Abstract
Anaphylaxis rarely manifests as a vasospastic acute coronary syndrome with or without the presence of underlying coronary artery disease. The variability in the underlying pathogenesis produces a wide clinical spectrum of this syndrome. We present three cases of anaphylactic acute coronary syndrome that display different clinical variants of this phenomenon. The main pathophysiological mechanism of the allergic anginal syndromes is the inflammatory mediators released during a hypersensitivity reaction triggered by food, insect bites, or drugs. It is important to appropriately recognize and treat Kounis syndrome in patients with exposure to a documented allergen.
Acute coronary syndrome accompanying mast cell activation from allergic, hypersensitivity, or anaphylactoid reactions was first described by Kounis and Zavras in 1991 and has been referred to as “allergic angina” or “allergic myocardial infarction” (1, 2). The mechanism of Kounis syndrome (KS) involves release of inflammatory cytokines through mast cell activation, which leads to coronary artery vasospasm and/or atheromatous plaque erosion or rupture (2). KS has been described with multiple conditions, including a variety of environmental exposures and drugs (3). More recently, variant presentations of allergic angina have been described. In this case series, we describe three variable case presentations of anaphylactic ST elevations, which include ST-elevation myocardial infarction (MI) in the presence of underlying coronary artery disease and ST-elevation MI without underlying coronary artery disease (pure vasospasm). We also briefly review the existing literature on KS.
CASE PRESENTATIONS
Case 1
A 64-year-old man with known peripheral arterial disease, chronic obstructive pulmonary disease, coronary artery disease, dyslipidemia, and hypertension presented with pruritus, chest pain, dyspnea, and nausea immediately after having dinner at a restaurant including pea salad. The initial electrocardiogram demonstrated ST-segment elevations in leads II, III, and aVF (Figure 1a). The patient had a prior known allergic reaction (type 1) to peas and peanuts. His initial vital signs were a temperature of 98°F, blood pressure of 81/60 mm Hg, respiration rate of 20 breaths/minute, and pulse of 91 beats/minute. Examination disclosed wheezing, generalized erythema, and weak peripheral pulses. His hemogram and basic chemistry panel were unremarkable except for a white blood cell count of 19,500/uL. Both the creatinine kinase and troponin I levels were normal (145 U/L, normal 24–204 U/L; and <0.30 ng/mL, normal <0.30 ng/mL, respectively).
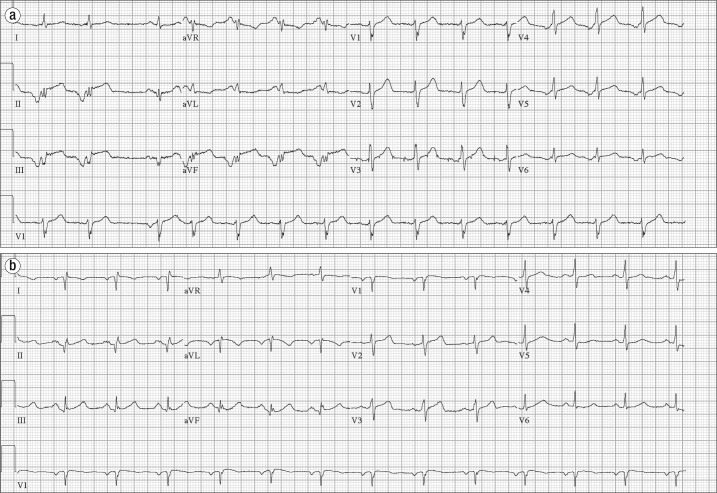
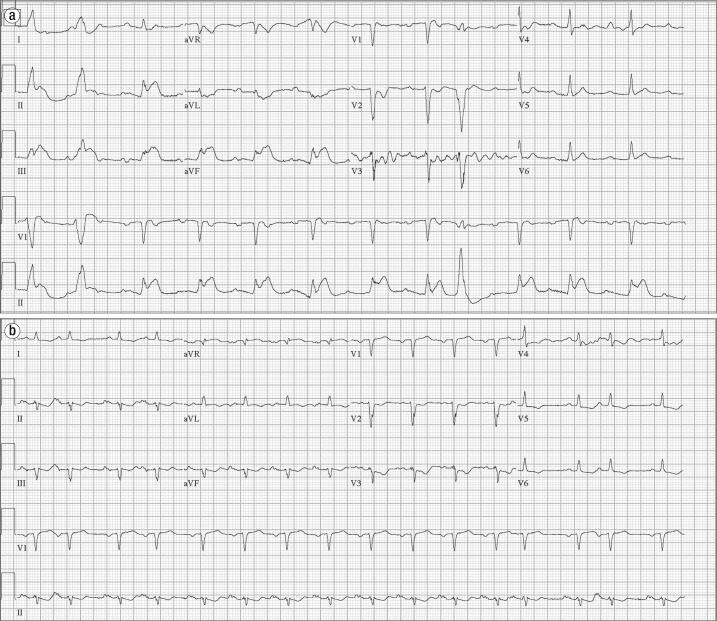
Figure 1.
Electrocardiograms in case 1. (a) Initial electrocardiogram demonstrates an ectopic atrial rhythm with an isolated sinus beat (third beat), inferior Q waves, and ST elevation in the inferior leads. (b) Repeat electrocardiogram shows Q waves in the inferior leads and resolution of inferior ST elevations. There is limb lead reversal.
Emergent coronary angiogram demonstrated moderate stenosis of the proximal and mid left anterior descending artery and the right coronary artery, with evidence of improvement in the stenosis with administration of intracoronary nitroglycerine (Figure 2). Diagnosis of coronary vasospasm was made secondary to anaphylactic reaction from pea ingestion. Treatment for anaphylaxis was initiated with intravenous hydrocortisone, diphenhydramine, and famotidine with improvement in pruritus, dyspnea, and chest discomfort. The patient's blood pressure also improved to 120/60 mm Hg. Repeat electrocardiogram showed resolution of ST segment elevations (Figure 1b). He was discharged home.
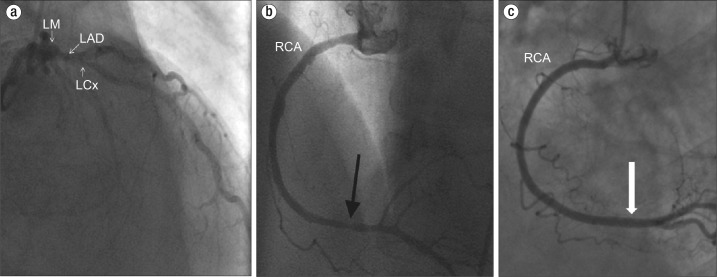
Figure 2.
Coronary angiogram in case 1. (a) A left anterior oblique cranial view shows left main coronary artery (LM), left circumflex artery (LCx) and left anterior descending artery (LAD). The arrow points to significant proximal LAD stenosis (a nonculprit lesion for the presentation). (b) The right coronary artery (RCA) shows evidence of distal stenosis (black arrow). (c) After administration of intracoronary nitroglycerine, the RCA stenosis demonstrated resolution consistent with coronary vasospasm (white arrow).
Case 2
A 54-year-old man with past Lyme disease, hypertension, hyperlipidemia, and gastroesophageal reflux disease presented after he was stung by a bee while working in the yard. He developed facial swelling and hypotension. Emergency medical services found him to be in respiratory distress associated with facial swelling. He was intubated and was administered intravenous diphenhydramine and methylprednisolone and inhaled albuterol. The initial electrocardiogram demonstrated ST elevations in the inferior leads (Figure 3a). The patient was started on intravenous fluids and heparin and was transferred to our hospital. On arrival, he was found to have transient complete heart block, which resolved after administration of diphenhydramine and methylprednisolone. Coronary angiography revealed severe 3-vessel disease but no acute culprit lesion (Figure 4). Based on the clinical data, coronary vasospasm as the likely cause was considered in retrospect. The ST elevations resolved on a repeat electrocardiogram (Figure 3b). He was seen by a cardiothoracic surgery team and was scheduled for elective bypass surgery.
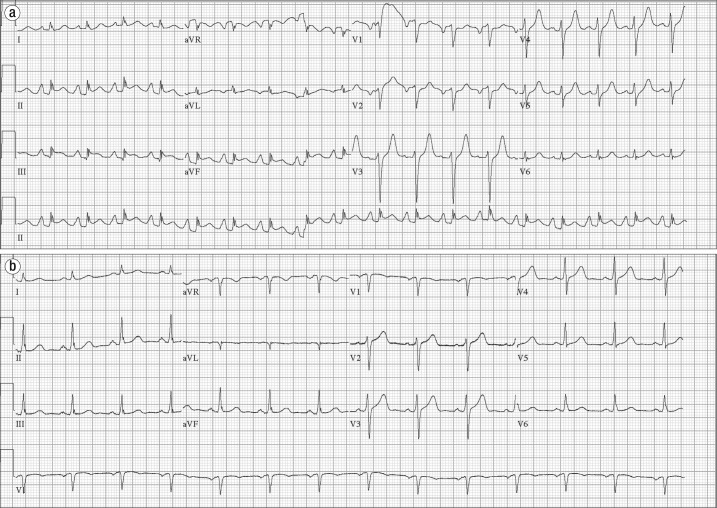
Figure 3.
Electrocardiograms in case 2. (a) Initial electrocardiogram demonstrates ST elevations in the inferior leads. (b) Repeat electrocardiogram demonstrates resolution of ST elevations.
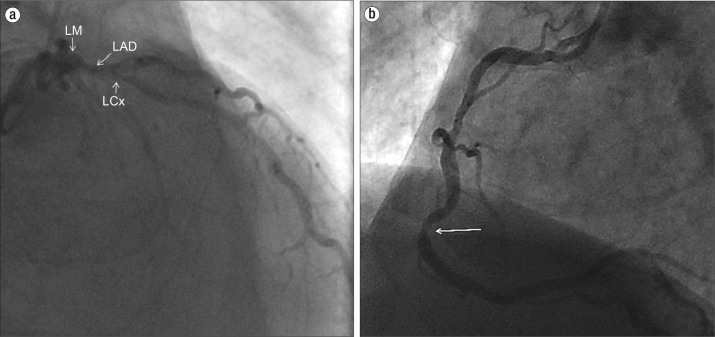
Figure 4.
Coronary angiogram in case 2. (a) Posteroanterior caudal view shows chronic multivessel coronary artery disease of the left main (LM) coronary artery, left anterior descending (LAD) artery, and left circumflex (LCx) artery (arrows). There was no acute culprit lesion. (b) Left anterior oblique projection of the right coronary artery (RCA) shows focal severe stenosis in the mid and distal RCA and mild to moderate atherosclerotic disease at other locations.
Case 3
A 75-year-old man with known diabetes mellitus, hypertension, hyperlipidemia, and abdominal aortic aneurysm presented to our hospital with dysuria and fever. His urinalysis was positive for leukocyte esterase, a few red blood cells, and multiple leucocytes. He was given intravenous ceftriaxone for suspected urinary tract infection. After the administration of ceftriaxone, he immediately developed a generalized erythematous macular rash, hypotension, and tachycardia followed by pulseless electrical activity and cardiac arrest. Advanced cardiovascular life support was employed, including the use of intravenous epinephrine per protocol. He had return of spontaneous circulation with complete neurological recovery. The electrocardiogram after return of the patient's spontaneous circulation revealed ST segment elevations in inferior leads (II, III, and aVF) (Figure 5a), leading to activation of the cardiac catheterization laboratory team. The patient also received intravenous diphenhydramine, famotidine, and methylprednisolone. Subsequent electrocardiograms taken 5 and 15 minutes later showed normalization of ST segments (Figure 5b). His hemogram and basic chemistry panel were normal; the creatinine kinase was 145 U/L, and troponin I, <0.30 ng/mL. The patient was admitted and successfully extubated after a day. His urinary tract infection resolved, and he was discharged home in a stable condition. Prior to discharge, he underwent an elective nuclear stress test, which showed a small fixed apical anterolateral wall defect with no evidence of ischemia.
Figure 5.
Electrocardiograms in case 3. (a) Initial electrocardiogram shows inferior lead ST elevations and premature ventricular complexes. (b) Repeat electrocardiogram demonstrates resolution of inferior lead ST elevations. Premature ventricular complexes and widespread T wave inversions are additional notable findings.
DISCUSSION
Three types of KS have been previously described (4). Type I includes patients with normal coronary arteries without predisposing factors for coronary artery disease in whom the acute allergic insult leads to coronary artery spasm with normal cardiac biomarkers or infarction with positive cardiac biomarkers. This variant represents a manifestation of endothelial dysfunction or microvascular angina (5). The type II variant includes patients with culprit but inactive preexisting atheromatous disease, in whom the allergic insult leads to plaque erosion or rupture, leading to acute myocardial infarction or coronary vasospasm with normal cardiac enzymes (4). The type III variant includes coronary artery stent thrombosis secondary to allergic reaction (4). The ischemia in allergic reaction is secondary to the release of inflammatory mediators, including histamine, tryptase, chymase, platelet-activating factor, cytokines, and prostaglandins, and leukotriene synthesis, which leads to coronary vasospasm (2). Vigorito et al previously reported a paradoxical vasoconstriction mediated by histamine-1 receptors (6). Multiple cases of KS have been reported in the literature (7). Our first two cases were a form of type 2 KS, and the third case represented a form of type 1 KS. The second patient experienced transient complete heart block with anaphylaxis and is the second such report of KS associated with complete heart block described in the literature (8).
Our cases further support KS as a distinct phenomenon. Some authors have argued against the vasospastic process being the dominant underlying pathophysiological explanation for KS. It is well known that anaphylaxis causes systemic vasodilation and decreased venous return secondary to increased vascular permeability and subsequently may lead to a depressed cardiac output, resulting in coronary hypoperfusion and myocardial damage. Thus, differentiating primary myocardial damage secondary to mast cell activation from global myocardial hypoperfusion can be challenging and remains an alternative potential explanation for allergic acute coronary syndrome (electrocardiographic ST elevations) (4). It is important to recognize these forms of KS to provide appropriate management and care. The diagnosis and treatment of KS can be indeed challenging, requiring attention to both the cardiac and anaphylactic pathophysiology concurrently.
Treatment of KS requires thoughtful use of several common drugs. Morphine, an important drug for treating acute chest pain, should be avoided in KS, as it may potentially stimulate histamine release and exacerbate the pathologic cascade in KS. Beta-blockers also may potentiate coronary vasospasm if used in an acute exacerbation of KS due to an unopposed alpha adrenergic action. Epinephrine, which is used routinely for the treatment of anaphylaxis, should also be used with cautionary monitoring, as it may potentially worsen coronary vasospasm and aggravate coronary ischemia in KS (4). The primary focus of treatment of KS should be directed towards the allergic insult and removal of the offending allergens.
References
- 1.Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45(2):121–128. [PubMed] [Google Scholar]
- 2.Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol. 2006;110(1):7–14. doi: 10.1016/j.ijcard.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues MC, Coelho D, Granja C. Drugs that may provoke Kounis syndrome. Braz J Anesthesiol. 2013;63(5):426–428. doi: 10.1016/j.bjan.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35(5):563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaidis LA, Kounis NG, Gradman AH. Allergic angina and allergic myocardial infarction: a new twist on an old syndrome. Can J Cardiol. 2002;18(5):508–511. [PubMed] [Google Scholar]
- 6.Vigorito C, Poto S, Picotti GB, Triggiani M, Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986;73(6):1175–1182. doi: 10.1161/01.cir.73.6.1175. [DOI] [PubMed] [Google Scholar]
- 7.Caglar FN, Caglar IM, Coskun U, Ugurlucan M, Okcun B. Kounis syndrome: myocardial infarction secondary to an allergic insult—a rare clinical entity. Acta Cardiol. 2011;66(4):559–562. doi: 10.1080/ac.66.4.2126625. [DOI] [PubMed] [Google Scholar]
- 8.Kounis NG. Caspofungin-induced fatal complete heart block: Another manifestation of Kounis syndrome. J Pharmacol Pharmacother. 2013;4(2):161–162. doi: 10.4103/0976-500X.110918. [DOI] [PMC free article] [PubMed] [Google Scholar]