Abstract
Endobronchial ultrasound (EBUS) plays a pivotal role in the minimally invasive staging of non–small cell lung cancer. The role of EBUS is progressively expanding to include the evaluation of peribronchial lesions, pulmonary nodules, and other mediastinal abnormalities. Recently, EBUS has assisted in the diagnosis of many other disease entities, including malignancies and various infections such as tuberculosis and sarcoidosis. This article reviews the indications and contraindications of EBUS, with emphasis on the technique and complications encountered during the procedure.
Endobronchial ultrasound (EBUS) combines bronchoscopy with ultrasound to enhance the definition of mediastinal structures and aid in visualization of parabronchial anatomy, reducing biopsy sampling errors due to superior site selection for transbronchial sampling (1). Over the last two decades, EBUS has emerged as a highly effective and minimally invasive technique for sampling peribronchial, mediastinal, and lung masses for pathologic examination. EBUS may provide rapid on-site results with relatively less expertise and has a very safe profile. It has been shown to be significantly cost-effective compared with prior gold standard techniques (2).
EBUS plays a role in the staging of non–small cell lung cancer (NSCLC) and the diagnostic evaluation of endobronchial lesions, peripheral pulmonary nodules, and mediastinal abnormalities (3, 4). Recently, use of EBUS has expanded for patients with sarcoidosis, tuberculosis, and lymphoma and in the workup of nonspecific mediastinal adenopathy (5–7). As chest computed tomography (CT) scans are being performed much more frequently than before, benign diseases and incidentalomas are being detected as well as early stage lung cancer (8). Benign diseases warrant the need for the least invasive technique of obtaining histologic diagnosis. For this reason, EBUS has become the staging tool of choice for mediastinal NSCLC and is currently being explored for many other disease entities. As medical diagnosis and therapy move towards less-invasive procedures that provide results equal or superior to those of more invasive procedures, additional uses of EBUS are emerging on a daily basis. EBUS can be used to characterize suspicious mucosal lesions, determine the extension of early lung cancer, and assess the involvement of the tracheobronchial wall or mediastinum in cases of advanced malignancy.
TECHNIQUE
EBUS may be performed under conscious sedation or general anesthesia depending on the anticipated length of the procedure. Local anesthetic may be administered to minimize cough, and the flexible bronchoscope is advanced for an initial airway exam. Bronchial segments and subsegments are identified, secretions are suctioned, and 1% to 2% lidocaine is administered to further minimize cough.
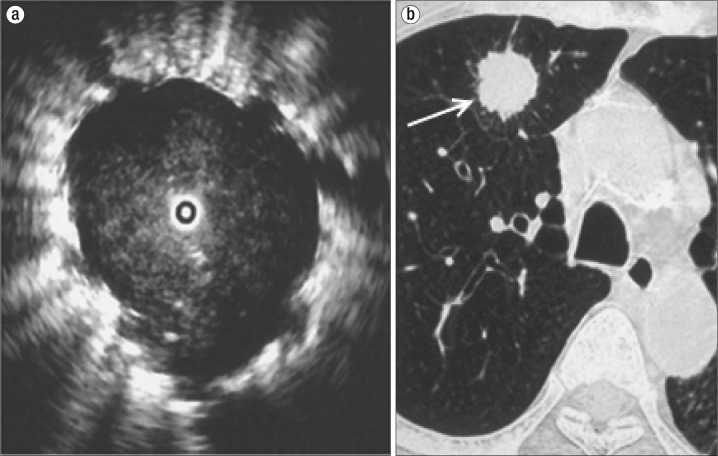
After initial airway examination, the flexible bronchoscope is removed and the EBUS bronchoscope is advanced. While EBUS is performed, the bronchoscopist simultaneously visualizes the ultrasonic and bronchoscopic views on display. EBUS can help differentiate normal parenchyma from malignant tissue by its sonographic appearance. The sonographic visualization of normal alveolar tissue is described as a “snowstorm” appearance (Figure 1). Figure 2a is a sonographic view of the peripheral pulmonary nodule visualized on the CT scan in Figure 2b.
Figure 1.
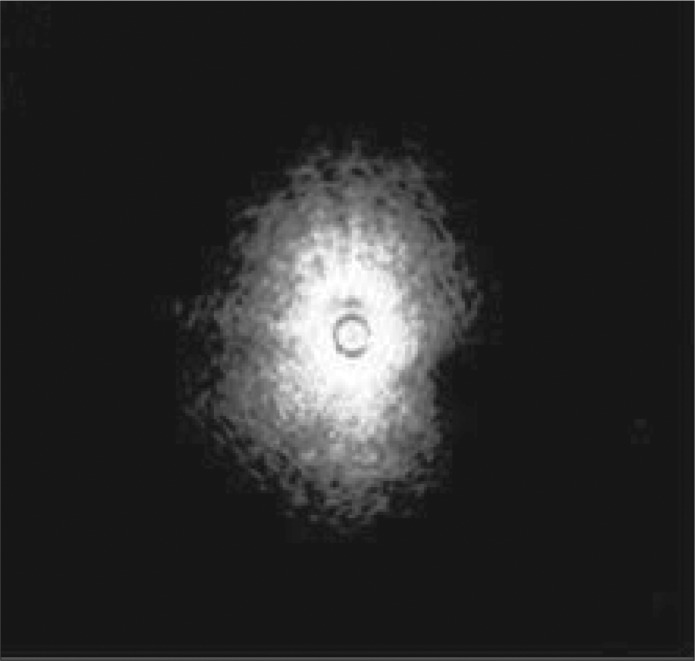
Endobronchial ultrasound of normal alveolar tissue described as a “snowstorm” appearance.
Figure 2.
(a) Imaging of a pulmonary nodule through endobronchial ultrasound, with a so-called “blizzard” appearance. (b) Axial CT of the lung nodule (arrow).
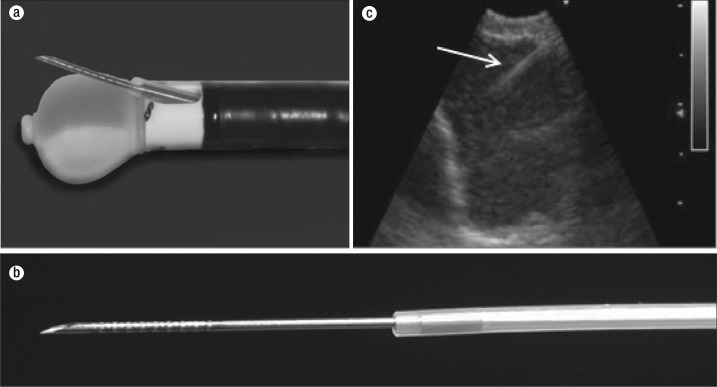
After sonographic confirmation of the biopsy site, the transbronchial needle aspiration (TBNA) needle is advanced through a 2.2 mm working channel on the bronchoscope (Figure 3a). This needle may be 21 or 22 gauge and can be advanced up to 40 mm. A stylet or wire is present in the needle at the time of insertion to clear tissue that may have collected while crossing the bronchial or tracheal wall. The distal end of the needle is grooved, rendering it hyperechoic and improving ultrasound visualization (Figure 3b). After the lymph node or tumor is punctured, the needle is connected to suction and excursions are made in the lymph node. Multiple punctures have been recommended to decrease sampling error.
Figure 3.
Endobronchial ultrasound (EBUS) bronchoscope. (a) The inflated balloon over the ultrasound transducer and a protracted biopsy needle through the EBUS working channel. (b) An EBUS biopsy needle with dimples for better sonographic visualization (arrow). (c) Real-time visualization of EBUS transbronchial aspiration needle advancement into the lymph node.
The samples obtained can either be analyzed on site by the use of rapid on-site evaluation (ROSE) or collected in saline or cell culture media. The application of ROSE has been shown to significantly lower the need for additional bronchoscopic procedures and puncture number (9). ROSE is particularly beneficial when performing EBUS in the operating room with an expected need for further invasive exploration or surgical resection. Procedure length varies by the number of lymph node stations sampled. When EBUS is performed in the outpatient setting, patients can be discharged home after they regain their gag reflex.
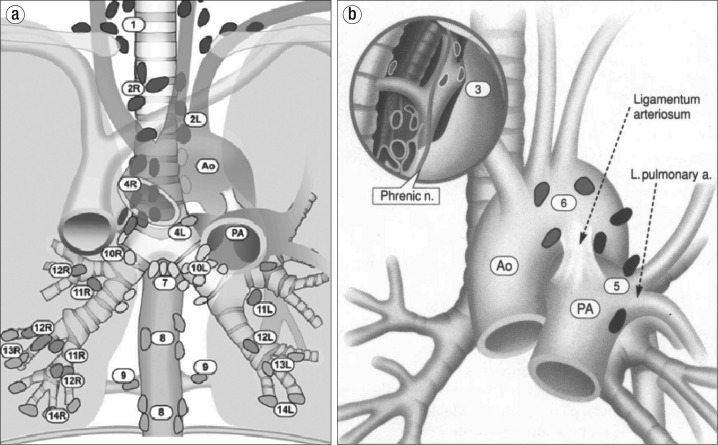
The anatomical lymph node stations that are accessible via EBUS are 2R, 2L, 3P, 4R, 4L and 7 (Figure 4a). A unique advantage of EBUS is the accessibility of stations 10R, 10L, 11R, and 11L, which are inaccessible by other invasive techniques (10). Endoscopic ultrasound (EUS) also provides access to 7, but in addition lymph node stations 8 and 9 can be accessed. EBUS may be performed safely in a single session alongside EUS (11, 12). Lymph node stations not accessible by EBUS are shown in Figure 4b. EBUS is preferred as a primary procedure when EUS is performed in the same session (13).
Figure 4.
Anatomical depiction of lymph node stations (a) accessible by EBUS and (b) not accessible by EBUS. Reproduced with permission from the American College of Chest Physicians via the Copyright Clearance Center: Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718–1723.
INDICATIONS
The role of diagnostic bronchoscopy has expanded with the introduction and rapid institution of ultrasound, giving the bronchologist vision beyond what the bronchoscope can see. EBUS was initially developed for the diagnosis and staging of NSCLC but is increasingly involved in nononcologic pulmonary disease states. The following are some of the indications and applications of EBUS.
Diagnosis, staging, and restaging of lung cancer
Mediastinoscopy has been the historic gold standard in lung cancer staging. Lung cancer staging has progressed from mediastinoscopy in the early 1950s to video-assisted thoracic surgery, video-assisted mediastinal lymphadenectomy, transcervical extended mediastinal lymphadenectomy, and TBNA (14). The introduction of EBUS improves the sensitivity and reliability of TBNA in the staging of lung cancer (1).
Appropriate staging of lung cancer is of utmost importance before initiation of treatment. Computed tomography (CT) and positron emission tomography (PET) have been inconsistent at accurately staging the mediastinum (15). Identification of suspicious mediastinal metastases by CT or PET should always be followed by histologic assessment of mediastinal and hilar lymph nodes for accurate staging. Minimally invasive modalities are preferred over more invasive modalities for the initial biopsy. However, sometimes more than one procedure will be necessary, particularly when initial tissue sampling provides inconclusive results or is insufficient for molecular characterization.
Histologic diagnosis is prudent in the diagnosis of NSCLC, as is disease staging, as therapy depends on the stage. In the absence of obvious metastatic disease, it may be important to biopsy mediastinal lymph nodes to accurately determine node status. The 5-year survival rate of 16% for bronchogenic carcinoma with lymph node metastases is very low compared to the rate of 49% in lung cancer without lymph node involvement (16).
Randomized trials have reported a low prevalence of metastatic disease in patients with suspected stage 1A NSCLC with peripheral tumors <3 cm. These patients may undergo primary tumor resection, and the need for preoperative mediastinal sampling is not well defined (17–20). Patients with suspected NSCLC stages IB, II, and III should undergo mediastinal sampling, the timing of which should be individualized according to patient characteristics and radiographic stage.
EBUS-TBNA is the preferred first step for large, centrally located tumors and for suspicious nodal involvement in the mediastinum. The most recent American College of Chest Physicians guidelines on the management and treatment of lung cancer recommend EBUS as the initial step in the diagnostic staging of lung cancer (21). The European Society of Thoracic Surgeons also recommends EBUS as a first choice for mediastinal staging in patients with NSCLC (14).
EBUS-TBNA has a reported high sensitivity to stage and diagnose NSCLC and is able to access more nodal stations than the prior gold standard, cervical mediastinoscopy (22). EBUS cannot sample all mediastinal lymph node stations and is usually combined with EUS for a more thorough and systematic sampling of the mediastinum. EBUS can access the anterosuperior mediastinum while EUS can access the posteroinferior mediastinum (23). Prior to the development of EBUS, mediastinoscopy was used to sample the mediastinum. Mediastinoscopy is an invasive procedure requiring general anesthesia, while EBUS can be performed as an outpatient procedure with conscious sedation and usually does not require postprocedure hospitalization. EBUS combined with EUS is essentially a less-invasive alternative to mediastinoscopy.
The mediastinum is restaged after induction therapy in patients with stage III NSCLC, as those with persistent mediastinal involvement have a worse prognosis than those with proven mediastinal downstaging. In the absence of an experienced surgeon, it is technically challenging and difficult to restage the mediastinum with mediastinoscopy in patients who had undergone a prior mediastinoscopy, as tissue planes are obliterated by fibrosis. EBUS is less invasive, more accurate, and can be performed time and time again without the limitations that prevent mediastinal restaging by mediastinoscopy. EBUS-TBNA is highly specific and sensitive in restaging the mediastinum after neoadjuvant chemotherapy.
EBUS is also able to obtain adequate samples to run immunohistochemistry and DNA analysis on the tissue obtained. There is a need to further subclassify cancer by driving mutations. Anaplastic lymphoma tyrosine kinase (ALK) and c-ros oncogene 1 receptor kinase (ROS1) are examples that can be specifically treated with targeted agents. Flow-cytometric analysis, DNA point mutations, and RNA analysis can be run on tissue specimens obtained by EBUS sampling to help individualize therapy for lung cancer with genetically based chemotherapy regimens (24).
Endobronchial lesions
EBUS allows for diagnosis and treatment as well as surveillance follow-up of pathologic processes. It extends the endoscopist's vision to allow evaluation of the tracheobronchial wall and parabronchial anatomy. Radiographically inapparent endobronchial lesions may be detected during bronchoscopy or by newer methods being developed to screen high-risk patients for lung cancer, such as autofluorescence bronchoscopy and confocal microscopic bronchoscopy (25, 26). EBUS has been shown to more accurately predict malignancy in endobronchial lesions. In a trial of 105 patients with central thoracic malignancies, EBUS was superior to chest CT in differentiating tumor invasion of the airway from tumor compression (25). EBUS not only provides a more accurate classification of mucosal lesions, but also contributes to therapy decisions (16).
Mediastinal and hilar lymphadenopathy
The most common indication for hilar or mediastinal lymph node sampling is as previously described: to assess lymph node involvement in lung cancer. There are many infectious, noninfectious, and malignant causes of hilar and mediastinal lymphadenopathy, such as sarcoidosis, reactive lymphadenopathy, and tuberculosis. Metastatic primary cancers, thymomas, and lymphoma are some malignant causes of hilar and mediastinal lymphadenopathy.
The incidence of sarcoidosis in the United States is high, and sarcoidosis-related mortality is increasing (27, 28). Currently, conventional bronchoscopy is regarded as the standard to demonstrate noncaseating granulomas in patients with suspected sarcoidosis. EBUS-TBNA has been used in patients with suspected sarcoidosis to obtain tissue diagnosis as well as exclude other pathologies such as tuberculosis and malignancy. Studies have shown a higher diagnostic yield and a lower complication rate in favor of EBUS-TBNA. It has also been shown that use of EBUS-TBNA can avoid further invasive procedures in patients with suspected sarcoidosis (29). EBUS-TBNA is critical in confirming a diagnosis of sarcoidosis as well as excluding other pathology that may require prompt initiation of therapy. Compared with conventional transbronchial biopsy, the use of EBUS has also resulted in greater diagnostic yield (30). EBUS-TBNA is a safe procedure with a high yield for the diagnosis of sarcoidosis (31).
Lymphoma
Mediastinoscopies or thoracotomies have been performed as the standard procedure to obtain a histologic diagnosis in patients with mediastinal lymphadenopathy and suspected lymphoma. These procedures require general anesthesia and carry immense risks. Mediastinoscopy also has limited access to perihilar lymph nodes. Conventional TBNA, though superior to mediastinoscopy, has been shown to be inferior to EBUS-TBNA due to a lower specificity and sensitivity (5). EBUS can be performed for a histologic diagnosis in suspected lymphoma, and fluorescence in situ hybridization can also be done on the samples obtained by EBUS to further characterize lymphoma subtypes (32).
LIMITATIONS OF EBUS
As with all medical technologic advances, EBUS has its limitations. In the instance of mediastinal staging in lung cancer, EBUS is not able to stage the entire mediastinum. EBUS is limited to the anterosuperior mediastinum, and EUS is often utilized to sample the posteroinferior mediastinum. EBUS and EUS can often be performed in the same session consecutively. EBUS is technically difficult to perform in some anatomic locations, such as the upper lobes, as scope angulation is required. Multiple studies have reported a good experience with the use of electromagnetic navigational bronchoscopy with EBUS in upper lobe lesions (33, 34).
Needle puncture can be technically difficult, specifically in the elderly who have narrow intercartilaginous spaces and cartilage calcification. A significant cough can limit the success of the procedure in patients undergoing bronchoscopy under conscious sedation.
With the growing development and application of EBUS, many chest physicians lack adequate training and experience. This may be influenced by the fact that a fully equipped EBUS bronchoscopy suite may cost several thousand dollars to establish. For this fairly large investment, there is minimal additional professional fee reimbursement and no additional facility fee reimbursement above standard bronchoscopy (35).
CONTRAINDICATIONS
Contraindications to EBUS are similar to those of bronchoscopy in general. Recent myocardial infarction or ischemia, poorly controlled heart failure, significant hemodynamic instability, chronic obstructive pulmonary disease or asthma exacerbations, or life-threatening cardiac dysrhythmias should delay an endobronchial procedure. Contraindications particular to EBUS-TBNA are related to coagulopathies (medication induced or inherent). The recommendation is to hold antiplatelet and anticoagulation agents prior to endoscopy to reduce bleeding risk (36).
COMPLICATIONS
Bronchoscopy is generally a safe procedure with a reported low complication rate (37–39). Complications related to the bronchoscopy procedure can be related to sedation for the procedure or directly from the bronchoscope. Complications related to sedation are hypoxemia and hypotension, while those related to bronchoscopy are bronchospasm, laryngospasm, nausea, vomiting, bleeding due to scope trauma, cardiac arrhythmias, and vasovagal syncope. Complications specifically related to EBUS-TBNA are pneumothorax and bleeding. There has been a single reported case of EBUS-TBNA needle breakage without event (40).
In summary, EBUS has become the standard of care and has rapidly attained a key status in the diagnosis and staging of various lung cancers, but in addition is also aiding and helping manage other pulmonary pathologies such as sarcoidosis, lymphoma, and in situ endobronchial lesions. The ability of EBUS to help perform mediastinal and transbronchial biopsies less invasively and with better sensitivity and specificity makes it more favorable than mediastinoscopy. EBUS and EBUS-TBNA, with convex probe or radial probe, have galvanized pulmonologists further into the care of thoracic malignancies, improving treatment selection based on genetics and helping stage the mediastinum in a minimally invasive, safer, and more effective fashion.
References
- 1.Jiang J, Browning R, Lechtzin N, Huang J, Terry P, Wang KP. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis. 2014;6(5):416–420. doi: 10.3978/j.issn.2072-1439.2014.03.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, Falzon M, Capitanio A, Shaw P, Morris S, Omar RZ, Janes SM. REMEDY Trial Investigators Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186(3):255–260. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, Fujisawa T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126(1):122–128. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 4.Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, Shibuya K, Iizasa T, Fujisawa T. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50(3):347–354. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Steinfort DP, Conron M, Tsui A, Pasricha SR, Renwick WE, Antippa P, Irving LB. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5(6):804–809. doi: 10.1097/jto.0b013e3181d873be. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro C, Oliveira A, Neves S, Campainha S, Nogueira C, Torres S, Brito MC, Almeida J, e Sá JM. Diagnosis of sarcoidosis in the endobronchial ultrasound-guided transbronchial needle aspiration era. Rev Port Pneumol. 2014;20(5):237–241. doi: 10.1016/j.rppneu.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Low SY, Koh MS, Ong TH, Phua GC, Anantham D. Use of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in the diagnosis of granulomatous mediastinal lymphadenopathy. Ann Acad Med Singapore. 2014;43(5):250–254. [PubMed] [Google Scholar]
- 8.Sarma A, Heilbrun ME, Conner KE, Stevens SM, Woller SC, Elliott CG. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142(3):750–760. doi: 10.1378/chest.11-2863. [DOI] [PubMed] [Google Scholar]
- 9.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, Ando M. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration. 2013;85(6):486–492. doi: 10.1159/000346987. [DOI] [PubMed] [Google Scholar]
- 10.Szlubowski A, Kuzdzał J, Kołodziej M, Soja J, Pankowski J, Obrochta A, Kopiński P, Zieliński M. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg. 2009;35(2):332–335. doi: 10.1016/j.ejcts.2008.09.022. discussion 335–336. [DOI] [PubMed] [Google Scholar]
- 11.Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, Lee HS, Kim MS, Lee JM, Nam BH, Zo JI. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010;138(4):795–802. doi: 10.1378/chest.09-2100. [DOI] [PubMed] [Google Scholar]
- 12.Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138(4):790–794. doi: 10.1378/chest.09-2149. [DOI] [PubMed] [Google Scholar]
- 13.Kang HJ, Hwangbo B, Lee GK, Nam BH, Lee HS, Kim MS, Lee JM, Zo JI, Lee HS, Han JY. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax. 2014;69(3):261–268. doi: 10.1136/thoraxjnl-2013-203881. [DOI] [PubMed] [Google Scholar]
- 14.De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Van Schil P, Venuta F, Waller D, Weder W, Zielinski M. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(5):787–798. doi: 10.1093/ejcts/ezu028. [DOI] [PubMed] [Google Scholar]
- 15.Yasufuku K, Nakajima T, Motoori K, Sekine Y, Shibuya K, Hiroshima K, Fujisawa T. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130(3):710–718. doi: 10.1378/chest.130.3.710. [DOI] [PubMed] [Google Scholar]
- 16.Goeckenjan G, Sitter H, Thomas M, Branscheid D, Flentje M, Griesinger F, Niederle N, Stuschke M, Blum T, Deppermann KM, et al. German Respiratory Society; German Cancer Society Prevention, diagnosis, therapy, and follow-up of lung cancer. Interdisciplinary guideline of the German Respiratory Society and the German Cancer Society—abridged version. Pneumologie. 2011;65(8):e51–e75. doi: 10.1055/s-0030-1256562. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Caro A, Garcia S, Reguart N, Arguis P, Sanchez M, Gimferrer JM, Marrades R, Lomeña F. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg. 2010;37(5):1168–1174. doi: 10.1016/j.ejcts.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Pozo-Rodríguez F, Martín de Nicolás JL, Sánchez-Nistal MA, Maldonado A, García de Barajas S, Calero-García R, Pozo MA, Martín-Escribano P, Martín-García I, García-Lujan R, Lopez-Encuentra A, Arenas de Pablo A. Accuracy of helical computed tomography and 18F-fluorodeoxyglucose positron emission tomography for identifying lymph node mediastinal metastases in potentially resectable non-small-cell lung cancer. J Clin Oncol. 2005;23(33):8348–8356. doi: 10.1200/JCO.2004.00.6361. [DOI] [PubMed] [Google Scholar]
- 19.Verhagen AF, Bootsma GP, Tjan-Heijnen VC, van der Wilt GJ, Cox AL, Brouwer MH, Corstens FH, Oyen WJ. FDG-PET in staging lung cancer: how does it change the algorithm? Lung Cancer. 2004;44(2):175–181. doi: 10.1016/j.lungcan.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Freixinet Gilart J, García PG, de Castro FR, Suárez PR, Rodríguez NS, de Ugarte AV. Extended cervical mediastinoscopy in the staging of bronchogenic carcinoma. Ann Thorac Surg. 2000;70(5):1641–1643. doi: 10.1016/s0003-4975(00)01825-7. [DOI] [PubMed] [Google Scholar]
- 21.Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):7S–37S. doi: 10.1378/chest.12-2377. [DOI] [PubMed] [Google Scholar]
- 22.Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, da Cunha Santos G, Geddie W, Boerner S, Le LW, Keshavjee S. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142(6):1393–1400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S, Hardee JN, Odell JA. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299(5):540–546. doi: 10.1001/jama.299.5.540. [DOI] [PubMed] [Google Scholar]
- 24.Stigt JA, Hart NA, Knol AJ, Uil SM, Groen HJ. Pyrosequencing analysis of EGFR and KRAS mutations in EUS and EBUS-derived cytologic samples of adenocarcinomas of the lung. J Thorac Oncol. 2013;8(8):1012–1018. doi: 10.1097/JTO.0b013e31829ce93e. [DOI] [PubMed] [Google Scholar]
- 25.Herth F, Ernst A, Schulz M, Becker H. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest. 2003;123(2):458–462. doi: 10.1378/chest.123.2.458. [DOI] [PubMed] [Google Scholar]
- 26.Chhajed PN, Shibuya K, Hoshino H, Chiyo M, Yasufuku K, Hiroshima K, Fujisawa T. A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. Eur Respir J. 2005;25(6):951–955. doi: 10.1183/09031936.05.00012504. [DOI] [PubMed] [Google Scholar]
- 27.Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011;305(4):391–399. doi: 10.1001/jama.2011.10. [DOI] [PubMed] [Google Scholar]
- 28.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Ichihara S, Moritani S. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg. 2012;143(6):1324–1329. doi: 10.1016/j.jtcvs.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 30.von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, et Veen JC, de Jong YP, van der Heijden EH, Tournoy KG, Claussen M, van den Blink B, Shah PL, Zoumot Z, Clementsen P, Porsbjerg C, Mauad T, Bernardi FD, van Zwet EW, Rabe KF, Annema JT. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013;309(23):2457–2464. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 31.Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, Iizasa T, Hiroshima K, Lam WK, Fujisawa T. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29(6):1182–1186. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 32.Nunez AL, Jhala NC, Carroll AJ, Mikhail FM, Reddy VV, Xian RR, Jhala DN. Endoscopic ultrasound and endobronchial ultrasound-guided fine-needle aspiration of deep-seated lymphadenopathy: Analysis of 1338 cases. Cytojournal. 2012;9:14. doi: 10.4103/1742-6413.95845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang HJ, Hwangbo B. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration. Tuberc Respir Dis (Seoul) 2013;75(4):135–139. doi: 10.4046/trd.2013.75.4.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong S, Ju H, Marshall H, Bowman R, Yang I, Ree AM, Saxon C, Fong KM. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis. 2012;4(2):173–185. doi: 10.3978/j.issn.2072-1439.2012.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010;182(5):589–597. doi: 10.1164/rccm.201002-0186CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst A, Eberhardt R, Wahidi M, Becker HD, Herth FJ. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006;129(3):734–737. doi: 10.1378/chest.129.3.734. [DOI] [PubMed] [Google Scholar]
- 37.Jin F, Mu D, Chu D, Fu E, Xie Y, Liu T. Severe complications of bronchoscopy. Respiration. 2008;76(4):429–433. doi: 10.1159/000151656. [DOI] [PubMed] [Google Scholar]
- 38.Facciolongo N, Patelli M, Gasparini S, Lazzari Agli L, Salio M, Simonassi C, Del Prato B, Zanoni P. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71(1):8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 39.Carr IM, Koegelenberg CF, von Groote-Bidlingmaier F, Mowlana A, Silos K, Haverman T, Diacon AH, Bolliger CT. Blood loss during flexible bronchoscopy: a prospective observational study. Respiration. 2012;84(4):312–318. doi: 10.1159/000339507. [DOI] [PubMed] [Google Scholar]
- 40.Özgül MA, Çetinkaya E, Tutar N, Özgül G. An unusual complication of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): the needle breakage. Ann Thorac Cardiovasc Surg. 2014;20(Suppl):567–569. doi: 10.5761/atcs.cr.12.02015. [DOI] [PubMed] [Google Scholar]