Abstract
Cognitive enhancement can be defined as the use of drugs and/or other means with the aim to improve the cognitive functions of healthy subjects in particular memory, attention, creativity and intelligence in the absence of any medical indication. Currently, it represents one of the most debated topics in the neuroscience community. Human beings always wanted to use substances to improve their cognitive functions, from the use of hallucinogens in ancient civilizations in an attempt to allow them to better communicate with their gods, to the widespread use of caffeine under various forms (energy drinks, tablets, etc.), to the more recent development of drugs such as stimulants and glutamate activators. In the last ten years, increasing attention has been given to the use of cognitive enhancers, but up to now there is still only a limited amount of information concerning the use, effect and functioning of cognitive enhancement in daily life on healthy subjects. The first aim of this paper was to review current trends in the misuse of smart drugs (also known as Nootropics) presently available on the market focusing in detail on methylphenidate, trying to evaluate the potential risk in healthy individuals, especially teenagers and young adults. Moreover, the authors have explored the issue of cognitive enhancement compared to the use of Anabolic Androgenic Steroids (AAS) in sports. Finally, a brief overview of the ethical considerations surrounding human enhancement has been examined.
Keywords: Anabolic androgenic steroids (AAS), cosmetic neurology, Human enhancement, methylphenidate, smart drugs
INTRODUCTION
Cognitive enhancement can be defined as the use of drugs and/or other means with the aim to improve the cognitive functions of healthy subjects in particular memory, attention, creativity and intelligence (intended as problem solving ability) in the absence of any medical indication [1, 2].
Currently, it represents one of the most debated topics in the neuroscience community. Human beings have always wanted to use substances to improve their cognitive functions, from the use of hallucinogens in ancient civilizations in an attempt to allow them to better communicate with their gods, to the widespread use of caffeine under various forms (energy drinks, tablets, etc.), to the more recent development of drugs such as stimulants and glutamate activators. According to many, this continued demand for substances to improve cognitive skills is a typically human feature and therefore cognitive enhancement can be considered as the application of new scientific advances to achieve the age-old desire of self-improvement [3-5].
In the last ten years, increasing attention has been given to the use of cognitive enhancers, but up to now there is still only a limited amount of information concerning the use, effect and functioning of cognitive enhancement in daily life on healthy subjects [6].
The first aim of this paper is to review current trends in the misuse of smart drugs (also known as Nootropics) presently available on the market focusing in detail on methylphenidate, trying to evaluate the potential risk in healthy individuals, especially teenagers and young adults.
Secondly, the authors will explore the issue of cognitive enhancement compared to the use of Anabolic Androgenic Steroids (AAS) in sports.
Finally, a brief overview of the ethical considerations surrounding human enhancement will be examined.
SMART DRUGS AS COGNITIVE ENHANCERS: THE EMBLEMATIC CASE OF METHYLPHENIDATE
Methylphenidate (Fig. 1) is a psycostimulant, present on the market with different trade names (e.g. Ritalin, Concerta, Methylin, Equasym XL). It was initially synthesized in 1944 by Leandro Panizzon and sold with the trade name of “Ritalin” by Ciba-Geigy Pharmaceutical Company in 1954 [7]. It was used at the beginning for the treatment of several conditions, such as: depressive states, psychosis associated to narcolepsy etc. [8]. Presently, methylphenidate is one of the most commonly prescribed drugs for the treatment of Attention deficit-hyperactivity disorder (ADHD) [9].
Fig. (1).
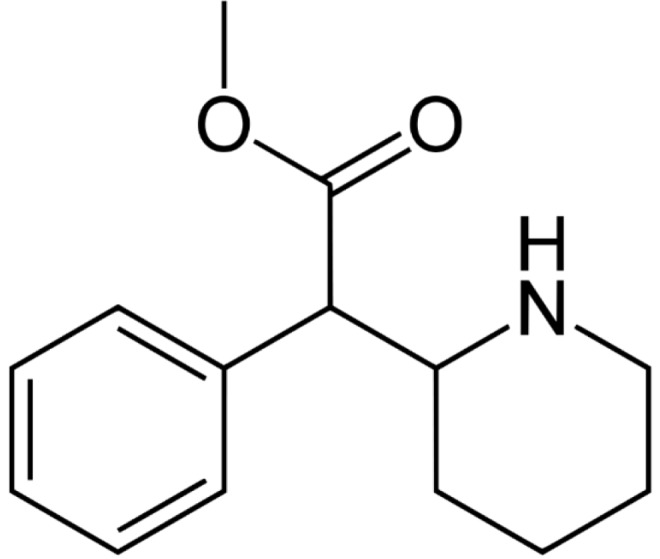
Chemical structure of Methylphenidate.
In order to understand the possible efficacy of this drug as a cognitive enhancer, a fundamental starting point is represented by its pharmacodynamics. Methylphenidate is a benzylpiperidine and phenethylamine derivative and it acts as a dopamine-norepinephrine reuptake inhibitor, by binding and blocking dopamine and norepinephrine transporters, which reuptake them within the presynaptic neuron after their release, although methylphenidate is most effective in modulating the levels of dopamine rather than norepinephrine [10, 11].
Numerous studies concerning the cognitive enhancing effects of methylphenidate on the normal brain have involved adult animals and humans [12-14]. At high doses (5–10 mg/kg), administered intraperitoneally in rats, there is an increase of the locomotor activity and an impairment of attention and performance, which affect those cognitive skills belonging to prefrontal cortex, whereas the intraperitoneal administration of low doses (0.5–2 mg/kg) improves cognitive performance and reduces motor activity. The administration of lower doses (0.25–1 mg/kg) of methylphenidate in healthy rats increases the attention skills without affecting the motor activity [15].
The potential effects of the psycostimulants (e.g. methylphenidate) and agents active on catecholamines, represent a plausible risk of cognitive enhancement. When the levels of dopamine and norepinephrine are optimal, they bind, showing a high affinity, respectively to D1-like receptor and α2 receptors, increasing the signal to noise ratio (S/N) in the prefrontal cortex. At higher doses, dopamine starts to bind to D2 receptors, while norepinephrine binds to α1 and β receptors; as a consequence there will be a reduction of S/N and the activation of neurons not involved in these tasks [16].
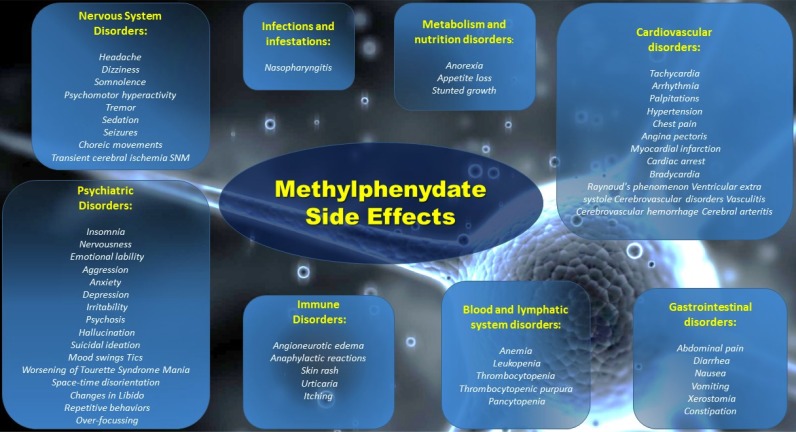
The abuse of methylphenidate among adolescents can be particularly dangerous and numerous side effects can arise after a prolonged use of this drug (see Fig. 2). Moreover, methylphenidate has an important role in regulating dopamine/norepinephrine levels, which can alter the maturation process of the prefrontal cortex, deeply active during that period of life [17].
Fig. (2).
Possible side effects after chronic use of Methylphenidate.
Lee et al [18] observed the effects of repeated methylphenidate exposure on the locomotor diurnal rhythm activity patterns of female adolescent Sprague–Dawley (SD) rats, which were divided into 4 groups: control, 0.6 mg/kg, 2.5 mg/kg, and 10 mg/kg methylphenidate group. The results obtained showed that the repeated administration of 2.5 mg/kg and 10 mg/kg of this drug was able to modify the locomotor diurnal rhythm patterns, suggesting that these doses exert long-term effects.
Urban et al [19] treated juvenile and adult SD rats with methylphenidate, and the neuronal excitability and synaptic transmission in pyramidal neurons of prefrontal cortex were examined. The results of this study showed that an intraperitoneal dose of 1 mg/kg, either single dose or chronic treatment produced significant depressive effects on pyramidal neurons by increasing hyperpolarization-activated currents in juvenile rat prefrontal cortex, whereas exerting excitatory effects in adult rats. Minimum clinically-relevant doses (from 0.03 to 0.3 mg/kg) also produced depressive effects in juvenile rats, in a linear dose-dependent manner. Function recovered within 1 week from chronic 1 mg/kg treatment, however chronic treatment with 3 and 9 mg/kg resulted in depression of prefrontal neurons lasting 10 weeks and more. According to this study the juvenile prefrontal cortex is supersensitive to methylphenidate, and the accepted therapeutic range for adults has been overestimated. Juvenile treatment with this drug could result in long-lasting, potentially permanent changes to excitatory neuron function in the prefrontal cortex of juvenile rats.
Therefore, the dosage of methylphenidate and the age of the subjects in relation to prefrontal cortical plasticity remain the key points, which should be better investigated with further studies involving healthy subjects, in order to clarify several aspects still not entirely understood.
Although according to numerous reports, methylphenidate is widely abused as a cognitive enhancer among healthy subjects, especially young adults and teenagers, it is very difficult to determine the actual trend of abuse [20]. First of all, it is necessary to distinguish the illicit from therapeutic use among people affected by ADHD or other pathologies requiring methylphenidate as therapy.
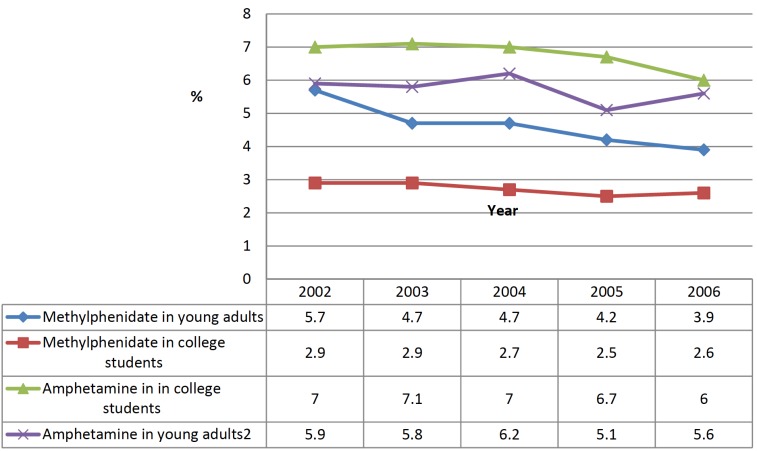
According to the U.S. Department of Health and Human Services, in monitoring the Future National Survey Results on Drug Use, 1975–2006 [21], the use of methylphenidate among college students was 3.9% in 2006, whereas in young adults it was 2.6%. It is interesting to underline a slight decrease in the use of this drug in both categories between 2002 and 2006 (see Fig. 3). Moreover, these trends of use if compared to other phenethylamines commonly abused, such as amphetamines, show that methylphenidate is less used both in college students and in young adults.
Fig. (3).
Trends of use of methylphenidate compared to amphetamine during the years 2002-2006.
Numerous other studies have investigated the misuse/abuse of methylphenidate in healthy subjects, but because of different methodologies, type of studies and statistical analysis they cannot be uniformly compared; Babcock and Byrne [22] showed the results of a study, in which the use of methylphenidate in a small New England college was investigated. According to this study about 16% of the student population had used the drug for recreational purposes and it is the most common among students (aged 18–24 years).
Teter et al [23] carried out a larger study at the University of Michigan, by administering a questionnaire. About 3% of the students had used or abused methylphenidate, the survey was completed within a year. Males and females presented nearly the same percentages of misuse/abuse. In addition, an association between misuse and attendance at parties was found.
White et al [24] report in their research that 16.2% of students have misused or abused stimulants. Of this group, 96% identified Ritalin as their stimulant of choice. The frequency of abuse in more than 50% of misusers was 2-3 times per year, in almost 34% it was monthly (1-2 times/month) and in 15.5% it was weekly (2-3 times/week).
Despite not allowing for comparative analysis, the above reported studies highlight a wide misuse/abuse of methylphenidate as a cognitive enhancer among teenagers, college students and young adults.
ANABOLIC ANDROGENIC STEROIDS MISUSE AND ABUSE
“Anabolic steroids” is the name given to synthetic variants of the male sex hormone testosterone. The comprehensive term for these compounds is anabolic-androgenic steroids (AASs): “anabolic” refers to muscle-building and “androgenic” refers to the increased male sexual features.
AASs can be used for clinical purposes in the treatment of several pathologies: several forms of anemia, acute and chronic wounds, severe burns, short stature, osteoporosis, hypogonadism (primary or secondary forms), AIDS wasting syndrome, catabolic states due to long-term corticosteroid therapy etc. [25-28].
Besides the numerous clinical applications of AAS, they are always more misused and abused by athletes as an ergogenic aid to improve their performance and/or physical shape [29].
Sports involving power production and strength training are the ones which commonly involve the use of AASs. Back in the 1950s, these compounds were used by a handful of professional athletes, but as they became more accessible and their use was extended to include also high school and college students [29-31]. Furthermore, as an ever increasing number of women use these compounds, the abuse of AASs can no longer be said to belong mostly to the male population [32].
The issue of AASs misuse and abuse creates some difficulties in order to make a proper and accurate estimation. Several aspects, such as the neurological effects of AASs are still under investigation. Moreover, in some cases, there is conflicting data, especially concerning psychological effects.
The abuser is placed under numerous influences and a very complex interconnection of forces, which cannot be ignored. The slogan “win at all costs” is the one that makes better this concept. Moreover, the significant appreciation of the society for winning athletes represents a further decisive factor for the consumption of these substances.
The issue of AAS use and abuse by athletes allows us to make numerous ethical considerations:
If on the one hand, the position of banning the use of AASs could be adopted, except for medical purposes, taking into consideration the numerous side effects associated with their use, on the other hand this stance may appear as “very parentalistic or paternalistic”, and it cannot be applied to “professional” athletes, who consider the use of AASs and their side effects as an equal exchange in order to succeed in sport [33]. This view point can be significantly internalized by athletes who follow tough training programs for several years in order to progress in levels of certain competitive sports only to discover that many of their rivals are using AASs, forcing upon them a difficult choice; either to decide to use AASs or to participate at a known disadvantage, or to withdraw from competition.
Generally in sports, athletes train in order to be better than other competitors, thereby beating their opponents. This advantage can be achieved through fair play, competitiveness, professional foul, or through the use of performance enhancing drugs like AASs [33]. This raises an ethical issue; if all athletes in a particular sport decide to consume AASs, would that not produce a fair competitive setting? The fairness would depend on whether all athletes were using a similar typology and quality of drugs [34]. If one argues that participants are free to choose to take part in competitive sports and are therefore free to use AASs or not, in spite of the possible side effects, then surely it can be regarded as an athlete’s right to choose [33-35].
Many argue that using AASs allows an athlete to perform beyond the natural limits of the human body. The counter argument could be that the widely accepted use of coaches, vitamins, or weight training, already pushes athletes beyond their natural limits [33]. Athletes are already able to perfect their performance as a result of scientifically prepared training programs. However, if it is agreed upon that sport is a test of character and greatness within the natural limitations of man, then AASs use would be seen as wrong because competitors would no longer be within this human state but in a pharmacologically-induced state [33].
AASs are able to modify the mood through numerous mechanisms [36-38]. Testosterone and AASs can act as a central monoamine oxidase inhibitor (MAOI).Therefore, AASs are considered mood enhancers, investigated in subjects affected by depression. Vogel et al [39] confronted the antidepressant effects of amitriptyline (range: from 75 mg/d to 300 mg/d) with those of mesterolone (range: from 100 mg/d to 550 mg/d) in a double-blind design with 34 male patients suffering with depression. According to this study [39] both drugs had a similar effect in diminishing the depressive symptoms and at the same time the mesterolone caused far less side effects than amitriptyline.
The neurologic and psychiatric consequences due to AAS abuse reported in adolescents and young adults require a careful evaluation.
Irving et al [40] performed a study in 4746 middle and high school students, in order to assess the prevalence of AAS use and to identify social, environmental and behavioral aspects concerning the health of students using these drugs. The results obtained show that AASs were more commonly used by males (5.4%), whereas the prevalence of use in females was 2.9%. In males, an association between AAS use and “poorer self-esteem and higher rates of depressed mood and attempted suicide, poorer knowledge and attitudes about health, greater participation in sports that emphasize weight and shape, greater parental concern about weight, and higher rates of disordered eating and substance use”, was found. In female students, the association between AAS use and the condition above reported was weaker, even if numerous common aspects between the two groups were highlighted.
Another study [41] conducted on 3054 high school students showed the association between the frequency of AAS use among students and high-risk behaviors, suggesting that AAS use is more a contributor to a "risk behavior syndrome" than a former of an isolated behavior.
Several cases reporting psychiatric symptoms in subjects using AASs are described in the literature [28, 42-44].
Thiblin et al [44] reported eight suicidal cases involving males aging from 21 to 33 years old, with a present or previous history of AAS use. In 5 cases, suicide was committed during the use of AAS, whereas in 2 cases it was committed after 2 and 6 months respectively following AAS discontinuation. Only in one case it was not possible to establish whether the subject was using or had recently stopped the use of AAS. In 5 males, their relatives had observed the onset of depressive symptoms associated to AAS discontinuation. In 4 cases, following a long use of AASs, the development of a depressive syndrome was noticed. Hypomania-like symptoms appeared in 2 cases right before committing suicide.
Only 1 out of the 8 cases examined by Thiblin et al [44] had developed suicidal thoughts prior to the consumption of AASs, however, the presence of suicidal risk factors, regardless of the use of anabolic steroids, was found in all cases. According to the authors of this study, psychiatric symptoms and conflicts because of a prolonged use of AASs can contribute to suicide in subjects with predisposing risk factors.
DISCUSSION AND CONCLUSIONS
Cognitive enhancers and AASs can be considered as the two sides of the same coin. Both of them represent the more general desire of “human enhancement” which can be defined as the wish to overcome, temporarily or permanently, the existing limits in the human body with natural or artificial methods. The use of artificial methods (including the use of drugs) in order to choose, vary and improve human features and capabilities beyond those that already exist in human beings, is the heart of this concept [45, 46].
In the era of “cosmetic neurology”, the first question that we ought to ask ourselves is, why should methylphenidate, be considered as a cognitive enhancer in the absence of scientific evidence? Many arguments can be provided; first of all, the one we think better suits this answer is “the sociological desire to find a pharmacological solution to social problems” [20]. But is this desire strong enough to support the wide spread of methylphenidate as a cognitive enhancer? And then, is its use among healthy subjects really so diffused?
The first question can be answered in this way, sustaining that “sociological desire” generates numerous expectations regarding what this compound can do, could do or even should do [47, 48]. These expectations have also promoted large speculation about the enhancement of different cognitive domains such as memory, attention and creativity. The overestimation of these expectations makes feasible what is only potentially hypothetical. Furthermore, another reason that supports the vast abuse of this drug rises from the mental association between the therapeutic use for ADHD treatment and its misuse in order to improve learning skills in healthy subjects. This improper use is based on exceeding the marked borderline between health and disease, which appears in the eyes of abusers increasingly blurred until it disappears. As a consequence, the word “treatment” might become a synonym of enhancement in the cognitive field [20, 48, 49].
Regarding the diffusion of methylphenidate among healthy subjects, no exhaustive conclusion can be formulated. Moreover, it is always necessary to contextualize the data available, not only by comparing its trends of abuse with other smart drugs, but also with several other drugs of abuse, which are constantly changing.
The expected cognitive advantages related to the use of methylphenidate offer an unfair advantage to those who use it. This “unfair advantage” becomes more evident in sport and the use of AASs represents a frank manifestation of this form of “cheating” [50, 51]. AASs have been abused over the time either alone or in association to several other prohibited substances especially among bodybuilders, such as gamma-hydroxybutyrate (GHB), which was thought to induce a significant release of growth hormone (GH) [52, 53].
We can argue that the thread that joins cognitive enhancers (including methylphenidate) and AASs in academia and in sport are the expected results and the Ovid’s maxim “exitus acta probat” (the result validates the deeds) highlights in fact this concept; the “exitus” is represented by human enhancement (both physical and mental), whereas the “acta” necessary to pursue this goal are represented by the misuse and abuse of these substances not taking into account the numerous established and potential side effects. The latter can represent the same criterion adopted in banning their use in sports, whether the risk is a real or only potential. This strategy has been applied by several organizations, such as: the World Anti-Doping Agency (WADA), Association of Tennis Professionals, Major League Baseball, Fédération Internationale de Football Association, the Olympics, the National Basketball Association, the National Hockey League, and the National Football League and many others [54-56].
The topic of cosmetic neurology is a controversial but emerging field, where medical therapies are used to enhance neurological functions [57]. The discussion above reported and the brief review of the literature in this field allow us almost to speculate: if on the one hand, cognitive enhancers may be intended as potential “steroids of the mind”, on the other hand, AASs can be considered “enhancers of the body”. This dualism may explain why there are the two sides of the same coin.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Lanni C, Lenzken S.C, Pascale A, Del Vecchio I, Racchi M, Pistoia F, Govoni S. Cognition enhancers between treating and doping the mind. Pharmacol. Res. 2008;57(3):196–213. doi: 10.1016/j.phrs.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy D. Just treat, or enhance? Science. 2004;304(5667):17. doi: 10.1126/science.304.5667.17. [DOI] [PubMed] [Google Scholar]
- 3.Rose S.P. ‘Smart drugs’: do they work? Are they ethical? Will they be legal? Nat. Rev. Neurosci. 2002;3(12):975–979. doi: 10.1038/nrn984. [DOI] [PubMed] [Google Scholar]
- 4.Farah M.J, Illes J, Cook-Deegan R, Gardner H, Kandel E, King P, Parens E, Sahakian B, Wolpe P.R. Neurocognitive enhancement: what can we do and what should we do? Nat. Rev. Neurosci. 2004;5(5):421–425. doi: 10.1038/nrn1390. [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee A. The promise and predicament of cosmetic neurology. J. Med. Ethics. 2006;32:110e13. doi: 10.1136/jme.2005.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Normann C, Berger M. Neuroenhancement: status quo and perspectives. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258: 110e14. doi: 10.1007/s00406-008-5022-2. [DOI] [PubMed] [Google Scholar]
- 7.Morton W.A, Stockton G.G. Methylphenidate abuse and psychiatric side effects. Prim. Care Companion J. Clin. Psychiatry. 2000;2(5):159–164. doi: 10.4088/PCC.v02n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard B.E, McCartan D, White J, King D.J. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum. Psychopharmacol. 2004;19(3):151–180. doi: 10.1002/hup.579. [DOI] [PubMed] [Google Scholar]
- 9.Lange K.W, Reichl S, Lange K.M, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2010;2(4):241–255. doi: 10.1007/s12402-010-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heal D.J, Pierce D.M. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006;20(9):713–738. doi: 10.2165/00023210-200620090-00002. [DOI] [PubMed] [Google Scholar]
- 11.Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br. J. Pharmacol. 2006;147(Suppl. 1):S82–S88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11(1-2):97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011;116(2):164–76. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markowitz J.S, DeVane C.L, Pestreich L.K, Patrick K.S, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J. Child Adolesc. Psychopharmacol. 2006;16(6):687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- 15.Mehta M.A, Sahakian B.J, Mavaddat N, Pickard J.D, Robbins T.W, Solanto M.V, Arnstenand A.F, Castellanos F.X. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD and experimental animals; pp. 303–331. [Google Scholar]
- 16.Arnsten A.F, Li B.M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Urban K.R, Gao W.J. Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain. Front. Syst. Neurosci. 2014;8:38. doi: 10.3389/fnsys.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M J, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology. 2009;57(3):201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Urban K.R, Waterhouse B.D, Gao W.J. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biol. Psychiatry. 2012;15(72):880–888. doi: 10.1016/j.biopsych.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Outram S.M. The use of methylphenidate among students: the future of enhancement? J. Med. Ethics. 2010;36(4):198–202. doi: 10.1136/jme.2009.034421. [DOI] [PubMed] [Google Scholar]
- 21.Johnston L.D, O’Malley P.M, Bachman J.G, Schulenberg J.E. Monitoring the future national survey results on drug use. College students and adults ages 19-45. National Institutes of Health. U.S. Department of Health and Human Services. 1975 - 2006;II doi: 10.1037/e567272009-001. [DOI] [Google Scholar]
- 22.Babcock Q, Byrne T. Student perceptions of methylphenidate abuse at a public liberal arts college. J. Am. Coll. Health. 2000;49(3):143–145. doi: 10.1080/07448480009596296. [DOI] [PubMed] [Google Scholar]
- 23.Teter C.J, McCabe S.E, Boyd C.J, Guthrie S.K. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609–617. doi: 10.1592/phco.23.5.609.34187. [DOI] [PubMed] [Google Scholar]
- 24.White BP, Becker-Blease K, Grace-Bishop K. Stimulantmedication use, misuse, andabuse in an undergraduate and graduate student sample. J. Am. Coll. Health. 2006;54:261e8. doi: 10.3200/JACH.54.5.261-268. [DOI] [PubMed] [Google Scholar]
- 25.Basaria S. Androgen abuse in athletes: detection and consequences. J. Clin. Endocrinol. Metab. 2010;95(4):1533–1543. doi: 10.1210/jc.2009-1579. [DOI] [PubMed] [Google Scholar]
- 26.Turillazzi E, Perilli G, Di Paolo M, Neri M, Riezzo I, Fineschi V. Side effects of AAS abuse: an overview. Mini Rev. Med. Chem. 2011;11(5):374–389. doi: 10.2174/138955711795445925. [DOI] [PubMed] [Google Scholar]
- 27.Casavant M.J, Blake K, Griffith J, Yates A, Copley L.M. Consequences of use of anabolic androgenic steroids. Pediatr. Clin. North Am. 2007;54(4):677–90. doi: 10.1016/j.pcl.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Kicman A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;54(4):677–90. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thein L.A, Thein J.M, Landry G.L. Ergogenic aids. Phys. Ther. 1995;75(5):426–439. doi: 10.1093/ptj/75.5.426. [DOI] [PubMed] [Google Scholar]
- 30.Rogol A.D, Yesalis C.E., III Clinical review 31: Anabolic-androgenic steroids and athletes: what are the issues? J. Clin. Endocrinol. Metab. 1992;74(3):465–469. doi: 10.1210/jcem.74.3.1740476. [DOI] [PubMed] [Google Scholar]
- 31.Kersey R.D. Anabolic-androgenic steroid use among california community college student-athletes. J. Athl. Train. 1996;31(3):237–241. [PMC free article] [PubMed] [Google Scholar]
- 32.Frounfelter G.G, Bradley-Popovich G.E. Ethical considerations regarding anabolic-androgenic steroid use emphasis on the exercise professional. PEP online. 2000;3:1–13. [Google Scholar]
- 33.Edgar A. Ethics of sports. Encyclopedia of Applied Ethics. Vol. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 34.Murray T. Sports. Encyclopedia of Bioethics. New York, NY: Simon and Schuster Macmillan; 1995. [Google Scholar]
- 35.Purtilo R. Ethical Dimensions in the Health Professions. 3rd ed. Philadelphia, PA: W.B. Saunders; 1999. [Google Scholar]
- 36.Talih F, Fattal O, Malone D., Jr Anabolic steroid abuse: psychiatric and physical costs. Cleve Clin. J. Med. 2007;74(5):341–352. doi: 10.3949/ccjm.74.5.341. [DOI] [PubMed] [Google Scholar]
- 37.Bahrke M.S, Yesalis C.E, III, Wright J.E. Psychological and behavioural effects of endogenous testosterone levels and anabolic-androgenic steroids among males. A review. Sports Med. 1990;10(5):303–337. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Graham M.R, Evans P, Davies B, Baker J.S. AAS, growth hormone, and insulin abuse: psychological and neuroendocrine effects. Ther. Clin. Risk Manag. 2008;4(3):587–597. doi: 10.2147/tcrm.s2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel W, Klaiber E.L, Broverman D.M. A comparison of the antidepressant effects of a synthetic androgen (mesterolone) and amitriptyline in depressed men. J. Clin. Psychiatry. 1985;46(1):6–8. [PubMed] [Google Scholar]
- 40.Irving L.M, Wall M, Neumark-Sztainer D, Story M. Steroid use among adolescents: findings from Project EAT. J. Adolesc. Health. 2002;30(4):243–252. doi: 10.1016/S1054-139X(01)00414-1. [DOI] [PubMed] [Google Scholar]
- 41.Middleman A.B, Faulkner A.H, Woods E.R, Emans S.J. ; Middleman A.B, Faulkner A.H, Woods E.R, Emans S.J, DuRant R.H. High-risk behaviors among high school students in Massachusetts who use anabolic steroids. Pediatrics. 1995;96(2 Pt 1):268–272. [PubMed] [Google Scholar]
- 42.Pope H.G, Jr, Katz D.L. Homicide and near-homicide by anabolic steroid users. J. Clin. Psychiatry. 1990;51(1):28–31. [PubMed] [Google Scholar]
- 43.Fudala P.J, Weinrieb R.M, Calarco J.S, Kampman K.M, Boardman C. An evaluation of anabolic-androgenic steroid abusers over a period of 1 year: seven case studies. Ann. Clin. Psychiatry. 2003;15(2):121–130. doi: 10.3109/10401230309085677. [DOI] [PubMed] [Google Scholar]
- 44.Thiblin I, Runeson B, Rajs J. Anabolic androgenic steroids and suicide. Ann. Clin. Psychiatry. 1999;11(4):223–231. doi: 10.3109/10401239909147074. [DOI] [PubMed] [Google Scholar]
- 45.Moore P. Enhancing Me: The Hope and the Hype of Human Enhancement. London: Science Museum TechKnow Series; 2008. pp. 195–198. [Google Scholar]
- 46.Bostrom N, Sandberg A. Cognitive enhancement: methods, ethics, regulatory challenges. Sci. Eng. Ethics. 2009;15(3):311–341. doi: 10.1007/s11948-009-9142-5. [DOI] [PubMed] [Google Scholar]
- 47.Brown N. Hope against hypedaccountability in biopasts, presents and futures. Sci. Studies. 2003:16–3e21. [Google Scholar]
- 48.Hedgecoe A, Martin P. The drugs don’t work: expectations and the shaping ofpharmacogenetics. Soc. Stud. Sci. 2003;33:327–e64. doi: 10.1177/03063127030333002. [DOI] [PubMed] [Google Scholar]
- 49.Rose N. The politics of life itself. Theory. Cult. Soc. 2001. pp. 18–1e30. [DOI]
- 50.Cakic V. Smart drugs for cognitive enhancement: ethical and pragmatic considerations in the era of cosmetic neurology. J. Med. Ethics. 2009;35:611–615. doi: 10.1136/jme.2009.030882. [DOI] [PubMed] [Google Scholar]
- 51.Foddy B, Savulescu J. In: Principles in health care ethics. New York: John Wiley and Sons; 2007. Ethics of performance enhancement in sport: drugs and genedoping; pp. 511–619. [Google Scholar]
- 52.Bertol E, Mari F, Vaiano F, Romano G, Zaami S, Baglìo G, Busardò F.P. Determination of GHB in human hair by HPLC-MS/MS: Development and validation of a method and application to a study group and three possible single exposure cases. Drug Test. Anal. doi: 10.1002/dta.1679. Jun 19, in press in press. [DOI] [PubMed] [Google Scholar]
- 53.Van Cauter E, Plat L, Scharf M.B, Leproult R, Cespedes S, L’Hermite-Balériaux M, Copinschi G. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J. Clin. Invest. 1997;100(3):745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Anti-Doping Agency. The 2014prohibited list. Available at: https://wada-main-prod.s3.amazonaws.com/resources/files/WADARevised-2014-Prohibited-List-EN.PDF . 2014. [[Accessed on: 23th August]].
- 55.Anti-Doping Regulations F.I. FIFA Anti-Doping Regulations. Available at: http://es.fifa.com/mm/document/afdeveloping/medical/50/29/56/fifadocregulations_09.01.09_e.pdf . [Accessed on: 20th July, 2014].
- 56.Olympic Movement Anti-Doping Code. Available at: http://www.medycynasportowa.pl/download/doping_code_e.pdf . 2014. [Accessed on: 29th July].
- 57.Muhammed K. Cosmetic neurology: the role of healthcare professionals. Med. Health Care Philos. 2014;17(2):239–240. doi: 10.1007/s11019-013-9497-x. [DOI] [PubMed] [Google Scholar]