Abstract
Objective:
Treatment of osteochondral defects remains a challenge in orthopedic surgery. The TruFit plug has been investigated as a potential treatment method for osteochondral defects. This is a biphasic scaffold designed to stimulate cartilage and subchondral bone formation. The aim of this study is to investigate clinical, radiological, and histological efficacy of the TruFit plug in restoring osteochondral defects in the joint.
Design:
We performed a systematic search in five databases for clinical trials in which patients were treated with a TruFit plug for osteochondral defects. Studies had to report clinical, radiological, or histological outcome data. Quality of the included studies was assessed.
Results:
Five studies describe clinical results, all indicating improvement at follow-up of 12 months compared to preoperative status. However, two studies reporting longer follow-up show deterioration of early improvement. Radiological evaluation indicates favorable MRI findings regarding filling of the defect and incorporation with adjacent cartilage at 24 months follow-up, but conflicting evidence exists on the properties of the newly formed overlying cartilage surface. None of the included studies showed evidence for bone ingrowth. The few histological data available confirmed these results.
Conclusion:
There are no data available that support superiority or equality of TruFit compared to conservative treatment or mosaicplasty/microfracture. Further investigation is needed to improve synthetic biphasic implants as therapy for osteochondral lesions. Randomized controlled clinical trials comparing TruFit plugs with an established treatment method are needed before further clinical use can be supported.
Keywords: TruFit plug, synthetic scaffold, cartilage, osteochondral defect
Introduction
The treatment of articular osteochondral defects remains a challenge in orthopedic surgery. The goal is to regenerate hyaline articular cartilage with effective load transmission, long-term resistance to wear, joint lubrication, and nutrition.1 Frequently used treatment options are debridement, microfracture, osteochondral auto- or allografts, or cell-based techniques such as autologous chondrocyte implantation.2 However, studies indicate the formation of a fibrocartilaginous tissue that leads to secondary arthritis.3
Microfracture, a bone marrow stimulation technique, has shown good clinical results.4,5 However, intralesional osteophytes can occur6 and create inferior mechanical stability of the osteochondral tissue. Follow-up studies on osteochondral autologous transplantation (OATS procedure), also known as mosaicplasty, also demonstrate failure of integration of the transplanted cartilage and adjacent cartilage, with signs of degeneration of the transplanted hyaline cartilage.7 In addition, osteochondral autograft transfer is limited by autograft availability and donor-site morbidity.8 Another concept is cell-based technologies, which include autologous chondrocyte implantation and matrix-induced autologous chondrocyte implantation. These involve two-staged operative procedures and are reserved for larger lesions and as a second-line treatment.5 These cell-based techniques are expensive and time consuming, although their superiority over microfracture has not been shown in smaller lesions.9,10
The TruFit Plug (Smith & Nephew, San Antonio, TX) has been used as a treatment method for primary osteochondral defects or for gap filling of donor sites during OATS procedures. The TruFit plug is a synthetic, acellular scaffold and is predominantly made from a polylactide-coglycolide copolymer. The scaffold consists of two “phases”. The bone phase contains calcium sulfate for stimulation of bone formation. Cartilage regeneration is instigated by the integration of cells and growth factors derived from the bone marrow that infiltrates the plug. Synthetic scaffolds such as the TruFit plug offer a number of potential benefits over traditional treatment options. The combination of marrow stimulation together with structural support can offer a benefit over microfracture. In the latter technique, bone marrow stem cells migrate in the fibrin network of a blood clot, but this “fibrin clot” is not mechanically stable enough to withstand tangential forces.11 The structural support property of a scaffold plug should prevent this problem. There is no donor-site morbidity as seen in the OATS procedure, and it requires only a single procedure instead of two-staged procedures for autologous chondrocyte implantation.
Williams and Gamradt2 examined the efficacy of this scaffold in defects in the femoral condyles and trochleae of goats. Gross observation showed good filling of osteochondral defects, good integration in the native cartilage, and histological observation showed a high percentage of hyaline-like cartilage and good bony restoration. The US Food and Drug Administration has approved this synthetic plug as an alternative treatment to backfill donor sites after an OATS procedure. Originally, the plug was designed for this purpose, but in Europe it has also been used for the treatment of acute focal articular cartilage or osteochondral defects.1,2,12
The aim of this study is to investigate the clinical, radiological, and histological efficacy of the TruFit plug in restoring osteochondral defects in the human joint, by performing a systematic review of clinical studies concerning the TruFit Plug.
Methods
Data Search Protocol
A systematic literature search of Embase (Embase and Medline), Medline (OVID-SP), Cochrane Central, Web of Science, and PubMed databases was performed for studies published up to September 2013. Main search items were TruFit plug, synthetic or polymer biphasic plug or scaffold, osteochondral defects.
The complete search strategy is shown in Supplementary Table 1. Additionally, reference lists of the selected articles were screened for further publications. Finally, additional data were acquired of one of the included studies (Hindle et al.13) after correspondence with the first author of this article via e-mail.
Study Selection and Eligibility Criteria
Articles were screened independently by their title and abstract by two observers. In case of disagreement, articles were discussed until agreement was reached.
Based on the following eligibility criteria, a selection was made:
Article written in English, French, Dutch, or Spanish
Full text had to be available
Human randomized controlled trials (RCT), clinical trials, or case series (n > 5)
Case reports, editorials, systematic reviews, and meta-analysis were excluded
Study subjects were patients treated with TruFit plug for osteochondral articular defects or gap filling of donor sites
Studies had to report clinical, histological, or radiological outcome data
Original postoperative data had to be available
Assessment of Quality
Methodological quality of the clinical studies was assessed using the PEDro Critical Appraisal Tool. This is a validated tool for quality assessment of clinical trials. It consists of 11 questions regarding recruitment, allocation, blinding, and data analysis aspects of clinical trials. Two observers independently assessed these criteria for each included study. Disagreements were solved in a single consensus meeting.
Data Extraction, Synthesis, and Analysis
Data were extracted by one observer and checked by a second observer. Data regarding the clinical outcome and radiological and histological information after the placement of a TruFit plug were extracted.
General information was collected about the study groups, such as age, gender, localization of the osteochondral defect, mean defect size, gradation of the defect, and number of used implants. Results from the early postoperative period (<6 months), intermediate postoperative period (6-24 months), and, if available, long-term follow-up results (>24 months) were gathered. Radiological information about defect filling, integration of newly formed cartilage with the adjacent cartilage, the cartilage surface quality, and the properties of the subchondral bone was extracted. If present, histological results after TruFit procedure were gathered and described.
Results
Characteristics and Methodological Quality of the Included Studies
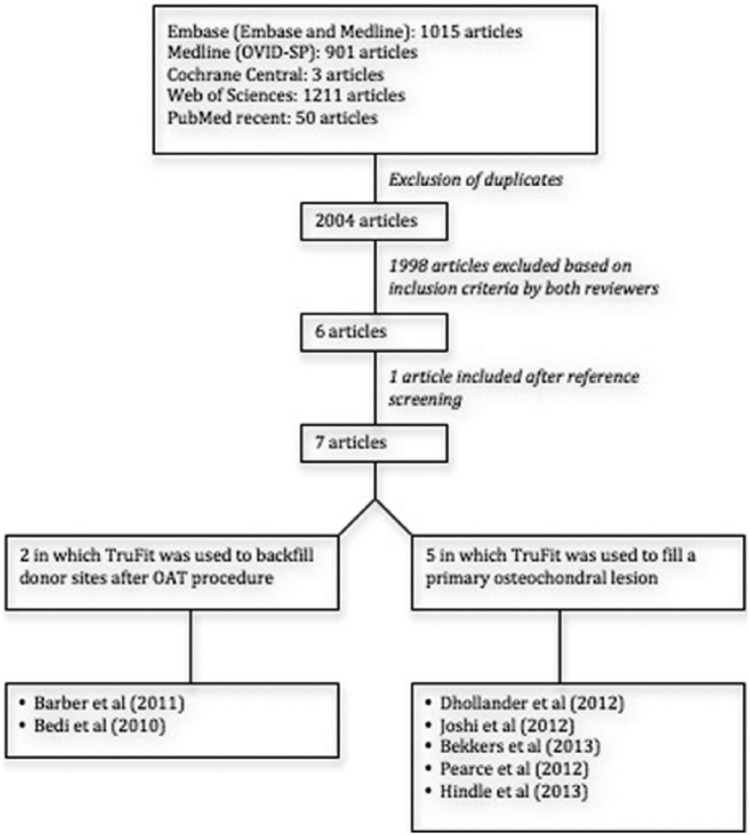
The initial search resulted in 2004 articles, of which 6 articles were selected based on the eligibility criteria. One article was added after reference screening of the included articles (Fig. 1).
Figure 1.
Study selection: Flow chart
A summary of the quality assessment results is presented in Supplementary Table 2. Only one included study13 attempted to compare with a control group or gold standard. However, quality assessment of this study was poor, and therefore, we gathered only information in the TruFit plug group. All studies were therefore considered observational studies with high risk of bias.
Patient characteristics of the included studies are shown in Table 1. The correspondence with the first author of Hindle et al.13 has resulted in adding the gender distribution and the distribution of defect localization in the TruFit group.
Table 1.
Study Group Description.
| Number of Treated Patients | Number of Dropouts | Age | Gender | Localization | Mean Defect Size | Gradation | Indication TruFit Plug | Number of Implants | Follow-Up Period | |
|---|---|---|---|---|---|---|---|---|---|---|
| Joshi et al.15 | 10 | 2 (at 12 mo FU) | 33.6 yr (17-49 yr) | 4 males, 6 females | Patella | 2.64 cm2 (1-5 cm2) | Outer bridge grade III or IV | Primary OC defects | 2 (1-4) | 24 mo |
| Dhollander et al.11 | 20 | 5 (at 12 mo FU) | 31.65 yr (17-53 yr) | 8 males, 12 females | 8 MFC, 4 LFC, 5 patella, 3 trochlea | 0.83 cm2 (0.38-1.58 cm2) | International Cartilage Repair Society grade III or IV | Primary OC defects | 17 with 1 plug, 3 with 2 plugs | 12 mo |
| Bekkers et al.16 | 13 | None | 32 ± 8 yr | Not reported | 7 MFC, 6 LFC | 1.9 ± 0.7 cm2 | Not reported | Primary OC defects | 4 with 1 plug, 6 with 2 plugs, 3 with 3 plugs | 12 ± 4 mo |
| Pearce et al.14 | 6 | None | Not reported | 5 males, 1 female | 5 medial talar dome, 1 distal tibia | Not reported | Not reported | Primary OC defects | Not reported | 12 mo |
| Hindle et al.13 | 35 | Not reported | 38.6 ± 13.3 yr | 23 males, 12 females | 32 MFC, 2 LFC, 1 trochlea | Not reported | Not reported | Primary OC defects | 2 or 3 | 22 ± 8.6 mo |
| Bedi et al.17 | 26 | Not reported | 28.72 yr (11-56 yr) | Not reported | Medial or lateral trochlear margin | Not reported | Not reported | Donor sites in OATS | 2 (1-5) | 21.3 mo (6-39 mo) |
| Barber et al.8 | 20 | 11 | 40 yr (26-58 yr) | 8 males, 1 females | Not reported | Not reported | Not reported | Donor sites in OATS | Not reported | Not reported |
FU = follow-up; MFC = medial femur condyle; LFC = lateral femur condyle; OC = osteochondral.
Clinical Outcome
Five included studies report clinical outcome after TruFit implantation as treatment of an osteochondral defect. As summarized in Table 1, study groups are in general similar regarding age, mean defect size, and defect gradation. There are differences in localization of the defect, some studies report the treatment of an osteochondral defect in patella, others in medial or lateral femoral condyles or in the trochlea. One study investigated the use of the TruFit plug for osteochondral defects in the ankle.14
Clinical Outcome in the Intermediate Postoperative Period (6-12 months Follow-Up)
As summarized in Table 2, all included studies show some form of improvement in clinical outcome at 12-month follow-up, compared to the preoperative status.
Table 2.
Clinical Outcome.
| Postoperative Complications | Intermediate Postoperative Period (12 mo) | Longer Postoperative Period (24 mo) | Revision Surgery | Number of Dropouts | |
|---|---|---|---|---|---|
| Joshi et al.15 | None | Improvement | Worsening | 70% at 24-m0 FU | 20% |
| Dhollander et al.11 | None | Modest improvement | Not reported | 20% at 12-mo FU | 25% |
| Bekkers et al.16 | None | Improvement | Not reported | None | None |
| Pearce et al.14 | None | Improvement | Not reported | None | None |
| Hindle et al.13 | One patient with a suspected infection | Improvement | Improvement | 25% at 22 ± 8.6 mo FU | Not reported for TruFit group |
FU = follow-up.
Joshi et al.15 reported improvement in 80% of the patients. Patients did not improve because of plateau fracture (10%), or a bone patellar fissure and a large cartilage injury (10%). The improvement was described either as excellent or good self-satisfaction of the patients, or as improvement in clinical outcome scores. A validated knee-specific scoring system was used, Knee Injury and Osteoarthritis Score (KOOS); a psychometric response scale for pain evaluation, Visual Analog Scale (VAS); and a health survey scale, Short Form 36 (SF-36). All these clinical outcome scores improved at 12-month follow-up compared with preoperative values.
Bekkers et al.16 had improvement in 85% of the patients, and they stated that mild knee complaints from the 15% nonsatisfied patients were probably not related to the implantation of the TruFit plug. No clinical outcome scores were used, but 85% of the patients were pain free and had full range of motion at maximum follow-up (12 ± 4 months).
Dhollander et al.11 reported modest improvement for 80% of the patients in clinical outcome at 12-month follow-up and 20% that showed persistent symptoms, which did not improve over time. This is the only study that reports clinical failure and the need for revision surgery at 12-month follow-up. The modest clinical improvement is defined as a modest improvement of the VAS score, a significant improvement in total KOOS and in all KOOS subdomain scores, and no observation of difference in the Tegner activity scale during 12 months of follow-up.
Pearce et al.14 had 100% satisfied patients and improvement of clinical outcome scores in all patients, although not all scores improved significantly. The American Orthopedic Foot and Ankle Society (AOFAS) Hindfoot score, the Ankle Osteoarthritis Scale (AOS), and SF-36 health survey were used. AOFAS and AOS disability improved significantly, AOS pain and SF-36 improved, but not significantly.
Clinical Outcome in Long-Term Follow-Up (16-24 months Follow-Up)
Further follow-up shows worsening of the clinical outcome scores because of pain and loss of knee function. Joshi et al.15 reported a follow-up of patients over a longer period than 12 months. In contrast to the 80% satisfied patients at 12-month follow-up, only 30% of the patients were still satisfied at 18 months, and no more than 10% at 24 months. Because of persistent pain and decrease of joint function, revision surgery was needed for 70% of the patients. Furthermore, 20% of the patients dropped out at 12-month follow-up.
Hindle et al.13 compared the clinical outcome of patients undergoing mosaicplasty and patients undergoing TruFit placement. The study described the improvement of clinical outcome scores after a mean follow-up period of 22 months (±8.6 months) in the TruFit group. It also compared the results with mosaicplasty and found better results for the mosaicplasty group. Patients undergoing mosaicplasty also returned earlier to their old sports activity level. A few important factors, such as defect localization, gender, and number of dropouts, were not separately mentioned for the TruFit group and the mosaicplasty group.
Radiological Evaluation
Filling of the Defect and Integration with Adjacent Cartilage
Radiological findings are summarized in Table 3. Dhollander et al.11 examined patients at 6 and 12 months of follow-up with MRI. In the early postoperative period (6-month follow-up), 61% of the patients showed complete filling of the defect. These results worsened during the intermediate postoperative period. Only 43% had complete filling of the defect at 12-month follow-up. None of the patients had a complete integration of the plug with adjacent cartilage either at 6-month follow-up or at 12-month follow-up.
Table 3.
Radiological Results.
| Early Postoperative Period (<6 mo) | Intermediate Postoperative Period (12 mo) | Longer Postoperative Period (16-24 mo) | |
|---|---|---|---|
| Filling of the defect | Mostly complete11,17 | Worsening results11,17 | Complete15,17 |
| Integration to border zone | Incomplete11,17 | Incomplete11,17 | Mostly complete15,17 |
| Properties of cartilage surface | Conflicting evidence8,11,14,15,17 | Conflicting evidence8,11,14,15,17 | Conflicting evidence8,11,14,15,17 |
| Subchondral bone | Not intact8,11,14,15 | Not intact8,11,14,15 | Not intact8,11,14,15 |
These results were confirmed by the study of Bedi et al.17 They evaluated patients that underwent the OATS procedure and had their donor sites backfilled with TruFit plugs. The favorable results at 6-month follow-up regarding the filling of the defect and the integration to the border zone worsened during the intermediate follow-up period. At 6 months, Bedi et al.17 had 78% patients with a complete filling of their defect, and at 12 months, only 52% of their patients had complete filling of the defect. Almost no patients had complete integration to the border zone at 6-month follow-up and at 12-month follow-up.
In the longer postoperative interval (16-24 months following surgery), Joshi et al.15 and Bedi et al.17 again found re-improvement of the radiological findings. There was complete filling of the defect in 90% of the patients in both studies and good integration to the border zone.
Cartilage Surface
Conflicting evidence was found evaluating the properties of the cartilage surface after TruFit plug placement (Table 3).
Joshi et al.15 described lesions of the surface in overlying predominant hyaline cartilage due to fibrillations and fissures at 24-month follow-up. Dhollander et al.11 also described a damaged surface due to fibrillations, fissures, and ulcerations on MRI at 12-month follow-up, but they did not mention which properties the cartilage surface had.
Pearce et al.14 performed an MRI at 12-month follow-up. They suggested a fibrous rather than a hyaline cartilage composition, because the qualitative T2 maps showed a disorganized pattern of T2 signal from the deep to superficial zones of the cartilage portion of the plug.
Also in the studies on patients that were treated with TruFit for backfilling of donor sites after OATS procedure, conflicting evidence was found regarding the properties of cartilage surface tissue.
A study performed by Barber et al.8 on nine patients that underwent the OATS procedure, with the donor sites backfilled with a TruFit plug, evaluated the patients with computed tomography (CT) over an interval of 2 to 63 months after surgery. They stated that any superficial soft tissue formation is most likely fibrous scar. In the study by Bedi et al.,17 the T2 relaxation times in the later follow-up period (>16 months postoperative) approached these of native hyaline cartilage, which suggest a collagen orientation more typical of hyaline cartilage.
Subchondral Bone
There was no evidence found to support osteoconductive bone ingrowth in any of the included studies (Table 3). Joshi et al.,15 Dhollander et al.,11 Pearce et al.,14 and Barber et al.8 only found bone edema, sclerosis, granulation tissue, and a cyst instead of subchondral bone ingrowth.
Histology
The few histology results available confirmed the findings in the radiological evaluation (Table 4).
Table 4.
Histological Results.
| Intermediate Postoperative Period (Dhollander et al.11) | Longer Postoperative Period (Joshi et al.15) | |
|---|---|---|
| Filling of the defect | Good filling of the defect | Not reported |
| Properties of cartilage surface | Fibrous vascularized tissue | High percentage of hyaline cartilage |
| Subchondral bone | Not reported | Bony cyst instead of bone ingrowth |
Dhollander et al.11 reoperated on three patients at 12-month follow-up because of persistent symptoms. Macroscopic evaluation showed good filling of the defect and no fissures in the underlying bone, nor ulcerations. Histological assessment on the biopsy specimen showed a vascularized and disorganized extracellular matrix of the repair tissue with the abundant presence of fibroblasts.
Joshi et al.15 revised seven patients because of persistent pain symptoms and loss of knee function at 24-month follow-up. A histological examination has been performed after implant removal at the time of revision surgery. Macroscopic evaluation showed soft tissue in the upper layer and a cyst in the deeper layer. Histological evaluation confirmed that the regenerated surface had a high percentage of hyaline cartilage, but a bony cyst was found instead of bony restoration.
It has to be noted that histological assessment was performed on patients who underwent revision surgery.
Discussion
We performed a systematic review of literature concerning the use of TruFit plugs to treat osteochondral defects in humans. This review was based on a very broad search strategy that was carried out in all relevant medical databases. Studies were assessed for quality, and all available data were extracted and summarized in a standardized way. Although the different study groups used different assessment tools for clinical outcome, all groups report improvement in clinical outcome in the intermediate postoperative period when comparing to preoperative status.11,13-16 However, these study groups were not compared to a control group, in which an improvement can be expected in the natural history after an acute trauma with an osteochondral lesion.18 Hindle et al.13 describe the only attempt to compare with a mosaicplasty, and they indicate improvement of clinical outcome scores at 22 months (±8.6 months) compared to preoperative status. They report less improvement compared to patients treated with mosaicplasty. Joshi et al.15 also describe a longer follow-up period of 24 months, with worsening of clinical outcome in almost all included patients. Carmont et al.,12 who reported a case of delayed incorporation of an articular cartilage defect treated with TruFit plugs, claim that alleviation and resumption of functional activity after 24 months of continued rehabilitation can still be expected. This is, however, contradicted by the study of Joshi et al.15
A summary of radiological findings shows favorable MRI findings at 6-month follow-up regarding filling of the defect and plug incorporation in the adjacent cartilage.11,15,17 These findings deteriorate in the intermediate postoperative period and improve again in a longer follow-up period. No studies found evidence for sufficient subchondral bone ingrowth, and conflicting evidence exists on the properties of newly formed cartilage. The histological results confirm these radiological findings, although bias may exist because histological examination could only be performed on clinical failures. MRI is easier to perform on all patients, but also has drawbacks, such as difficulties to interpret the actual properties of the newly formed cartilage.
Joshi et al.15 imputed the early clinical improvement of their patients to the formation of predominant hyaline cartilage during the first 12 months, which partially restored the cartilage injury. Then, radiological and histological data indicated a deterioration of the newly formed cartilage. This could be explained by the lack of subchondral bone formation, which is shown in different studies. It is probably crucial for the newly formed cartilage to achieve mechanical characteristics that match those of native cartilage.15 As the bone formation is poor after treatment with a TruFit plug, its use in osteochondral repair is questionable. Even more, a deep lesion is made in the subchondral bone, which makes revision surgery more difficult. Future designs of synthetic biphasic scaffolds should focus further on establishing subchondral bone that has the biomechanical and structural potential to support cartilage formation.19
We only included seven articles in this review. It is likely that a negative publication bias exists. Furthermore, during our search, we found an AAOS Instructional Course Letter by Williams and Gamradt, in which the authors mention good results with the use of TruFit plug in 100 patients. However, these results were never published and no data can be found concerning this prospective observational study. Therefore, we were not able to include this study. Ideally, randomized controlled clinical trials should be performed that compare TruFit plug with one of the established techniques, such as microfracture or OATS procedure, in lesions similar in size and location, and with no prior surgery or associated procedures. So far, it is unclear how the clinical evolution is compared to the traditional treatment strategies. Only one study compared the TruFit technique with an established technique, the OATS procedure.13 This was a retrospective analysis, without randomization and without prescriptive protocol or clear inclusion criteria for patients.
Because the included clinical trials described rather small groups, even with a control group it would be difficult to gain useful definitive data.
Study groups were in general similar regarding age, mean defect size, defect gradation, and number of implants used. However, the studies included in this review had different methodological designs, inclusion criteria, aims, and used different clinical and radiological assessment tools, making meta-analysis of results impossible. There were large differences in localization (different joints, different location within joint) that could affect the outcome of the treatment. The quality and quantity of new tissue development not only depends on the characteristics of the implant but also on the biological environment, such as the blood supply or weight bearing function. Besides the use of the TruFit plug in the knee or ankle joint, one case report by Vundelinckx et al.20 indicates that it is technically feasible to use the TruFit plug also in the hip.
Conclusion
This review describes the current available evidence for the treatment of osteochondral defects with a TruFit plug. These data do not support superiority of the TruFit plug in terms of clinical improvement at follow-up compared to conservative treatment or other cartilage techniques. The aim of this biphasic scaffold is to regenerate both hyaline cartilage formation and subchondral bone ingrowth, but conflicting evidence exists on the properties of the newly formed cartilage, and none of the studies could provide evidence for osteoconductive bone ingrowth. Further in vitro and in vivo works are needed to improve synthetic biphasic implants as therapy for osteochondral lesions. Well-designed, large-scale, randomized controlled trials are needed to investigate the value of future synthetic biphasic plug before it can be implemented in clinical practice.
Supplementary Material
Supplementary Material
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Acknowledgments and Funding: The authors thank Gerdien B. De Jonge for her support during the extensive search in medical databases. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study did not require an ethical approval.
References
- 1. Melton JTK, Wilson AJ, Chapman-Sheath P, Cossey AJ. TruFit CB bone plug: chondral repair, scaffold design, surgical technique and early experiences. Expert Rev Med Devices. 2010;7(3):333-41. [DOI] [PubMed] [Google Scholar]
- 2. Williams RJ, Gamradt SC. Articular cartilage repair using a resorbable matrix scaffold. Instr Course Lect. 2008;57:563-71. [PubMed] [Google Scholar]
- 3. Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-60. [DOI] [PubMed] [Google Scholar]
- 4. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 5. Bekkers JEJ, Inklaar M, Saris DBF. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(1 suppl):1485-555. [DOI] [PubMed] [Google Scholar]
- 6. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tibesku CO, Szuwart T, Kleffner TO, Schlegel PM, Jahn UR, Van Aken H, et al. Hyaline cartilage degenerates after autologous osteochondral transplantation. J Orthop Res. 2004;22(6):1210-4. [DOI] [PubMed] [Google Scholar]
- 8. Barber FA, Dockery WD. A computed tomography scan assessment of synthetic multiphase polymer scaffolds used for osteochondral defect repair. Arthroscopy. 2011;27(1):60-4. [DOI] [PubMed] [Google Scholar]
- 9. Wasiak J, Clar C, Villaneuva E. Autologous cartilage implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2006;(3):CD003323. [DOI] [PubMed] [Google Scholar]
- 10. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-64. [DOI] [PubMed] [Google Scholar]
- 11. Dhollander AAM, Liekens K, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, et al. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy. 2012;28(2):225-33. [DOI] [PubMed] [Google Scholar]
- 12. Carmont MR, Carey-Smith R, Saithna A, Dhillon M, Thompson P, Spalding T. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy. 2009;25(7):810-4. [DOI] [PubMed] [Google Scholar]
- 13. Hindle P, Hendry JL, Keating JF, Biant LC. Autologous osteochondral mosaicplasty or TruFit plugs for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1235-40. [DOI] [PubMed] [Google Scholar]
- 14. Pearce CJ, Gartner LE, Mitchell A, Calder JD. Synthetic osteochondral grafting of ankle osteochondral lesions. Foot Ankle Surg. 2012;18(2):114-8. [DOI] [PubMed] [Google Scholar]
- 15. Joshi N, Reverte-Vinaixa M, Diaz-Ferreiro EW, Dominguez-Oronoz R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am J Sports Med. 2012;40(6):1289-95. [DOI] [PubMed] [Google Scholar]
- 16. Bekkers JEJ, Bartels LW, Vincken KL, Dhert WJA, Creemers LB, Saris DBF. Articular cartilage evaluation after TruFit plug implantation analyzed by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Am J Sports Med. 2013;41(6):1290-5. [DOI] [PubMed] [Google Scholar]
- 17. Bedi A, Foo LF, Williams RJ, III, Potter HG. The maturation of synthetic scaffolds for osteochondral donor sites of the knee. An MRI and T2-mapping analysis. Cartilage. 2010;1(1):20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cain EL, Clancy WG. Treatment algorithm for osteochondral injuries of the knee. Clin Sports Med. 2001;20(2):321-42. [DOI] [PubMed] [Google Scholar]
- 19. Guzman-Morales J, Lafantaisie-Favreau CH, Chen G, Hoemann CD. Subchondral chitosan/blood implant-guided bone plate resorption and woven bone repair is coupled to hyaline cartilage regeneration from microdrill holes in aged rabbit knees. Osteoarthritis Cartilage. 2014;22(2):323-33. [DOI] [PubMed] [Google Scholar]
- 20. Vundelinckx B, De Mulder K, De Schepper J. Osteochondral defect in femoral head: Trufit implantation under fluoroscopic and arthroscopic control. Acta Orthop Belg. 2012;78(6):796-9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.