Abstract
Epidemiologic studies report a protective association between non-steroidal anti-inflammatory drug (NSAID) use and hormone receptor-positive breast cancer risk, a finding consistent with NSAID-mediated suppression of aromatase-driven estrogen biosynthesis. However, the association between NSAID use and breast cancer-specific mortality is uncertain and it is unknown whether this relationship differs by hormone receptor status. This study comprised 935 invasive breast cancer cases, of which 490 were estrogen receptor (ER)-positive, enrolled between 1996 and 2001 in the Carolina Breast Cancer Study. Self-reported NSAID use in the decade prior to diagnosis was categorized by duration and regularity of use. Differences in tumor size, stage, node, and receptor status by NSAID use were examined using Chi-square tests. Associations between NSAID use and breast cancer-specific mortality were examined using age- and race-adjusted Cox proportional hazards analysis. Tumor characteristics did not differ by NSAID use. Increased duration and regularity of NSAID use was associated with reduced breast cancer-specific mortality in women with ER-positive tumors (long-term regular use (≥8 days/month for ≥ 3 - years) versus no use; hazard ratio (HR) 0.48; 95 % confidence interval (CI) 0.23–0.98), with a statistically significant trend with increasing duration and regularity (p-trend = 0.036). There was no association for ER-negative cases (HR 1.19; 95 %CI 0.50–2.81; p-trend = 0.891). Long-term, regular NSAID use in the decade prior to breast cancer diagnosis was associated with reduced breast cancer-specific mortality in ER-positive cases. If confirmed, these findings support the hypothesis that potential chemopreventive properties of NSAIDs are mediated, at least in part, through suppression of estrogen biosynthesis.
Keywords: Non-steroidal anti-inflammatory drugs, Breast cancer-specific mortality, Duration, Estrogen receptor, Regularity
Introduction
Non-steroidal anti-inflammatory drug (NSAID) use is associated with 10–20 % reduced risk of breast cancer [1–3], with similar effect estimates for aspirin use alone [1–5]. A previous analysis using data from the Carolina Breast Cancer Study reported that NSAID use was associated with reduced breast cancer incidence [6], and several other epidemiologic studies have reported that this protective effect of NSAID use on breast cancer risk is strongest for hormone receptor-positive tumors [7–10].
The anti-inflammatory properties of NSAIDs are mediated via cyclooxygenase (COX) inhibition which in turn reduces prostaglandin levels, resulting in down-regulation of the aromatase pathway and decreased estrogen biosynthesis [11, 12]. The inverse association between NSAID use and risk of hormone receptor-positive breast cancer is consistent with this NSAID-mediated suppression of estrogen biosynthesis. However, evidence for an association between NSAID use and breast cancer-specific mortality is conflicting. Although one study reported that aspirin use was associated with reduced breast cancer-specific mortality [13], three studies reported no association between NSAID or aspirin use and breast cancer-specific mortality [14–16]. Only a single study examined the association between aspirin use and breast cancer-specific mortality by estrogen receptor (ER) status and found no evidence of differential effects by ER status [13]. Notably, these studies were limited by small numbers of breast cancer-specific deaths [14, 16], incomplete NSAID exposure assessment, focusing on aspirin and/or ibuprofen use only [13, 16], or examination of regularity of NSAID use without taking duration of use into account [14].
The objective of this current study was to examine the association between use of prescription and non-prescription NSAIDs and breast cancer-specific mortality within the population-based Carolina Breast Cancer Study. Given evidence that NSAIDs reduce estrogen biosynthesis via inhibition of aromatase activity, we hypothesized that there would be a stronger protective association between NSAID use and risk of breast cancer-specific mortality in women with ER-positive tumors.
Methods
Study population
The Carolina Breast Cancer Study is a population-based, case–control study conducted in North Carolina (NC) between 1993 and 2001 [17]. This study was approved by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill and informed consent was obtained from each participant. The present study includes 935 women with invasive breast cancer who were interviewed between 1996 and 2001 and provided information on NSAID use. Briefly, cases of invasive breast cancer were identified using rapid case ascertainment in cooperation with the NC Central Cancer Registry. All women who agreed to participate were interviewed in person by a registered nurse using a standardized questionnaire to collect information on established and suspected breast cancer risk factors, including prescription and non-prescription medication use. Tumor size, stage, and lymph node status were abstracted from medical records. Estrogen receptor (ER) and progesterone receptor (PR) status were abstracted from medical records for 80 % of participants [18]. For the remaining 20 % of participants, tumor tissue was sectioned and stained for ER and PR at the Immunohistochemistry Core Laboratory at the University of North Carolina.
Exposure assessment
Women were shown photographs of commonly used NSAIDs and asked to report non-prescription and prescription NSAID use during the past decade. Assessment of duration and regularity of NSAID use is described in detail by Moorman et al. [6]. Briefly, women who reported NSAID use ≥8 days a month for ≥3 months were categorized as regular users. Regular users were further categorized by duration of use (<3 vs. ≥3 years). Women who reported NSAID use <3 months or who reported sporadic use (≤7 days a month) regardless of duration were categorized as occasional users. Acetaminophen has a COX-independent mechanism of action, so was included in the “no use” category. Women were also asked the reason for NSAID use, and could select multiple reasons from a list which included arthritis, bursitis or rheumatism, back pain, surgical/dental pain, menstrual cramps, injury, or other.
Outcome assessment
Vital status was determined through December 31, 2011 through linkage with the National Death Index, which provided date and cause of death for each individual. Using international classification of disease (ICD) codes, we categorized cause of death as either breast cancer-specific (ICD-9 code 174.9 or ICD-10 code 50.9) or other cause of death based on the first listed primary cause of death.
Statistical analysis
Age and race were selected a priori to be potential confounders of the association between NSAID use and risk of breast cancer-specific mortality. We also considered the following variables as potential confounders: education level, body mass index (BMI), and menopausal status. Chi-square tests were used to examine the distribution of these potential confounders according to NSAID use and to examine differences in tumor characteristics between NSAID users and non-users.
Individuals who died of causes other than breast cancer were censored at time of death and living individuals were censored on December 31, 2011. We modeled breast cancer-specific survival curves according to categories of duration and regularity of NSAID use versus no use using the Kaplan–Meier method and we compared survival curves using log-rank analysis. The proportional hazards assumption was met for each variable. We then conducted Cox proportional hazards analysis to test the association between categories of duration and regularity of NSAID use versus no use and risk of breast cancer-specific mortality among all invasive cases and within strata defined by hormone receptor status. We tested for linear trend with increasing duration and regularity of NSAID use using by assigning the median value to each NSAID category. We stratified both by ER status (ER + vs. ER–) and by positive hormone receptor status (ER + or PR + vs. ER– and PR–). Hazard ratios (HRs) were adjusted for age and race (African American (AA), non-AA). We also explored the effect of adjusting HRs for menopausal status (pre-, postmenopausal) and education level (< high school, high school graduate, > high school). Given the lack of association between NSAID use and BMI in this population, models were not adjusted for BMI. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). Statistical tests were two-sided, and p values < 0.05 were considered statistically significant.
Results
Baseline characteristics of breast cancer cases stratified by NSAID use
The majority of study participants reported NSAID use; 84 (11.5 %) breast cancer survivors and 25 (12.1 %) women who died of breast cancer were categorized as non-users. Breast cancer cases who reported NSAID use were younger than non-users (p = 0.029), had a higher level of education (p = 0.029) and were more likely to be premenopausal (p = 0.029; Table 1). There were no differences in race or BMI according to NSAID use (p = 0.259 and p = 0.634, respectively).
Table 1.
Characteristics of invasive breast cancer cases in the Carolina Breast Cancer Study Phase 2 according to NSAID use
| No use of NSAIDs, n (%) | Use of NSAIDs, n (%) | p value* | |
|---|---|---|---|
| Race | |||
| Non-African American | 51 (46.8) | 434 (52.5) | 0.259 |
| African American | 58 (53.2) | 392 (47.5) | |
| Age at selection | |||
| < 40 | 9 (8.3) | 128 (15.5) | 0.029 |
| 40-49 | 37 (33.9) | 292 (35.4) | |
| 50-59 | 22 (20.2) | 194 (23.5) | |
| 60-74 | 41 (37.6) | 212 (25.7) | |
| Education | |||
| < High school | 27 (24.8) | 143 (17.3) | 0.029 |
| High school graduate | 35 (32.1) | 219 (26.5) | |
| > High school | 47 (43.1) | 464 (56.2) | |
| Body mass index (kg/m2) | |||
| < 25 | 35 (34.0) | 259 (31.8) | 0.634 |
| 25-29 | 32 (31.1) | 231 (28.4) | |
| 30+ | 36 (35.0) | 324 (39.8) | |
| Missing | 6 | 12 | |
| Menopausal status | |||
| Premenopausal | 39 (35.8) | 387 (46.9) | 0.029 |
| Postmenopausal | 70 (64.2) | 439 (53.1) |
Chi-square test
We compared reasons for NSAID use between categories of duration and regularity of NSAID use (Online Resource 1). Relative to occasional users, long-term regular NSAID users were significantly more likely to cite chronic conditions including arthritis, bursitis or rheumatism (50.3 % of long-term regular users versus 14.8 % of occasional users; p < 0.0001), and back pain (27.1 % of long-term regular users versus 17.6 % of occasional users; p = 0.006) as reasons for NSAID use. Conversely, occasional users were more likely to cite acute conditions including menstrual cramps (18.6 % of occasional users versus 11 % of long-term regular users; p = 0.009) and headache (68.2 % of occasional users versus 51.9 % of long-term regular users; p < 0.0001).
Tumor characteristics of NSAID users
Although there was a suggestion that ER-positive and ER or PR-positive breast cancer was less common among NSAID users, these associations were not statistically significant (p = 0.149 and p = 0.135, respectively; Table 2). There were no strong or significant associations between NSAID use and tumor stage, node status or tumor size (all p > 0.5; Table 2).
Table 2.
Tumor characteristics of invasive breast cancer cases in the Carolina Breast Cancer Study Phase 2 according to NSAID use
| No use of NSAIDs, n (%) | Use of NSAIDs, n (%) | p value* | |
|---|---|---|---|
| Tumor stage | |||
| Stage I | 48 (44.9) | 328 (41.5) | 0.515 |
| Stage II | 42 (39.3) | 355 (44.9) | |
| Stage III | 15 (14.0) | 84 (10.6) | |
| Stage IV | 2 (1.9) | 23 (2.9) | |
| Missing | 2 | 36 | |
| Node status | |||
| Negative | 74 (67.9) | 533 (64.7) | 0.510 |
| Positive | 35 (32.1) | 291 (35.3) | |
| Missing | 0 | 2 | |
| Tumor size | |||
| ≤ 2 cm | 52 (50.5) | 406 (52.3) | 0.939 |
| > 2–5 cm | 38 (36.9) | 279 (35.9) | |
| > 5 cm | 13 (12.6) | 92 (11.8) | |
| Missing | 6 | 49 | |
| ER status | |||
| Positive | 64 (62.1) | 426 (54.6) | 0.149 |
| Negative | 39 (37.9) | 354 (45.4) | |
| Missing | 6 | 46 | |
| ER/PR status | |||
| ER + or PR+ | 72 (69.9) | 485 (62.3) | 0.135 |
| ER– and PR– | 31 (30.1) | 293 (37.7) | |
| Missing | 6 | 48 |
Chi-square test
NSAID use and breast cancer-specific mortality
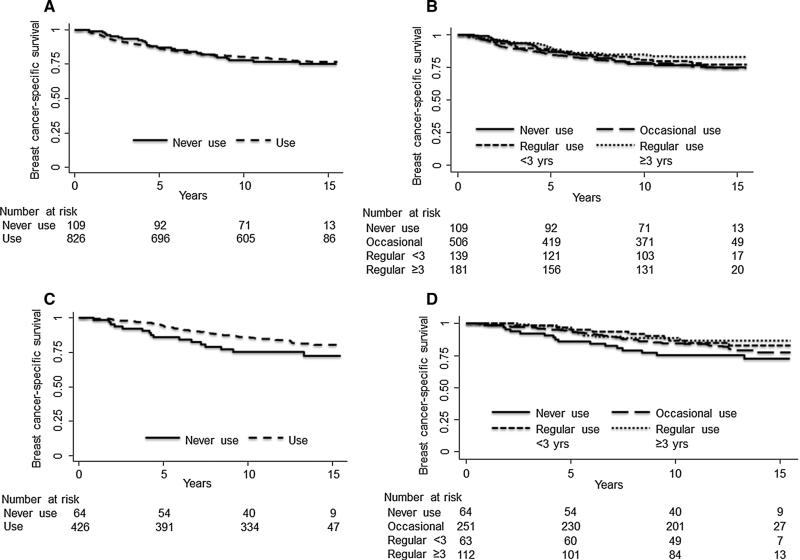
Overall, 181 (21.9 %) NSAID users and 25 (22.9 %) non-users died of breast cancer (log-rank p = 0.808; Fig. 1a) during a median follow-up period of 13 years (interquartile range; 8–14 years). Among all invasive cases, increased duration and regularity of NSAID use was not associated with breast cancer-specific mortality on unadjusted analysis (log-rank p = 0.221; Fig. 1b). After adjusting for age and race, although there was no association between NSAID ever use and risk of breast cancer-specific mortality (use versus no use; HR 0.93; 95 %CI 0.61–1.41; Table 3), there was a suggestion that increasing duration and regularity of NSAID use was associated with a reduced hazard ratio of breast cancer-specific mortality, although this was not significant (NSAID use ≥8 days/month for ≥3 years versus no use; HR 0.71; 95 %CI 0.42–1.21; p-trend = 0.105; Table 3).
Fig. 1.
Kaplan—Meier plots showing breast cancer-specific mortality by NSAID use versus no use among a all invasive cases and b ER-positive cases, and categories of duration and regularity of NSAID use among c all invasive cases and d among ER-positive cases
Table 3.
Associations between NSAID use and risk of breast cancer-specific mortality among invasive cases in the Carolina Breast Cancer Study Phase 2, overall and stratified by estrogen receptor status
| All invasive cases |
||
|---|---|---|
| n, cases (deaths) | HR* (95 % CI) | |
| NSAID use | ||
| No use | 109 (25) | 1.00 (ref) |
| Use | 826 (181) | 0.93 (0.61–1.41) |
| NSAID regularity and duration of use | ||
| No use | 109 (25) | 1.00 (ref) |
| Occasional | 506 (122) | 1.02 (0.66–1.59) |
| Regular < 3 years | 139 (30) | 0.90 (0.53–1.53) |
| Regular ≥ 3 years | 181 (29) | 0.71 (0.42–1.21) |
| p-trend | 0.105 | |
| ER-positive cases n, cases (deaths) HR* (95 % CI) | ER-negative cases n, cases (deaths) HR* (95 % CI) | |||
|---|---|---|---|---|
| NSAID use | ||||
| No use | 64 (16) | 1.00 (ref) | 39 (8) | 1.00 (ref) |
| Use | 426 (76) | 0.63 (0.37-1.09) | 354 (100) | 1.46 (0.71-3.01) |
| NSAID regularity and duration of use | ||||
| No use | 64 (16) | 1.00 (ref) | 39 (8) | 1.00 (ref) |
| Occasional | 251 (52) | 0.73 (0.41–1.29) | 222 (65) | 1.56 (0.74–3.28) |
| Regular <3 years | 63 (10) | 0.57 (0.26–1.25) | 68 (20) | 1.47 (0.65–3.34) |
| Regular ≥3 years | 112 (14) | 0.48 (0.23–0.98) | 64 (15) | 1.19 (0.50–2.81) |
| p-trend | 0.036 | 0.891 | ||
HRs adjusted for age and race
Among ER-positive cases only (n = 490), 76 (17.8 %) NSAID users and 16 (25.0 %) non-users died of breast cancer (log-rank p = 0.089; Fig. 1c). Increased duration and regularity of NSAID use was not associated with breast cancer-specific mortality on unadjusted analysis (log-rank p = 0.141; Fig. 1d). However, after adjusting for age and race, increased duration and regularity of NSAID use was significantly associated with a reduced hazard ratio for ER-positive breast cancer-specific mortality (regular use ≥3 years versus no use; HR 0.48; 95 %CI 0.23–0.98; Table 3), with a significant trend across categories of increasing duration and regularity of NSAID use (p-trend = 0.036). When duration and regularity categories were collapsed to create a single NSAID use category, there was a reduced hazard ratio among women with ER-positive tumors (use versus no use; HR 0.63; 95 %CI 0.37–1.09; Table 3). In contrast, among women with ER-negative breast cancer, use of NSAIDs had an elevated, but imprecise hazard ratio (use versus no use; HR 1.46; 95 %CI 0.71–3.01; Table 3) and there was no association between increased duration and regularity of NSAID use and breast cancer-specific mortality (regular use ≥3 years versus no use; HR 1.19; 95 %CI 0.50–2.81; p-trend = 0.891). Further adjustment of our models for menopausal status and education level had no appreciable effect on our estimates (data not shown). Finally, these associations were similar when we stratified women into groups based on both ER and progesterone receptor (PR) positivity (ER- or PR-positive versus ER- and PR-negative; data not shown).
Discussion
Evidence from preclinical models [19, 20] and epidemiologic studies [3] supports a potential role for NSAIDs in breast cancer chemoprevention. Using data from the population-based Carolina Breast Cancer Study, we found that increasing duration and regularity of NSAID use within the decade prior to diagnosis was associated with reduced breast cancer-specific mortality in women with ER-positive tumors. This association was not observed among women with ER-negative tumors, suggesting that the protective effect of NSAID use on breast cancer-specific mortality may be limited to hormone-dependent breast cancer. These results are consistent with the hypothesis that the potential chemopreventive and tumor suppressive properties of NSAIDs are mediated, at least in part, through suppression of estrogen biosynthesis.
NSAIDs inhibit activity of COX-2, a key enzyme in prostaglandin synthesis with an established role in inflammation and carcinogenesis [21]. While normal breast tissue expresses low levels of COX-2, approximately 40 % of invasive breast tumors overexpress this enzyme [11, 22] and elevated levels are associated with increased risk of breast cancer-specific mortality [23]. Given that COX-2-mediated prostaglandin production promotes estrogen biosynthesis via up regulation of the aromatase pathway [12], there is biologic rationale to support a role for NSAIDs in ER-positive breast cancer [11]. Indeed, NSAID use is associated with reduced serum estradiol levels in women with breast cancer [24], which may impact growth of estrogen-responsive tumors. Several studies have found the protective effect of NSAID use on breast cancer incidence to be restricted to hormone receptor-positive breast cancer [7–9], although others reported no difference in this association according to hormone receptor status [25–31].
Few studies have examined the association between NSAID use and breast cancer-specific mortality, and only one conducted stratified analysis by ER status [13]. While this prior study found no evidence of effect modification by ER status (p-interaction = 0.52), their results were limited to assessment of aspirin use only and there was a reduced sample size for which ER status was available [13]. In this study, we were able to examine all prescription and non-prescription NSAIDs using a population-based dataset of over 900 incident cases over 13 years of follow-up.
Our results should be considered in light of the study's strengths and limitations. First, confounding by indication is an important consideration in observational studies of NSAID use. To ascertain whether long-term regular NSAID users were more likely to suffer from chronic conditions, we documented the reason for NSAID use. As anticipated, we found that long-term regular users were more likely to use NSAIDs to control symptoms of arthritis, relative to occasional users. While a possible association between arthritis and increased risk of breast cancer-specific mortality has not been consistently reported [32, 33], such an association would likely result in uncontrolled bias in our results. However, the direction of bias would likely be toward the null and, as such, our analysis may have underestimated the strength of the association between long-term regular NSAID use and risk of breast cancer-specific mortality. Moreover, a previous study found that a diagnosis of arthritis did not modify the association between NSAID use and breast cancer risk [25]. Second, we lacked treatment data in Carolina Breast Cancer Study Phase 2, and so could not examine interactions between NSAID use and breast cancer treatment. Third, we did not collect information on post-diagnosis NSAID use. However, long-term regular users were more likely to use NSAIDs to control chronic conditions, suggesting they would be likely to continue regular NSAID use post-diagnosis. While occasional users who reported NSAID use for menstrual cramps may have been less likely to continue NSAID use if they experienced treatment-induced menopause, the majority of occasional users cited headache as the reason for NSAID use, and this condition would not be expected to be altered by breast cancer diagnosis or treatment. Thus, while we would expect similar patterns of NSAID use post-diagnosis, future studies should explore the association between post-diagnosis NSAID use and risk of breast cancer-specific mortality.
These limitations are balanced by an important strength of this study. Most previous studies did not collect complete NSAID use information, focusing solely on aspirin and ibuprofen use [13, 15, 16]. Incomplete NSAID use data may attenuate the association between NSAID use and breast cancer, since many “unexposed” women may have taken NSAIDs that were not ascertained during data collection, particularly given the widespread use of NSAIDs. In this study, we attempted to collect complete information on all prescription and non-prescription NSAID use, and improved recall to the best of our ability by showing photographs of commonly used NSAIDs to each participant. This study is also strengthened by long follow-up and by inclusion of a large number of African American women, allowing our results to be generalized to a diverse population of women.
This is the first study, to our knowledge, to report a protective association between NSAID use and breast cancer-specific mortality which is limited to ER-positive tumors. This finding is supported by extensive preclinical and epidemiologic evidence for a role of COX-2 inhibition in ER-positive breast cancer. Several clinical trials in breast cancer patients have suggested that combining COX-2 inhibitors with aromatase inhibitors may improve efficacy of aromatase inhibitors in patients with ER-positive tumors [34, 35]. However, while these trials support a role for targeting COX-2 in breast cancer treatment, the clinical utility of selective COX-2 inhibitors is limited due to increased risk of serious cardiovascular events [36, 37]. Thus, if confirmed, our results provide additional rationale for exploring a potential role for NSAIDs in breast cancer treatment.
Acknowledgments
Funding This work was supported by a SPORE in Breast Cancer [P50-CA058223] and the University Cancer Research Fund, University of North Carolina at Chapel Hill.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
E. H. Allott, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
C.-K. Tse, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
A. F. Olshan, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
L. A. Carey, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
P. G. Moorman, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC, USA
M. A. Troester, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
References
- 1.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84:1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3(1):28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 4.Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 5.Luo T, Yan HM, He P, et al. Aspirin use and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2012;131:581–587. doi: 10.1007/s10549-011-1747-0. [DOI] [PubMed] [Google Scholar]
- 6.Moorman PG, Grubber JM, Millikan RC, et al. Association between non-steroidal anti-inflammatory drugs (NSAIDs) and invasive breast cancer and carcinoma in situ of the breast. Cancer Causes Control. 2003;14:915–922. doi: 10.1023/b:caco.0000007973.59863.66. [DOI] [PubMed] [Google Scholar]
- 7.Terry MB, Gammon MD, Zhang FF, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;29:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 8.Marshall SF, Bernstein L, Anton-Culver H, et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97:805–812. doi: 10.1093/jnci/dji140. [DOI] [PubMed] [Google Scholar]
- 9.Gill JK, Maskarinec G, Wilkens LR, et al. Nonsteroidal antiinflammatory drugs and breast cancer risk: the multiethnic cohort. Am J Epidemiol. 2007;166:1150–1158. doi: 10.1093/aje/kwm195. [DOI] [PubMed] [Google Scholar]
- 10.Gierach GL, Lacey JV, Jr, Schatzkin A, et al. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health-AARP Diet and Health Study. Breast Cancer Res. 2008;10:R38. doi: 10.1186/bcr2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe LR, Subbaramaiah K, Brown AM, et al. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Agarwal VR, Mendelson CR, et al. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 13.Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair CK, Sweeney C, Anderson KE, et al. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat. 2007;101:191–197. doi: 10.1007/s10549-006-9277-x. [DOI] [PubMed] [Google Scholar]
- 15.Wernli KJ, Hampton JM, Trentham-Dietz A, et al. Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors. Pharmacoepidemiol Drug Saf. 2011;20:131–137. doi: 10.1002/pds.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Brasky TM, Nie J, et al. Use of nonsteroidal anti-inflammatory drugs and survival following breast cancer diagnosis. Cancer Epidemiol Biomark Prev. 2012;21:239–242. doi: 10.1158/1055-9965.EPI-11-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 20.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang D, Scollard D, Byrne J, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 23.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 24.Hudson AG, Gierach GL, Modugno F, et al. Nonsteroidal anti-inflammatory drug use and serum total estradiol in post-menopausal women. Cancer Epidemiol Biomark Prev. 2008;17:680–687. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- 25.Kirsh VA, Kreiger N, Cotterchio M, et al. Nonsteroidal antiinflammatory drug use and breast cancer risk: subgroup findings. Am J Epidemiol. 2007;166:709–716. doi: 10.1093/aje/kwm216. [DOI] [PubMed] [Google Scholar]
- 26.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Coogan PF, Palmer JR, et al. Use of nonsteroidal antiinflammatory drugs and risk of breast cancer: the Case-Control Surveillance Study revisited. Am J Epidemiol. 2005;162:165–170. doi: 10.1093/aje/kwi182. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs EJ, Thun MJ, Connell CJ, et al. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomark Prev. 2005;14:261–264. [PubMed] [Google Scholar]
- 29.Brasky TM, Bonner MR, Moysich KB, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) and breast cancer risk: differences by molecular subtype. Cancer Causes Control. 2011;22:965–975. doi: 10.1007/s10552-011-9769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SM, Cook NR, Manson JE, et al. Low-dose aspirin and breast cancer risk: results by tumour characteristics from a randomised trial. Br J Cancer. 2008;98:989–991. doi: 10.1038/sj.bjc.6604240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardia A, Olson JE, Vachon CM, et al. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat. 2011;126:149–155. doi: 10.1007/s10549-010-1074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson RE, Flatt SW, Saquib N, et al. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122:859–865. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji J, Liu X, Sundquist K, et al. Survival of cancer in patients with rheumatoid arthritis: a follow-up study in Sweden of patients hospitalized with rheumatoid arthritis 1 year before diagnosis of cancer. Rheumatology (Oxford) 2011;50:1513–1518. doi: 10.1093/rheumatology/ker143. [DOI] [PubMed] [Google Scholar]
- 34.Chow LW, Yip AY, Loo WT, et al. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol. 2008;111:13–17. doi: 10.1016/j.jsbmb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Lustberg MB, Povoski SP, Zhao W, et al. Phase II trial of neoadjuvant exemestane in combination with celecoxib in post-menopausal women who have breast cancer. Clin Breast Cancer. 2011;11:221–227. doi: 10.1016/j.clbc.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]