There is mounting evidence that stress during pregnancy can have detrimental effects on gestation and birth. Existing studies indicate that prenatal stress may increase levels of circulating inflammatory markers that are associated with prematurity and pregnancy complications, suggesting that stress-related changes in the cytokine milieu may increase the risk of poor pregnancy outcome. Previous studies, however, have not clearly connected stress during pregnancy to changes in inflammatory mediators and, in turn, to clinically-relevant outcomes such as premature delivery. The present study sought to directly connect prenatal stress and changes in inflammatory markers to preterm delivery and gestational age at birth (GAB). A sample of 173 women was recruited during the first trimester of pregnancy and followed through delivery. Overall stress, pregnancy-specific distress, and inflammatory markers were assessed early and later in pregnancy, and the predictive value of these measures for preterm birth and GAB was determined. There were significant differences in pregnancy-specific distress, IL-6, and TNF-α between women who delivered prematurely versus those who delivered at term, and elevated levels of pregnancy-specific distress, IL-6, and TNF-α were predictive of shortened GAB overall. Importantly, in many cases, the effects of overall stress and pregnancy-specific distress on GAB were mediated by levels of circulating inflammatory markers. Collectively, these data provide strong evidence that prenatal stress experiences can affect the timing of parturition via alterations in circulating inflammatory mediators, and underscore the need for ongoing research aimed at further understanding the mechanisms and effects of prenatal stress on maternal and infant health.
Maternal stress in pregnancy is a potentially important factor in fetal development, and there is growing evidence that psychosocial, cultural, and environmental stressors experienced during gestation can be detrimental to pregnancy outcome and infant health. Studies have shown that both stress and pregnancy-specific distress are related to poor pregnancy outcomes (i.e. Arck 2010;Maina et al. 2008;Zhu et al. 2010), and a primary finding is that prenatal stress can increase prematurity and the incidence of infants born at low birth weight (LBW) for their gestational age (Nkansah-Amankra et al. 2010;Orr et al. 1992;Wadhwa et al. 1993). Despite improvements in the practice and accessibility of prenatal care, the rate of preterm birth (< 37 weeks gestation) remains relatively high in the US (12-13%), and infants born even moderately prematurely can be affected by a variety of ongoing behavioral, developmental, and health challenges (Batton et al. 2011;Dong and Yu 2011;Goldenberg et al. 2008) Notably, shortened gestational age at birth, even within the “at term” range (37-40 weeks) has been associated with suboptimal brain development and altered cognitive development (Davis et al. 2011;Yang et al. 2010). Given that nearly 40% of cases of preterm birth lack a clear etiology (Goldenberg et al. 2008), understanding the impact of stress as a potential contributor to gestational age at birth and premature delivery is essential.
Normal pregnancy requires a balance of aspects of the immune, endocrine, and nervous systems that delicately shifts through the course of pregnancy to support maternal and fetal well-being, and it is likely that perturbation of this balance increases the risk of poor pregnancy outcomes (Arck 2010;Arck 2001). Support for this notion comes from observations that women who experience activation of the immune system via viral or bacterial infection during pregnancy are prone to pregnancy complications including preterm delivery and exhibit elevated levels of proinflammatory cytokines even after the infection has been resolved (Gibbs et al. 1992;Gomez et al. 1995;Gotsch et al. 2008;McGregor et al. 2000;Wadhwa et al. 2001). Interestingly, studies in non-pregnant populations have shown that stress experiences can elevate inflammatory markers such as IL-6 and TNF-α even in the absence of infection (Dunn AJ 1993;Maes et al. 1998;Rozlog LA et al. 1999), and as such, it has been hypothesized that maternal psychosocial stress affects pregnancy outcome by altering inflammatory markers via neurochemicals that are released as part of the physiological response to stress (Coussons-Read et al. 2007;Dunkel Schetter 2011;Knackstedt et al. 2005;Ruiz and Avant 2005). Previous work in our laboratory and others has suggested that stress-related changes in endocrine and immune function may play a role in how stress alters the course of pregnancy, although to date, no published studies have directly connected these factors to prematurity or pregnancy complications. Thus far, studies show that psychosocial stress is associated with elevations in circulating levels of inflammatory markers that have been associated with poor outcome, including C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (Coussons-Read et al. 2007;Pearce et al. 2010;Ruiz et al. 2003). Although these studies were not able to clearly link stress-related changes in these inflammatory markers to poor pregnancy outcomes such as prematurity, they did establish that prenatal stress perturbs the critical balance of immune responsiveness necessary for uncomplicated pregnancy. Given our understanding of the balance between the endocrine, immune, and nervous systems required for reproductive health and pregnancy success, stress-related changes in these systems increase the risk of adverse birth outcomes.
Stress, broadly defined in the context of neural-immune relationships, often refers to events, situations, emotions, and interactions which are perceived as negatively affecting the well being of the individual or which cause responses perceived as harmful (Dantzer 1989;Maes et al. 1998;Orr et al. 1992). Psychosocial stressors are life experiences, such as changes in personal life, relationships, job status, housing, and family makeup, which require adaptive coping behavior on the part of an individual (Orr et al. 1992;Yali and Lobel 1999). Much of the current research on how pregnancy is affected by stress has focused on stress as defined in the above context with particular emphasis on psychosocial stressors (Coussons-Read et al. 2007;Dunkel Schetter 2011;Dunkel-Schetter and Glynn 2011;Gennaro and Fehder 1996;Ruiz and Fullerton 1999). It is this conceptualization which is the basis for the construct of “overall stress” utilized in the present study. This approach to examining the effects of stress on pregnancy is certainly valid, but there are aspects of maternal stress during pregnancy which are not captured by the “overall stress” construct. Although overall stress certainly affects women during pregnancy, there are pregnancy-specific stressors which can create distress for expectant mothers, and “pregnancy-specific distress” related to worry about prenatal screenings and concerns about infant health and development, may occur along with “overall stress” as described above (Lobel et al. 2008;Woods-Giscombe et al. 2010). Studies have shown that both overall stress and pregnancy-specific distress are associated with increased occurrence of maternal anxiety during pregnancy as well as higher rates of preterm delivery and unplanned cesarean sections (Glynn et al. 2008;Lobel et al. 2008;Saunders et al. 2006).
There is growing evidence that the mechanism of the effects of stress and distress on pregnancy outcome involves activation of the hypothalamo-pituitary-adrenal (HPA) axis. Several studies have shown that women who encounter psychosocial stress or distress during pregnancy have higher levels of ACTH, CRH, and cortisol than non-stressed pregnant women (McLean and Smith 2001;Parker and Douglas 2010;Wadhwa et al. 1993). Additional evidence that HPA axis activity may play a critical role in preterm birth comes from findings that women experiencing preterm labor and delivery have significantly higher levels of plasma cortisol and CRH prior to onset of labor than women who deliver normally (Field et al. 2008;Pearce et al. 2010). A major component of the HPA response to stress is release of CRH in the hypothalamus, which regulates the peripheral aspects of the stress response. The placenta also produces CRH, which increases exponentially over gestation (Vitoratos et al. 2006;Wadhwa 2005), and considerable interest has focused on the role of stress-related CRH production in modulation of labor and delivery because CRH may act as a signal for normal labor and stress-related may induce premature labor (Kalantaridou et al. 2010;McLean et al. 1995;Stamatelou et al. 2009). Studies indicate that maternal stress and distress increase CRH levels in pregnancy, and given the role CRH plays in regulation of parturition, it is likely that stress-related increases in CRH contribute to premature labor (Kramer et al. 2009). Others studies show that CRH upregulates the inflammatory response including release of the proinflammatory cytokines assessed in the present study, suggesting a further pathway through which HPA axis activity is connected to pregnancy outcome (Kalantaridou et al. 2010;Pearce et al. 2010).
Although previous work has suggested that prenatal stress and elevated inflammatory markers may be related to poor pregnancy outcomes (i.e. Coussons-Read et al. 2005;Parker and Douglas 2010;Woods et al. 2010), no definitive connections between maternal stress, elevated inflammatory markers, and pregnancy outcomes such as preterm birth have been established. The present study begins to fill this gap by longitudinally assessing overall stress, pregnancy-specific distress, and inflammatory markers, and examining their relationships to one another and to preterm delivery and gestational age at birth (GAB). Two primary hypotheses were tested. First, it was expected that elevated overall stress, pregnancy-specific distress, and inflammatory markers would be associated with preterm birth and shortened GAB. The second hypothesis was that the effect(s) of elevated overall stress and/or pregnancy-specific distress on GAB would be mediated by levels of inflammatory markers.
Methods
Subjects
Two hundred twenty-five women were recruited for this study. The sample consisted of pregnant women (ages 18-45), without a history of current or past drug use, who were recruited by study staff at the Denver Health and Hospital Authority (DHHA) Medical Center in Denver, CO. Both primi- and multiparous women were recruited between 10 and 18 weeks of gestation. The only exclusion criteria were a current diagnosis of mental illness or substance abuse, or classification as a “high risk” pregnancy by clinic personnel (i.e. recurrent spontaneous abortion, current cancer, autoimmune disorders, and infection at intake). Visits occurred at normally scheduled obstetrics clinic appointments between February 2009 and July 2010. Fifty-two women were dropped from the final sample because they did not complete the protocol. Reasons for non-completion included moving away from the area or moving care to another clinic (20), hospitalization, drug use, or delivery prior to the second time point or fetal demise (7), failure to appear for the later experimental time point (18), or unanticipated delivery of twins (7; determined via ultrasound after study enrollment), resulting in a final sample of 173 women. A power analysis determined that a sample size of 173 has 80% power to detect an R2= 0.189 attributed to up to 6 predictor variables using an F-test with α=0.05. Final sample characteristics are displayed in Table 1. The ethnic mix of the sample (66% Latina) is in line with that of the population served by DHHA (70% Latina).
Table 1. Demographic characteristics of the final sample (n=173).
| Maternal Age (range) |
Race/Ethnicity | Years of education (range) |
Employment status |
Marital status (current) |
|||
|---|---|---|---|---|---|---|---|
| 27.49 (18-43) |
Caucasian: | 14% | 13.09 (12-18) |
Not Employed: |
27% | Never married: | 20% |
| Hispanic: | 67% | Student: | 5% | Living w/partner: |
38% | ||
| Native American: |
2% | Homemaker: | 32% | Married: | 35% | ||
| African American: |
11% | Part-time: | 14% | Divorced: | 1% | ||
| Asian American: |
0% | Full-time: | 22% | Separated: | 6% | ||
| Multi-Racial: | 6% | ||||||
Procedure
Once in early pregnancy (14-18 weeks) and again later in pregnancy (28-32 weeks), women completed assessments of overall stress and pregnancy-specific distress and provided a blood sample. These time points were selected based on our previous work showing that although stress and inflammatory markers were correlated in early and later pregnancy, these relationships were not observed during mid-pregnancy (Coussons-Read et al. 2007). Since preterm birth was the primary outcome measure in the present work, the “late” pregnancy assessment was timed between 28 and 32 weeks of gestation in order to increase the probability of capturing complete data from women who would deliver prematurely (prior to 37 weeks of gestation). To maximize sample size, the analyses are conducted using measures of stress, distress, and inflammatory markers averaged across any of the two time periods available. The inclusion of early and late measures also allow separate assessments of any differential impact on gestational age and prematurity of heightened levels of stress, distress and inflammatory markers early vs. late in pregnancy. Both survey instruments were translated into Spanish for use in non-English speaking Latinas using a decentered translation process. Subjects completed the surveys during regularly scheduled prenatal checkups. Whenever possible, the blood draws corresponded with regularly scheduled prenatal blood tests (triple screen at 14-18 weeks; gestational diabetes screen at 28-32 weeks), and an additional 10 ml of blood were collected to complete the study in addition to what was required for prenatal care. All blood sampling occurred between 8 and 11am to account for circadian rhythmicity and diurnal variation in the measures of interest.
Psychosocial Assessments
Revised Pregnancy Distress Questionnaire: The Revised Pregnancy Distress Questionnaire NUPDQ) was used to assess pregnancy-related distress. The original version of the Prenatal Distress Questionnaire (PDQ) is a 12-item measure self-administered during mid-pregnancy (Yali and Lobel 1999). The revised measure (NUPDQ,Lobel et al. 2008) was modified for interview format and in this study, was administered in early (14-18 weeks) and later (28-32 weeks) pregnancy. Some items are repeated in each assessment; others are assessed once, representing unique stressors that usually arise during a specific period of pregnancy (Lobel et al. 2008). For example, in early pregnancy, women tend to be concerned about physical symptoms such as nausea and vomiting, whereas later they are typically more concerned with the health of the baby. Respondents are asked to indicate if they are currently feeling bothered, upset, or worried about different aspects of pregnancy on a 3-point scale ranging from “not at all” (0) to “very much” (2). Since the total number of items in each assessment varies (early = 9 items; late = 17 items), an average pregnancy-specific distress score at each time point is calculated for each respondent by summing item responses and dividing by the total number of items at that time point. In addition, distress scores from the two time points are averaged to produce a mean level of pregnancy-specific distress across pregnancy that also ranges from 0 to 2.
Denver Maternal Health Assessment: The Denver Maternal Health Assessment (DMHA) was used to gather demographic information, and a measure of overall stress. The DMHA was adapted from a validated questionnaire developed by Meikle, Orleans, Leff, Shain, and Gibbs (Meikle et al. 1995) and is a valid and reliable instrument for assessing maternal overall stress (measured through a combination of daily stress experiences and life events) among Latinas, African Americans, and Caucasians (Coussons-Read et al. 2005;Coussons-Read et al. 2007;Leff et al. 1992;Meikle et al. 1995). The DMHA also includes a module that collects demographic information as well as one that is focused on perceived self-efficacy. Only the overall stress and demographic modules of the DMHA were used in the present study, however, due to concerns about the degree to which the concepts in the self-efficacy module would apply to and be culturally-valid for the Latina portion of the sample. The demographic module of the DMHA collects data about subject marital status, race, ethnicity, age, income, and education and was administered to subjects only at study enrollment. Multiple choice options are provided for the demographic variables (i.e. “never married”, “living with a partner”, “married”, “divorced”, and “separated”), and women were required to select only one option. The overall stress module of the DMHA is comprised of a daily stress scale and a series of major life events items, and was administered to women at both study visits.
A primary component of the overall stress portion of the DMHA is assessment of the participants’ levels of stress experiences and feelings about stress. Items on the daily stress scale use a 5-point, Likert scale with responses ranging from “not a stress at all” to “major stress”. Participants can also mark the does not apply response, which excludes that item from the analysis. These are representative questions from the DMHA: “The following refer to stresses in your life in the last couple of months. The following is a list of things that can be stressful in day-to-day life. Please respond to each item by saying if it was not a stress, was a mild stress, a moderate stress, or a big stress. If the item does not apply to you, please say so.” This direction to subjects is followed by 58 major life events items to which they respond on a Likert scale (1= does not apply, to 5= big stress). Example items are “your job”, “not having a job”, “your children”, “your marriage”, “your spouse/partner”, “time for yourself”, “your weight”, “saving money”, “your neighborhood”, and “the news”. Women’s responses were entered into an Excel spreadsheet, and each response was assigned a numerical value. These scores were summed to produce a summary “overall stress” score for each woman at each time point. These scores were averaged to provide global measures of overall stress across pregnancy ranging from 1 to 52.
Blood Sample Collection
A 10 ml sample of maternal blood was collected during routine venipuncture at each office visit. Blood was drawn into a red-top clotting tube for serum collection for serum cytokine and CRP assessments. Blood collections coincided with regularly scheduled blood tests in prenatal care (i.e. 16-18 weeks of gestation, triple screen). For serum extraction, blood samples were collected in non-heparinized Vacutainer tubes and allowed to clot at room temperature for 30 minutes after collection. Samples were centrifuged at 2000rpm for 30 minutes in a clinical centrifuge. One milliliter aliquots were collected using a sterile at −70° C Pasteur pipet into cryovials and frozen until analysis.
Inflammatory Marker Assessments
Commercially available enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems) were used to quantify CRP, IL-6 and TNF-α in the circulation. Undiluted serum samples were tested in duplicate and according to the directions provided by the manufacturer. Optical density at 450 nm was assessed using an automatic microplate reader (LabSystems MultiSkan), and the amount of inflammatory marker in each sample was determined using the standard curve generated with each assay according to the manufacturer’s instructions. Samples were frozen until the study was completed, and all samples were run together to avoid problems with assay drift and interassay variability. ELISA kits from the same manufacturer’s lot were used for all assays for all measures. In practice, these assays show minimal variability between the standard curves (less than 6% variability) in our laboratory. The mean of the duplicates was used as the unit of analysis for statistical evaluation of these data.
Gestational Age at Birth
Review of maternal medical charts was used to collect information about GAB. In addition to examining GAB as a continuous variable, we also created a dichotomous variable for Preterm Birth. Determination of preterm birth was based on GAB (in weeks), which was established based on last menstrual period and confirmed with ultrasound dating for all subjects. Preterm birth was identified as infants born <37 weeks of gestation and term birth was defined as birth at or beyond 37 weeks of gestation.
Data Disposition and Analyses
All statistical assessments were made using a computerized program for data analysis (SPSS, IBM SPSS Inc.). Given the a priori hypotheses about the directions of all of the relationships to be examined, we utilized 1-tailed analyses and 90% confidence intervals for all of the statistical tests reported here. Repeated measures ANOVAs were used to assess the effect of early and later pregnancy on levels of inflammatory markers and overall stress in women delivering a preterm or term infant. Pregnancy-specific distress was not included in the repeated measures analysis, however, because although the construct of distress is consistent, the actual items that create the distress score early in pregnancy are different from those in later pregnancy. One-tailed independent samples t-tests were used to compare demographic characteristics (maternal age, ethnicity, marital status, years of education, household income, number of prior pregnancies, BMI at study enrollment, and occurrence of bacterial infection during pregnancy), early and late levels of inflammatory mediators, overall stress, and pregnancy-specific distress in women who delivered preterm versus those who delivered at term.
As mentioned previously, 52 women in the original sample failed to complete the protocol, and were dropped from the analyses. In addition, early in the study, a group of blood samples was improperly processed, and as such 18 subjects lacked inflammatory marker data for the early time point. To assess the sensitivity of the results to this sample reduction, we reestimate our models by assigning these women the mean value for the sample and then created a dichotomous indicator for missing data. By including this indicator as a control in the regressions, subjects with a missing variable did not contribute to the estimate of that coefficient, but were still part of the sample and contribute to our ability to detect effects of other variables. The results are qualitatively similar to those that drop observations with missing data that are presented here. Because one of the dependent variables of interest is dichotomous, we also re-estimate the equations for preterm birth with a logit model. These results are unchanged from the ordinary least squares linear probability models results reported here.
Multiple regression analyses were conducted to examine the direct contributions of the psychosocial and inflammatory marker variables to the occurrence of preterm birth and GAB. Three regression analyses were conducted to examine these relationships. We first estimate the model using values that reflect the average scores for time-varying covariates. We then test whether these relationships vary during pregnancy by estimating separate models early and late in pregnancy. For each regression analysis, maternal bacterial infection in pregnancy was entered in step 1, the inflammatory mediators were entered in steps 2, 3, and 4, and overall stress and pregnancy-specific distress were entered in steps 5 and 6. The order of entry of the variables into the regression equations was based on a priori hypotheses that after controlling for maternal bacterial infection during pregnancy, the inflammatory markers would have the largest influence on preterm birth and GAB, followed by levels of pregnancy-specific distress and overall stress.
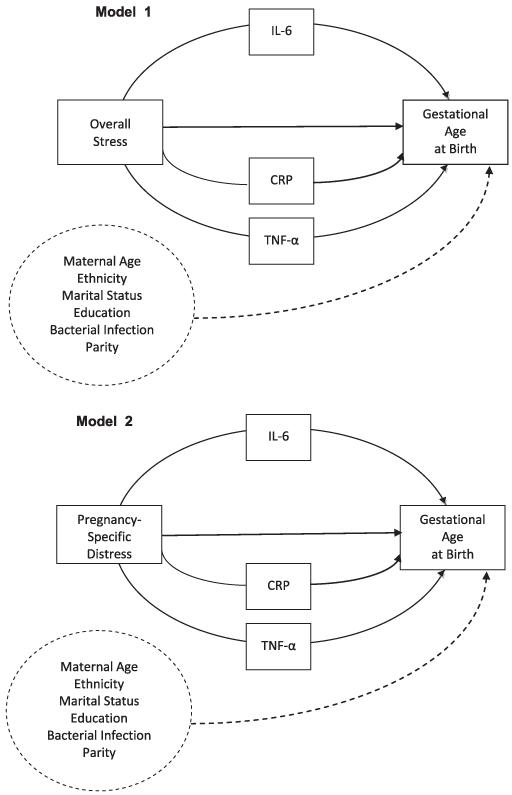
In order to examine the potential role of indirect effects of overall stress, pregnancy-specific distress, and inflammatory markers in determining GAB, mediation analyses were conducted in SPSS (Preacher and Hayes 2008). This approach permits examination of the degree to which the effects of a predictor variable on an outcome variable are due to the effects of one or more mediating variables while controlling for the effects of covariates. It is important to note, however, that this approach diverges from the “causal steps approach” to meditational analyses (i.e. Baron and Kenney 1986) in favor of focusing on the product of the path coefficients that comprise a potential mediated relationship (Hayes 2009;Preacher and Hayes 2004;Rucker et al. 2011). This approach has gained substantial support over the past several years it provides increased power for detecting meditation over the causal steps approach and directly tests the indirect (mediated) pathway rather than inferring it based on significance of all of the constituent pathways in a model (Fritz and MacKinnon 2007;Hayes 2009;Preacher and Hayes 2008). Our application of this approach in the present study is illustrated in Figure 1, which emphasizes that the analyses focus on the significance of the entire indirect effect rather than on the significance of some or all of the constituent paths (Hayes 2009;Rucker et al. 2011, A. Hayes, personal communication). Another benefit of this approach is that it enables assessment of mediated effects in smaller samples through a bootstrapping method in which the original data are sampled (with replacement) 5000 times. This procedure generates a set of ordered indirect effect coefficients, around which confidence intervals are created. Because the hypothesized meditational pathways were directional, we utilized a 90% confidence interval in our analyses (retrieved August 31, 2011 from http://www.afhayes.com/macrofaq.html). Significant meditational relationships are identified by cases in which zero does not fall within the 90% confidence interval produced for each analysis. Such cases indicate that the proposed mediator variable (IL-6, TNF-α, or CRP) significantly contributed to the effect of the predictor variable (overall stress or pregnancy specific distress) and the outcome variable (GAB) at the standard Type I error rate of α = .10. Our hypotheses led us to test 2 mediation models (Figure 1) in which we expected that the effect of each predictor variable (Model 1:Overall Stress; Model 2: Pregnancy-Specific Distress) on GAB (the outcome variable) would occur through levels of the inflammatory markers IL-6, TNF-α, and/or CRP (the mediating variables), controlling for the potential effects of maternal age, ethnicity, BMI at study enrollment, education, parity, and the occurrence of bacterial infection during pregnancy (covariates). We used the continuous measure of GAB as the dependent variable in these analyses rather than the dichotomous variable of preterm birth due to the low frequency of preterm birth in the sample as a whole. As in earlier analyses, we first estimate models that average time-varying variables throughout pregnancy and then estimate separate models early and late in pregnancy.
Figure 1.
Proposed meditational models of the role of inflammatory markers in the relationship between prenatal Overall Stress (Model 1) and Pregnancy-Specific Distress (Model 2) and Gestational Age at Birth
Results
The rate of preterm delivery in the final sample was 9.9% (17 preterm births out of a total of 173). When women who gave birth to twins were included, this number increased to 11.1%, which is consistent with the rate of premature delivery in the state of Colorado, reported to be 11.4% in 2008 (Retrieved June 20, 2011, from www.marchofdimes.com/peristats). All the preterm births occurred in Caucasian and Latina women, who comprised 81% of the sample, and as such, ethnicity rather than race was included as a covariate in the subsequent analyses. There were no significant differences in the other demographic or maternal subject variables (age, number of previous births [parity], BMI at study enrollment, total years of education, ethnicity, total household income, marital status) between women who delivered at term or prematurely (Table 2). T-tests indicated that maternal bacterial infection during pregnancy was strongly associated with preterm delivery. Bacterial infection was assessed through chart extraction of information that laboratory tests had confirmed bacterial infection at some time during the pregnancy. Such infections occurred in 14 of the 173 women (8% of the sample). This rate of occurrence is slightly below the rate of asymptomatic urinary tract infection in pregnancy (10-15%) (Gilstrap and Ramin 2001), suggesting that an additional 3-8 women in the sample may have had an undiagnosed urinary tract infection.
Table 2. Demographic characteristics of women who delivered preterm (n=17) or term (n=156). Significance levels for t-tests examining differences in demographic variables between women who delivered prematurely and at term are included.
| Term Deliveries | Preterm Deliveries | t | p | |
|---|---|---|---|---|
|
Maternal Age
(Mean ± SD) |
27.33 (5.86) |
28.47 (7.74) |
.585 | .566 |
|
Years of Education
(Mean ± SD) |
14.35 (1.57) |
14.23 (1.46) |
.324 | .747 |
|
Household Income
(Mean ± SD) |
$28,610 (14,920) |
$25,000 (14,190) |
−.919 | .359 |
|
Prior Pregnancies
(Mean ± SD) |
3.85 (2.82) |
3.32 (2.10) |
1.010 | .314 |
|
BMI at Enrollment
(Mean ± SD) |
26.34 (8.21) |
28.24 (8.53) |
.743 | .459 |
| Ethnicity (% of sample) | .338 | .736 | ||
| Caucasian | 26% | 24% | ||
| Latina/Hispanic | 74% | 76% | ||
| Marital Status (% of sample) | −1.125 | .263 | ||
| Never Married | 25% | 29% | ||
| Living w/Partner | 33% | 47% | ||
| Married | 35% | 18% | ||
| Divorced | 1% | 6% | ||
| Separated | 6% | 0% | ||
| Confirmed Bacterial Infection in Pregnancy (% of sample) | 4.85 | .000 | ||
| Yes | 7% | 35% | ||
| No | 93% | 65% | ||
Table 3 illustrates the results of independent samples t-tests conducted on inflammatory markers, stress, and distress in early and late pregnancy and across pregnancy in women delivering preterm or term infants. Levels of inflammatory markers, stress and distress are uniformly lower for women whose pregnancy went to term than for women who delivered preterm, but these differences do not always reach conventional levels of significance. T-tests indicated that early in pregnancy, levels of overall stress, IL-6, and TNF-α differed between women who delivered preterm infants compared to those who did not. Later and averaged across pregnancy, pregnancy-specific distress, IL-6 and TNF-α differed between women who ultimately delivered their infants prematurely and those who delivered at term.
Table 3. Descriptive statistics and independent samples t-test results for inflammatory and psychosocial variables in women delivering prematurely (n=17) or at term (n=156) early (14-18 weeks’ gestation), later (28-32 weeks’ gestation) or averaged across pregnancy. Significant differences at the .10 level are indicated by an asterisk.
| Preterm Delivery | Term Delivery | |||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | p | t (173) | |
|
Averaged Across
Pregnancy | ||||||
| Mean IL-6 | 9.58 | 1.57 | 8.22 | 1.55 | .001 | 3.348* |
| Mean TNF-α | 2.03 | 1.33 | 1.09 | .910 | .004 | 2.936* |
| Mean CRP | 62.6 | 21.0 | 50.0 | 28.1 | .075 | 1.788 |
| Mean Pregnancy-Specific Distress |
.771 | .435 | .598 | .337 | .009 | 3.604* |
| Mean Overall Stress | 24.0 | 12.23 | 17.6 | 11.2 | .113 | 1.592 |
| Early Pregnancy | ||||||
| Early IL-6 | 9.20 | 1.67 | 8.145 | 1.55 | .009 | 2.633* |
| Early TNF-α | 1.99 | 1.23 | 1.30 | 1.05 | .024 | 2.282* |
| Early CRP | 64.2 | 23.7 | 51.7 | 29.9 | .098 | 1.663 |
| Early Pregnancy-Specific Distress |
.833 | .520 | .712 | .371 | .103 | 1.728 |
| Early Overall Stress | 24.4 | 10.7 | 17.7 | 11.3 | .026 | 2.243* |
| Late Pregnancy | ||||||
| Late IL-6 | 9.59 | 1.99 | 8.28 | 1.57 | .002 | 3.182* |
| Late TNF-α | 2.04 | 1.01 | 1.34 | .839 | .012 | 3.206* |
| Late CRP | 61.0 | 22.8 | 48.1 | 26.1 | .053 | 1.948 |
| Late Pregnancy-Specific Distress |
.710 | .350 | .484 | .302 | .011 | 2.578* |
| Late Overall Stress | 23.7 | 13.7 | 17.5 | 11.1 | .061 | 1.893 |
Table 4 shows the results of correlational analyses of the relationships among overall stress, pregnancy-specific distress, IL-6, TNF-α, CRP and GAB early, late, and averaged across pregnancy. The range of GAB in the sample was 29.6 weeks to 41.6 weeks (Mean= 38.6, SD=1.82). As shown in the table, although not uniformly significant, there were positive relationships between the psychosocial variables and the inflammatory markers early, late and across pregnancy, as well as significant negative relationships between the psychosocial variables and inflammatory markers and GAB.
Table 4. Pearson correlations between the psychosocial variables of overall stress, pregnancy-specific distress, and the inflammatory markers (a) averaged across pregnancy,(b) early in pregnancy, and (c) late in pregnancy.
| 4a | |||||
|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | |
| 1. Mean Overall Stress | 1 | .565** .000 |
.022 .381 |
−.008 .458 |
.225** .001 |
|
2. Mean Pregnancy
Distress |
.565** .000 |
1 | −.065 .187 |
−.017 .409 |
.184** .006 |
| 3. Mean IL-6 | .022 .382 |
−.065 .187 |
1 | .363** .000 |
.132* .034 |
| 4. Mean CRP | −.008 .458 |
−.017 .409 |
.363** .000 |
1 | .100 .085 |
| 5. Mean TNF-a | .225** .001 |
.184** .006 |
.132* .034 |
.100 .085 |
1 |
|
Gestational Age at
Birth |
−.098 .105 |
−.195** .003 |
−.293** .000 |
−.186** .010 |
−.281** .000 |
| 4b | |||||
|---|---|---|---|---|---|
| 1. | 2. | 3 | 4. | 5. | |
| 1. Early Overall Stress | 1 | .512** .000 |
.105 .080 |
.006 .467 |
.273** .000 |
|
2. Early Pregnancy
Distress |
.512** .000 |
1 | −.061 .206 |
−.032 .334 |
.192** .005 |
| 3. Early IL-6 | .105 .080 |
−.061 .206 |
1 | .334** .000 |
.113 .063 |
| 4. Early CRP | .006 .467 |
−.032 .334 |
.334** .000 |
1 | .065 .191 |
| 5. Early TNF-α | .273** .000 |
.192** .005 |
.113 .063 |
.065 .191 |
1 |
|
Gestational Age at
Birth |
−.158* .021 |
−.136* .041 |
−.274** .000 |
−.218** .003 |
−.269** .000 |
| 4c | |||||
|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | |
| 1. Late Overall Stress | 1 | .484** .000 |
.020 .407 |
.050 .280 |
.195* .011 |
|
2. Late Pregnancy
Distress |
.484** .000 |
1 | −.023 .395 |
.040 .319 |
.150* .040 |
| 3. Late IL-6 | .020 .407 |
−.023 . 395 |
1 | .299** .000 |
.184* .015 |
| 4. Late CRP | .050 .280 |
.040 .319 |
.299** .000 |
1 | .197* .010 |
| 5. Late TNF-α | .195* .011 |
.150* .040 |
.184* .015 |
.197* .010 |
1 |
|
Gestational Age at
Birth |
−.158 .062 |
−.239** .003 |
−.289** .000 |
−.133 .063 |
−.268** .001 |
Correlation is significant at the 0.01 level
Correlation is significant at the 0.05 level (1-tailed).
Table 5 a-c displays the results of the multivariate regression analyses of the effects of maternal bacterial infection, IL-6, TNF-α, CRP, pregnancy-specific distress and overall stress on both preterm birth and GAB averaged across pregnancy (5a), and assessed early (5b), and late (5c) in pregnancy. After controlling for maternal infection during pregnancy, regression analyses showed that elevated circulating IL-6 and TNF-α and levels of distress averaged across pregnancy were significantly associated with the occurrence of preterm birth (R2adj=.23, F(5,159)= 11.369, p<.000). The “adjusted r-squared” values take the number of degrees of freedom in each model into account to provide a more conservative estimate of R2. Tables 5b and 5c report estimates of the effects of inflammatory markers, stress and distress early and late in pregnancy on pregnancy outcomes. The estimates reported in 5b suggest that elevated levels of IL-6 and TNF-α early in pregnancy are associated with preterm birth (R2adj=.16, F(1,156)=15.992, p<.000). The estimates in Table 5c showed that the combination of elevated distress, IL-6, and TNF-α later in pregnancy was significantly predictive of preterm birth (R2adj=.26, F(5,122)=8.810, p<.000). Similar patterns of the predictive value of pregnancy-specific distress, IL-6, and TNF-α were observed in regression analyses focusing on the continuous outcome variable of GAB (reported in the second column of Table 5a-5c). Examination of these estimates indicates, for example, that an increase of one standard deviation (SD) unit in average pregnancy-specific distress translates into a reduction of roughly 25% of an SD unit in weeks of gestation.
Table 5. Regression analyses of the relative contributions of maternal infection, pregnancy-specific distress (distress), overall stress (stress), and the inflammatory markers to the occurrence of preterm birth and gestational age at birth (GAB) early (5a), late (5b), and averaged across (5c) pregnancy. Significant effects (p<.10) are indicated by bold typeface.
| 5a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Step | Variables entered | df | Δ R 2 | β | P | Δ R 2 | β | P |
| Preterm Birth | GA | |||||||
| 1 | Infection | 1, 163 | .145 | .330 | .000 | .128 | −.302 | .000 |
| 2 | Mean IL-6 | 1, 162 | .054 | .225 | .001 | .054 | −.219 | .001 |
| 3 | Mean TNF-α | 1, 161 | .020 | .122 | .043 | .027 | −.152 | .020 |
| 4 | Mean CRP | 1, 160 | .001 | .029 | .620 | .003 | −.044 | .471 |
| 5 | Mean Distress | 1, 159 | .029 | .196 | .017 | .028 | −.233 | .017 |
| 6 | Mean Stress | 1, 158 | .000 | .048 | .577 | .008 | −.111 | .195 |
| 5b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Step | Variables entered | df | Δ R 2 | β | P | Δ R 2 | β | P |
| Preterm Birth | GAB | |||||||
| 1 | Infection | 1, 157 | .132 | .326 | .000 | .122 | −.286 | .000 |
| 2 | Early IL-6 | 1, 156 | .038 | .167 | .039 | .047 | −.176 | .008 |
| 3 | Early TNF-α | 1, 155 | .007 | .057 | .331 | .019 | −.118 | .247 |
| 4 | Early CRP | 1, 154 | .003 | .063 | .442 | .0113 | −.109 | .426 |
| 5 | Early Distress | 1, 153 | .010 | .068 | .338 | .0110 | −.100 | .174 |
| 6 | Early Stress | 1, 152 | .012 | .060 | .514 | .0002 | −.011 | .339 |
| 5c | ||||||||
|---|---|---|---|---|---|---|---|---|
| Step | Variables entered | df | Δ R 2 | β | P | Δ R 2 | β | P |
| Preterm Birth | GAB | |||||||
| 1 | Infection | 1, 127 | .133 | .315 | .000 | .097 | −.260 | .000 |
| 2 | Late IL-6 | 1, 126 | .066 | .217 | .002 | .093 | −.291 | .000 |
| 3 | Late TNF-α | 1, 125 | .026 | .155 | .018 | .032 | −.156 | .025 |
| 4 | Late CRP | 1, 124 | .005 | .066 | .314 | .000 | −.011 | .811 |
| 5 | Late Distress | 1, 123 | .028 | .151 | .034 | .041 | −.201 | .011 |
| 6 | Late Stress | 1, 122 | .004 | .001 | .664 | .000 | −.002 | .983 |
Tests of the hypothesized meditational models (Figure 1) demonstrated significant meditated effects of overall stress and pregnancy-specific distress on GAB by inflammatory markers. Table 6 shows the results of the bootstrapping analyses conducted to test Model 1 and Model 2 averaged across pregnancy and early and later in pregnancy. Overall, the data showed that levels of circulating inflammatory markers partially mediated the effects of stress and distress on GAB early, and averaged across pregnancy, although these effects are best described as inconsistent mediation (MacKinnon et al. 2000). In some cases, elevated inflammatory markers acted as mediators by augmenting the effect of the stress variables on GAB, while in other cases, a suppressive effect was evident. For example, as noted by the negative confidence interval reported in Table 6, TNF-α was found to play a mediational role in the effect of overall stress early in pregnancy on GAB. The total effect of stress on GAB was −0.0118 and was found to be mediated by TNF-α (β = −0.0052, p<.10). The constituent paths show a significant positive link between overall stress and TNF-α (β = .022, p = 0.01) and a significant path linking higher TNF-α to lower GAB (β = −.233, p = .0795). These analyses also showed that pregnancy-specific distress had a significant negative impact on GAB via levels of IL-6 early and averaged across pregnancy. In this case, however, levels of IL-6 appeared to suppress the negative effect of distress on GAB. For example, the overall effect of mean pregnancy-specific distress on GAB (β = −.833) reflects some suppression by lower levels of IL-6 (β = .2709, p< .10).
Table 6. Results of the multiple mediation analyses of the hypothesized mediating roles of IL-6, TNF-α, and CRP in the effects of Overall Stress (Model 1) and Pregnancy-Specific Distress (Model 2) on gestational age at birth early, late, and averaged across pregnancy. Significant mediating relationships are indicated by 90% confidence intervals that do not include zero (indicated by an asterisk). Confidence intervals are biascorrected and based on 5000 bootstrapped samples.
| Model | Bootstrapped 90% CI estimates |
|---|---|
| Averaged Across Pregnancy | |
|
| |
| Model 1 | |
| Overall Stress →IL-6→ Gestational Age | [−.0041, .0073] |
| Overall Stress →TNF-a→ Gestational Age | [−.0114, .0006] |
| Overall Stress →CRP→ Gestational Age | [−.0001, .0113] |
| Model 2 | |
| Pregnancy Distress →IL-6→ Gestational Age | [.0905, .5980]* |
| Pregnancy Distress →TNF-a→ Gestational Age | [−.3473, .0787] |
| Pregnancy Distress →CRP→ Gestational Age | [−.0197, .2987] |
| Early Pregnancy | |
|
| |
| Model 1 | |
| Overall Stress →IL-6→ Gestational Age | t [−.0059, .0056] |
| Overall Stress →-TNF-α-→ Gestational Age | [−.0174, -.0008]* |
| Overall Stress →CRP→ Gestational Age | [.0010, .0086] |
| Model 2 | |
| Pregnancy Distress →IL-6-→ Gestational Age | [.0122, .4413]* |
| Pregnancy Distress →-TNF-a-→ Gestational Age | [−.3221, .0039] |
| Pregnancy Distress →CRP-→ Gestational Age | [−.0023, .2747] |
| Late Pregnancy | |
|
| |
| Model 1 | |
| Overall Stress →-IL-6→ Gestational Age | [−.0068, .0034] |
| Overall Stress →TNF-α-→ Gestational Age | [−.0031, .0070] |
| Overall Stress →CRP-→ Gestational Age | [−.0009, .0084] |
| Model 2 | |
| Pregnancy Distress →IL-6-→ Gestational Age | [−.0144, .3258] |
| Pregnancy Distress →-TNF-α-→ Gestational Age | [−.1930, .2612] |
| Pregnancy Distress →CRP-→ Gestational Age | [−.0650, .2693] |
Discussion
The present study provides substantive evidence that elevated overall stress and pregnancy-specific distress and increased inflammatory cytokines in maternal circulation are predictive of preterm birth and shortened GAB. The data first demonstrate that there are significant elevations in pregnancy-specific distress and inflammatory markers in women who deliver prematurely compared to those who deliver at term, second, confirms that overall stress and pregnancy-specific distress are related to elevated inflammatory markers, preterm birth, and shortened GAB, and finally, demonstrates a significant predictive pathway from psychological stress to levels of inflammatory markers and, in turn, to GAB. These findings are important given that both preterm delivery and shortened GAB are associated with a variety of persistent behavioral, cognitive, and health challenges for infants born prior to 40 weeks gestation (Davis et al. 2011;Dong and Yu 2011;Samra et al. 2011;Yang et al. 2010). These data help to fill in the gaps in our understanding of the relevance of psychosocial factors such as stress and distress and related changes in the inflammatory milieu for pregnancy outcome, and raise additional questions for future studies.
Although previous work showed that overall stress and pregnancy-related distress are related to preterm birth (i.e. Dunkel Schetter 2011;Glynn et al. 2008;Lobel et al. 2008), the present report is the first to clearly connect overall stress and pregnancy-specific distress, increased inflammatory mediators, and shortened gestational age at birth. First, regression analyses showed that there were significant direct effects of pregnancy-specific stress or distress, IL-6, and TNF-α early, later, and averaged across pregnancy on both the occurrence of preterm birth and GAB (Table 5), and second, meditational analyses showed that in several instances, TNF-α served as a partial mediator of the effects of overall stress on GAB, with higher levels of TNF-α contributing to the effects of stress in shortening GAB (Table 6). This is a key finding as it provides the first demonstration of a pathway between psychosocial stress, increased inflammatory markers, and shortened GAB. This initial confirmation of this pathway is an essential step in understanding the how interactions between psychological factors and physiological correlates of stress and inflammation affect pregnancy outcome, and eventually, developing interventions to support healthy pregnancies.
Interestingly, although levels of IL-6 partially mediated the effects of pregnancy-specific distress on GAB in the present study, the direction of these relationships is best characterized as “inconsistent mediation” or “suppression” (MacKinnon et al. 2000). As hypothesized, levels of IL-6 partially mediated the effect of pregnancy-specific distress on GAB averaged across and early in pregnancy. In both cases, however, higher levels of distress were associated with lower, rather than higher, IL-6 and, in turn, less impact on GAB. Although this relationship is still a mediated one, the role played by IL-6 in the relationship between the independent variable (distress) and the dependent variable (GAB) is described as suppression (MacKinnon et al. 2000). There is no existing data framework in which to consider these findings as no previous studies have examined the effects of pregnancy-specific distress on inflammatory markers or how inflammatory markers may be involved in the effects of pregnancy-specific distress on complications and outcome.
Statisticians are often concerned about Type I errors in models that test multiple hypotheses such as those used here. One adjustment to address this concern was developed by Simes (Simes 1986) and applies a more conservative p-value criterion to determine significance recognizing that the models test three mediational hypotheses in each time period. It should be noted that if we apply this substantially more conservative condition to the mediation results in the present data, we are not able to reject the null hypothesis that there are no mediating effects of inflammatory markers on the relationship between stress or distress and GAB although these effects are statistically significant under our chosen p-value. As such, although our planned analyses provide initial support for the hypothesis that the effects of stress and distress during pregnancy on GAB are partially mediated by inflammatory markers; these results must be interpreted with caution until the relationships in question can be examined in larger samples.
The above data also suggest that “pregnancy-specific distress” may have characteristics that differentiate it even further from “overall stress” than those described in this report. For example, although pregnancy-specific distress as assessed here is associated with poor outcome, other studies suggest that is the more specific construct of “pregnancy-specific anxiety”, which we did not explicitly assess, is a stronger predictor of increased risk of complications (Dunkel Schetter 2011). Others have shown that trait anxiety can play a role during pregnancy and suggest that it is important to examine “pregnancy-specific” affective states in the context of maternal “trait” characteristics (Pluess et al. 2010). Although the present study did not assess trait anxiety or pregnancy-specific anxiety, future work will do so to clarify the above findings.
We hypothesized that, consistent with our previous work, elevated stress and distress would be associated with higher levels of IL-6, CRP, and TNF-α, and that all of these would be related to shortened GAB. Correlational analyses confirmed that there were significant relationships in the predicted directions between overall stress, pregnancy-specific distress, IL-6, CRP, and TNF-α and GAB early, late, and/or averaged across pregnancy (Table 4). Together, these analyses show that there are reliable and consistent relationships between elevated inflammatory markers and lower GAB, and that higher pregnancy-specific throughout pregnancy is related to lower GAB. These data are consistent with other work showing connections between stress and shortened GAB and with other studies showing that elevated inflammatory markers are also connected to preterm birth and shortened GAB (Nkansah-Amankra et al. 2010;Ruiz et al. 2003;Wadhwa et al. 2001;Zhu et al. 2010. Moreover, there were significant differences in levels of pregnancy-specific distress, IL-6, and TNF-α in women who delivered preterm infants compared to those who delivered their infants after 37 weeks of gestation (Table 3). These data are consistent with previous work showing relationships between IL-6 and TNF-α and prematurity (Curry et al. 2007;Zhang et al. 2000), and add to a growing body of work showing that these inflammatory cytokines play an important role in the timing of parturition. Interestingly, the CRP data in the present work were less clear. Specifically, although elevated CRP was correlated with shortened GAB (Table 4) it did not mediate between stress and pregnancy-specific distress and GAB (Table 6), regression analyses did not show any significant direct effects of CRP on GAB or the occurrence of preterm birth per se (Table 5). This may not be surprising given that the literature on the relationship between circulating CRP and preterm birth somewhat mixed, with some studies showing this connection (Pearce et al. 2010), and others showing no relationship between CRP and preterm birth (Kramer et al. 2010). Clearly, additional work is needed to tease apart the role of CRP in gestation and stress-related pregnancy complications.
It is important to note that the significant connections between prenatal stress, inflammatory markers, and GAB demonstrated here remained significant even after accounting maternal BMI at study enrollment, marital status, income, education, parity, and maternal age in the regression analyses. In addition, although bacterial infection during pregnancy was strongly related to preterm delivery and GAB, the relationships between pregnancy-specific distress, inflammatory markers, preterm birth, and GAB remained significant when bacterial infection was taken into account (Table 5). This is an important observation as it indicates that although maternal infection has long been identified as a risk factor for premature delivery (Gibbs et al. 1992;Mazor et al. 1998;Romero et al. 1989), elevated inflammatory markers and elevated maternal stress and distress on their own are directly connected to preterm birth and shortened GAB. A limitation of the present data, however, is that we did not assess the effect of the timing of prenatal infection on outcome, but rather looked at bacterial infection during gestation a dichotomous variable with no temporal component. This approach provides an incomplete picture as it may be that women experiencing infections earlier in pregnancy, for example, have increased distress as a result and that the effect of distress and inflammatory markers becomes more pronounced. In addition, it is likely that some women in the sample had undiagnosed bacterial infections. Despite these limitations, given that nearly 40% of preterm deliveries without clear predisposing conditions (retrieved June 27, 2011 from http://www.marchofdimes.com/mission/prematurity_indepth.html), the observation that overall stress, pregnancy-specific distress are linked to GAB via elevated inflammatory markers has important clinical significance.
The approach used in this study was to assess overall stress, pregnancy-specific distress, and inflammatory mediators in early (14-18 weeks of gestation) and later (28-32 weeks of gestation) pregnancy and relate these to GAB and preterm birth. These time points were selected on the basis of previous work from our laboratory indicating that mid-pregnancy is relatively quiescent, and that stress-related changes in inflammatory markers were not observed during this period (Coussons-Read et al. 2007). Although justified on the basis of our prior work, the two time points selected in the present work do not provide a complete picture of the relationships between psychosocial factors, changes in inflammatory markers, and GAB. For example, although our previous work and that of others has shown increases in circulating IL-6 between early and late pregnancy (Coussons-Read et al. 2005;Coussons-Read et al. 2007;Curry et al. 2008), this was not evident in the present study (p=.131). The present data, however, are consistent with recent work showing that in cultured lymphocytes from healthy pregnant women, levels of IL-6 remain relatively stable between early and late pregnancy, with a trend toward declining rather than increasing (Denney et al. 2011). It may be that part of this discrepancy is that although the early time point used here did occur in early pregnancy, the “late” time point is not as late in gestation as in our previous work. For example, in our prior work, the “late” assessment occurred between 34-38 weeks of gestation (Coussons-Read et al. 2007). Given that a primary outcome measure in the present work, however, was preterm birth, we decided to place our “late” assessment earlier, at 28-32 weeks of gestation, in hopes of capturing this datapoint for women prior to premature delivery. This strategy was successful, but has the clear drawback of falling short of a truly late pregnancy assessment. These discrepancies, coupled with the work of Denney at al. (Denney et al. 2011), underscore the need for additional work aimed at describing normative patterns of cytokine production throughout healthy pregnancies both in culture and in the circulation.
A shortcoming of our previous studies was that the DMHA, although valid and effective for assessing overall stress, does not address the unique stresses and worries that can accompany pregnancy. The present work expanded our assessment of stress to include the Revised Pregnancy Distress Questionnaire (NUPDQ), which was developed to better capture the complexities stress related to pregnancy per se (Lobel et al. 2008). The inclusion of the NUPDQ in the present work and the finding that increased serum levels of inflammatory mediators were observed in women reporting high overall stress and pregnancy-specific distress across pregnancy adds to our previous work (Coussons-Read et al. 2005;Coussons-Read et al. 2007) by showing that not only is overall stress related to changes in inflammatory markers in pregnancy, but importantly, pregnancy-specific distress was also associated with elevated inflammatory markers (Table 4). If therapeutic approaches or behavioral interventions are to be effective in alleviating these effects, they must recognize the multi-dimensional nature of stress and distress experienced by women in the prenatal period.
In conclusion, the present study confirms a predictive relationship between overall stress, pregnancy-specific distress, inflammatory markers, preterm birth, and GAB, but it also raises additional questions about the mechanisms of these effects and the role of maternal ethnicity and culture in these phenomena. Future work must focus on the cultural context in which women from different racial and ethnic groups experience stress during pregnancy, as well as how individual differences in constructs such as resilience may play a role in these effects. Determination of the mechanism of these effects is currently underway, with many researchers addressing the role of the HPA axis in the effects of stress on pregnancy outcome (i.e. Giurgescu 2009;Kramer et al. 2009;Wadhwa 2005). These lines of investigation along with an eye toward developing effective behavioral interventions to help women experiencing stress and distress in pregnancy will create a framework in which clinicians can identify women who are at risk for stress-related preterm delivery and intervene to support healthy pregnancies for all women.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Elevated psychological stress, distress, IL-6, and TNF-α during pregnancy are predictive of preterm delivery and shortened gestational age at birth.
References
- 1.Arck P. Stress during pregnancy: maternal endocrine-immune imbalances and fetal health. Journal of Reproductive Immunology. 2010;86:11. [Google Scholar]
- 2.Arck PC. Stress and pregnancy loss: Role of immune mediators, hormones and neurotransmitters. American Journal of Reproductive Immunology. 2001;46:117–123. doi: 10.1111/j.8755-8920.2001.460201.x. [DOI] [PubMed] [Google Scholar]
- 3.Baron RM, Kenney DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 4.Batton B, Burnett C, Verhulst S, Batton D. Extremely preterm infant mortality rates and cesarean deliveries in the United States. Obstetrics and Gynecology. 2011;118:43–48. doi: 10.1097/AOG.0b013e318221001c. [DOI] [PubMed] [Google Scholar]
- 5.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 7.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. Journal of Reproductive Immunology. 2008;77:152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, Hougaard D, Olsen J, Thorsen P. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- 10.Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, Hobel C, Sandman CA. Children’s Brain Development Benefits from Longer Gestation. Frontiers in psychology. 2011;2:1. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of child psychology and psychiatry, and allied disciplines. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53:170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Yu JL. An overview of morbidity, mortality and long-term outcome of late preterm birth. World journal of pediatrics : WJP. 2011;7:199–204. doi: 10.1007/s12519-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 14.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu. Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 15.Dunkel-Schetter C, Glynn L. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary researchers. In: Contrada R, Baum A, editors. Handbook of Stress Science. Springer; New York: 2011. pp. 321–343. [Google Scholar]
- 16.Dunn AJ. Infection as a stressor: a cytokine-mediated activation of the hypothalam-pituitary-adrenal axis? Ciba Found Symp. 1993;172 doi: 10.1002/9780470514368.ch11. discussion 239-242. [DOI] [PubMed] [Google Scholar]
- 17.Field T, Diego M, Hernandez-Reif M. Prenatal dysthymia versus major depression effects on the neonate. Infant behavior & development. 2008;31:190–193. doi: 10.1016/j.infbeh.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz MS, MacKinnon DP. Required Sample Size to Detect the Mediated Effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gennaro S, Fehder WP. Stress, immune function, and reationship to pregnancy outcome. Nursing clinics of North America. 1996;31:293–302. [PubMed] [Google Scholar]
- 20.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. American Journal of Obstetrics and Gynecology. 1992:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 21.Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs. 2009;38:377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 22.Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of Perceived Stress and Anxiety in Pregnancy Predicts Preterm Birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clinics in Perinatology. 1995;22:281–342. [PubMed] [Google Scholar]
- 25.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, Vaisbuch E, Than NG, Mazaki-Tovi S, Chaiworapongsa T, Mazor M, Yoon BH, Edwin S, Gomez R, Mittal P, Hassan SS, Sharma S. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: A role for interleukin-10. Journal of Maternal-Fetal & Neonatal Medicine. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- 27.Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Knackstedt MK, Hamelmann E, Arck PC. Mothers in stress: consequences for the offspring. Am J Reprod Immunol. 2005;54:63–69. doi: 10.1111/j.1600-0897.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- 30.Leff M, Orleans M, Haverkamp AD, Baron AE, Alderman BW, Freedman WL. The association of maternal low birthweight and infant low birthweight in a racially mixed population. Paediatr Perinat Epidemiol. 1992;6:51–61. doi: 10.1111/j.1365-3016.1992.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 31.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 32.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the Mediation, Confounding and Suppression Effect. Prevention Science. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes M, Song C, Lin A, de Jongh R, Van Gastel A, Kenis G, Bosmans E, DeMeester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. Th effects of psychological stress on humans: increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 34.Maina G, Saracco P, Giolito MR, Danelon D, Bogetto F, Todros T. Impact of maternal psychological distress on fetal weight, prematurity and intrauterine growth retardation. J Affect Disord. 2008;111:214–220. doi: 10.1016/j.jad.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Mazor M, Furman B, Bashiri A. Cytokines in preterm parturition. Gynecol Endocrinol. 1998;12:421–427. doi: 10.3109/09513599809012845. [DOI] [PubMed] [Google Scholar]
- 36.McGregor JA, French JI, Lawellin D, Todd JK. Preterm birth and infection: pathogenic possibilities. Am J Reprod Immunol Microbiol. 2000;16:123–132. doi: 10.1111/j.1600-0897.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 37.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nature medicine. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 38.McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- 39.Meikle SF, Orleans M, Leff M, Shain R, Gibbs RS. Women’s resons for not seeking prenatal care: Racial and ethnic factors. Birth. 1995;22:81–86. doi: 10.1111/j.1523-536x.1995.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 40.Nkansah-Amankra S, Luchok KJ, Hussey JR, Watkins K, Liu X. Effects of maternal stress on low birth weight and preterm birth outcomes across neighborhoods of South Carolina, 2000-2003. Matern Child Health J. 2010;14:215–226. doi: 10.1007/s10995-009-0447-4. [DOI] [PubMed] [Google Scholar]
- 41.Orr ST, James SA, Casper R. Psychosocial stressors and low birthweight: development of a questionnaire. Developmental and Behavioral Pediatrics. 1992;13:343–347. [PubMed] [Google Scholar]
- 42.Parker VJ, Douglas AJ. Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. J Reprod Immunol. 2010;85:86–92. doi: 10.1016/j.jri.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Pearce BD, Grove J, Bonney EA, Bliwise N, Dudley DJ, Schendel DE, Thorsen P. Interrelationship of cytokines, hypothalamic-pituitary-adrenal axis hormones, and psychosocial variables in the prediction of preterm birth. Gynecol Obstet Invest. 2010;70:40–46. doi: 10.1159/000284949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pluess M, Bolten M, Pirke KM, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. 2010;83:169–175. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 46.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 48.Rozlog LA, Kiecolt-Glaser JK, Marucha PT, Sheridan JF, Glaser R. Stress and immunity: implications for viral disease and wound healing. J Periodontol. 1999;70:786–792. doi: 10.1902/jop.1999.70.7.786. [DOI] [PubMed] [Google Scholar]
- 49.Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass. 2011;5:359–371. [Google Scholar]
- 50.Ruiz RJ, Fullerton JT. The measurement of stress in pregnancy. Nurs Health Sci. 1999;1:19–25. doi: 10.1046/j.1442-2018.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz RJ, Avant KC. Effects of maternal prenatal stress on infant outcomes: a synthesis of the literature. Adv Nurs Sci. 2005;28:345–355. doi: 10.1097/00012272-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz RJ, Fullerton J, Dudley DJ. The interrelationship of maternal stress, endocrine factors and inflammation on gestational length. Obstet Gynecol Surv. 2003;58:415–428. doi: 10.1097/01.OGX.0000071160.26072.DE. [DOI] [PubMed] [Google Scholar]
- 53.Samra HA, McGrath JM, Wehbe M. An integrated review of developmental outcomes and late-preterm birth. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN / NAACOG. 2011;40:399–411. doi: 10.1111/j.1552-6909.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- 54.Saunders TA, Lobel M, Veloso C, Meyer BA. Prenatal maternal stress is associated with delivery analgesia and unplanned cesareans. J Psychosom Obstet Gynaecol. 2006;27:141–146. doi: 10.1080/01674820500420637. [DOI] [PubMed] [Google Scholar]
- 55.Simes RJ. An Improved Bonferroni Procedure for Multiple Tests of Significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 56.Stamatelou F, Deligeoroglou E, Farmakides G, Creatsas G. Abnormal progesterone and corticotropin releasing hormone levels are associated with preterm labour. Ann Acad Med Singapore. 2009;38:1011–1016. [PubMed] [Google Scholar]
- 57.Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G. "Reproductive" corticotropin-releasing hormone. Ann N Y Acad Sci. 2006;1092:310–318. doi: 10.1196/annals.1365.029. [DOI] [PubMed] [Google Scholar]
- 58.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern. Child Health J. 2001;5:119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 60.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, Hobel CJ, Chicz-DeMet A, Dunkel-Schetter C, Garite TJ, Glynn L. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr. Perinat. Epidemiol. 2001;15(Suppl 2):17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 61.Wadhwa PD, Sandman C, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. American Journal of Obstetrics and Gynecology. 1993;169:858–866. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 62.Woods SM, Melville JL, Guo Y, Fan MY, Gavin A. Psychosocial stress during pregnancy. Am J Obstet Gynecol. 2010;202:61. doi: 10.1016/j.ajog.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woods-Giscombe CL, Lobel M, Crandell JL. The impact of miscarriage and parity on patterns of maternal distress in pregnancy. Res Nurs Health. 2010;33:316–328. doi: 10.1002/nur.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J Psychosom Obstet Gynaecol. 1999;20:39–52. doi: 10.3109/01674829909075575. [DOI] [PubMed] [Google Scholar]
- 65.Yang S, Bergvall N, Cnattingius S, Kramer MS. Gestational age differences in health and development among young Swedish men born at term. International journal of epidemiology. 2010;39:1240–1249. doi: 10.1093/ije/dyq070. [DOI] [PubMed] [Google Scholar]
- 66.Yang S, Platt RW, Kramer MS. Variation in child cognitive ability by week of gestation among healthy term births. American journal of epidemiology. 2010;171:399–406. doi: 10.1093/aje/kwp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Wang L, Zhao Y, Kang J. Changes in cytokine (IL-8, IL-6 and TNF-alpha) levels in the amniotic fluid and maternal serum in patients with premature rupture of the membranes. Chung Hua I. Hsueh Tsa Chih (Taipei) 2000;63:311–315. [PubMed] [Google Scholar]
- 68.Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. 2010;203:34. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]