Abstract
Boerhaave's syndrome, or spontaneous esophageal rupture, is a rare condition that classically presents with Mackler's triad of vomiting, subcutaneous emphysema, and severe sudden onset of chest pain and requires immediate medical attention. Approximately 90% of the perforations occur at the left lateral aspect of the distal esophagus, causing a left-sided pleural effusion. Less than 10% of patients have bilateral effusions, and few patients have a right-sided pleural effusion only. We present the case of a 59-year-old man with spontaneous esophageal rupture. His clinical presentation is of interest since he had no inciting event for spontaneous esophageal rupture and had a delayed presentation with a right-sided hydropneumothorax.
Boerhaave's syndrome, or spontaneous esophageal rupture, is a life-threatening condition that requires prompt diagnosis and treatment. Delayed diagnosis may result in serious complications, including mediastinitis, pneumonitis, pericarditis, empyema, and death. We report an atypical presentation of Boerhaave's syndrome with no vomiting, with progressive right-sided chest pain and shortness of breath of 2 weeks' duration, and with a right-sided hydropneumothorax.
CASE REPORT
A 59-year-old man with a history of hypertension presented with worsening right-sided chest pain and progressive shortness of breath of 2 weeks' duration. The patient had reported mild constant right-sided chest pain over the past year. The chest pain was pleuritic in nature. He denied fevers, chills, productive cough, nausea, and vomiting. The patient had a history of upper gastrointestinal bleeding 4 months prior to presentation while taking meloxicam. He underwent esophagogastroduodenoscopy, which showed benign esophageal ulcers in the middle thoracic esophagus from 26 to 29 cm and in the distal esophagus from 33 to 36 cm, as well as chronic gastritis. He took oral omeprazole 20 mg twice a day and stopped taking meloxicam. He started to take one or two 7.5 mg tablets of meloxicam a day to relieve the chest pain without taking omeprazole for 1 week before his current presentation. He denied reflux symptoms, a history of heavy lifting or straining, a history of chest trauma, peptic ulcer disease, or alcohol or steroid use.
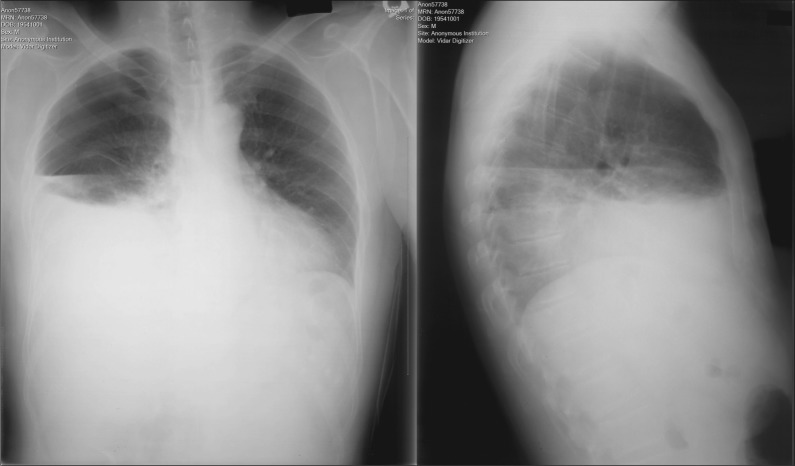
His blood pressure was 114/66 mm Hg; heart rate, 123 beats/min; respiratory rate, 21 breaths/min; and temperature, 36.7°C (98°F). His body mass index was 20.3 kg/m2. The jugular veins were not distended. Breath sounds were decreased in the right lower field, with corresponding dullness on percussion. There was no murmur, subcutaneous emphysema, or Hamman's crunch. The abdomen was soft without tenderness. The bowel sounds were normal. Initial laboratory tests revealed the following: white blood cell count, 26,570/µL with 95% polymorphonuclear cells; hemoglobin, 10.7 g/dL; blood urea nitrogen, 31 mg/dL; creatinine, 0.9 mg/dL; and albumin, 2.7 g/dL. All other laboratory results were unremarkable. His initial chest radiograph revealed a right-sided hydropneumothorax (Figure 1).
Figure 1.
Chest radiograph demonstrates right hydropneumothorax.
A thoracostomy tube was placed into the right chest, resulting in the drainage of 1 L of brownish purulent material. Pleural fluid analysis showed a pH of 7.2. Gram stain of the pleural fluid showed a few white blood cells; many Gram-positive cocci in pairs, chains, and clusters; moderate Gram-positive rods; few Gram-negative rods; and few yeast. Computed tomography (CT) of the chest revealed moderate right pleural effusion, small right pneumothorax, consolidation/atelectasis in the right lower lobe with air bronchograms, and thickening of the distal esophagus and gastroesophageal junction.
The patient had video-assisted thorascopic surgery 2 days after admission. Gastrointestinal contents were found in the pleural cavity. At this point, the diagnosis of Boerhaave's syndrome was suspected, and the procedure was converted to an open thoracotomy. The patient had a hiatal hernia and a distal esophagus perforation measuring approximately 2.5 cm. A distal esophageal resection and gastrostomy tube placement were performed. The biopsy report of the distal esophagus showed intestinal metaplasia and ulcer without dysplasia or malignancy.
The patient's postoperative course was complicated by sepsis and acute renal failure. The pleural culture grew Citrobacter freundii; blood cultures were negative. He was continued on meropenem and fluconazole. His renal function returned to normal. The chest tubes, drains, and gastrostomy tube were discontinued, and the patient was discharged on day 19 of admission.
DISCUSSION
Boerhaave's syndrome is a rare condition, with an incidence of 7.4 per 10 million per year (1). It is associated with substantial morbidity and has a mortality rate of at least 20% (1–3). This condition is caused by an acute increase in intraluminal esophageal pressure as the result of retching or vomiting causing perforation at the weak point of the esophageal wall, which is the left posterolateral wall of the distal esophagus (4). There are anatomical reasons to explain this location, including thinning of the muscle in the distal esophagus, weakening of its wall as a result of vessels and nerves entering it, anterior angulation at the left diaphragmatic crus, and lack of adjacent supporting structures (5). The classical clinical presentation, Meckler's triad, is severe vomiting and retching followed by severe sudden onset of chest pain and subcutaneous emphysema (6). However, some reviews point out that the presence of the entire triad is rare, and reliance on a classic presentation may lead to delayed diagnosis, resulting in mediastinitis, pneumonitis, pericarditis, empyema, and increased mortality (5, 7).
Mediastinal pleura rupture from acute intraabdominal pressure elevation or digestion by gastric contents will lead to leakage of air and fluid into the pleural space, causing pleural effusion, pneumothorax, or hydropneumothorax. Approximately 90% of patients have left-sided pleural effusions, 5% to 10% of patients have bilateral effusions, and few patients have an effusion on the right side only (8). Only five cases have been reported to present with right-sided effusions in the past 10 years (Table 1) (9–12).
Table 1.
Patients with spontaneous esophageal perforation who presented with right-sided pleural effusions, 2005 to 2015
| First author, year of publication (ref) | Age (years) | Gender | Underlying disease | Vomiting prior to presentation | Perforation length | Days from presentation to diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Rokszin, 2011 (9) | 53 | Male | GERD, BE, rectal cancer | Yes | 5–7 mm | – | Endoscopic closure | Survived |
| Hingston, 2010 (10) | 84 | Female | Osteopenia treated with alendronic acid 5 h before presentation | Yes | 6 cm | 3 | Gastric port and bilateral chest tube drainage | Survived |
| Cascio, 2010 (11) | 45 | Female | Alcoholism, pregnancy | No | – | 8 | Right chest tube drainage; primary repair of esophagus | Died |
| Khan, 2005 (12) | 61 | Male | Spontaneous esophageal rupture 26 mo earlier, BE | Yes | 2 cm | – | Primary repair of esophagus | Survived |
| Khan, 2005 (12) | 51 | Male | Spontaneous esophageal rupture 27 mo earlier, BE | Yes | 3 cm | – | Conservative treatment | Survived |
BE indicates Barrett esophagus; GE, gastroesophageal; GERD, gastroesophageal reflux disease; –, no information available.
The diagnosis of Boerhaave's syndrome is made primarily from clinical presentation and radiographic evidence. A posteroanterior and lateral chest radiograph is the best method to see indirect signs of esophageal perforation, since up to 90% of patients have abnormal chest x-ray findings, including pleural effusion, pneumothorax, hydropneumothorax, pneumomediastinum, widening mediastinum, and subcutaneous emphysema. However, an immediate chest x-ray after esophageal perforation may be normal, as a pneumomediastinum will take at least an hour to develop and a pleural effusion may take several hours to become discernable (13). A water-soluble contrast swallow study is recommended to confirm the diagnosis by showing leakage of contrast into the mediastinum and/or pleural cavity and to identify the anatomical site of perforation. CT of the chest and upper abdomen with oral contrast can also show the site of perforation and the degree of contamination. Given current access to CT and oral contrast studies, the diagnosis of esophageal rupture was missed and found only at autopsy in only 17% of patients in a recent study (1).
Early recognition and operative repair are the mainstays of treatment and provide better outcomes. Dasari and colleagues summarized 27 case series using esophageal stents in patients with esophageal anastomotic leaks and benign perforations. These series included 340 patients, and technical and clinical success rates with stenting were 91% and 81%, respectively. However, 187 patients required thoracotomy procedures, including chest tubes and surgery (14). Salo reported his experience with Boerhaave's syndrome over a three-decade period. His approach includes early antibiotics, a CT study of the thorax and upper abdomen, emergency endoscopy to evaluate the rupture and tissue vitality, and primary esophageal repair with fundic reinforcement when possible. Mortality has fallen from 50% to 5%; all aspects of current management appear to have contributed to this improvement (15). Conservative treatment can be an option for selected patients with small and contained intrathoracic esophageal ruptures (16). Unusual presentations should be kept in mind while evaluating patients with hydropneumothorax, even on the right side.
The only risk factor that might explain esophageal rupture in our patient is nonsteroidal antiinflammatory drug use with a history of esophageal ulcer when he was taking this drug in the past. Our patient had a delayed presentation with a right-sided hydropneumothorax, required surgery, and had a prolonged 19-day hospital course.
References
- 1.Vidarsdottir H, Blondal S, Alfredsson H, Geirsson A, Gudbjartsson T. Oesophageal perforations in Iceland: a whole population study on incidence, aetiology and surgical outcome. Thorac Cardiovasc Surg. 2010;58(8):476–480. doi: 10.1055/s-0030-1250347. [DOI] [PubMed] [Google Scholar]
- 2.Ryom P, Ravn JB, Penninga L, Schmidt S, Iversen MG, Skov-Olsen P, Kehlet H. Aetiology, treatment and mortality after oesophageal perforation in Denmark. Dan Med Bull. 2011;58(5):A4267. [PubMed] [Google Scholar]
- 3.Bhatia P, Fortin D, Inculet RI, Malthaner RA. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg. 2011;92(1):209–215. doi: 10.1016/j.athoracsur.2011.03.131. [DOI] [PubMed] [Google Scholar]
- 4.Garas G, Zarogoulidis P, Efthymiou A, Athanasiou T, Tsakiridis K, Mpaka S, Zacharakis E. Spontaneous esophageal rupture as the underlying cause of pneumothorax: early recognition is crucial. J Thorac Dis. 2014;6(12):1655–1658. doi: 10.3978/j.issn.2072-1439.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagminas L, Silverman RA. Boerhaave's syndrome presenting with abdominal pain and right hydropneumothorax. Am J Emerg Med. 1996;14(1):53–56. doi: 10.1016/S0735-6757(96)90016-9. [DOI] [PubMed] [Google Scholar]
- 6.Curci JJ, Horman MJ. Boerhaave's syndrome: the importance of early diagnosis and treatment. Ann Surg. 1976;183(4):401–408. doi: 10.1097/00000658-197604000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones WG, 2nd, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg. 1992;53(3):534–543. doi: 10.1016/0003-4975(92)90294-e. [DOI] [PubMed] [Google Scholar]
- 8.Janjua KJ. Boerhaave's syndrome. Postgrad Med J. 1997;73(859):265–270. doi: 10.1136/pgmj.73.859.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rokszin R, Simonka Z, Paszt A, Szepes A, Kucsa K, Lazar G. Successful endoscopic clipping in the early treatment of spontaneous esophageal perforation. Surg Laparosc Endosc Percutan Tech. 2011;21(6):e311–312. doi: 10.1097/SLE.0b013e31823118ee. [DOI] [PubMed] [Google Scholar]
- 10.Hingston CD, Saayman AG, Frost PJ, Wise MP. Boerhaave's syndrome—rapidly evolving pleural effusion: a radiographic clue. Minerva Anestesiol. 2010;76(10):865–867. [PubMed] [Google Scholar]
- 11.Cascio A, Barone M, Micali V, Iaria C, Delfino D, David A, Monaco M, Monaco F. On a fatal case of Candida krusei pleural empyema in a pregnant woman with spontaneous esophagus perforation. Mycopathologia. 2010;169(6):451–455. doi: 10.1007/s11046-010-9277-6. [DOI] [PubMed] [Google Scholar]
- 12.Khan OA, Barlow CW, Weeden DF, Amer KM. Recurrent spontaneous esophageal rupture. Eur J Cardiothorac Surg. 2005;28(1):178–179. doi: 10.1016/j.ejcts.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Wu JT, Mattox KL, Wall MJ., Jr Esophageal perforations: new perspectives and treatment paradigms. J Trauma. 2007;63(5):1173–1184. doi: 10.1097/TA.0b013e31805c0dd4. [DOI] [PubMed] [Google Scholar]
- 14.Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259(5):852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 15.Salo J, Sihvo E, Kauppi J, Rasanen J. Boerhaave's syndrome: lessons learned from 83 cases over three decades. Scand J Surg. 2013;102(4):271–273. doi: 10.1177/1457496913495338. [DOI] [PubMed] [Google Scholar]
- 16.Cameron JL, Kieffer RF, Hendrix TR, Mehigan DG, Baker RR. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg. 1979;27(5):404–408. doi: 10.1016/s0003-4975(10)63335-8. [DOI] [PubMed] [Google Scholar]