Abstract
Anabolic androgenic steroids (AAS) are some of the most common performance enhancing drugs (PED) among society. Despite the broad spectrum of adverse effects and legal consequences, AAS are illicitly marketed and distributed in many countries. To circumvent existing laws, the chemical structure of AAS is modified and these designer steroids are sold as nutritional supplements mainly over the Internet. Several side effects are linked with AAS abuse. Only little is known about the pharmacological effects and metabolism of unapproved steroids due to the absence of clinical studies. The large number of designer steroid findings in dietary supplements and the detection of new compounds combined with legal loopholes for their distribution in many countries show that stricter regulations and better information policy are needed.
Keywords: AAS, designer steroids, dietary supplements, performance enhancing drugs.
INTRODUCTION
The use of anabolic androgenic steroids is a widespread issue not only among athletes but also in recreational sports as well as in the general population. In elite sports, the use of anabolic agents is prohibited by the World Anti-Doping Agency (WADA). Its list of prohibited substances covers exogenous synthetic steroids as well as endogenous androgens and precursors of androgens [1]. The majority of adverse analytical findings in sports reported by WADA laboratories is associated with anabolic steroids [2]. An ever-increasing trend for attractive body appearance and better sports performance along with less effort is recognized in athletes and nonathletes. A large part of unapproved steroids is marketed as ‘dietary supplements’ in dubious stores or over the Internet. These products include common AAS, designer steroids and/or prohormones, often without proper labeling of the content. Even cross-contaminations of non-hormonal dietary supplements with steroids have been detected [3]. The illusion that these products have none or minimal side effects (or can be controlled by the users), along with the easy access of performance or image enhancing drugs, increases the willingness to take ‘pills for everything’ [4].
Data from the US suggest that 1-3 % of the inhabitants use anabolic steroids [5, 6]. The lifetime prevalence of AAS use in the US was estimated in a recent study with about 3-4 million [7], whereby also recently another study showed that AAS use is even prevalent in men >40 years [8], probably describing aging long-term user. A recent meta-analysis of 187 studies predicts for the first time a global lifetime prevalence with an estimated value of 3.3 % [9]. Pope et al. published an extensive review about performance enhancing drugs and their health consequences earlier this year [10]. This review focuses on the steroidal findings of androgens in dietary supplements.
STEROID HORMONES
Steroid hormones are lipophilic substances derived from cholesterol and are subgrouped based on their pharmacological profile and their receptor binding affinity as sex steroids (androgens, estrogens, gestagens) and corticosteroids (gluco-, and mineralocorticoids). After cleavage of the side chain of cholesterol by cytochrome P450 enzyme CYPscc pregnenolone represents the basic pregnane. Pregnenolone is the general precursor for the biosynthesis of androgens, estrogens and corticosteroids. Corticosteroids are mainly synthesized in the adrenal gland and regulate water and mineral transport (mineralcorticoids), energy metabolism and immune and stress response (glucocorticoids). The loss of C20/C21 from the molecule leads to the androgens and estrogens. The female sex steroids estrogens and gestagens are important for the development of female sex characteristics, the regulation of the menstrual cycle and maintenance of pregnancy. They also play a role in the regulation of spermatogenesis and sperm maturation in males [11]. Estrogens originate from androgens. The enzyme complex aromatase, CYP19, produces etradiol and estrone from testosterone and androstenedione respectively. Androgens represent the male sex steroids and are discussed in more detail below. In addition to the above mentioned functions, steroids play an important role in the brain: Peripheral steroids that are involved in brain functioning, so-called neuroactive steroids, and neurosteroids that are only synthesized within the central nervous system (Fig. 2).
Fig. (2).
Structures of some important neurosteroids: (I) Pregnenolone, (II) DHEA, (III) Allopregnanolone, (IV) Tetrahydrodeoxycorticosterone.
Androgens
The pharmacology of androgens and the androgen receptor has been extensively reviewed [12, 13]. Therefore, a very brief overview is given in here. Androgens are male sex hormones that promote androgenic and anabolic effects. Besides testosterone and its 5α-hydrogenated form, the more active metabolite 5α-dihydrostestosterone (5α-DHT), also other endogenous androgens like androstenedione, dehydro-epiandrosterone (DHEA) and its sulfate (DHEAS), and androstenediol play important roles in humans. In men, testosterone is mainly produced in the Leydig cells of the testis. The ovaries in women produce androstenedione and testosterone [14]. The biosynthesis of androgens in the testis and ovaries is regulated by the hypothalamic-pituitary-gonadal (HPG) axis. The anterior pituitary induces the production of testosterone through the secretion of luteinizing hormone (LH) after activation through gonadotropin releasing hormone (GnRH) by the hypothalamus. Negative feedback through testosterone is mediated through decreasing the release of GnRH by the hypothalamus as well as decreasing the sensitivity of the pituitary to GnRH. The weaker androgens are synthesized in both sexes in the adrenal cortex, regulated by adrenocorticotropic hormone (ACTH). In the bloodstream testosterone is predominantly bound to proteins like the sex hormone binding globulin (SHBG) or albumin. Transported to its target tissues, free testosterone mainly diffuses into the cells where it mediates its action itself by binding to the androgen receptor (AR) or is reduced by 5α-reductase to 5α-DHT or is aromatized by aromatase to estradiol.
Androgen Receptor Mediated Action
Androgens mainly exert their effects via the androgen receptor, a member of the nuclear receptor superfamily [15, 16]. AR’s are located in the cytosol stabilized by heat shock proteins (Hsp) and other chaperones (e.g. p23) [17]. After binding of an androgen this complex dissociates due to conformational changes within the receptor, which is then able to interact with coregulators that allow the AR to migrate into the cell nucleus and form a homodimer [13]. After dimerization the receptor complex binds to androgen response elements (AREs), specific promoter regions of the target genes and acts as a ligand dependent DNA-binding transcription factor. Heterodimers with ERα (estrogen receptor α) or orphan nuclear receptors are also possible but not so common and address different target genes [18]. AR action can be regulated by allosteric modulation or phosphorylation of the AR itself [19, 20] as well as by coregulators. These coregulators largely influence the androgen receptor and other steroid hormone receptors. Recent reviews summarized the importance of steroid receptor coregulators (SRC) [21, 22] acting as coactivators or corepressors by altering ligand selectivity, modification of DNA or histones, or acting as promoters [13, 23]. Differences in coregulator expression in androgen target tissues helps to understand the various effects of androgens.
Non-Classical Actions
Besides the classical, genomic mechanism of action, steroid hormones are reported to act via non-genomic pathways [24, 25]. It is known that estrogens can interfere with the orphan G-protein coupled receptor (GPCR) GPR30, an intracellular trans-membrane receptor, and cause rapid steroid hormone actions [26]. Effects of rapid estrogen action on the behavior in humans have been reviewed recently [27]. Progesterone and androgens have the ability to interact via non-genomic pathways as well [28, 29]. Non-classical testosterone actions were observed mediating their effects in several tissues (reproductive, cardiovascular, immune and musculoskeletal systems) [29, 30]. Signaling pathways via a SHBG receptor or interaction of androgens with tyrosine kinases which alter coregulator function by phosphorylation, as well as membrane associated ARs affecting intracellular Ca2+ levels or allosteric modulation of GABAA receptors are discussed [18, 31-33]. Recently, a group supported the assumption of rapid androgen signaling mediated via a membrane bound GPCR [34].
PHYSIOLOGICAL EFFECTS OF AAS
As the name implies, androgens play a key role in reproduction, sexual maturation and differentiation in males, but also have an important impact in normal human development and physiology in general. Reviewers showed the importance of the AR and androgen action in several tissues [35]. Depending on the target tissue, different androgens are the main endogenous ligand. The existence of 5α-reductase is a sign for effects mostly derived from 5α-DHT whereas aromatase activity is an indication that besides testosterone estrogens may play a role in signaling. Studies using AR knockout mice helped to discover the importance of estrogen action in androgen target tissues. Investigations using AR knockout mice (ARKO) have been reviewed by Chang et al. [36], showing many key roles and locations of AR and its significance in production and maturation of immune cells, bone mineralization, and muscle growth, regulation of insulin sensitivity and glucose homeostasis in brain and liver, cutaneous wound healing and cardiovascular diseases. As mentioned above the androgens testosterone and 5α-DHT regulate the development of the testis, spermatogenesis and differentiation of secondary male sex characteristics, whereby even the female sex steroid estrogen is involved in regulation of spermatogenesis and sperm maturation [11].
The anabolic effects of androgens are an increase of skeletal muscle mass and strength whereby the mechanism of action is not fully understood. AR mediated actions as well as AR independent pathways are suggested [37]. ARs are expressed in several cell types in human skeletal muscle, including satellite cells, fibroblasts, CD34+ precursor cells, vascular endothelial, smooth muscle cells, and mast cells [38]. In the skeletal muscle testosterone itself seems to be the acting hormone because no 5α-reductase activity is observed in these cells [12]. It induces hypertrophy of type 1 and 2 fibers and increases the number of muscle progenitor cells (satellite cells) and promotes their myogenic differentiation [38]. These changes in skeletal muscle lead to improved muscle strength and leg power [39, 40].
Besides AR both estrogen receptor isoforms ERα and ERβ are expressed in human skeletal muscle [41]. ERβ is also reported to mediate anabolic effects [42]. Another effect of androgens is the up-regulation of insulin-like growth factor-I receptor (IGF-IR) [43].
Additionally androgens affect bone maturation. Thereby AR action is important for trabecular and cortical bone maintenance, whereas for the latter estrogens play a part as well [44]. Evidence is emerging that androgens may protect men against osteoporosis via maintenance of cancellous bone mass and expansion of cortical bone. This effect was mediated by the AR and ERα [45, 46].
Recent epidemiological data showed a correlation between low testosterone levels in men and incidence of cardiovascular disease and stroke [47].
Behavioral effects of androgens include sexual behavior, cognitive abilities, aggression and mood [12, 48]. In adults, testosterone levels showed a positive correlation with good mood and negative correlations with anxiety and depression [12]. A clinical study in hypogonadal men showed a connection between testosterone administration and decrease in depressive symptoms [49]. High testosterone levels are often linked with aggressive behavior without resolved mechanism. A recent animal study with castrated macaques showed that androgens stimulated serotonin related gene expression [50], contradicting earlier studies that found low serotonin being one reason for androgen induced aggression [51]. Neuroprotective effects are currently discussed. The neural brain AR in rats increased remyelination, and aging men with low testosterone showed signs of Alzheimers disease [52, 53]. Other important target tissues of androgens are the liver, kidney, skin, and fat tissue. Adipose tissue has a considerable aromatase activity. Thereby the androgen testosterone is converted into estradiol. 5α-DHT is mostly inactivated due to 3α-hydroxy steroid dehydrogenase (3α-HSD) activity in adipose tissue [54]. An increase in androgen levels throughout gestation is likely to be important for establishment and maintenance of pregnancy and initiation of parturition [55].
ADVERSE EFFECTS
However, AAS abuse is associated with various adverse events in different androgen target tissues and has been reviewed extensively elsewhere [10, 56]. Pope et al. categorize adverse events in cardiovascular, neuroendocrine, neuropsychiatric, hepatic, musculoskeletal, kidney, immune and dermatologic effects. Common side effects of AAS abuse include virilization and menstrual irregularities in females, gynaecomastia mediated by an excess of estrogenic metabolites and decreased sperm count and testicle size in men, infertility, altered lipid metabolism, decreased glucose tolerance, hypertrophy of sebaceous gland, acne, hair loss and liver toxicity in both sexes. In adolescents an acceleration of bone maturation and premature closure of the epiphysis lead to cessation of the longitudinal growth.
Additionally, adverse cardiovascular effects after AAS abuse are widely reported [57-60]. Recent studies repeatedly confirmed this. A forensic study in polydrug abusing AAS users showed an obvious number of cardiovascular diseases [61]. Again, case reports revealed the connection between myocardial infarction [62], myocardial hypertrophy and hypertension with AAS abuse [63]. A group postulated possible subclinical markers for these cardiac effects. They associated long-term supraphysiological doses of AAS with higher values of intra- and interatrial electrochemical delay in healthy young bodybuilders [64]. Another research group confirmed in a cross-sectional study increased markers of cardiovascular risk and endothelial dysfunction [65]. To investigate how endothelial dysfunction might arise after AAS use, a supraphysiological dose of testosterone was administered to 17 young males. Decreased urinary nitric oxide levels and decreased antioxidative capacity were detected as well as lower expression of endothelial NO synthase in a cell model [66]. A mechanism how AAS may influence cardiovascular health was postulated recently. In this study, the androgen fluoxymesterone has shown to inhibit 11β-hydroxysteroid dehydrogenase, which may lead to decreased glucocorticoid inactivation and therefore to cortisol-induced mineralcorticoid receptor activation ending up in electrolyte disturbances. This could cause possible hypertension [67]. A recent study in recreational sports was conducted in 17 men given either the prohormone 3β-hydroxy-5α-androst-1-en-17-one or placebo where the treated group showed negative effects on cardiovascular and liver parameters [68].
The central nervous system is another important target for AAS side effects. There are several studies describing psychiatric symptoms after AAS abuse like major mood disorders, aggressive behavior, dependence syndrome, or cognitive effects [51, 69]. Neuroprotective as well as neurotoxic effects of testosterone are discussed. Apoptotic effects of AAS are present in several cell culture models regarding different cell types [70, 71]. Neurotoxic effects due to apoptotic mechanisms are emerging [72]. A study using neuronal cells treated with testosterone or AAS showed neurotoxic effects via a genomic as well as a non-genomic pathway with a membrane-bound AR being the more potent target [73]. The administration of supraphysiological doses of metandienone and 17α-methyltestosterone promotes apoptotic pathways in AR expressing neuron cells only [74]. Cognitive deficits in a human study after long-term high-dose AAS exposure were investigated recently [75]. Increased sensitivity towards psychopathological disorders has been reviewed in regard of pubertal AAS use in preclinical models and humans [76].
Major mood disorders are connected with AAS use as is shown in humans [77] and animals. An animal study in rats showed that nandrolone and stanozolol administration resulted in pathophysiological signs of major depressive disorder like decreased levels of brain-derived neurotrophic factor (BDNF), lowered expression of glucocorticoid receptors, and an increase in corticosterone levels (the stress hormone in rodents) whereas the antidepressant chlorimipramine antagonized these effects [78]. Investigations of depressive but not anxious symptoms linked to changes in dopaminergic, serotonergic and noradrenergic neurotransmission of nandrolone exposed rats [79] are partly inconsistent with a recent study describing serotonergic and noradrenergic changes in neurotransmission as well as depressive and anxious behavior in rats [80].
Observations of aggressive behavior caused by AAS use are described in human and animal studies [81, 82]. Aggression might be linked with anxiety in animals and involves several brain circuits that are affected by AAS [83]. Animal studies showed impact on the glutamate, the GABAergic, the serotonergic and dopaminergic system as well as on tachykinin and encephalin pathways [84-88]. A recent controlled study in healthy young males investigated the potential of testosterone towards aggressive behavior. A testosterone baseline was established after administering a GnRH antagonist (cetrorelix acetate). Physiological doses of testosterone rapidly increased the response of neural circuits mediating threat processing and aggressive behavior, probably via non-genomic pathways [89].
Steroid dependence is another severe and perhaps underestimated adverse event of AAS abuse. Data from the Anabolic 500 survey showed that almost one quarter of AAS users were dependent on these drugs [90]. The relatively high prevalence of AAS use in the US (about 4 mio.) is accompanied by roughly 1 million users who might have experienced AAS dependence [7].
DESIGNER ANDROGENS
Androgenic anabolic steroids are derived from the endogenous androgens testosterone and 5α-DHT. Chemical modifications of these molecules aimed towards more selective anabolic properties, higher oral bioavailability, optimized pharmacokinetics and minimized estrogenic side effects. Attempts to separate androgenic from anabolic activity entirely failed. However, there are some AAS with a relatively high anabolic-androgenic index (e.g. stanozolol, nandrolone), which means the anabolic effects exceed the androgenic effects but are still inseparable. 17α-Methylation enables oral administration by decreasing inactivation during first pass metabolism however liver toxicity increases at the same time [56]. Reduction of estrogen-related side effects can be achieved by elimination of the C19-methyl group, introduction of a 1,2-double bond or methylation at C2, not allowing the aromatase enzyme complex for aromatization of the A-ring, but still cannot be avoided completely [91-94]. Further modifications include methylation, halogenation or hydroxylation at C-1, C-2, C-4, C-6, C-7, or C-11, or introduction of additional double bonds in ring A, B and/or C, as well as attachment of an additional ring at C-2/C-3 [12, 95] (Fig. 1).
Fig. (1).
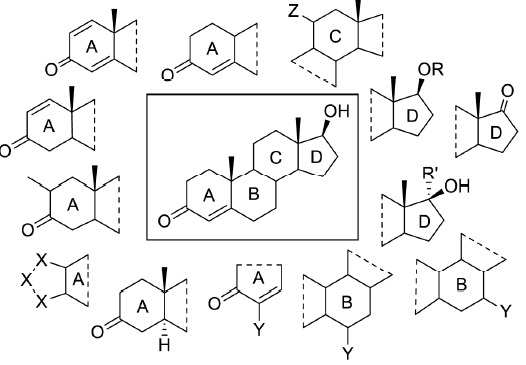
Modifications of the testosterone molecule to yield designer anabolics. Chemical structure of testosterone (center) with examples for possible modifications of the molecule (R: acyl; R‘: alkyl; X: carbon, nitrogen, oxygen; Y: methyl, hydroxyl, oxo, bromo, chloro; Z: oxo, hydroxyl).
Precursors of active hormones with only little intrinsic effects are often called prohormones. Today, this term is mainly used for prohormones of anabolic androgenic steroids with testosterone or nortestosterone being the corresponding active hormones (e.g. 19-norandrostenedione, 4-androstenediol).
The inhibition of the enzyme complex aromatase is therapeutically used in estrogen depending cancer [96]. In combination with anabolic steroids they are administered in order to reduce side effects like gynocomastia resulting from estrogenic metabolites [91]. Additionally, they are discussed to increase endogenous testosterone levels by reducing its metabolic conversion and thereby leading to muscle hypertrophy [97]. Aromatase inhibitors (as well as other anti-estrogens) are prohibited substances in sports according to regulations of the World Anti-Doping Agency [1], and can be subgrouped into steroidal (type-I, e.g. exemestane) and non-steroidal (type-II, e.g. anastrozole) inhibitors [98, 99]. Several steroids appeared on the dietary supplement market, that are advertised to result in aromatase inhibition (e.g. formestane, androst-4-ene-3,6,17-trione, androsta-1,4,6-triene-3,17-dione, and 6α-methylandrost-4-ene-3,17-dione) [100-102]. The chemical structures of steroidal aromatase inhibitors are closely related to those of anabolic androgenic steroids.
More recently so-called selective androgen receptor modulators (SARMs, examples in Fig. 3) got the attraction of the pharmaceutical industry. They promote full agonistic action in muscles and bones while only partial or antagonistic effects in the prostate [103]. Like aromatase inhibitors, they are divided in steroidal (e.g. 7α-methyl-19-nortestosterone) and non-steroidal (e.g. Andarine) types. A recent review updates the developments of SARMs [104]. Some clinical trials involving non-steroidal SARMs are currently ongoing but approved drugs are still missing. Nevertheless they are listed on the WADA prohibited list and adverse analytical findings in doping control already occurred (S-4, Andarine) [105]. Positive findings of Andarine (S4) as well as MENT (7α-methyl-19-nortestosterone) and its prodrug 7α-methyl-19-norandrost-4-ene-3,17-dione in ‘dietary supplements’ were also reported recently [106, 107].
Fig. (3).
Structures of steroidal and non-steroidal SARMs: (I) Andarine/S-4, (II) MENT, (III) MK-0773.
DESIGNER SUPPLEMENTS
To avoid legal consequences with regard to trading or using androgenic anabolic steroids, chemical modifications of existing approved or prohibited substances have been realized [95]. Only testosterone, nortestosterone, dihydrochlormethyltestosterone (DHCMT), metenolon, metandienone, methyltestosterone, oxandrolone, fluoxymesterone, stanozolol, formestane, 5α-DHT, and DHEA, have ever been approved for therapeutical use in humans. Aside from these substances any institution has never approved other steroids, and therefore their effects, side effects or metabolism are not well investigated. In addition to the above mentioned effects and side effects of AR agonists the designer steroids may also display side effects related to an activation of other steroid receptors, e.g. the glucocorticoid receptor (GR) in case of tetrahydro-gestrinone [108]. The first designer steroid that has never been marketed as approved drug before was DHCMT, which has been used in regard to doping purposes by athletes. In the early 2000s norbolethone, tetrahydrogestrinone (THG, ‘The Clear’) and desoxymethyltestosterone (madol, DMT) were marketed as ‘undedectable’ steroids by the US ‘nutritional supplement’ company BALCO whereby the identification of their structures started in 2002 [109-112]. Since then, an increasing number of designer steroids appeared, mainly marketed as supplements (Table 1). Only recently methylstenbolone was discovered as a new designer steroid [113].
Table 1.
Findings of caffeine effects on main neurodegenerative diseases.
| Chemical Name (IUPAC) | Trivial Name | References |
|---|---|---|
| 17β -Hydroxy-2α ,17α -dimethyl-5a-androstan-3-one 2) | Methasterone | [122,129-134] |
| 17β -Hydroxy-17α -methyl-5α -androst-1-en-3-one 1) | [131] | |
| 4,17β -Dihydroxyandrost-4-en-3-one 1) | 4-Hydroxytestosterone | [135,136] |
| 5α -Androstane-3β ,17α -diol | [137] | |
| Androst-4-ene-3β ,17α -diol | [137] | |
| 5β -Androst-1-ene-3β ,17β -diol | [137] | |
| 5β -Androst-1-ene-3α ,17β -diol | [137] | |
| 17β -Hydroxy-5α -androstano-[3,2-c]-pyrazol 2) | Prostanozol | [130] |
| 6α -Methylandrost-4-ene-3,17-dione 2) | [102, 130, 132] | |
| 3β -Hydroxy-5β -androstan-17-one | Epietiocholanolone | [132] |
| 17β -Hydroxy-17α -methyl-5β -androstan-3-one | 5β -Mestanolone | [132] |
| 17α -Methyl-5α -androst-2-en-17β -ol | Desoxymethyltestosterone, DMT, Madol | [132, 138, 139] |
| 4-Chloro-17α -methylandrost-4-ene-3α ,17β -diol | [133] | |
| Androst-4-ene-3,6,17-trione | 6-Oxoandrostenedione, 6-Oxo | [140, 141] |
| Androsta-1,4,6-triene-3,17-dione 2) | Androstatrienedione | [142-144] |
| 3β -Hydroxyandrost-4-ene-7,17-dione | 7-Keto-DHEA | [145] |
| 6α /β -Bromoandrost-4-ene-3,17-dione | 6-Bromandrostenedione | [139, 146] |
| Estra-4,9-diene-3,17-dione | Trenbolox | [123, 139, 147] |
| 17β -Acetoxy-17α -methylandrost-5-ene-3β ,7β -diol | MbAEt | [139] |
| 3β -Hydroxy-5β -androstan-17-one | Epiandrosterone | [139] |
| 2α ,3α -Epithio-17α -methylandrostan-17β -ol 2) | 2a,3a-Epithio-17a-methyletiocholanol | [123, 139] |
| 3-Tetrahydropyranoloandrost-4-ene-6,17-dione | 6-KetoDHEA | [139] |
| Androsta-1,4-diene-3,17-dione | Boldione | [139] |
| 3α -Acetoxy-5α -androstan-17-one | Androsterone acetate | [139] |
| 5α -Androst-1-ene-3β ,17β -diol 1) | [139] | |
| 17β -Hydroxy-17α -methyl-5á-androstan-3-oxime 2) | Mestanolon-oxim | [139] |
| 5α -Androstane-3,17-dione-bis-oxim | [139] | |
| 17β -Hydroxy-5α -androstano-[3,2-c]-isoxazol 2) | [123, 139, 147-149] | |
| 17β -Hydroxy-5α -androstano-[2,3-d]-isoxazol 2) | [123, 139, 147-149] | |
| 6ξ-Hydroxyandrost-4-ene-3,17-dione | [123, 142] | |
| 3ξ-Hydroxyandrost-4-ene-6,17-dione | 6,17-Keto-etiocholeve-3-ol-tetrahydropyranol | [144] |
| 4,17α -Dimethylestra-1,3,5-trien-17β -ol | M1,4ADD (Methyl-1,4-androstadiene-3,17-diol) | [150] |
| 17β -Hydroxy-17α -methylandrosta-4,6-dien-3-one | Jungle Warfare | [123, 139, 151] |
| 3β -Hydroxy-5α -androst-1-en-17-one | 1-Androsterone, 1-DHEA | [139, 152, 153] |
listed in the ‘Anabolic Steroid Control Act’, 2listed in the ‘Designer Anabolic Steroid Control Act 2014’.
The legal status of designer steroids differs from country to country. In most countries, they are regarded as controlled substances. In the US most anabolic steroids are classified as schedule III controlled substances, which means that already possession is classified as offense [114]. In 2005 prohormones were also included in the list of controlled substances by the ‘Anabolic Steroid Control Act’ [115]. By the term ‘chemically and pharmacologically related to testosterone (other than estrogens, progestins, corticosteroids, and dehydroepiandrosterone)’ designer steroids may also be regarded as controlled substances. Recent developments in updating the legal status of designer steroids are ongoing. A new version of the designer steroid control act was introduced to the US senate in 2014 literally naming 27 new steroids [116].
The legal status of dietary supplements is also not clear in several countries. In the United States, the ’Dietary Supplement Health and Education Act’ (DSHEA) classifies dietary supplements as a subcategory of food, allowing manufacturers to distribute their products without submitting proof of safety or efficacy to the Food and Drug Administration (FDA). Potential ingredients are vitamins, minerals, botanicals, amino acids, concentrates, metabolites, extracts, etc. Therefore it is possible to sell AAS containing supplements until the FDA or another administrative agency classifies otherwise. In the European Union, the European Food Safety Authority (EFSA) defines dietary supplements as ‚concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities’ [117]. Medicinal products are defined as ‚any substance or combination of substances presented as having properties for treating or preventing disease in human beings; or any substance or combination of substance which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis’ [118, 119]. Hence, dietary supplements are categorized as foodstuff in almost every country and a strict discrimination from pharmaceuticals remains complex. Often, the difficulty in classifying these products exists not because of the ingredients but of its presentation [120].
Several investigators showed the presence of designer steroids, prohormones, aromatase inhibitors, and SARMs in products marketed as dietary supplements (Table 1). Probably due to insufficient quality control procedures during the production of steroid containing preparations cross-contaminations have been detected in non-hormonal dietary supplements as well [3, 121]. Only recently contaminated nutritional supplements appeared where vitamin B capsules contained the designer steroids methylstenbolone and methasterone [122]. Supplements containing steroids that are not or falsely labelled have also been identified [123]. Dimethazine (17β-hydroxy-2α,17β-dimethyl-5α-androstan-3-one-azine), a steroid dimer consisting of two methasterone (17β-hydroxy-2α,17α-dimethyl-5a-androstan-3-one) molecules linked with an azine group, was detected in a dietary supplement [124]. A case report shows severe adverse events in connection with this steroid [125]. Phytoecdysteroids show anabolic effects with very little androgenic action and may therefore represent a new therapeutical as well as a doping alternative [126]. Effects on skeletal muscle seem to be mediated via ERβ [127]. Like aromatase inhibitors, selective estrogen receptor modulators (SERMs) are administered by AAS user to reduce androgenic side effects. For instance tamoxifen was found recently in a dietary supplement [128].
The detection of designer steroids remains a challenging task because often reference material is hardly commercially available.
CONCLUSION
The large number of positive findings of designer steroids in dietary supplements together with the discovery of new structures shows the importance of further research in this field. The easy access of designer steroids and readiness of many users to take supplements with often unknown content needs to be stricter regulated by authorities. Immense health risks may arise from the ingestion of underinvestigated ingredients. The complex production and trade of dietary supplements needs collaborative international intervention.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.World Anti-Doping Agency. The World Anti-Doping Code The 2014 Prohibited List. http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/2014/WADA-prohibited-list-2014-EN.pdf . 2014. pp. 1–10.
- 2.World Anti-Doping Agency. Adverse analytical findings reported by accredited laboratories. [Online] http://www.wada-ama.org/Documents/Resources/Testing-Figures/WADA-2013-Anti-Doping-Testing-Figures-LABORATORY-REPORT.pdf . 2014.
- 3.Geyer H., Parr M.K., Koehler K., Mareck U., Schänzer W., Thevis M. Nutritional supplements cross-contaminated and faked with doping substances. J. Mass Spectrom. 2008;43(7):892–902. doi: 10.1002/jms.1452. [DOI] [PubMed] [Google Scholar]
- 4.Graham M.R., Ryan P., Baker J.S., Davies B., Thomas N.E., Cooper S.M., Evans P., Easmon S., Walker C.J., Cowan D., Kicman A.T. Counterfeiting in performance- and image-enhancing drugs. Drug Test. Anal. 2009;1(3):135–142. doi: 10.1002/dta.30. [DOI] [PubMed] [Google Scholar]
- 5.Kashkin K.B., Kleber H.D. Hooked on hormones? An anabolic steroid addiction hypothesis. JAMA. 1989;262(22):3166–3170. doi: 10.1001/jama.1989.03430220089036. [DOI] [PubMed] [Google Scholar]
- 6.Tokish J.M., Kocher M.S., Hawkins R.J. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am. J. Sports Med. 2004;32(6):1543–1553. doi: 10.1177/0363546504268041. [DOI] [PubMed] [Google Scholar]
- 7.Pope H.G., Jr, Kanayama G., Athey A., Ryan E., Hudson J.I., Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans. Current best estimates. Am J Addict. 2014;23(4):371–377. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip E.J., Trinh K., Tenerowicz M.J., Pal J., Lindfelt T.A. Perry P.J. Characteristics and Behaviors of Older Male Anabolic Steroid Users. J. Pharm. Pract. 2014. [DOI] [PubMed]
- 9.Sagoe D., Molde H., Andreassen C.S., Torsheim T., Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014;24(5):383–398. doi: 10.1016/j.annepidem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Pope H.G., Jr, Wood R.I., Rogol A., Nyberg F., Bowers L., Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr. Rev. 2014;35(3):341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreau S., Wolczynski S., Galeraud-Denis I. Aromatase. oestrogens and human male reproduction. Philosophical Transactions of the Royal Soc. B: Biol. Sci. 2010;365(1546) doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kicman A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto T., Sakari M., Okada M., Yokoyama A., Takahashi S., Kouzmenko A., Kato S. The androgen receptor in health and disease. Annu. Rev. Physiol. 2013;75(1):201–224. doi: 10.1146/annurev-physiol-030212-183656. [DOI] [PubMed] [Google Scholar]
- 14.Burger H.G. Androgen production in women. Fertil. Steril. 2002;77(4):S3-5. doi: 10.1016/S0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander S.P., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Spedding M., Peters J.A., Harmar A.J., Collaborators C., CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: nuclear hormone receptors. Br. J. Pharmacol. 2013;170(8):1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard D. Chaperoning steroid hormone action. Trends Endocrinol. Metab. 2006;17(6):229–235. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Bennett N.C., Gardiner R.A., Hooper J.D., Johnson D.W., Gobe G.C. Molecular cell biology of androgen receptor signalling. Int. J.Biochem. Cell Biol. 2010;42(6):813–-827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R., McEwan I.J. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr. Rev. 2012;33(2):271–299. doi: 10.1210/er.2011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigel N.L., Moore N.L. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol. Endocrinol. 2007;21(10):2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 21.Heemers H.V., Tindall D.J. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 2007;28(7):778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 22.Stashi E., York B., O'Malley B.W. Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends Endocrinol. Metabolism. 2014;25(7):337–347.. doi: 10.1016/j.tem.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinlein C.A., Chang C. Androgen receptor (AR) coregulators: an overview. Endocr. Rev. 2002;23(2):175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 24.Norman A.W., Mizwicki M.T., Norman D.P. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat. Rev. Drug Discov. 2004;3(1):27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 25.Losel R., Wehling M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003;4(1):46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 26.Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 27.Laredo S.A., Villalon Landeros R., Trainor B.C. Rapid effects of estrogens on behavior: environmental modulation and molecular mechanisms. Front. Neuroendocrinol. 2014;35(4):447–458. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh M., Su C., Ng S. Non-genomic mechanisms of progesterone action in the brain. Front. Neurosci. 2013;7:159. doi: 10.3389/fnins.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian H.C., Rolls N.J., Morris J.F. Nongenomic actions of testosterone on a subset of lactotrophs in the male rat pituitary. Endocrinology. 2000;141(9):3111–3119. doi: 10.1210/endo.141.9.7662. [DOI] [PubMed] [Google Scholar]
- 30.Rahman F., Christian H.C. Non-classical actions of testosterone: an update. Trends Endocrinol. Metab. 2007;18(10):371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Heinlein C.A., Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 32.Foradori C.D., Weiser M.J., Handa R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008;29(2):169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadopoulou N., Papakonstanti E.A., Kallergi G., Alevizopoulos K., Stournaras C. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life. 2009;61(1):56–61. doi: 10.1002/iub.150. [DOI] [PubMed] [Google Scholar]
- 34.Shihan M., Bulldan A., Scheiner-Bobis G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with GnI 11. Biochim. Biophys. Acta. 2014;1843(6):1172–1181. doi: 10.1016/j.bbamcr.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2014;63(2 ):142–148. doi: 10.1016/j.maturitas.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Chang C., Yeh S., Lee S.O., Chang T.M. Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl. Recept Signal. 2013;11(e001) doi: 10.1621/nrs.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharmacol. 2008;154(3):522–528. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha-Hikim I., Taylor W.E., Gonzalez-Cadavid N.F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004;89(10):5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 39.Herbst K.L., Bhasin S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7(3):271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Bhasin S., Storer T.W., Berman N., Callegari C., Clevenger B., Phillips J., Bunnell T.J., Tricker R., Shirazi A., Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 41.Wiik A., Ekman M., Johansson O., Jansson E., EsbjArnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem. Cell Biol. 2009;131(2):181–189. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 42.Velders M., Schleipen B., Fritzemeier K.H., Zierau O., Diel P. Selective estrogen receptor-I activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26(5):1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 43.Pandini G., Mineo R., Frasca F., Roberts C.T., Jr, Marcelli M., Vigneri R., Belfiore A. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005;65(5):1849–1857. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 44.Sinnesael M., Claessens F., Boonen S., Vanderschueren D. Novel insights in the regulation and mechanism of androgen action on bone. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20(3):240–244. doi: 10.1097/MED.0b013e32835f7d04. [DOI] [PubMed] [Google Scholar]
- 45.Vanderschueren D., Vandenput L., Boonen S., Lindberg M.K., Bouillon R., Ohlsson C. Androgens and bone. Endocr. Rev. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 46.Manolagas S.C., O'Brien C.A., Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013;9(12):699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quillinan N., Deng G., Grewal H., Herson P.S. Androgens and stroke: good, bad or indifferent? Exp. Neurol. 2014;259:10–15. doi: 10.1016/j.expneurol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubinow D.R., Schmidt P.J. Androgens, brain, and behavior. Am. J. Psychiatry. 1996;153(8):974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 49.Giltay E.J., Tishova Y.A., Mskhalaya G.J., Gooren L.J., Saad F., Kalinchenko S.Y. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J. Sex. Med. 2010;7(7):2572–2582. doi: 10.1111/j.1743-6109.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 50.Bethea C.L., Coleman K., Phu K., Reddy A.P., Phu A. Relationships between androgens, serotonin gene expression and innervation in male macaques. Neuroscience. 2014;274:341–356. doi: 10.1016/j.neuroscience.2014.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark A.S., Henderson L.P. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci. Biobehav. Rev. 2003;27(5):413–436. doi: 10.1016/S0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 52.Hussain R., Ghoumari A.M., Bielecki B., Steibel J., Boehm N., Liere P., Macklin W.B., Kumar N., Habert R., Mhaouty-Kodja S., Tronche F., Sitruk-Ware R., Schumacher M., Ghandour M.S. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136(Pt 1):132–146. doi: 10.1093/brain/aws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vest R.S., Pike C.J. sex steroid hormones, and Alzheimer's disease. Horm. Behav. 2013;63(2):301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Reilly M.W., House P.J., Tomlinson J.W. Understanding androgen action in adipose tissue. J. Steroid Biochem. Mol. Biol. 2014;143:277–284. doi: 10.1016/j.jsbmb.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Makieva S., Saunders P.T., Norman J.E. Androgens in pregnancy: roles in parturition. Hum. Reprod. Update. 2014;20(4):542–559. doi: 10.1093/humupd/dmu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shahidi N.T. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001;23(9):1355–1390. doi: 10.1016/S0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- 57.Achar S., Rostamian A., Narayan S.M. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am. J. Cardiol. 2010;106(6):893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krieg A., Scharhag J., Albers T., Kindermann W., Urhausen A. Cardiac tissue Doppler in steroid users. Int. J. Sports Med. 2007;28(8):638–643. doi: 10.1055/s-2007-964848. [DOI] [PubMed] [Google Scholar]
- 59.Angell P.J., Chester N., Sculthorpe N., Whyte G., George K., Somauroo J. Performance enhancing drug abuse and cardiovascular risk in athletes: implications for the clinician. Br. J. Sports Med. 2012;46(Suppl 1):i78–84. doi: 10.1136/bjsports-2012-091186. [DOI] [PubMed] [Google Scholar]
- 60.Kanayama G., Pope H.G., Jr Illicit use of androgens and other hormones: recent advances use of androgens and other hormones: recent advances. Curr. Opin. Endocrinol.Diabetes Obes. 2012;19(3):211–219. doi: 10.1097/MED.0b013e3283524008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darke S., Torok M., Duflou J. Sudden or unnatural deaths involving anabolic-androgenic steroids. J. Forensic Sci. 2014;59(4):1025–1028. doi: 10.1111/1556-4029.12424. [DOI] [PubMed] [Google Scholar]
- 62.Peoples K., Kobe D., Campana C., Simon E. Hyperhomocysteinemia-induced myocardial infarction in a young male using anabolic steroids. Am. J. Emerg. Med. 2014;32(8):948.e1–948.e2. doi: 10.1016/j.ajem.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Quintana E., Saiz-Udaeta B., Marrero-Negrin N., Lopez-MA(c)rida X., Rodriguez-Gonzalez F., Nieto-Lago V. Androgenic anabolic steroid, cocaine and amphetamine abuse and adverse cardiovascular effects. Int. J. Endocrinol. Metab. 2013;11(4):e8755. doi: 10.5812/ijem.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akcakoyun M., Alizade E., Gundogdu R., Bulut M., Tabakci M.M., Acar G., Avci A., Simsek Z., Fidan S., Demir S., Kargin R., Emiroglu M.Y. Long-term anabolic androgenic steroid use is associated with increased atrial electromechanical delay in male bodybuilders. Biomed. Res. Int. 2014. (Article ID 451520), 8 pages. [DOI] [PMC free article] [PubMed]
- 65.Severo C.B., Ribeiro J.P., Umpierre D., Da Silveira A.D., Padilha M.C., De Aquino Neto F.R., Stein R. Increased atherothrombotic markers and endothelial dysfunction in steroid users. Eur. J. Prev. Cardiol. 2013;20(2):195–201. doi: 10.1177/2047487312437062. [DOI] [PubMed] [Google Scholar]
- 66.Skogastierna C., Hotzen M., Rane A., EkstrAm L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur. J. Prev. Cardiol. 2013;21(8):1049–1054. doi: 10.1177/2047487313481755. [DOI] [PubMed] [Google Scholar]
- 67.FA1/4rstenberger C., Vuorinen A., Da Cunha T., Kratschmar D.V., Saugy M., Schuster D., Odermatt A. The anabolic androgenic steroid fluoxymesterone inhibits 11I -hydroxysteroid dehydrogenase 2-dependent glucocorticoid inactivation. Toxicol. Sci. 2012;126(2):353–361. doi: 10.1093/toxsci/kfs022. [DOI] [PubMed] [Google Scholar]
- 68.Granados J., Gillum T.L., Christmas K.M., Kuennen M.R. Prohormone supplement 3beta-hydroxy-5alpha-androst-1-en-17-one enhances resistance training gains but impairs user health. J. Appl. Physiol. (1985) 2014;116(5):560–569. doi: 10.1152/japplphysiol.00616.2013. [DOI] [PubMed] [Google Scholar]
- 69.Kanayama G., Hudson J.I., Pope H.G., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98(1-2):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaugg M., Jamali N.Z., Lucchinetti E., Xu W., Alam M., Shafiq S.A., Siddiqui M.A. Anabolic-androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J. Cell Physiol. 2001;187(1):90–95. doi: 10.1002/1097-4652(2001)9999:9999. [DOI] [PubMed] [Google Scholar]
- 71.Janjic M.M., Stojkov N.J., Andric S.A., Kostic T.S. Anabolicandrogenic steroids induce apoptosis and NOS2 (nitric-oxide synthase 2) in adult rat Leydig cells following in vivo exposure. Reprod. Toxicol. 2012;34(4):686–693. doi: 10.1016/j.reprotox.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Cunningham R.L., Giuffrida A., Roberts J.L. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology. 2009;150(12):5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caraci F., PistarA V., Corsaro A., Tomasello F., Giuffrida M.L., Sortino M.A., Nicoletti F., Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J. Neurosci. Res. 2011;89(4):592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- 74.Basile J.R., Binmadi N.O., Zhou H., Yang Y.H., Paoli A., Proia P. Supraphysiological doses of performance enhancing anabolicandrogenic steroids exert direct toxic effects on neuron-like cells. Front. Cell Neurosci. 2013;69 doi: 10.3389/fncel.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanayama G., Kean J., Hudson J.I., Pope H.G., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013;130(1-3):208–214. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cunningham R.L., Lumia A.R., McGinnis M.Y. Androgenic anabolic steroid exposure during adolescence: ramifications for brain development and behavior. Horm. Behav. 2013;64(2):350–356. doi: 10.1016/j.yhbeh.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanayama G., Hudson J.I., Pope H.G., Jr Illicit anabolic-androgenic steroid use. Horm. Behav. 2010;58(1):111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matrisciano F., Modafferi A.M., Togna G.I., Barone Y., Pinna G., Nicoletti F., Scaccianoce S. Repeated anabolic androgenic steroid treatment causes antidepressant-reversible alterations of the hypothalamic-pituitary-adrenal axis, BDNF levels and behavior. Neuropharmacology. 2010;58(7):1078–1084. doi: 10.1016/j.neuropharm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Zotti M., Tucci P., Colaianna M., Morgese M.G., Mhillaj E., Schiavone S., Scaccianoce S., Cuomo V., Trabace L. Chronic nandrolone administration induces dysfunction of the reward pathway in rats. Steroids. 2013;79(7-13) [PubMed] [Google Scholar]
- 80.Rainer Q., Speziali S., Rubino T., Dominguez-Lopez S., Bambico F.R., Gobbi G., Parolaro D. Chronic nandrolone decanoate exposure during adolescence affects emotional behavior and monoaminergic neurotransmission in adulthood. Neuropharmacology. 2014;83:79–88. doi: 10.1016/j.neuropharm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Pope H.G., Jr, Kouri E.M., Hudson J.I. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch. Gen. Psychiatry. 2000;57(2):133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 82.Steensland P., Blakely G., Nyberg F., Fahlke C., Pohorecky L.A. Anabolic androgenic steroid affects social aggression and fear-related behaviors in male pair-housed rats. Horm. Behav. 2005;48(2):216–224. doi: 10.1016/j.yhbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Oberlander J.G., Henderson L.P. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012;35(6):382–392. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Grevès P., Huang W., Johansson P., Thornwall M., Zhou Q., Nyberg F. Effects of an anabolic-androgenic steroid on the regulation of the NMDA receptor NR1, NR2A and NR2B subunit mRNAs in brain regions of the male rat. Neurosci. Lett. 1997;226(1):61–64. doi: 10.1016/S0304-3940(97)00244-9. [DOI] [PubMed] [Google Scholar]
- 85.Henderson L.P., Penatti C.A., Jones B.L., Yang P., Clark A.S. Anabolic androgenic steroids and forebrain GABAergic transmission. Neuroscience. 2006;138(3):793–799. doi: 10.1016/j.neuroscience.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 86.Keleta Y.B., Lumia A.R., Anderson G.M., McGinnis M.Y. Behavioral effects of pubertal anabolic androgenic steroid exposure in male rats with low serotonin. Brain Res. 2007;1132(1):129–138. doi: 10.1016/j.brainres.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 87.Kindlundh A.M., Lindblom J., Bergstrom L., Wikberg J.E., Nyberg F. The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. Eur. J. Neurosci. 2001;13(2):291–296. doi: 10.1046/j.0953-816X.2000.01402.x. [DOI] [PubMed] [Google Scholar]
- 88.Hallberg M., Johansson P., Kindlundh A.M., Nyberg F. Anabolic-androgenic steroids affect the content of substance P and substance P(1-7) in the rat brain. Peptides. 2000;21(6):845–852. doi: 10.1016/S0196-9781(00)00218-7. [DOI] [PubMed] [Google Scholar]
- 89.Goetz S.M., Tang L., Thomason M.E., Diamond M.P., Hariri A.R., Carre J.M. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol. Psychiatry. 2014;76(4):324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ip E.J., Lu D.H., Barnett M.J., Tenerowicz M.J., Vo J.C., Perry P.J. Psychological and physical impact of anabolic-androgenic steroid dependence. Pharmacotherapy. 2012;32(10):910–919. doi: 10.1002/j.1875-9114.2012.01123. [DOI] [PubMed] [Google Scholar]
- 91.Llewellyn W. Molecular Nutrition LLc: Jupiter, FL. 2009. Anabolics. [Google Scholar]
- 92.Yoshiji S., Yamamoto T., Okada H. [Aromatization of androstenedione and 19-nortestosterone in human placenta, liver and adipose tissues]. Nippon Naibunpi Gakkai Zasshi. 1986;62(1):18–25. doi: 10.1507/endocrine1927.62.1_18. Aromatization of androstenedione and 19-nortestosterone in human placenta, liver and adipose tissues. [DOI] [PubMed] [Google Scholar]
- 93.Kuhl H., Wiegratz I. Can 19-nortestosterone derivatives be aromatized in the liver of adult humans? Are there clinical implications? Climacteric. 2007;10(4):344–353. doi: 10.1080/13697130701380434. [DOI] [PubMed] [Google Scholar]
- 94.Kicman A.T. Biochemical and Physiological Aspects of Endogenous Androgens. In: Thieme D., Hemmersbach P., editors. Doping in Sports: Biochemical Principles, Effects and Analysis. Vol. 195. Springer Berlin Heidelberg; 2010. pp. 25–64. [DOI] [Google Scholar]
- 95.Parr M.K., Schänzer W. Detection of the misuse of steroids in doping control. J. Steroid Biochem. Mol. Biol. 2010;121(3-5):528–537. doi: 10.1016/j.jsbmb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Wiseman L.R., Goa K.L. Formestane. A review of its pharmacological properties and clinical efficacy in the treatment of postmenopausal breast cancer. Drugs Aging. 1996;9(4):292–306. doi: 10.2165/00002512-199609040-00006. [DOI] [PubMed] [Google Scholar]
- 97.Willoughby D.S., Wilborn C., Taylor L., Campbell W. Eight weeks of aromatase inhibition using the nutritional supplement Novedex XT: effects in young, eugonadal men. Int. J. Sport Nutr. Exerc. Metab. 2007;17(1):92–108. doi: 10.1123/ijsnem.17.1.92. [DOI] [PubMed] [Google Scholar]
- 98.Miller W.R. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003;30(4) Suppl. 14:3–11. doi: 10.1016/S0093-7754(03)00302-6. [DOI] [PubMed] [Google Scholar]
- 99.Mokbel K. The evolving role of aromatase inhibitors in breast cancer. Int. J. Clin. Oncol. 2002;7(5):279–283. doi: 10.1007/s101470200040. [DOI] [PubMed] [Google Scholar]
- 100.Geyer H., Koehler K., Mareck U., Parr M.K., Schänzer W.M. Nutritional supplements cross-contaminated and faked with prohormones, "classic" anabolic steroids and "designer steroids"; In: Effectivness of the Antidoping fight; 2007. pp. 95–99. [Google Scholar]
- 101.Kohler M., Parr M.K., Opfermann G., Thevis M., Schlorer N., Marner F.J., Schanzer W. Metabolism of 4-hydroxyandrostenedione and 4-hydroxytestosterone: Mass spectrometric identification of urinary metabolites. Steroids. 2007;72(3):278–286. doi: 10.1016/j.steroids.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 102.Parr M.K., Kazlauskas R., SchlArer N., Opfermann G., Piper T., Schulze G., Schänzer W. 6alpha-Methylandrostenedione: gas chromatographic mass spectrometric detection in doping control. Rapid Commun. Mass Spectrom. 2008;22(3):321–329. doi: 10.1002/rcm.3367. [DOI] [PubMed] [Google Scholar]
- 103.Bhasin S., Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12(3):232–240. doi: 10.1097/MCO.0b013e32832a3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haendler B., Cleve A. Recent developments in antiandrogens and selective androgen receptor modulators. Mol. Cell. Endocrinol. 2012;352(1-2):79–91. doi: 10.1016/j.mce.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 105.Starcevic B., Ahrens B.D., Butch A.W. Detection of the selective androgen receptor modulator S-4 (Andarine) in a doping control sample. Drug Test. Anal. 2013;5(5):377–379. doi: 10.1002/dta.1466. [DOI] [PubMed] [Google Scholar]
- 106.Kohler M., Thomas A., Geyer H., Petrou M., Schänzer W., Thevis M. Confiscated black market products and nutritional supplements with non-approved ingredients analyzed in the Cologne Doping Control Laboratory 2009. Drug Test. Anal. 2010;2(11-12):533–537. doi: 10.1002/dta.186. [DOI] [PubMed] [Google Scholar]
- 107.Nair V., Campbell T., Eichner D. Evolution of the supplement industry: a case study with MENT.; 32nd Cologne Workshop on Dope Analysis; 2014. [Google Scholar]
- 108.Friedel A., Geyer H., Kamber M., Laudenbach-Leschowsky U., Schänzer W., Thevis M., Vollmer G., Zierau O., Diel P. Tetrahydrogestrinone is a potent but unselective binding steroid and affects glucocorticoid signalling in the liver. Toxicol. Lett. 2006;164(1):16–23. doi: 10.1016/j.toxlet.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Catlin D.H., Ahrens B.D., Kucherova Y. Detection of norbolethone, an anabolic steroid never marketed, in athletes' urine. Rapid Commun. Mass Spectrom. 2002;16(13):1273–1275. doi: 10.1002/rcm.722. [DOI] [PubMed] [Google Scholar]
- 110.Knight J. Drugs bust reveals athletes' secret steroid. Nature. 2003;425(6960):752. doi: 10.1038/425752a. [DOI] [PubMed] [Google Scholar]
- 111.Catlin D.H., Sekera M.H., Ahrens B.D., Starcevic B., Chang Y.C., Hatton C.K. Tetrahydrogestrinone: discovery, synthesis, and detection in urine. Rapid Commun. Mass Spectrom. 2004;18(12):1245–049. doi: 10.1002/rcm.1495. [DOI] [PubMed] [Google Scholar]
- 112.Sekera M.H., Ahrens B.D., Chang Y.C., Starcevic B., Georgakopoulos C., Catlin D.H. Another designer steroid: discovery, synthesis, and detection of 'madol'(tm) in urine. Rapid Commun. Mass Spectrom. 2005;19(6):781–784. doi: 10.1002/rcm.1858. [DOI] [PubMed] [Google Scholar]
- 113.Cavalcanti Gde.A., Leal F.D., Garrido B.C., Padilha M.C., de Aquino Neto F.R. Detection of designer steroid methylstenbolone in ?onutritional supplement ?? using gas chromatography and tandem mass spectrometry: elucidation of its urinary metabolites. Steroids. 2013;78(2):228–233. doi: 10.1016/j.steroids.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 114.US Drug Enforcement Administration. In; US Drug Enforcement Administration. 1996;2005 [Google Scholar]
- 115.US Drug Enforcement Administration. Anabolic Steroids Control Act. 2004;2005 [Google Scholar]
- 116.US Drug Enforcement Administration. Designer Anabolic Steroid Control Act of 2014. 2014.
- 117.European Parliament and the Council of the European Union. Approximation of the laws of the Member States relating to food supplements. In: Official J. Eur. Commun. 2002;L 183:51–57. [Google Scholar]
- 118.European Parliament and the Council of the European Union. Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal of the European Communities. 2001;L(311):67–128. [Google Scholar]
- 119.Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal of the European Communities. 2004;L(136):34–57. [Google Scholar]
- 120.Coppens P., da Silva M.F., Pettman S. European regulations on nutraceuticals, dietary supplements and functional foods: a framework based on safety. Toxicology. 2006;221(1):59–74. doi: 10.1016/j.tox.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 121.Geyer H., Parr M.K., Mareck U., Reinhart U., Schrader Y., Schanzer W. Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids - results of an international study. Int. J. Sports Med. 2004;25(2):124–129. doi: 10.1055/s-2004-819955. [DOI] [PubMed] [Google Scholar]
- 122.Tran B., Spink D., Aldous K., Ahmad N. Detection and Identification of Anabolic Steroids in Over-the-counter Nutritional Supplements. Indianapolis: International Association for Food Protection; 2014. [Google Scholar]
- 123.Parr M.K., Haenelt N., FuAYhAller G., Flenker U., Rodchenkov G., Opfermann G., Schänzer W. Recent steroid findings in "designer supplements". In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. In: Recent Advances in Doping Analysis (17) Vol. 17. Cologne: Sport & Buch Strauss; 2009. pp. 71–80. [Google Scholar]
- 124.Geldof L., Lootens L., Van Lyseheth J., Meuleman P., Leroux-Roels G., Deventer K., Van Eenoo P. Metabolic study of a steroid product containing dimethazine using human liver microsomes and a chimeric mouse model; 32nd Cologne Workshop on Dope Analysis; 2014. [Google Scholar]
- 125.Agbenyefia P., Arnold C.A., Kirkpatrick R. Cholestatic Jaundice With the Use of Methylstenbolone and Dymethazine, Designer Steroids Found in Super DMZ Rx 2.0 “Nutritional Supplement”: A Case Report. J. Investig. Med. High Impact Case Rep. 2014;2(2) doi: 10.1177/2324709614532800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zwetsloot K.A., Shanely A.R., Merritt E.K., McBride J.M. A Novel, Non-Androgenic Alternative for Muscle Health and Performance. J. Steroids Hormonal Sci. 2013;s12(1):10–12. [Google Scholar]
- 127.Parr M.K., Zhao P., Haupt O., Ngueu S.T., Hengevoss J., Fritzemeier K.H., Piechotta M., Schlorer N., Muhn P., Zheng W.Y., Xie M.Y., Diel P. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol. Nutr. Food Res. 2014;58(9):1861–1872. doi: 10.1002/mnfr.201300806. [DOI] [PubMed] [Google Scholar]
- 128.Evans-Brown M., KimergA rd A., McVeigh J., Chandler M., Brandt S.D. Is the breast cancer drug tamoxifen being sold as a bodybuilding dietary supplement? BMJ. 2014;348:g1476. doi: 10.1136/bmj.g1476. [DOI] [PubMed] [Google Scholar]
- 129.Ayotte C., Gouderault D., Cyr D., Gauthier P., Larochelle D., Poirer D. Characterisation of chemical and pharmacological properties of new steroids related to athletes. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 14. Cologne: Sport & Buch Strauss; 2006. pp. 151–150. [Google Scholar]
- 130.Kazlauskas R. Miscellaneous projects in sports drug testing at the National Measurement Institute, Australia, 2005. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 14. Cologne: Sport & Buch Strauss; 2006. pp. 129–140. [Google Scholar]
- 131.Parr M.K., Opfermann G., Schänzer W. Detection of new 17-alkylated anabolic steroids on WADA 2006 list. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 14. Cologne: Sport & Buch Strauss; 2006. pp. 249–258. [Google Scholar]
- 132.Rodchenkov G., Sobolevsky T., Sizoi V. New designer anabolic steroids from internet. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 14. Cologne: Sport & Buch Strauss; 2006. pp. 141–150. [Google Scholar]
- 133.van Eenoo P., Spaerkeer A., Lootens L., Van Thuyne W., Deventer K., Delbeke F. Results of several (small) research projects at DoCoLab in 2005. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 14. Cologne: Sport & Buch Strauss; 2006. pp. 79–88. [Google Scholar]
- 134.Geldof L., Lootens L., Polet M., Eichner D., Campbell T., Nair V., BotrA" F., Meuleman P., Leroux-Roels G., Deventer K., Eenoo P.V. Metabolism of methylstenbolone studied with human liver microsomes and the uPA(+/+) -SCID chimeric mouse model. Biomed. Chromatogr. 2014;28(7):974–985. doi: 10.1002/bmc.3105. [DOI] [PubMed] [Google Scholar]
- 135.Parr M.K., Opfermann G., Schänzer W. Analytical Properties of 4-Hydroxysteroids and some Esters. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 12. Cologne: Sport & Buch Strauss; 2004. pp. 129–139. [Google Scholar]
- 136.Kohler M., Parr M.K., Opfermann G., Thevis M., Schlörer N., Marner F.J., Schänzer W. Metabolism of 4-hydroxyandrostenedione and 4-hydroxytestosterone: Mass spectrometric identification of urinary metabolites. Steroids. 2007;72(3):278–286. doi: 10.1016/j.steroids.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 137.Parr M.K., Geyer H., Opfermann G., Schänzer W. Prescription drugs and new anabolic steroids in nutritional supplements. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent advances in doping analysis. Vol. 12. Cologne: Sport & Buch Strauss; 2004. pp. 71–80. [Google Scholar]
- 138.Okano M., Sato M., Ikekita A., Kageyama S. Analysis of non-ketoic steroids 17alpha-methylepithiostanol and desoxymethyl- testosterone in dietary supplements. Drug Test. Anal. 2009;1(11-12):518–525. doi: 10.1002/dta.72. [DOI] [PubMed] [Google Scholar]
- 139.Kazlauskas R., Hasick N. ASDTL Supplements Project 2010. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 19. Cologne: Sport & Buch Strauss; 2011. pp. 10–14. [Google Scholar]
- 140.Van Eenoo P., Mikulèíková P., Deventer K., VanThuyne W., Delbeke F. Metabolism, excretion and detection of androst-4-ene-3,6,17-trione. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 13. Cologne: Sport & Buch Strauss; 2005. pp. 57–64. [Google Scholar]
- 141.Van Thuyne W., Van Eenoo P., Mikulèíková P., Deventer K., Delbeke F.T. Detection of androst-4-ene-3,6,17-trione (6-OXO) and its metabolites in urine by gas chromatography-mass spectrometry in relation to doping analysis. Biomed. Chromatogr. 2005;19(9):689–695. doi: 10.1002/bmc.496. [DOI] [PubMed] [Google Scholar]
- 142.Kazlauskas R. Supplements & WADA List. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. 15. Cologne: Sport & Buch Strauss; 2007. pp. 31–40. [Google Scholar]
- 143.Parr M.K., Opfermann G., Piper T., Schlörer N., Fuβhöller G., Thomas A., Thevis M., Schänzer W. Characterisation of steroid metabolites recently detected in doping control analyses. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 15. Cologne: Sport & Buch Strauss; 2007. pp. 143–152. [Google Scholar]
- 144.Parr M.K., Fusshöller G., Schänzer N., Opfermann G., Piper T., Rodchenkov G., Schaänzer W. Metabolism of androsta-1,4,6-triene-3,17-dione and detection by gas chromatography/mass spectrometry in doping control. Rapid Commun. Mass Spectrom. 2009;23(2):207–218. doi: 10.1002/rcm.3861. [DOI] [PubMed] [Google Scholar]
- 145.Delbeke F.T., Van Eenoo P., Van Thuyne W., Desmet N. Prohormones and sport. J. Steroid Biochem. Mol. Biol. 2002;83(1-5):245–251. doi: 10.1016/S0960-0760(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 146.van Eenoo P., Lootens L., van Thuyne W., Deventer K., Pozo-Mendoza O., Delbeke F.T. Results of several (small) research projects at DoCoLab in 2006. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 15. Cologne: Sport & Buch Strauss; 2007. pp. 41–48. [Google Scholar]
- 147.Parr M.K., Geyer H., GA1/4tschow M., Haenelt N., Opfermann G., Piper T., Thevis M., Schänzer W. New steroids on the “supplement” market. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 16. Cologne: Sport & Buch Strauss; 2008. pp. 73–82. [Google Scholar]
- 148.Sobolevsky T., Virus E., Semenistaya E., Kachala V., Kachala I., Rodchenkov G. Orastan-A: Structural elucidation and detection in urine. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advance in Doping Analysis. Vol. 16. Cologne: Sport & Buch Strauss; 2008. pp. 337–340. [Google Scholar]
- 149.Parr M.K., GA1/4tschow M., Daniels J., Opfermann G., Thevis M., Schänzer W. Identification of steroid isoxazole isomers marketed as designer supplement. Steroids. 2009;74(3):322–328. doi: 10.1016/j.steroids.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 150.Parr M.K., Opfermann G., Schlörer N., Geyer H., Rataj F., Zierau O., Diel P., Schänzer W. Sense or nonsense of prohormone designing: Reduced metandienone as supplement. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis. Vol. 19. Cologne: Sport & Buch Strauss; 2011. pp. 15–23. [Google Scholar]
- 151.Parr M.K., Fusshöller G., Schlörer N., Opfermann G., Parr M.K., Fusshöller G., Schäzer N., Opfermann G., Geyer H., Rodchenkov G., Schänzer W. Detection of Delta6-methyltestosterone in a "dietary supplement" and GC-MS/MS investigations on its urinary metabolism. Toxicol. Lett. 2011;201(2):101–104. doi: 10.1016/j.toxlet.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 152.Parr M.K., Westphal F., Sönnichsen F., Geyer H., Schänzer W. 1-DHEA identification in seized dietary supplement. In: Schänzer W., Geyer H., Gotzmann A., Mareck U., editors. Recent Advances in Doping Analysis (18). Vol. 18. Cologne: Sport & Buch Strauss; 2010. pp. 241–244. [Google Scholar]
- 153.Parr M.K., Opfermann G., Geyer H., Westphal F., SAnnichsen F.D., Zapp J., Kwiatkowska D., Schänzer W. Seized designer supplement named ?o1-Androsterone ??: identification as 3I -hydroxy-5I -androst-1-en-17-one and its urinary elimination. Steroids. 2011;76(6):540–547. doi: 10.1016/j.steroids.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 154.Brooker L., Parr M.K., Cawley A., Flenker U., Howe C., Kazlauskas R., Schänzer W., George A. Development of criteria for the detection of adrenosterone administration by gas chromatography-mass spectrometry and gas chromatography-combustion-isotope ratio mass spectrometry for doping control. Drug Test. Anal. 2009;1(11-12):587–595. doi: 10.1002/dta.108. [DOI] [PubMed] [Google Scholar]
- 155.Krug O., Thomas A., Walpurgis K., Piper T., Sigmund G., Geyer H., Schänzer W., Thevis M. Mass spectrometric analysis of black market products by HPLC-(HR)MS, GC-(HR)MS and 1D-gel electrophoresis-UPLC-MSn. In: Schänzer W., Thevis M., Geyer H., Mareck U., editors. Recent Advances in Doping Analysis (21). Vol. 21. Cologne: Sport & Buch Strauss; 2013. pp. 169–172. [Google Scholar]


