersen K.H. Psychiatric symptoms associated with anabolic steroids: a controlled, retrospective study. Ann. Clin. Psychiatry. 1990;2:11–17. doi: 10.3109/10401239009150000. [DOI] [Google Scholar]
- 47.Freinhar J.P., Alvarez W. Androgen-induced hypomania. J. Clin. Psychiatry. 1985;46(8):354–355. [PubMed] [Google Scholar]
- 48.Hall R.C., Popkin M.K., Stickney S.K., Gardner E.R. Presentation of the steroid psychoses. J. Nerv. Ment. Dis. 1979;167(4):229–236. doi: 10.1097/00005053-197904000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Pärssinen M., Kujala U., Vartiainen E., Sarna S., Seppälä T. Increased premature mortality of competitive powerlifters suspected to have used anabolic agents. Int. J. Sports Med. 2000;21(3):225–227. doi: 10.1055/s-2000-304. [DOI] [PubMed] [Google Scholar]
- 50.Kanayama G., Hudson J.I., Pope H.G., Jr Illicit anabolic-androgenic steroid use. Horm. Behav. 2010;58(1):111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberlander J.G., Henderson L.P. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012;35(6):382–392. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trenton A.J., Currier G.W. Behavioural manifestations of anabolic steroid use. CNS Drugs. 2005;19(7):571–595. doi: 10.2165/00023210-200519070-00002. [DOI] [PubMed] [Google Scholar]
- 53.Henderson L.P., Penatti C.A., Jones B.L., Yang P., Clark A.S. Anabolic androgenic steroids and forebrain GABAergic transmission. Neuroscience. 2006;138(3):793–799. doi: 10.1016/j.neuroscience.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 54.Kindlundh A.M.S., Rahman S., Lindblom J., Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci. Lett. 2004;356(2):131–134. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 55.Kurling S., Kankaanpaa A., Ellermaa S., Karila T., Seppala T. The effect of sub-chronic nandrolone decanoate treatment on dopaminergic and serotonergic neuronal systems in the brains of rats. Brain Res. 2005;1044(1):67–75. doi: 10.1016/j.brainres.2005.02.071. [DOI] [PubMed] [Google Scholar]
- 56.Penatti C.A., Costine B.A., Porter D.M., Henderson L.P. Effects of chronic exposure to an anabolic androgenic steroid cocktail on alpha5-receptor-mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009;161(2):526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossbach U.L., Steensland P., Nyberg F., Le Grevès P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. Biochem. Biophys. Res. Commun. 2007;357(4):1028–1033. doi: 10.1016/j.bbrc.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 58.Thiblin I., Finn A., Ross S.B., Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br. J. Pharmacol. 1999;126(6):1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanayama G., Kean J., Hudson J.I., Pope H.G., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013;130(1-3):208–214. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pieretti S., Mastriota M., Tucci P., Battaglia G., Trabace L., Nicoletti F., Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rats. Med. Sci. Sports Exerc. 2013;45(1):29–35. doi: 10.1249/MSS.0b013e31826c60ea. [DOI] [PubMed] [Google Scholar]
- 61.Scaccianoce S., Caruso A., Miele J., Nistico’ R., Nicoletti F. Potential neurodegenerative effect of anabolic androgenic steroid abuse. J. Biol. Regul. Homeost. Agents. 2013;27(2) Suppl.:107–114. [PubMed] [Google Scholar]
- 62.McEwen B.S. Neural gonadal steroid actions. Science. 1981;211(4488):1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- 63.McEwen B.S. How do sex and stress hormones affect nerve cells? Ann. N.Y. Acad. Sci. 1994;743:1–16. doi: 10.1111/j.1749-6632.1994.tb55784.x. [DOI] [PubMed] [Google Scholar]
- 64.Fink G., Rosie R., Sheward W.J., Thomson E., Wilson H. Steroid control of central neuronal interactions and function. J. Steroid Biochem. Mol. Biol. 1991;40(1-3):123–132. doi: 10.1016/0960-0760(91)90175-5. [DOI] [PubMed] [Google Scholar]
- 65.Melcangi R.C., Panzica G.C. Neuroactive steroids: old players in a new game. Neuroscience. 2006;138(3):733–739. doi: 10.1016/j.neuroscience.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 66.Saartok T., Dahlberg E., Gustafsson J.A. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114(6):2100–2106. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- 67.Roselli C.E. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res. 1998;792(2):271–276. doi: 10.1016/S0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 68.Fragkaki A.G., Angelis Y.S., Koupparis M., Tsantili-Kakoulidou A., Kokotos G., Georgakopoulos C. Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure. Steroids. 2009;74(2):172–197. doi: 10.1016/j.steroids.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Lippi G., Franchini M., Banfi G. Biochemistry and physiology of anabolic androgenic steroids doping. Mini Rev. Med. Chem. 2011;11(5):362–373. doi: 10.2174/138955711795445952. [DOI] [PubMed] [Google Scholar]
- 70.Melcangi R.C., Cavarretta I.T.R., Ballabio M., et al. Peripheral nerves: a target for the action of neuroactive steroids. Brain Res. Rev. 2005;48(2):328–338. doi: 10.1016/j.brainresrev.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 71.Belelli D., Herd M.B., Mitchell E.A., Peden D.R., Vardy A.W., Gentet L., Lambert J.J. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138(3):821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Frye C.A., Rhodes M.E., Petralia S.M., Walf A.A., Sumida K., Edinger K.L. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138(3):1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henderson V.W. Estrogen - containing hormone therapy and Alzheimer’s disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138(3):1031–1039. doi: 10.1016/j.neuroscience.2005. [DOI] [PubMed] [Google Scholar]
- 74.Henderson L.P., Jorge J.C. Steroid modulation of GABAA receptors: CNS roles in reproduction, dysfunction and drug abuse. In: Maue R.A., editor. Advances in molecular and cell biology: molecular insights into ion channel biology in health and disease. Vol. 32. Amsterdam: Elsevier; 2004. pp. 217–249. [Google Scholar]
- 75.Gao B., Moore R.Y. The sexually dimorphic nucleus of the hypothalamus contains GABA neurons in rat and man. Brain Res. 1996;742(1-2):163–171. doi: 10.1016/S0006-8993(96)01005-0. [DOI] [PubMed] [Google Scholar]
- 76.Sagrillo C.A., Selmanoff M. Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the diagonal band of broca and the sexually dimorphic nucleus of the preoptic area. J. Neuroendocrinol. 1997;9(9):699–706. doi: 10.1046/j.1365-2826.1997.00630.x. [DOI] [PubMed] [Google Scholar]
- 77.Penatti C.A., Porter D.M., Jones B.L., Henderson L.P. Sex specific effects of chronic anabolic androgenic steroid treatment on GABAA receptor expression and function in adolescent mice. Neuroscience. 2005;135(2):533–543. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 78.Oberlander J.G., Porter D.M., Penatti C.A., Henderson L.P. Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain. J. Neuroendocrinol. 2012;24(1):202–214. doi: 10.1111/j.1365-2826.2011.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winters S.J. Androgens: endocrine physiology and pharmacology. NIDA Res. Monogr. 1990;102:113–130. [PubMed] [Google Scholar]
- 80.Ryan K.J. Biological aromatization of steroids. J. Biol. Chem. 1959;234(2):268–272. [PubMed] [Google Scholar]
- 81.Quincey R.V., Gray C.H. The metabolism of [1,2-3H]17-α-methyltestosterone in human subjects. J. Endocrinol. 1967;37(1):37–55. doi: 10.1677/joe.0.0370037. [DOI] [PubMed] [Google Scholar]
- 82.Dimick D.F., Heron M., Baulieu E.E., Jayle M.F. A comparative study of the metabolic fate of testosterone, 17 α-methyl-testosterone. 19-nor-testosterone. 17 α-methyl-19-nor-testosterone and 17 α-methylestr-5(10)-ene-17 β-ol-3-one in normal males. Clin. Chim. Acta. 1961;6:63–71. doi: 10.1016/0009-8981(61)90037-7. [DOI] [PubMed] [Google Scholar]
- 83.Schänzer W. Metabolism of anabolic androgenic steroids. Clin. Chem. 1996;42(7):1001–1020. [PubMed] [Google Scholar]
- 84.Fragkaki A.G., Angelis Y.S., Tsantili-Kakoulidou A., Koupparis M., Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J. Steroid Biochem. Mol. Biol. 2009;115(1-2):44–61. doi: 10.1016/j.jsbmb.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Penatti C.A., Porter D.M., Henderson L.P. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J. Neurosci. 2009;29(40):12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luzardo O.P., Machín R.P., Díaz-Chico B.N., Fernández L. Photoaffinity labeling identification of a specific binding protein for the anabolic steroids stanozolol and danazol: an oligomeric protein regulated by age, pituitary hormones, and ethinyl estradiol. Endocrinology. 2000;141(9):3377–3387. doi: 10.1210/endo.141.9.7667. [DOI] [PubMed] [Google Scholar]
- 87.Clark A.S., Jones B.L., Yang P., Henderson L.P. 2004. Anabolic androgenic steroids and the brain: novel actions at the GABAA receptor and on GABAA receptor mediated-behaviors. [Google Scholar]
- 88.Clark A.S., Costine B.A., Jones B.L., Kelton-Rehkopf M.C., Meerts S.H., Nutbrown-Greene L.L., Penatti C.A., Porter D.M., Yang P., Henderson L.P. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126(1):122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 89.Henderson L.P. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52(7):1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinna G., Costa E., Guidotti A. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc. Natl. Acad. Sci. USA. 2005;102(6):2135–2140. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinna G., Agis-Balboa R.C., Pibiri F., Nelson M., Guidotti A., Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem. Res. 2008;33(10):1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 92.Baulieu E.E., Robel P. Non-genomic mechanisms of action of steroid hormones. Ciba Found Symp. 1995;191(10):24–37. doi: 10.1002/9780470514757.ch3. [DOI] [PubMed] [Google Scholar]
- 93.Foradori C.D., Weiser M.J., Handa R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008;29(2):169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kicman A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michels G., Hoppe U.C. Rapid actions of androgens. Front. Neuroendocrinol. 2008;29(2):182–198. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Lieberherr M., Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J. Biol. Chem. 1994;269(10):7217–7223. [PubMed] [Google Scholar]
- 97.Gorczynska E., Handelsman D.J. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995;136(5):2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 98.Steinsapir J., Socci R., Reinach P. Effects of androgen on intracellular calcium of LNCaP cells. Biochem. Biophys. Res. Commun. 1991;179(1):90–96. doi: 10.1016/0006-291X(91)91338-D. [DOI] [PubMed] [Google Scholar]
- 99.Benten W.P., Lieberherr M., Stamm O., Wrehlke C., Guo Z., Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol. Biol. Cell. 1999;10(10):3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benten W.P., Lieberherr M., Sekeris C.E., Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407(2):211–214. doi: 10.1016/S0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- 101.Benten W.P., Lieberherr M., Giese G., Wrehlke C., Stamm O., Sekeris C.E., Mossmann H., Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13(1):123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 102.Foradori C.D., Werner S.B., Sandau U.S., Clapp T.R., Handa R.J. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149(1):155–164. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 103.Vicencio J.M., Estrada M., Galvis D., Bravo R., Contreras A.E., Rotter D., Szabadkai G., Hill J.A., Rothermel B.A., Jaimovich E., Lavandero S. Anabolic androgenic steroids and intracellular calcium signaling: a mini review on mechanisms and physiological implications. Mini Rev. Med. Chem. 2011;11(5):390–398. doi: 10.2174/138955711795445880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berridge M.J., Bootman M.D., Lipp P. Calcium-a life and death signal. Nature. 1998;395(6703):645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 105.Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog. Brain Res. 2009;175:443–452. doi: 10.1016/S0079-6123(09)17529-5. [DOI] [PubMed] [Google Scholar]
- 106.Pang Z.P., Sudhof T.C. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 2010;22(4):496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kemmerling U., Muñoz P., Müller M., Sánchez G., Aylwin M.L., Klann E., Carrasco M.A., Hidalgo C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium. 2007;41(5):491–502. doi: 10.1016/j.ceca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 108.Marchenko S.M., Thomas R.C. Nuclear Ca2+ signalling in cerebellar Purkinje neurons. Cerebellum. 2006;5(1):36–42. doi: 10.1080/14734220600554438. [DOI] [PubMed] [Google Scholar]
- 109.Abramov A.Y., Duchen M.R. Impaired mitochondrial bioenergetics determines glutamate-induced delayed calcium deregulation in neurons. Biochim. Biophys. Acta. 2010;1800(3):297–304. doi: 10.1016/j.bbagen.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 110.Vicencio J.M., Lavandero S., Szabadkai G. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 2010;47(2):112–121. doi: 10.1016/j.ceca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 111.Harr M.W., Distelhorst C.W. Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb. Perspect. Biol. 2010;2(10):a005579. doi: 10.1101/cshperspect.a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cabeza M., Flores M., Bratoeff E., de la Peña A., Mendez E., Ceballos G. Intracellular Ca2+ stimulates the binding to androgen receptors in platelets. Steroids. 2004;69(11-12):767–772. doi: 10.1016/j.steroids.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Estrada M., Espinosa A., Müller M., Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 114.Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Barone M.V., Ametrano D., Zannini M.S., Abbondanza C., Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gong Y., Blok L.J., Perry J.E., Lindzey J.K., Tindall D.J. Calcium regulation of androgen receptor expression in the human prostate cancer cell line LNCaP. Endocrinology. 1995;136(5):2172–2178. doi: 10.1210/endo.136.5.7720667. [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto A., Arai Y., Urano A., Hyodo S. Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm. Behav. 1994;28(4):357–366. doi: 10.1006/hbeh.1994.1032. [DOI] [PubMed] [Google Scholar]
- 117.Rubinow D.R., Schmidt P.J. Androgens, brain, and behavior. Am. J. Psychiatry. 1996;153(8):974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 118.Cunningham R.L., Lumia A.R., McGinnis M.Y. Androgenic anabolic steroid exposure during adolescence: ramifications for brain development and behavior. Horm. Behav. 2013;64(2):350–356. doi: 10.1016/j.yhbeh.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Estrada M., Uhlen P., Ehrlich B.E. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J. Cell Sci. 2006;119(Pt 4):733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 120.Goldberg J.L., Barres B.A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 121.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., Hengartner M., Knight R.A., Kumar S., Lipton S.A., Malorni W., Nuñez G., Peter M.E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G., Nomenclature Committee on Cell Death 2009 Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V.L., El-Deiry W.S., Fulda S., Gottlieb E., Green D.R., Hengartner M.O., Kepp O., Knight R.A., Kumar S., Lipton S.A., Lu X., Madeo F., Malorni W., Mehlen P., Nuñez G., Peter M.E., Piacentini M., Rubinsztein D.C., Shi Y., Simon H.U., Vandenabeele P., White E., Yuan J., Zhivotovsky B., Melino G., Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Janjic M.M., Stojkov N.J., Andric S.A., Kostic T.S. Anabolicandrogenic steroids induce apoptosis and NOS2 (nitric-oxide synthase 2) in adult rat Leydig cells following in vivo exposure. Reprod. Toxicol. 2012;34(4):686–693 . doi: 10.1016/j.reprotox.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Riezzo I., De Carlo D., Neri M., Nieddu A., Turillazzi E., Fineschi V. Heart disease induced by AAS abuse, using experimental mice/rats models and the role of exercise-induced cardiotoxicity. Mini Rev. Med. Chem. 2011;11(5):409–424. doi: 10.2174/138955711795445862. [DOI] [PubMed] [Google Scholar]
- 126.Shokri S., Aitken R.J., Abdolvahhabi M., Abolhasani F., Ghasemi F.M., Kashani I., Ejtemaeimehr S., Ahmadian S., Minaei B., Naraghi M.A., Barbarestani M. Exercise and supraphysiological dose of nandrolone decanoate increase apoptosis in spermatogenic cells. Basic Clin. Pharmacol. Toxicol. 2010;106(4):324–330. doi: 10.1111/j.1742-7843.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 127.Tousson E., Alm-Eldeen A., El-Moghazy M. p53 and Bcl-2expression in response to boldenone induced liver cells injury. Toxicol. Ind. Health. 2011;27(8):711–718. doi: 10.1177/0748233710395350. [DOI] [PubMed] [Google Scholar]
- 128.Fineschi V., Di Paolo M., Neri M., Bello S., D’Errico S., Dinucci D., Parente R., Pomara C., Rabozzi R., Riezzo I., Turillazzi E. Anabolic steroid- and exercise-induced cardio-depressant cytokines and myocardial β1 receptor expression in CD1 mice. Curr. Pharm. Biotechnol. 2011;12(2):275–284. doi: 10.2174/138920111794295792. [DOI] [PubMed] [Google Scholar]
- 129.Wiren K.M., Toombs A.R., Semirale A.A., Zhang X. Osteoblast and osteocyte apoptosis associated with androgen action in bone: requirement of increased Bax/Bcl-2 ratio. Bone. 2006;38(5):637–651. doi: 10.1016/j.bone.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 130.Zaugg M., Jamali N.Z., Lucchinetti E., Xu W., Alam M., Shafiq S.A., Siddiqui M.A. Anabolic-androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J. Cell Physiol. 2001;187(1):90–95. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1057>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 131.Abu-Shakra S., Alhalabi M.S., Nachtman F.C., Schemidt R.A., Brusilow W.S. Anabolicsteroids induce injury and apoptosis of differentiated skeletal muscle. J. Neurosci. Res. 1997;47(2):186–197. [PubMed] [Google Scholar]
- 132.Becker C., Riedmaier I., Reiter M., Tichopad A., Groot M.J., Stolker A.A., Pfaffl M.W., Nielen M.F., Meyer H.H. Influence of anabolic combinations of an androgen plus an estrogen on biochemical pathways in bovine uterine endometrium and ovary. J. Steroid Biochem. Mol. Biol. 2011;125(3-5):192–201. doi: 10.1016/j.jsbmb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Lopes R.A., Neves K.B., Pestana C.R., Queiroz A.L., Zanotto C.Z., Chignalia A.Z., Valim Y.M., Silveira L.R., Curti C., Tostes R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Heart Circ. Physiol. 2014;306(11):H1485–H1494. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 134.D’Ascenzo S., Millimaggi D., Di Massimo C., Saccani-Jotti G., Botrè F., Carta G., Tozzi-Ciancarelli M.G., Pavan A., Dolo V. Detrimental effects of anabolic steroids on human endothelial cells. Toxicol. Lett. 2007;169(2):129–136. doi: 10.1016/j.toxlet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Fanton L., Belhani D., Vaillant F., Tabib A., Gomez L., Descotes J., Dehina L., Bui-Xuan B., Malicier D., Timour Q. Heart lesions associated with anabolic steroid abuse: comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp. Toxicol. Pathol. 2009;61(4):317–323. doi: 10.1016/j.etp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 136.Estrada M., Varshney A., Ehrlich B.E. Elevated testosterone induces apoptosis in neuronal cells. J. Biol. Chem. 2006;281(35):25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- 137.Orlando R., Caruso A., Molinaro G., Motolese M., Matrisciano F., Togna G., Melchiorri D., Nicoletti F., Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–29. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 138.Olney J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(3880):719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 139.Olney J.W., Wozniak D.F., Farber N.B. Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies. Arch. Neurol. 1997;54(10):1234–1240. doi: 10.1001/archneur.1997.00550220042012. [DOI] [PubMed] [Google Scholar]
- 140.Lipton S.A., Rosenberg P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 141.Lipton S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 142.Chen H.S., Lipton S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006;97(6):1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham R.L., Giuffrida A., Roberts J.L. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology. 2009;150(12):5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caraci F., Pistarà V., Corsaro A., Tomasello F., Giuffrida M.L., Sortino M.A., Nicoletti F., Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J. Neurosci. Res. 2011;89(4):592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- 145.Tugyan K., Ozbal S., Cilaker S., Kiray M., Pekcetin C., Ergur B.U., Kumral A. Neuroprotective effect of erythropoietin on nandrolone decanoate-induced brain injury in rats. Neurosci. Lett. 2013;533:28–33. doi: 10.1016/j.neulet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 146.Basile J.R., Binmadi N.O., Zhou H., Yang Y.H., Paoli A., Proia P. Supraphysiological doses of performance enhancing anabolic-androgenic steroids exert direct toxic effects on neuron-like cells. Front. Cell. Neurosci. 2013;7:69. doi: 10.3389/fncel.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heinlein C.A., Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 148.Nicotera P., Lipton S.A. Excitotoxins in neuronal apoptosis and necrosis. J. Cereb. Blood Flow Metab. 1999;19(6):583–591. doi: 10.1097/00004647-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 149.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 150.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 151.Muchowski P.J. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35(1):9–12. doi: 10.1016/s0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 152.Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 153.Beal M.F. Experimental models of Parkinson’s disease. Nat. Rev. Neurosci. 2001;2(5):325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 154.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 155.Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S.A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. USA. 1995;92(16):7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nicotera P., Bernassola F., Melino G. Nitric oxide (NO), a signaling molecule with a killer soul. Cell Death Differ. 1999;6(10):931–933. doi: 10.1038/sj.cdd.4400583. [DOI] [PubMed] [Google Scholar]
- 157.Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29(3-4):222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 158.Nakamura T., Cho D.H., Lipton S.A. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp. Neurol. 2012;238(1):12–21. doi: 10.1016/j.expneurol.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakamura T., Lipton S.A. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer’s and Parkinson’s diseases. 2010. [DOI] [PMC free article] [PubMed]
- 160.Heikkila R., Cohen G. Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science. 1971;172(3989):1257–1258. doi: 10.1126/science.172.3989.1257. [DOI] [PubMed] [Google Scholar]
- 161.Kumar R., Agarwal A.K., Seth P.K. Free radical-generated neurotoxicity of 6-hydroxydopamine. J. Neurochem. 1995;64(4):1703–1707. doi: 10.1046/j.1471-4159.1995.64041703.x. [DOI] [PubMed] [Google Scholar]
- 162.Oishi T., Hasegawa E., Murai Y. Sulfhydryl drugs reduce neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse. J. Neural Transm. Park. Dis. Dement. Sect. 1993;6(1):45–52. doi: 10.1007/BF02252622. [DOI] [PubMed] [Google Scholar]
- 163.Przedborski S., Kostic V., Jackson-Lewis V., Naini A.B., Simonetti S., Fahn S., Carlson E., Epstein C.J., Cadet J.L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 1992;12(5):1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abdel-Salam O.M. The paths to neurodegeneration in genetic Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2014;13(9):1485–1512. doi: 10.2174/1871527313666140806142955. [DOI] [PubMed] [Google Scholar]
- 165.Gan X., Wu L., Huang S., Zhong C., Shi H., Li G., Yu H., Howard Swerdlow R., Chen Xi. Oxidative stressmediated activation of extracellular signal-regulated kinase scontributes to mild cognitive impairment-related mitochondrial dysfunction. [Jul 23];Free Radic. Biol. Med . 2014 doi: 10.1016/j.freeradbiomed.2014.07.021. pii: S0891-5849(14)00338-4 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Radi E., Formichi P., Battisti C., Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014;42(Suppl. 3):S125–S152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 167.McCord M.C., Aizenman E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014;6:77. doi: 10.3389/fnagi.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie A., Gao J., Xu L., Meng D. Shared mechanisms of neurodegeneration in Alzheimer's disease and Parkinson's disease. Biomed. Res. Int. 2014 doi: 10.1155/2014/648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bonda D.J., Wang X., Lee H.G., Smith M.A., Perry G., Zhu X. Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci. Bull. 2014;30(2):243–252. doi: 10.1007/s12264-013-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bhasker A.S., Sant B., Yadav P., Agrawal M., Lakshmana Rao P.V. Plant toxin abrin induced oxidative stress mediated neurodegenerative changes in mice. Neurotoxicology. 2014;44:194–203. doi: 10.1016/j.neuro.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 171.Deng Y., Jiao C., Mi C., Xu B., Li Y., Wang F., Liu W., Xu Z. Melatonin Inhibits Manganese-Induced Motor Dysfunction and Neuronal Loss in Mice: Involvement of Oxidative Stress and Dopaminergic Neurodegeneration. Mol. Neurobiol. 2014 doi: 10.1007/s12035-014-8789-3. [DOI] [PubMed] [Google Scholar]
- 172.Kraemer B.R., Snow J.P., Vollbrecht P., Pathak A., Valentine W.M., Deutch A.Y., Carter B.D. A role for the p75 neurotrophin receptor in axonal degeneration and apoptosis induced by oxidative stress. J. Biol. Chem. 2014;289(31):21205–21216. doi: 10.1074/jbc.M114.563403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chege P.M., McColl G. Caenorhabditis elegans: a model to investigate oxidative stress and metal dyshomeostasis in Parkinson’s disease. Front. Aging Neurosci. 2014;6:89. doi: 10.3389/fnagi.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nirmaladevi D., Venkataramana M., Chandranayaka S., Ramesha A., Jameel N.M., Srinivas C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014;34(7):973–985. doi: 10.1007/s10571-014-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Riezzo I., Turillazzi E., Bello S., Cantatore S., Cerretani D., Di Paolo M., Fiaschi A.I., Frati P., Neri M., Pedretti M., Fineschi V. Chronic nandrolone administration promotes oxidative stress, induction of pro-inflammatory cytokine and TNF-α mediated apoptosis in the kidneys of CD1 treated mice. Toxicol. Appl. Pharmacol. 2014;280(1):97–106. doi: 10.1016/j.taap.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 176.Neri M., Bello S., Bonsignore A., Cantatore S., Riezzo I., Turillazzi E., Fineschi V. Anabolic androgenic steroids abuse and liver toxicity. Mini Rev. Med. Chem. 2011;11(5):430–437. doi: 10.2174/138955711795445916. [DOI] [PubMed] [Google Scholar]
- 177.Cerretani D., Neri M., Cantatore S., Ciallella C., Riezzo I., Turillazzi E., Fineschi V. Looking for Organ Damages Due to Anabolic-androgenic Steroids (AAS): is Oxidative Stress the Culprit? Mini Rev. Org. Chem. 2014;10(4):393–399. doi: 10.2174/1570193X113106660025. [DOI] [Google Scholar]
- 178.Holmes S., Abbassi B., Su C., Singh M., Cunningham R.L. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154(11):4281–4292. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cunningham R.L., Singh M., O’Bryant S.E., Hall J.R., Barber R.C. Oxidative stress, testosterone, and cognition among Caucasian and Mexican-American men with and without Alzheimer’s disease. J. Alzheimers Dis. 2014;40(3):563–573. doi: 10.3233/JAD-131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cunningham R.L., Macheda T., Watts L.T., Poteet E., Singh M., Roberts J.L., Giuffrida A. A. Androgens esacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Horm. Behav. 2011;60(5):617–624. doi: 10.1016/j.yhbeh.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Doyle K.P., Simon R.P., Stenzel-Poore M.P. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mehta S.L., Manhas N., Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 183.Mayeux R., Marder K., Cote L.J., Denaro J., Hemenegildo N., Mejia H., Tang M.X., Lantigua R., Wilder D., Gurland B., Hauser A. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988-1993. Am. J. Epidemiol. 1995;142(8):820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 184.Hofman A., Collette H.J., Bartelds A.I. Incidence and risk factors of Parkinson’s disease in The Netherlands. Neuroepidemiology. 1989;8(6):296–299. doi: 10.1159/000110197. [DOI] [PubMed] [Google Scholar]
- 185.Van Den Eeden S., Tanner C., Bernstein A., et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 186.Hammond J., Le Q., Goodyer C., Gelfand M., Trifiro M., LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001;77(5):1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 187.Nguyen T.V., Yao M., Pike C.J. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J. Neurochem. 2005;94(6):1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 188.Ramsden M., Shin T.M., Pike C.J. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122(3):573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 189.Nguyen T.V., Jayaraman A., Quaglino A., Pike C.J. Androgens selectively protect against apoptosis in hippocampal neurons. J. Neuroendocrinol. 2010;22(9):1013–1022. doi: 10.1111/j.1365-2826.2010.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chisu V., Manca P., Lepore G., Gadau S., Zedda M., Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alphatubulin in a mouse neuroblastoma cell line. Arch. Ital. Biol. 2006;144(2):63–73. [PubMed] [Google Scholar]
- 191.Chisu V., Manca P., Zedda M., Lepore G., Gadau S., Farina V. Effects of testosterone on differentiation and oxidative stress resistance in C1300 neuroblastoma cells. Neuroendocrinol. Lett. 2006;27(6):807–812. [PubMed] [Google Scholar]
- 192.Ahlbom E., Grandison L., Bonfoco E., Zhivotovsky B., Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur. J. Neurosci. 1999;11(4):1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 193.Ahlbom E., Prins G.S., Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. doi: 10.1016/S0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 194.Matrisciano F., Modafferi A.M., Togna G.I., Barone Y., Pinna G., Nicoletti F., Scaccianoce S. Repeated anabolic androgenic steroid treatment causes antidepressant-reversible alterations of the hypothalamic-pituitary-adrenal axis, BDNF levels and behavior. Neuropharmacology. 2010;58(7):1078–1084. doi: 10.1016/j.neuropharm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 195.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tucci P., Morgese M.G., Colaianna M., Zotti M., Schiavone S., Cuomo V., Trabace L. Neurochemical consequence of steroid abuse: stanozolol-induced monoaminergic changes. Steroids. 2012;77(3):269–275. doi: 10.1016/j.steroids.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 197.Tirassa P., Thiblin I., Agren G., Vigneti E., Aloe L., Stenfors C. 1997. [DOI] [PubMed]
- 198.Handa R.J., Pak T.R., Kudwa A.E., Lund T.D., Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm. Behav. 2008;53(5):741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dodge T., Hoagland M.F. The use of anabolic androgenic steroids and polypharmacy: a review of the literature. Drug Alcohol Depend. 2011;114(2-3):100–109. doi: 10.1016/j.drugalcdep.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schulze J.J., Rane A., Ekström L. Genetic variation in androgen disposition: implications in clinical medicine including testosterone abuse. Expert Opin. Drug Metab. Toxicol. 2009;5(7):731–744. doi: 10.1517/17425250902976862. [DOI] [PubMed] [Google Scholar]
- 201.Townsend D.M. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007;7(6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xiong Y., Uys J.D., Tew K.D., Townsend D.M. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011;15(1):233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Virmani A., Ali S.F., Binienda Z.K. Neuroprotective strategies in drug abuse-evoked encephalopathy. Ann. N. Y. Acad. Sci. 2010;1199:52–68. doi: 10.1111/j.1749-6632.2009.05171.x. [DOI] [PubMed] [Google Scholar]
- 204.Akram Y., Copello A., Moore D. Family-based interventions for substance misuse: a systematic review of systematic reviews--protocol. Syst. Rev. 2014;3:90. doi: 10.1186/2046-4053-3-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Uys J.D., Mulholland P.J., Townsend D.M. Glutathione and redox signaling in substance abuse. Biomed. Pharmacother. 2014;68(6):799–807. doi: 10.1016/j.biopha.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Antonelli M.C., Guillemin G.J., Raisman-Vozari R., Del-Bel E.A., Aschner M., Collins M.A., Tizabi Y., Moratalla R., West A.K. New strategies in neuroprotection and neurorepair. Neurotox. Res. 2012;21(1):49–56. doi: 10.1007/s12640-011-9265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


