Abstract
Ornithine transcarbamoylase deficiency is the most common inherited urea cycle disorder. In adults, its phenotypes are diverse. In asymptomatic patients with late presentations, symptom onset is often associated with a precipitating factor. We present a case of a woman with urea cycle disorder diagnosed after an acute peptic ulcer bleed and fasting.
Urea cycle disorders are commonly seen in the pediatric population. Several enzymes are involved in this cycle, and a defect in any of them can lead to specific laboratory abnormalities and variable clinical presentations. Deficiency of the enzyme ornithine transcarbamoylase (OTC) is the most common inherited urea cycle disorder. In adults, its phenotypes range from severe encephalopathy to subtle psychiatric manifestations to apparently asymptomatic states. Among asymptomatic patients with late presentations, symptom onset may be associated with a precipitating factor, such as trauma, surgery, infection, and/or increased protein intake. We present a case of a woman who had undiagnosed urea cycle disorder that was unmasked by an acute peptic ulcer bleed and fasting for several days.
CASE REPORT
A 35-year-old white woman with prior peptic ulcer disease and menorrhagia was taken to a local emergency department for an altered level of consciousness and transferred to our medical intensive care unit. She had been alone for 5 days when her family found her lethargic, confused, and minimally responsive. The patient apparently had been withdrawn for 1 year with low self-esteem, occasionally stating that she wished she were dead. Home medications included pantoprazole and norgestimate/ethinyl estradiol. She was currently single and unemployed and had few friends and no children. Examination disclosed normal vital signs. Neurological examination revealed a minimally responsive woman without apparent focal deficits and normal reflexes. She had no nuchal rigidity. Initial laboratory work showed a normal metabolic panel, thyroid function tests, and liver function tests, including aminotransferases, albumin, bilirubin, and prothrombin time. She had anemia with a hemoglobin of 8.1 g/dL, a guaiac-positive stool, and a ferritin level of 8 ng/mL (reference range, 12–150 ng/mL). A urine drug screen was negative; acetaminophen, alcohol, and salicylate levels were undetectable. Computed tomography (CT) and magnetic resonance images of the head were within normal limits.
On the night of admission, the patient had an episode of hematemesis, and her hemoglobin dropped to 6.6 g/dL. The next morning she had decerebrate posturing to pain, a decline in responsiveness, twitching movements in her left leg, and plantar flexion of both feet. A repeat CT scan was normal, and electroencephalography did not reveal any subclinical epileptiform discharges. She was intubated, and an esophagogastroduodenoscopy showed an actively bleeding duodenal ulcer that was cauterized.
Her ammonia level was 593 mcg/dL (reference range, 15–45 mcg/dL). The patient was started on lactulose, rifaximin, and a low-protein diet. Her subsequent ammonia level in 4 hours dropped to 266 mcg/dL, which continued to improve over the next few hours. A liver ultrasound was within normal limits, and her liver enzymes were normal throughout her hospitalization. She gradually improved, with her ammonia level returning toward normal over the next several days. She had a plasma citrulline of 20 nmol/mL (reference range, 30–45 nmol/mL) and a urine orotic acid level of 1.8 mmol/mol creatinine (reference range, 0.4–1.2 mmol/mol creatinine), levels consistent with OTC deficiency. She was discharged home on lactulose and a low-protein diet.
DISCUSSION
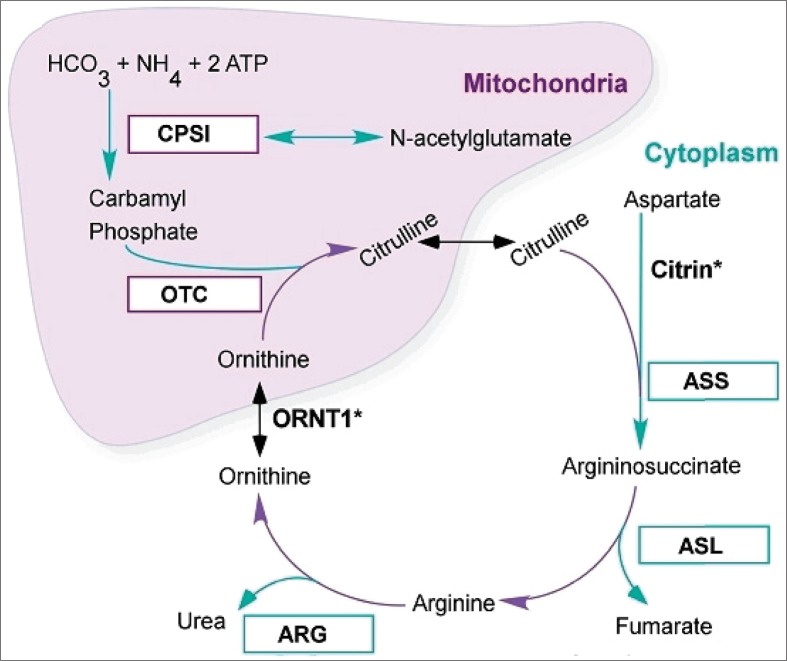
The urea cycle is the metabolic pathway that converts nitrogen to urea for excretion from the body (Figure 1). A deficiency in one of the enzymes in this pathway causes a urea cycle disorder (1), and all deficiencies except arginase cause severe hyperammonemia. The amino acid products of endogenous and exogenous protein digestion are degraded by hepatic transamination and oxidative deamination to produce ammonia, which is then converted to urea via the six enzymes of the urea cycle (Table) and excreted by the kidneys (2). The most common genetic disorder of the urea cycle is a deficiency in OTC (3). Partial OTC deficiency has a variable presentation (4), but its symptoms are caused by hyperammonemia secondary to accumulation of precursors of urea, primarily ammonia and glutamine (2). The clinical presentation of hyperammonemia caused by OTC deficiency varies with the age of the patient. In infants, hyperammonemia is associated with lethargy, poor sucking response, vomiting, hypotonia, and seizures (5). In adults, its phenotypes are diverse, ranging from severe encephalopathy to apparently asymptomatic states (6). The OTC gene is encoded on the X chromosome and is expressed in the mitochondrial matrix of the small intestine and liver, where it catalyzes the synthesis of citrulline from carbamoyl phosphate and ornithine (2).
Figure 1.
Urea cycle disorders. ARG indicates arginase; ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; CPS1, carbamoylphosphate synthetase 1; ORNT1, ornithine transporter 1; OTC, ornithine transcarbamoylase.
Table.
Urea cycle defects
| Enzyme deficiency | Location | Incidence | Genetics | Presentation | Distinct labs |
|---|---|---|---|---|---|
| 1. CPS1: Carbamoyl phosphate synthetase | Mitochondria | 1:1,300,000 | AR | ↑↑NH3 in newborns | +NH3, low CIT, low ARG, normal urine OA |
| 2. OTC: Ornithine transcarbamylase | Mitochondria | 1:56,500 0–30% EA | X-linked dominant | Risk of ↑NH3 high in pregnancy | +OR, +UR, +UOA, low plasma CIT |
| 3. ASS: Argininosuccinic acid synthetase (citrullinemia) | Cytosol | 1:250,000 | AR | Some nitrogen incorp in UC | ++CIT, absent AS, fibroblast EA |
| 4. ASL: Argininosuccinate lyase (argininosuccinic aciduria) | Cytosol | 1:218,750 | AR | Rap in infants, Rx ARG | +CIT, +ASA |
| 5. ARG: Arginase (hyperargininemia) | Cytosol | 1:950,000 | AR | Often normal NH3, spasticity, ataxia | +ARG |
| 7. NAGS: N-acetylglutamate synthetase | Mitochondria | 1:2,000,000 | AR | ↑NH3 may respond to NAG | +NH3 |
| 8. ORNT1: Ornithine transporter | Gene mutation | Membrane transporter | +NH3,+OR, +homocitrulline |
AR indicates autosomal recessive; ARG, arginine; AS, argininosuccinate; ASA, argininosuccinate acid; CIT, citrulline; EA, enzyme assay; NAG, N-acetylglutamate; NH3, ammonia; OA, orotic acid; OR, ornithine; UC, urea cycle; UOA, urine orotic acid; UR, uracil.
OTC deficiency has an estimated incidence of 1 in 82,000 live births in the USA and is the only X-linked urea cycle disorder (2). The biochemical and molecular bases of OTC deficiency have a wide spectrum of genetic defects, resulting in different phenotypes (7). Mutations that abolish all enzyme activity are found in the neonatal-onset group; mutations causing partial enzyme deficiency are found in the late-onset group (8). Among asymptomatic patients with late presentation, symptom onset is often associated with a precipitating factor, such as infection, trauma, medications (sodium valproate), surgery, pregnancy, excess protein intake, and physiological stress (9, 10). Patients with partial urea cycle disorders may exhibit psychological or cognitive difficulties, presumably due to chronic low-level hyperammonemia, before their presentation with a hyperammonemic crisis (11, 12). Some patients report self-selection of vegetarian diets, stating that high protein intake makes them sick (11). Due to its diverse phenotypic presentations and rarity in the adult population, hyperammonemic crisis resulting from a partial urea cycle disorder is probably underdiagnosed. An elevated serum ammonia level, particularly with normal liver enzymes and the absence of relevant medications, should suggest an inborn error of metabolism and prompt treatment of the hyperammonemic state followed by measurement of plasma amino acid and urine orotic acid levels.
There are case reports in which neuropsychiatric and/or neurodevelopmental disturbances represented the initial manifestations and main clinical signs of the disease. Mental retardation, attention-deficit hyperactivity disorder, oppositional defiant disorder, and problems in communication, such as autistic-like behavior, have been reported. Delirium, confusion, and incoherent speech can occur. Moreover, episodes of depressed consciousness were misdiagnosed in one series and erroneously interpreted as psychiatric disorders. These patients responded well to dietary modifications and ammonia-lowering therapy (13). Our patient had recent neuropsychiatric problems, including regression, lack of attention leading to job loss, and introverted behavior, but a formal psychiatric diagnosis was not available.
Due to the rarity of these disorders, most physicians have relatively little experience with management. Treatment for acute hyperammonemia should be started before the precise diagnosis is made to reduce production of nitrogenous waste and to lower plasma ammonia levels quickly. This is achieved by withdrawing all protein for 24 to 48 hours, supplying a hypercaloric, protein-free solution with insulin to enhance anabolism (14), infusing arginine, and exploiting alternative pathways for excretion of waste nitrogen. A loading dose of L-arginine hydrochloride, sodium benzoate, and sodium phenyl acetate is given intravenously over 90 minutes, followed by sustained infusion over 24 hours until the patient is no longer hyperammonemic and oral therapy can be tolerated. Hemodialysis should be considered in patients with persistently high ammonia levels (15).
References
- 1.Ah Mew N, Lanpher BC, Gropman A, Chapman KA, Simpson KL. Urea Cycle Disorders Consortium, Summar ML. Urea cycle disorders overview. 2003 Apr 29 [updated 2014 Sep 11] In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews® [Internet] Seattle, WA: University of Washington, Seattle; p. 1993–2015. Available at http://www.ncbi.nlm.nih.gov/books/NBK1217/; accessed April 8, 2015. [PubMed] [Google Scholar]
- 2.Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- 3.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105(1):e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- 4.Tuchman M, Jaleel N, Morizono H, Sheehy L, Lynch MG. Mutations and polymorphisms in the human ornithine transcarbamylase gene. Hum Mutat. 2002;19(2):93–107. doi: 10.1002/humu.10035. [DOI] [PubMed] [Google Scholar]
- 5.Kang ES, Snodgrass PJ, Gerald PS. Ornithine transcarbamylase deficiency in the newborn infant. J Pediatr. 1973;82(4):642–649. doi: 10.1016/s0022-3476(73)80590-6. [DOI] [PubMed] [Google Scholar]
- 6.McCullough BA, Yudkoff M, Batshaw ML, Wilson JM, Raper SE, Tuchman M. Genotype spectrum of ornithine transcarbamylase deficiency: correlation with the clinical and biochemical phenotype. Am J Med Genet. 2000;93(4):313–319. doi: 10.1002/1096-8628(20000814)93:4<313::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Tuchman M, McCullough BA, Yudkoff M. The molecular basis of ornithine transcarbamylase deficiency. Eur J Pediatr. 2000;159(Suppl 3):S196–S198. doi: 10.1007/pl00014402. [DOI] [PubMed] [Google Scholar]
- 8.Tuchman M, Morizono H, Rajagopal BS, Plante RJ, Allewell NM. The biochemical and molecular spectrum of ornithine transcarbamylase deficiency. J Inherit Metab Dis. 1998;21(Suppl 1):40–58. doi: 10.1023/a:1005353407220. [DOI] [PubMed] [Google Scholar]
- 9.Gordon N. Ornithine transcarbamylase deficiency: a urea cycle defect. Eur J Paediatr Neurol. 2003;7(3):115–121. doi: 10.1016/s1090-3798(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 10.Ellaway CJ, Bennetts B, Tuck RR, Wilcken B. Clumsiness, confusion, coma, and valproate. Lancet. 1999;353(9162):1408. doi: 10.1016/s0140-6736(99)01433-6. [DOI] [PubMed] [Google Scholar]
- 11.Summar ML, Barr F, Dawling S, Smith W, Lee B, Singh RH, Rhead WJ, Sniderman King L, Christman BW. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin. 2005;21(4 Suppl):S1–S8. doi: 10.1016/j.ccc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Lien J, Nyhan WL, Barshop BA. Fatal initial adult-onset presentation of urea cycle defect. Arch Neurol. 2007;64(12):1777–1779. doi: 10.1001/archneur.64.12.1777. [DOI] [PubMed] [Google Scholar]
- 13.Serrano M, Martins C, Pérez-Dueñas B, Gómez-López L, Murgui E, Fons C, García-Cazorla A, Artuch R, Jara F, Arranz JA, Häberle J, Briones P, Campistol J, Pineda M, Vilaseca MA. Neuropsychiatric manifestations in late-onset urea cycle disorder patients. J Child Neurol. 2010;25(3):352–358. doi: 10.1177/0883073809340696. [DOI] [PubMed] [Google Scholar]
- 14.Singh RH. Nutritional management of patients with urea cycle disorders. J Inherit Metab Dis. 2007;30(6):880–887. doi: 10.1007/s10545-007-0718-4. [DOI] [PubMed] [Google Scholar]
- 15.Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med. 2007;356(22):2282–2292. doi: 10.1056/NEJMoa066596. [DOI] [PubMed] [Google Scholar]