Abstract
Objective
To compare the outcome of matrix-induced autologous chondrocyte implantation (MACI) and bone marrow aspirate concentrate (BMAC)–derived multipotent stem cells (MSCs) implantation in patellofemoral chondral lesions, using the same HYAFF11 scaffold.
Methods
From January 2005 to December 2010, 37 patients with patellofemoral chondral lesions were prospectively followed up, for a minimum of 3 years; 19 of these patients were treated with MACI and 18 with BMAC. Radiographs, magnetic resonance imaging, and clinical scores (International Knee Documentation Committee, Knee Injury and Osteoarthritis Outcome Score, visual analog scale, and Tegner) were collected preoperatively, at 2-year and final follow-up. Five patients of MACI and 6 of the BMAC group underwent second-look arthroscopy; 4 patients of each group consented to a concomitant biopsy.
Results
No adverse reactions or postoperative infections were noted. Baseline characteristics were similar in both groups (P > 0.05). Both groups showed significant improvement in all scores, from preoperative to final follow-up (P = 0.001), but there was no significant difference in improvement between the 2 groups, except for the IKDC subjective score (P = 0.015), which favored the BMAC group. Deterioration in MACI and improvement in BMAC group scores were noticed, from 2-year to final follow-up, but was nonsignificant. MACI patients with trochlear lesions showed better results than patellar lesions, while location was not a prognostic factor in the BMAC group. MRI showed complete filling of the defects in 76% of patients in MACI and 81% of patients in BMAC, and histological analysis revealed hyaline-like features.
Conclusion
Both techniques are viable and effective for large patellofemoral chondral lesions at minimum 3-year follow-up.
Keywords: matrix-induced autologous chondrocyte implantation (MACI), multipotent stem cell (MSC), bone marrow aspirate concentrate (BMAC), HYAFF11, patellofemoral chondral defects
Introduction
Patellofemoral chondral lesions are difficult-to-treat entities that often affect young and active patient population and often remain challenging, especially large full-thickness lesions.1-4 Moreover, cartilage has limited intrinsic healing potential because of the fact that it is isolated from systemic regulation and lacks vessels and nerve supply, thus contributing to healing difficulties. Over the years, autologous chondrocyte implantation (ACI) has been established as a good treatment option to deal with large full-thickness chondral lesions.5,6 The first-generation ACI technique was complex, required periosteal tissue harvest and a meticulous sewing of the patch over the defect to ensure a “water-tight” closure preventing spillage of the chondrocytes7; furthermore, it was associated with donor site morbidity due to periosteal patch retrieval.5,7 Second-generation ACI was introduced when periosteum was exchanged with a resorbable membrane out of collagen. The evolution of this technique represents the third-generation ACI that consists of either chondrocytes grown on scaffold carriers or within a porous matrix.8 The third generation ACI was named matrix-induced autologous chondrocyte implantation (MACI) for its recourse to a matrix seeded with chondrocytes.8,9 However, the utilized term MACI in current study should be distinguished from the MACI product, which is a commercial name of a membrane.
The use of a 3-dimensional scaffold for chondrocyte culture was developed with the joint aim of improving the biological performance of chondrogenic autologous cells and also to simplify surgical technique as it avoids the use of the periosteal flap.10 Previous studies1,9 showed good results with various scaffolds in patellofemoral chondral lesions, improvement at medium-term follow-up with a repair tissue similar to normal cartilage, which was stable mechanically and histologically.
Hyaluronic acid (HA) is a naturally occurring molecule present in all soft tissues that plays an essential role in the maintenance of the normal extracellular matrix structure. The use of this molecule in cartilage lesion treatment has been shown to provide good results, since it favors the formation of new cartilage tissue.1,2 Scaffolds based on HYAFF 11, a derivative of HA, has shown to induce successful tissue-engineered repair of cartilage. MACI technique provides a user-friendly application of graft and shorter surgical time as there is no need for periosteal tissue harvest; however, MACI still requires 2 surgical procedures.1,2,9,13 In this approach, characterized viable autologous chondrocytes expanded in vitro were seeded and cultured on the HYAFF 11 nonwoven scaffold for implantation.1,2,9,11-13
Research is now progressing toward the possibility of performing a single-step procedure. In this regard, the use of bone marrow aspirate concentrate (BMAC), which contains multipotent stem cells (MSCs) and growth factors, could represent a possible solution.14,15 MSCs are a suitable option to repair cartilage defects because of their differentiation potential toward different tissues, including cartilage.14-16 Several studies have already demonstrated their ability to produce cartilage both in vitro17 and in animal models.18,19 Easy availability, coupled with the self-renewal capacity and multilineage differentiation potential of MSCs to cartilage tissue, offers a promising option in cartilage surgery.20,21 MSC interaction with a nonwoven scaffold was found suitable for tissue repair; the HYAFF 11 scaffold supported the adhesion, migration and proliferation of MSCs, as well as the synthesis and delivery of extracellular matrix components under static culture conditions.22-25 Additionally, the specific ability of MSCs on the HYAFF 11 scaffold to differentiate into chondrocytes was made evident by the expression and production of specific extracellular matrix molecules.26 Unlike MACI, MSC isolation does not require healthy cartilage tissue harvesting and thus bypasses the first surgical step required for cartilage biopsy and subsequent chondrocyte cultivation. Recent studies26-28 have reported that 1-step technique with bone marrow-derived MSC implantation could be an efficient alternative for cartilage repair, permitting marked improvement of functional scores and hyaline-like cartilage repair, while overcoming the drawbacks of previous techniques.
The purpose of the present study was to compare the clinical outcomes of 2 similar groups of patients with patellofemoral full-thickness cartilage lesions, treated with MACI or BMAC, employing the same scaffold. To our knowledge, this is the first study that reports such a comparison. Our hypothesis was that MSC implantation using the same matrix could be as effective as the MACI technique with chondrocytes cultivation.
Materials and Methods
From January 2005 to December 2010, 67 patients were treated in our institute for full-thickness patellofemoral chondral defects; amongst them 37 fulfilled the criteria of this study and were prospectively followed up: 19 patients were treated with MACI with HYAFF11 as a seeded scaffold and 18 underwent BMAC surgery with HYAFF11 as nonseeded scaffold (1-step surgery). Ten of the patients of the MACI group were previously assessed in another study.
The inclusion criteria of this study were age between 30 and 60 years and body mass index (BMI) between 20 and 30 kg/m2; patients with grade 4 cartilage lesions as per the International Cartilage Repair Society (ICRS) classification of patella or trochlea, with size ≥4 cm2; clinical symptoms of pain, swelling, locking or giving way; patients treated by a mini-arthrotomy approach with the same hyaluronan scaffold. In case of coexisting knee pathologies such as tibiofemoral axial malalignment, patellofemoral maltracking, and ligamentous insufficiency, they were treated during the same surgical procedure. The exclusion criteria were uncorrected malalignment, ligament insufficiency or patellofemoral maltracking; deep osteochondral lesions requiring bone grafting; tricompartmental arthritis, osteonecrosis; patients with other general medical conditions (diabetes, rheumatoid arthritis, etc); multiple, recent (<3 months) intra-articular injections with steroids; deformity or osteoarthritis at ipsilateral and contralateral hip, knee, or ankle joints; noncompliance to follow our rehabilitation protocol.
Functional evaluation was performed using various scoring systems. Visual analog scale (VAS) for pain (0 = no pain, 10 = worst pain), International Knee Documentation Committee (IKDC),29,30 Knee injury and Osteoarthritis Outcome Score (KOOS),31 and Tegner32 scores were documented preoperatively, at 2-year and at final follow-up. Radiographic and magnetic resonance imaging (MRI) results were collected preoperatively, at 2-year and final follow-up. Standard radiographic evaluation included standing anteroposterior (AP) long-leg views, including hips and ankles, standing AP/lateral views of the knee, skyline patellofemoral views, and standing views with the knee bent at 45°. Two independent musculoskeletal radiologists who were blinded to the clinical history of the patients evaluated the MRI scans based on system developed by Henderson et al.33 Features of the graft that were assessed included the extent of filling of the defect by repair tissue, integration of the graft with native cartilage to the subchondral bone, and presence of edema or cysts. The extent of under filling or hypertrophy of the graft in the defect and changes on the articular surface were compared between the preoperative and final MRI findings.
All the procedures were performed by the senior author and all patients followed the same 4-phase rehabilitation protocol.1,28 All patients gave informed consent prior to inclusion in the study. The study was conducted with the principles of the Declaration of Helsinki (World Medical Association) and approved by our institutional ethics committee.
Procedure in the MACI Group
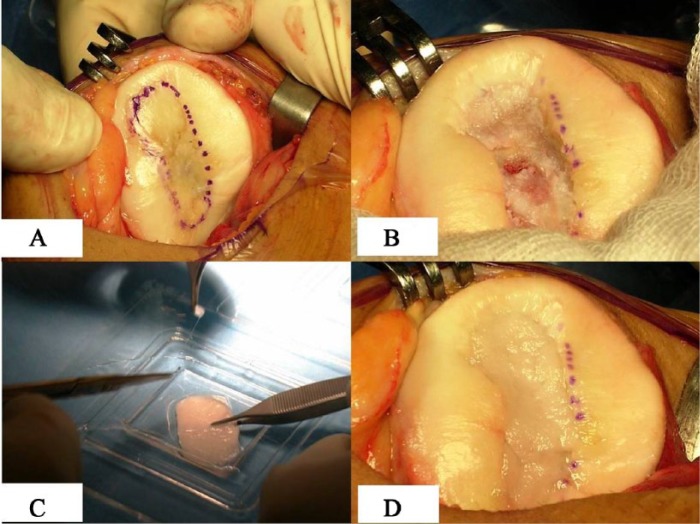
Patients underwent the standard 2-step surgical technique which has already been described1,9; cartilage biopsies were obtained from the minimally weightbearing portions of the knee (intercondylar notch) and the biopsy material was sent to the laboratory for in vitro isolation and expansion of autologous chondrocytes. Subsequently, the cells were seeded into the HYAFF 11 scaffold (Hyalograft C scaffold, Anika Therapeutics Srl, Abano Terme, Italy) for 2 weeks. At a second stage, implantation was carried out through a mini-arthrotomy approach (Figs. 1A-D).
Figure 1.
Matrix-induced autologous chondrocyte implantation (MACI) procedure: (A) outlined patellar lesion, (B) prepared bed of lesion in patella, (C) graft templating with cultured chondrocytes, (D) graft implantation.
Procedure in BMAC group
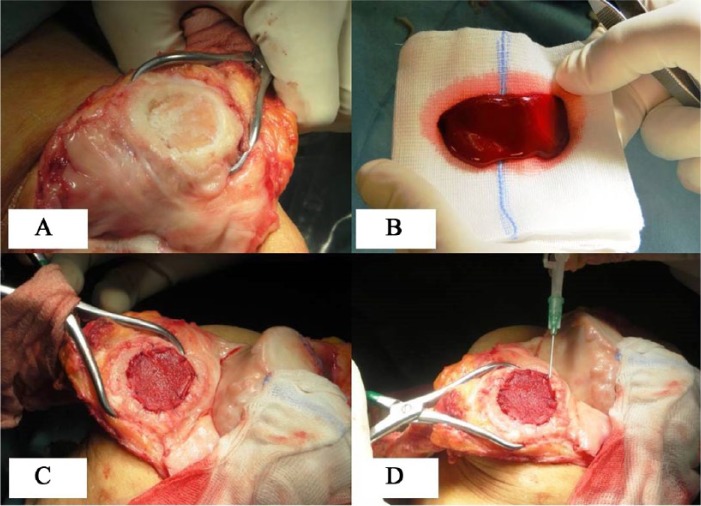
Arthroscopic evaluation and debridement were initially performed to assess the condition of the joint and to confirm the MRI findings with regard to the size and location of cartilage defects. Subsequently, approximately 60 mL of bone marrow was harvested from the ipsilateral iliac crest using a dedicated aspiration kit and centrifuged using a commercially available system (BMAC Harvest Smart PreP2 System, Harvest Technologies, Plymouth, MA) to obtain a concentration of bone marrow cells 4 to 6 times the baseline value. With the use of the Batroxobin enzyme (Plateltex Act, Plateltex SRO, Bratislava, Slovakia), the bone marrow concentrate was activated to produce a sticky clot material. The knee was then approached with mini-arthrotomy and the chondral defects were prepared in a similar way as described before in the MACI group procedure (Fig. 2A and B). A 3-dimensional HYAFF 11 scaffold (Hyalofast, Anika Therapeutics, Srl, Abano Terme, Italy) was fashioned according to the defect size. The prepared clot was then implanted into the prepared cartilage defect and covered with a presized scaffold (Fig. 2C), which was anchored to the surrounding cartilage using a polydioxanone suture (PDS II 6-0, Ethicon, Somerville, NJ) and sealed with fibrin glue (Tissucol, Baxter Spa, Rome, Italy) (Fig. 2D).
Figure 2.
Bone marrow aspirate concentrate (BMAC) implantation: (A) prepared bed of lesion in patella, (B) BMAC clot after activation, (C) clot pasted and scaffold stabilized, (D) fibrin seal.
Second-Look Arthroscopy and Biopsy
A second-look arthroscopy was performed in patients of the groups who underwent surgical treatment on the same knee for hardware removal or gave their consent while undergoing surgical procedure in the contralateral knee. During the procedures, the grafts were inspected and evaluated using the ICRS cartilage repair assessment scoring system (degree of defect fill, graft integration to adjacent normal articular surface, and gross appearance of the graft surface). Samples were then sent for histological and immunohistochemical analyses for collagen type; the assessors of the histologies were blinded to the utilized technique. Frozen sections of the specimens were stained with hematoxylin and eosin for general histological analysis. Safranin O was used for evaluation of proteoglycans. On the basis of this analysis, the type of tissue repair was classified as hyaline-like, fibrocartilage, or mixed tissues. Integration of graft to adjacent normal articular cartilage was also noted.
Statistical Analysis
The statistical analysis was performed by an independent statistician using SPSS 20 (IBM SPSS Statistics V20.0, Armonk, NY) and EPI info (Centers for Disease Control and Prevention. Atlanta, GA) statistical software. Pearson’s χ2 analysis was done to assess homogeneity between group characteristics and concomitant procedures. The general linear model for repeated measure test was used to investigate within time variations for continuous variables (age, weight, and lesion size, KOOS, VAS, and IKDC subjective scores) for all patients in both groups and subgroups. A post hoc test, with a Bonferroni adjustment for pairwise comparisons within time, was used to investigate improvement and/or deterioration for each variable and between subgroups. The nonparametric Friedman test was used to detect within-time significant differences in ordinal variables (Tegner and IKDC objective scores), while the post hoc nonparametric Wilcoxon rank test was used with a Bonferroni adjustment of the significance level. The Mann–Whitney test and Kruskal–Wallis test were used to assess differences in improvement of continuous data between the 2 groups while the χ2 trend test was used for intergroup comparisons for IKDC objective and Tegner score. Multivariate analysis was performed to assess whether the size, number and location of lesions and patient age were relevant and whether the presence of concomitant surgical procedures affected outcomes. The Kruskal–Wallis nonparametric test was used for comparison of more than two subgroups, with the post hoc nonparametric Mann–Whitney U test with a Bonferroni adjustment of the significance level; only the Mann–Whitney U test with a Bonferroni adjustment was used when there were two evaluated subgroups.
Differences in outcomes between the groups over time were also analyzed by univariate analysis of variance for repeated measures with the Bonferroni post hoc test. The model was adjusted for baseline characteristics (age, BMI, and lesion size) by multivariate analysis of variance, while a 2-factor (time × group) analysis was performed to evaluate the overall group effect, overall time effect, and interaction between group and time.
All continuous data are expressed in terms of mean and standard deviation of the mean. As for significance values, P values <0.05 were considered significant.
Results
All patients were available at 2-year and final follow-up (minimum of 3 years). Average follow-up was 59.69 months for the MACI (range 48.2-74.7 months) and 54.16 months for the BMAC group (range 38-77.8 months). Average lesion size was 7.12 cm2 and 5.54 cm2 for MACI and BMAC patients respectively. Total lesion area was 9.73 ± 6.09 cm2/patient (in MACI group) and 10.48 ± 6.01 cm2/patient (in BMAC group). The total number of lesions was 26 in MACI group and 34 in BMAC group; higher number of lesions in BMAC group brought the average total lesion area/patient size larger in BMAC group. Two patients in each group presented with kissing patellofemoral lesions. Chi-square and t tests did not reveal any significant difference between the groups in terms of age, BMI, lesion size, etiology, and concomitant procedures (P > 0.05). Demographic data (mean age, average total lesion area, and BMI) and group characteristics are summarized in Table 1.
Table 1.
Demographic Data and Concomitant Procedures.
| Value | MACI (Mean ± SD) | BMAC (Mean ± SD) | P Valuea |
|---|---|---|---|
| Age (years) | 43.10 ± 5.81 | 45.5 ± 7.55 | 0.286 |
| BMI (kg/m2) | 24.31 ± 1.37 | 24.77 ± 2.75 | 0.520 |
| Total lesion area (cm2)/patient | 9.73 ± 6.09 | 10.48 ± 6.01 | 0.673 |
| Features | MACI (n) | BMAC (n) | P Valueb |
| Average Lesion Size (cm2)/lesion | 7.12 | 5.54 | 0.174 |
| Total number of lesions | 26 | 34 | — |
| Total number of patients | 19 | 18 | — |
| Males/females | 9/10 | 10/8 | 0.6184 |
| Right/left knee | 7/12 | 7/11 | 0.8979 |
| Age (years) | |||
| ≤45 | 11 | 8 | 0.4132 |
| >45 | 8 | 10 | |
| Etiology | |||
| Traumatic | 12 | 13 | 0.5560 |
| Degenerative | 7 | 5 | |
| Lesion size (cm2) | |||
| ≤10 | 13 | 12 | 0.9092 |
| >10 | 6 (multiple = 4) | 6 (multiple = 5) | |
| Lesion number | |||
| Single | 12 (7 PAT, 5 TRO) | 8 (6 PAT, 2 TRO) | 0.2536 |
| Multiple | 7 (lesion >10 cm2 =4)c | 10 (lesion >10 cm2 =6)d | |
| Concomitant surgery | |||
| PF realignment | 8 | 5 | 0.4663 |
| HTO | 3 | 5 | |
| ACLR | 1 | 2 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; BMI = body mass index; PAT = patellar; TRO = trochlear; PF = patellofemoral; HTO = high tibial osteotomy; ACLR = anterior cruciate ligament reconstruction.
Continuous variables calculated by Student’s t test.
Pearson χ2 test.
One patient had associated lesion on medial and 1 patient on lateral femoral condyle; 2 patients had kissing patellofemoral lesions.
Two patients had associated lesions on medial and 1 patient on lateral femoral condyle; 2 patients had kissing patellofemoral lesions.
No adverse reactions or postoperative infections were reported. No major (>15° compared with the opposite healthy knee) limitation of range of motion was noted at 2-year and final follow-up. However, 1 patient of each group required debridement and mobilization due to intra-articular adhesions, at 7 and 6 months postoperatively, but full range of motion was restored in both cases.
Clinical Outcome
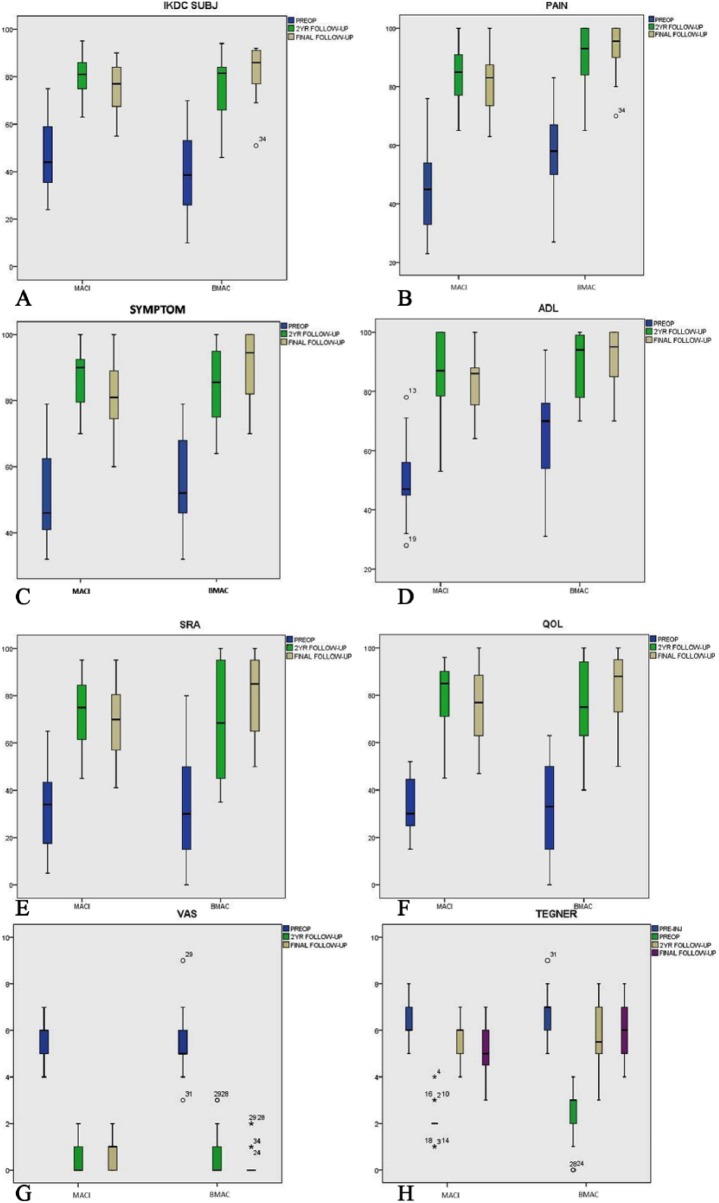
All patients within the 2 groups showed significant improvement in all the evaluated scores at 2-year and final follow-up, when compared with their respective preoperative scores (P = 0.001) (Table 2). However, statistical analysis revealed nonsignificant deterioration in the MACI group (P = 0.999) and improvement in BMAC group (P = 0.999) from 2 years to final follow-up in all scores. Difference in improvement between the 2 groups was nonsignificant (P > 0.05), with the exception of IKDC subjective score (P = 0.015). Average IKDC subjective score was significantly improved in both the MACI and BMAC groups (P = 0.001) (Table 2, Fig. 3A). Preoperative IKDC objective scores in MACI group were 9C (abnormal), 10D (severely abnormal) and in BMAC group were 8C and 10D. At final follow-up, 18 patients in MACI group scored normal (10A), or nearly normal (8B), while patients in the BMAC group scored normal (14A) and nearly normal (4B).
Table 2.
Clinical Outcome.
| MACI |
BMAC |
Within Time Improvement for MACI (P Value) |
Within Time Improvement for BMAC (P Value) |
MACI vs. BMAC (P value)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scoresa | Preop | 2-Year Follow-up | Final Follow-up | Preop | 2-Year Follow-up | Final Follow-up | Preop vs. 2 Years | Preop vs. Final | 2 Years vs. Final | Preop vs. 2 Years | Preop vs. Final | 2 Years vs. Final | |
| IKDC Objective | 9C, 10D | 15A, 4B | 10A, 8B, 1C | 8C, 10D | 12A, 6B | 14A, 4B | 0.0001 | 0.0001 | 0.07217 | 0.0001 | 0.0001 | 0.4630 | 0.12 |
| IKDC Subjectivec | 46.37 ± 14.44 | 81.05 ± 8.31 | 75.70 ± 9.85 | 38.78 ± 19.18 | 74.67 ± 13.90 | 82.52 ± 10.72 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.015 |
| KOOS Pain | 44.26 ± 14.46 | 83.26 ± 10.59 | 80.73 ± 11.79 | 56.44 ± 14.13 | 90.33 ± 10.15 | 93.50 ± 8.22 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.336 |
| KOOS Symptoms | 50.53 ± 13.22 | 86.05 ± 9.47 | 81.05 ± 11.04 | 56.39 ± 14.86 | 84.94 ± 11.92 | 90.61 ± 10.85 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.430 |
| KOOS ADL | 50.42 ± 12.50 | 85.94 ± 13.66 | 82.15 ± 11.29 | 64.17 ± 17.35 | 88.67 ± 10.90 | 92.11 ± 9.02 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.461 |
| KOOS SRA | 32.21 ± 16.92 | 71.42 ± 14.16 | 68.84 ± 15.25 | 33.33 ± 22.09 | 68.78 ± 23.36 | 79.72 ± 17.37 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.173 |
| KOOS QOL | 33.63 ± 10.74 | 79.26 ± 15.09 | 76.10 ± 16.90 | 32.83 ± 18.31 | 76.00 ± 18.52 | 84.00 ± 14.81 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.107 |
| Tegnerc | 2.1 ± 0.73d | 5.57 ± 0.83 | 5.26 ± 1.14 | 2.33 ± 1.18d | 5.61 ± 1.41 | 6.05 ± 1.10 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.220 |
| VAS | 5.53 ± 0.90 | 0.47 ± 0.61 | 0.84 ± 0.68 | 5.33 ± 1.32 | 0.72 ± 1.01 | 0.33 ± 0.68 | 0.0001 | 0.0001 | 0.9999 | 0.0001 | 0.0001 | 0.9999 | 0.418 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; VAS = visual analog scale; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life; Pre-op = preoperative values.
Values are expressed as mean ± standard deviation unless otherwise indicated. Post hoc test with a Bonferroni adjustment (paired t test) for pairwise comparisons.
Nonparametric Friedman test and χ2 trend tests.
Preinjury scores in the MACI group was 6.52 ± 0.84 (P = 0.001 with final follow-up) and the BMAC group was 6.94 ± 0.93 (P > 0.05 with final follow-up).
Mann–Whitney test was used for intergroup comparisons (preoperative to final follow-up); for IKDC objective and Tegner scores, χ2 trend test was used.
Figure 3.
Boxplots describing the mean values of the evaluated scores for both groups: (A) IKDC subjective, (B) KOOS pain, (C) KOOS symptoms, (D) KOOS ADL, (E) KOOS SRA, (F) KOOS QOL, (G) VAS, and (H) Tegner scores. MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; pre-op = preoperative values; pre-inj = preinjury values; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life; VAS = visual analog scale.
Pain and symptoms significantly improved in both groups and all patients returned to their previous daily activities. Average KOOS scales (Fig. 3B-F) were significantly improved (P = 0.001), while VAS score (Fig. 3G) was significantly reduced (P = 0.001) for both MACI and BMAC patients (Table 2). However, only 31% of patients in the MACI group and 34% of patients in the BMAC group returned to their previous level of activity (recreational sports). Average Tegner score (Fig. 3H) in the MACI and BMAC groups improved from 2.11 ± 0.73 and 2.33 ± 1.18, preoperatively to 5.26 ± 1.14 and 6.05 ± 1.10, respectively, at final follow-up (P = 0.001).
Clinical Outcome in Subgroups
We also studied the difference in improvement in subgroups in correlation to age, etiology, lesion size, number, location, concomitant surgeries, and previous surgeries (Tables 3-6). The difference in improvement in the subgroups according to age or etiology was nonsignificant (P = 0.999) for both groups. Interestingly, MACI group patients presenting with traumatic lesions showed better results than those with degenerative etiology, though nonsignificant (P = 0.999) (Table 3).
Table 3.
Clinical Outcome at Final Follow-up: Age and Etiology Subgroups.
| Age |
Etiology |
|||||||
|---|---|---|---|---|---|---|---|---|
| MACI Group |
BMAC Group |
MACI Group |
BMAC Group |
|||||
| Scoresa | ≤45 Years | >45 Years | ≤45 Years | >45 Years | Trauma | Degenerative | Trauma | Degenerative |
| IKDC Subjective | 77.10 ± 9.21 | 73.77 ± 11.00 | 84.18 ± 13.47 | 81.19 ± 8.45 | 77.19 ± 8.96 | 73.14 ± 11.48 | 82.07 ± 12.30 | 87.68 ± 5.69 |
| IKDC Objective | 6A, 5B | 4A, 3B, 1C | 8A, 2B | 6A, 2B | 9A, 3B | 1A, 5B, 1C | 8A | 5A, 5B |
| KOOS Pain | 82.36 ± 14.00 | 81.75 ± 8.77 | 94.25 ± 10.16 | 92.9 ± 6.82 | 80.91 ± 13.37 | 80.42 ± 9.43 | 94.40 ± 8.76 | 93.15 ± 8.35 |
| KOOS Symptom | 83.64 ± 13.64 | 82.75 ± 6.47 | 90.75 ± 11.19 | 90.5 ± 11.18 | 80.66 ± 13.49 | 81.71 ± 5.55 | 92.6 ± 12.79 | 89.85 ± 10.49 |
| KOOS ADL | 82.27 ± 13.48 | 82 ± 8.26 | 93.63 ± 9.89 | 90.90 ± 8.59 | 82 ± 12.49 | 82.42 ± 9.82 | 91.2 ± 10.94 | 92.46 ± 8.67 |
| KOOS SRA | 72.81 ± 18.4 | 71.62 ± 9.98 | 87.38 ± 13.25 | 73.6 ± 18.42 | 68.5 ± 17.94 | 69.42 ± 10.35 | 75 ± 19.68 | 81.54 ± 16.89 |
| KOOS QOL | 78.18 ± 21.43 | 77.35 ± 8.68 | 89.88 ± 13.04 | 79.3 ± 15.07 | 76.08 ± 20.45 | 76.14 ± 9.52 | 81.2 ± 13.21 | 85.08 ± 15.75 |
| Tegner | 5.9 ± 1.22 | 5.5 ± 1.06 | 6 ± 1.06 | 6 ± 1.19 | 5.33 ± 1.37 | 5.14 ± 0.69 | 6 ± 1.08 | 5.81 ± 1.51 |
| VAS | 0.72 ± 0.78 | 1 ± 0.53 | 0.25 ± 0.46 | 0.40 ± 0.84 | 0.75 ± 0.75 | 1 ± 0.57 | 0.80 ± 1.09 | 0.55 ± 0.37 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; VAS = visual analog scale; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life.
All scores are final follow-up scores with mean ± standard deviation values. The difference in improvement in the subgroups are nonsignificant (P = 0.999) in all scores, for both MACI and BMAC patients; exceptions are reported in text.
Table 4.
Clinical Outcome at Final Follow-up: Lesion Size and Number Subgroups.
| Lesion Size |
Lesion Number |
|||||||
|---|---|---|---|---|---|---|---|---|
| MACI Group |
BMAC Group |
MACI Group |
BMAC Group |
|||||
| Scoresa | ≤10 cm2 | >10 cm2 | ≤10 cm2 | >10 cm2 | Single | Multiple | Single | Multiple |
| IKDC Subjective | 76.39 ± 8.57 | 74.20 ± 13.01 | 84.67 ± 11.27 | 78.22 ± 8.84 | 78.33 ± 7.35 | 71.19 ± 12.43 | 89.12 ± 2.32 | 77.2 ± 11.97 |
| IKDC Objective | 9A, 4B | 1A, 4B, 1C | 11A, 1B | 3A, 3B | 9A, 3B | 1A, 5B, 1C | 8A | 5A, 5B |
| KOOS Pain | 83.92 ± 10.24 | 73.83 ± 12.85 | 94.67 ± 8.42 | 91.17 ± 8.01 | 83.08 ± 12.25 | 68.71 ± 10.57 | 98.38 ± 2.38 | 89.6 ± 9.24 |
| KOOS Symptom | 84.07 ± 9.83 | 74.5 ± 11.46 | 91.42 ± 11.15 | 89.00 ± 11.06 | 83.66 ± 10.89 | 68 ± 10.44 | 94.88 ± 8.61 | 87.2 ± 11.66 |
| KOOS ADL | 85.84 ± 9.27 | 74.16 ± 11.87 | 94.50 ± 8.44 | 87.33 ± 8.86 | 85.08 ± 11.74 | 69.29 ± 9.34 | 98.13 ± 2.64 | 87.30 ± 9.51 |
| KOOS SRA | 71.23 ± 15.31 | 63.66 ± 15.10 | 83.17 ± 14.50 | 72.83 ± 21.85 | 69.92 ± 16.95 | 62 ± 12.85 | 89.38 ± 9.03 | 72 ± 18.9 |
| KOOS QOL | 78.76 ± 17.56 | 70.33 ± 15.17 | 84.92 ± 16.04 | 82.17 ± 13.19 | 76 ± 19.15 | 71.29 ± 14.77 | 90.25 ± 9.34 | 79 ± 16.85 |
| Tegner | 5.61 ± 1.04 | 4.5 ± 1.04 | 6.16 ± 0.93 | 5.83 ± 1.47 | 5 ± 1.12 | 4.57 ± 1.13 | 6.37 ± 0.91 | 5.8 ± 1.22 |
| VAS | 0.69 ± 0.63 | 1.16 ± 0.75 | 0.17 ± 0.38 | 0.67 ± 1.03 | 0.66 ± 0.65 | 1.71 ± 0.95 | 0.13 ± 0.35 | 0.50 ± 0.85 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; VAS = visual analog scale; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life.
All scores are final follow-up scores with mean ± standard deviation values. The difference in improvement in the subgroups are nonsignificant (P = 0.999) in all scores, for both MACI and BMAC patients; exceptions are reported in text.
Table 5.
Clinical Outcome at Final Follow-up: Lesion Location.
| Location |
||||
|---|---|---|---|---|
| MACI Group |
BMAC Group |
|||
| Scoresa | Patellar | Trochlear | Patellar | Trochlear |
| IKDC Subjective | 74.19 ± 6.32 | 84.12 ± 4.10 | 89.19 ± 2.23 | 89 ± 3.56 |
| IKDC Objective | 4A, 3B | 5A | 6A | 2A |
| KOOS Pain | 75.57 ± 9.43 | 93.6 ± 6.54 | 98.33 ± 2.65 | 98.5 ± 2.12 |
| KOOS Symptom | 76.71 ± 8.17 | 93.4 ± 4.77 | 94.16 ± 9.88 | 97 ± 4.24 |
| KOOS ADL | 78.28 ± 10.04 | 94.6 ± 5.81 | 97.50 ± 2.81 | 100 ± 0 |
| KOOS SRA | 59.14 ± 12.34 | 85 ± 8.60 | 87.5 ± 9.87 | 95 ± 0 |
| KOOS QOL | 63.14 ± 13.47 | 94 ± 6.51 | 91.16 ± 9.78 | 87.5 ± 10.60 |
| Tegner | 5.14 ± 1.34 | 6 ± 0.70 | 6 ± 0.63 | 7.5 ± 0.70 |
| VAS | 1 ± 0.57 | 0.2 ± 0.44 | 0.16 ± 0.40 | 0 ± 0 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; VAS = visual analog scale; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life.
All scores are final follow-up scores with mean ± standard deviation values. The difference in improvement in the subgroups are nonsignificant (P = 0.999) in all scores, for both MACI and BMAC patients; exceptions are reported in text.
Table 6.
Clinical Outcome at Final Follow-up: With Concomitant Surgery.
| MACI Group |
BMAC Group |
|||||
|---|---|---|---|---|---|---|
| Scoresa | HTO | Realignment | None | HTO | Realignment | None |
| IKDC Subjective | 82.5 ± 8.86 | 72.58 ± 10.56 | 77.48 ± 9.22 | 82.63 ± 4.82 | 84.96 ± 6.17 | 80.19 ± 12.95 |
| IKDC Objective | 1A,1B,1C | 4A,4B | 5A,2B | 4A,1B | 5A | 2A,2B |
| KOOS Pain | 85 ± 6.24 | 76.12 ± 9.24 | 84.85 ± 15.60 | 93.4 ± 8.23 | 95.83 ± 6.64 | 93 ± 6.78 |
| KOOS Symptom | 84 ± 8.71 | 76.88 ± 9.97 | 84.57 ± 13.50 | 92.6 ± 12.79 | 92.67 ± 11.63 | 90.75 ± 5.96 |
| KOOS ADL | 84 ± 5.29 | 78.62 ± 10.97 | 85.85 ± 14.02 | 90.2 ± 10.13 | 95.67 ± 6.83 | 91 ± 7.78 |
| KOOS SRA | 74.33 ± 7.50 | 61.75 ± 11.18 | 73 ± 20.26 | 73 ± 17.53 | 86 ± 17.32 | 79.25 ± 21.56 |
| KOOS QOL | 80 ± 7 | 70 ± 18.79 | 79.71 ± 20.26 | 81.00 ± 12.94 | 88.5 ± 19.73 | 83.25 ± 9.25 |
| Tegner | 5.33 ± 1.52 | 4.87 ± 0.99 | 5.71 ± 1.25 | 6.4 ± 1.14 | 5.6 ± 0.89 | 6.25 ± 1.70 |
| VAS | 0.66 ± 0.57 | 0.87 ± 0.64 | 0.85 ± 0.89 | 0.8 ± 1.09 | 0 ± 0 | 0 ± 0 |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; VAS = visual analog scale; IKDC = International Knee Documentation Committee; KOOS = Knee Injury Osteoarthritis Outcome Score; ADL = activities of daily living; SRA = sports and recreational activities; QOL = quality of life; HTO = high tibial osteotomy.
All scores are final follow-up scores with mean ± standard deviation values. The difference in improvement in the subgroups are nonsignificant (P = 0.999) in all scores, for both MACI and BMAC patients; exceptions are reported in text.
In the MACI group, patients presenting with lesions ≤10 cm2 improved at 2-year follow-up, while patients presenting with lesions >10 cm2 deteriorated in all scores. However, significant deterioration (P = 0.005) in lesions >10 cm2 subgroup occurred only in VAS score. In the BMAC group, there was no significant difference in all the evaluated scores between patients presenting with lesions larger or smaller than 10 cm2 (P = 0.999) (Table 4). In the MACI group, patients with single lesions showed significantly improvement (P = 0.005) at 2-year follow-up in KOOS (pain and symptoms) and VAS scores; patients with multiple lesions deteriorated more, from 2-year to final follow-up, with significant deterioration (P = 0.005) in their VAS score. In the BMAC group, patients with single lesions showed nonsignificant difference in all scores at all follow-ups; significant difference in improvement was found only in KOOS, sports and recreational activities (SRA) and Tegner scores (Table 4).
Patients in the MACI group presenting with patellar lesions showed significant deterioration (P < 0.05) in all scores except KOOS activities of daily living (ADL) score, from 2-year to final follow-up, but deterioration of scores in patients presenting with trochlear lesions was nonsignificant (P = 0.999). In the BMAC group, patients presenting with trochlear lesions showed nearly the same improvement as the subgroup with patellar lesions, in almost all scores, at final follow-up (P = 0.999) (Table 5). In addition, there was no significant difference in improvement in clinical outcome between patients who had undergone a concomitant procedure or not; or between patients who underwent various associated procedures, in both groups (P > 0.05) (Table 5).
Regression Analysis
A statistically significant benefit of BMAC was observed throughout the entire postoperative period for IKDC subjective, KOOS symptoms, KOOS SRA, and VAS scores (P < 0.001, P = 0.001, P = 0.002, and P = 0.004 by repeated-measures analysis, respectively). Repeated-measures analysis (repeated-measured multivariate analysis of covariance) yielded a statistically significant difference of IKDC subjective, KOOS symptom, KOOS SRA, and VAS scores for the time-by-treatment interaction (P < 0.001, P = 0.001, P = 0.002, and P = 0.004 by repeated-measures analysis, respectively).
Second-Look Arthroscopy and Histologic Findings
Second-look arthroscopy was performed in patients who required surgical treatment on the same knee for hardware removal or gave their consent while undergoing surgical procedure in the contralateral knee. However, in 2 patients, second-look arthroscopy was performed during debridement and mobilization for range of motion limitation. Second-look arthroscopy was performed in 5 patients treated with MACI and 6 patients of the BMAC group at a mean follow-up of 14 and 13.2 months, respectively. Among them, concomitant biopsy from the regenerated tissue was taken in 4 patients in the MACI group, and from 4 patients in the BMAC group, with informed consent. Second-look arthroscopic evaluation of the grafted areas demonstrated smooth surface, stable graft on probing, good fill, well-integrated tissue repair with the surrounding cartilage and normal to nearly normal cartilage according to the ICRS visual scoring system in both groups (Table 7, Fig. 4A and B). In the biopsies done, the different zones (superficial, middle, deep, and calcified) could be identified in both groups, with hyaline-like, fibrocartilage, or mixed characteristics (Table 8, Fig. 5A and B).
Table 7.
Second-Look Arthroscopy and Histology Findings.
| Cases | Lesion Location | Lesion Size (cm2) | Time (Months) | Reason | Histological Grading | ICRS Cartilage Repair Assessment Scorea | Overall Repair Assessment Gradea | |
|---|---|---|---|---|---|---|---|---|
| MACI | 1 | TRO | 4.5 | 7 | Debridement/mobilization | Mixed | 8 | II |
| 2 | PAT | 12 | 11 | Contralateral knee surgery | No biopsy | 11 | II | |
| 3 | PAT | 4 | 11 | Hardware removal (HTO) | Hyaline-like/fibrocartilage | 9 | II | |
| 4 | TRO | 6 | 29 | Hardware removal (HTO) | Hyaline-like/fibrocartilage | 10 | II | |
| 5 | TRO | 4.5 | 12 | Hardware removal (HTO) | Mixed | 11 | II | |
| BMAC | 1 | TRO | 5 | 12 | Hardware removal (HTO) | Hyaline-like/fibrocartilage | 11 | II |
| 2 | PAT | 6.75 | 24 | Hardware removal (HTO) | Hyaline | 12 | I | |
| 3 | PAT | 8 | 12 | Contralateral knee surgery | No biopsy | 11 | II | |
| 4 | PAT | 8 | 6 | Debridement/mobilization, meniscectomy | Hyaline-like/fibrocartilage | 11 | II | |
| 5 | PAT | 4 | 12 | Contralateral knee surgery | Mixed | 11 | II | |
| 6 | PAT | 6 | 13 | Hardware removal (HTO) | No biopsy | 11 | II | |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; HTO = high tibial osteotomy; PAT = patella; TRO = trochlea; ICRS = International Cartilage Repair Society.
Grade I = normal (12); grade II = nearly normal (11-8); grade III = abnormal (7-4); grade IV = severely abnormal (3-1).
Figure 4.
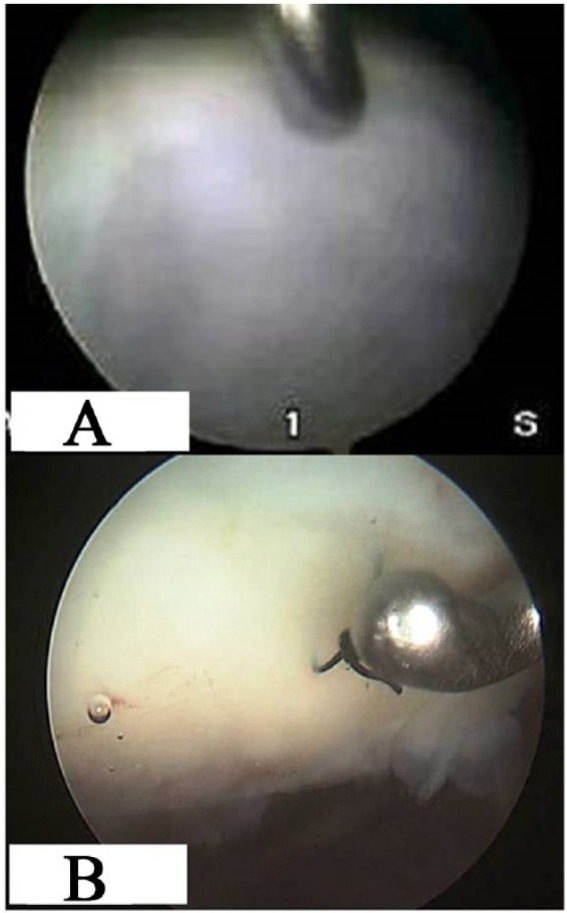
Second-look arthroscopy: (A) Trochlear after matrix-induced autologous chondrocyte implantation (MACI) at 29-month follow-up and (B) patellar after bone marrow aspirate concentrate (BMAC) implantation at 24 month follow-up.
Table 8.
Biopsy Specimens Reports.
| Histopathologya |
|||||||
|---|---|---|---|---|---|---|---|
| Cases | Surface | Structure | Proteoglycans | Cells | Subchondral Bone | Immunohistochemistry | |
| MACI | 1 | Smooth | Not well organized | Not represented | Not represented | Active remodeling | Col 1: E/C, Col 2: I/C |
| 3 | Smooth | Well organized and slightly fibrous | Represented | Columnar disorganized distribution | Active remodeling | Col 1: few positive cells, Col 2: E/C | |
| 4 | Smooth | Well organized | Represented | Columnar homogenous distribution | Active remodeling | Col 1: absent, Col 2: E/C | |
| 5 | Smooth | Well organized | Represented | Columnar homogenous distribution | Active remodeling | Col 1: absent, Col 2: E/C | |
| BMAC | 1 | Smooth | Well organized | Represented | Columnar homogenous distribution | Active remodeling | Col 1: absent, Col 2: E/C |
| 2 | Smooth | Well organized | Represented | Columnar homogenous distribution | Active remodeling | Col 1: absent, Col 2: E/C | |
| 4 | Smooth | Not well organized | Not represented | Not represented | Active remodeling | Col 1: E/C, Col 2: I/C | |
| 5 | Smooth | Well organized and slightly fibrous | Represented | Columnar homogenous distribution | Active remodeling | Col 1: absent, Col 2: E/C | |
MACI = matrix-induced autologous chondrocyte implantation; BMAC = bone marrow aspirate concentrate; Col = collagen type; E/C = extracellular; I/C = intracellular.
Hematoxylin and eosin or safranin-O staining.
Figure 5.
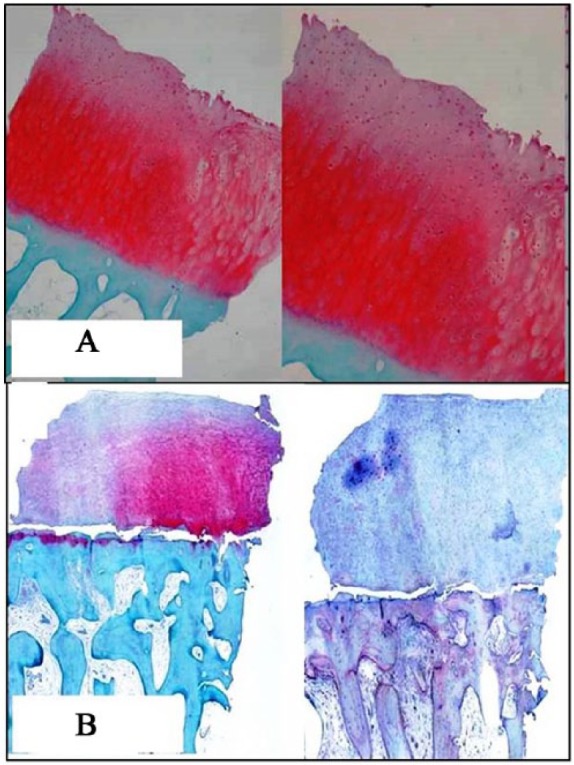
Histological results showing nearly normal cartilage appearance and dominance of type II collagen: (A) 29 months after matrix-induced autologous chondrocyte implantation (MACI)—case 4 in Table 7 and (B) 24 months after multipotent stem cells (MSCs) implantation—case 2 in Table 7.
Magnetic Resonance Imaging Findings
At final follow-up, MRI findings were available in 16 (84.2%) patients of MACI group and in 16 (88.8%) patients of BMAC group (5 patients refused due to claustrophobia). In the BMAC group, 81% of patients showed complete or near complete (>50%) filling of the defect while in the MACI group, 76% had complete or near complete fill (Fig. 6A and B). No signs of hypertrophy were identified in either group. Integration with adjacent cartilage was complete in 88.2% patients in the MACI group and 93.7% patients in the BMAC group with restoration of the cartilage layer over the subchondral bone in both groups. Two patients in each group presented with subchondral edema; however, we did not find cysts or sclerosis of subchondral bone in either of the groups.
Figure 6.
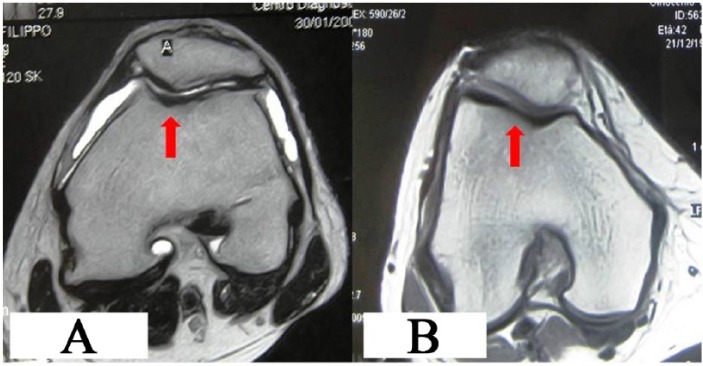
Magnetic resonance imaging results at 5 years showing good fill on the defect, with no effusion and none or slight bone edema in trochlear regions after (A) matrix-induced autologous chondrocyte implantation (MACI) and (B) bone marrow aspirate concentrate (BMAC) implantation.
In comparison with the MRI findings at 2 years, no documented deterioration was detected, while the newly formed tissue was still maturing or stabilized at final follow-up.
Discussion
In this study, we analyzed and compared the outcomes of 2 groups of patients presenting with full-thickness chondral lesions of the patellofemoral joint, treated either with MACI or BMAC implantation, using the same hyaluronan scaffold. To our knowledge, this is the first and only study that compares the efficacy of the 2-step technique of cartilage implantation (MACI) versus a single-step technique (BMAC) for full-thickness patellofemoral lesions, at medium-term follow-up. Only 1 article has been published so far, reporting comparison between first-generation ACI and BMAC for chondral lesions of knee.34 The strengths of this study were strict selection of patients, use of similar baseline characteristics (age, BMI, lesion size, etiology, and concomitant procedures) to decrease the confounding effect of these variables, all surgeries performed by the same surgeon, trained independent observers for collection of scores, treatment of concomitant pathologies at the same surgery, and similar rehabilitation protocols for both groups.
The weakness of this study was the nonrandom selection of the patient population; however, this investigation can provide useful information about the comparative efficacy of these 2 emergent treatments for large full-thickness patellofemoral chondral lesions. Another limitation is that only a small number of patients consented to a second-look arthroscopy and biopsy. The possible confounding factor of addressing associated pathologies concomitantly was investigated, but statistical analysis did not reveal any significant difference in improvement between patients who had undergone a concomitant procedure or not, or between patients who underwent various associated procedures, for both the groups. In our study, associated knee pathologies were addressed concomitantly in 12 patients in each group, to create an essential mechanical environment to protect the implanted cells and provide long-term stability of the outcome. The need to address associated pathological conditions such as tibiofemoral axis malalignment, patellofemoral maltracking and ligamentous insufficiency, in a previous or concomitant cartilage repair procedure, is widely recognized.35,36 Articular cartilage restoration techniques with concomitant correction of tibiofemoral axis malalignment provide greater survival at medium and long-term follow-up.36-37 Similarly, concomitant patellofemoral maltracking correction reduces overloading of the lateral patellofemoral joint and therefore reduces the risk of future cartilage injuries.38
Statistical analysis showed significant improvement for both MACI and BMAC groups in all scores at final follow-up. Moreover, the obtained clinical outcomes were consistent with MRI, second-look arthroscopy, and tissue biopsy findings. Nonsignificant deterioration in MACI patients clinical scores was noted from 2-year to final follow-up, especially in patients presenting with degenerative-etiology lesions; however, patients still scored significantly higher from baseline values, indicating the maintenance of good outcome at medium-term follow-up, which is consistent with the findings of previous studies.39-41 In a previous study,1 a significant decline from 2 to 5 years was reported in MACI patients presenting with degenerative-etiology lesions, while in another study39 a higher number of failures was reported in patients treated with MACI for degenerative lesions. However, other authors reported that degenerative joint environment does not inhibit the regenerative process, and even patients with early osteoarthritis can benefit from cartilage treatments with no inhibition.42,43 A longer follow-up of our MACI group is needed, in order to confirm whether this deterioration will be significantly increased. On the other hand, as in other recent studies,27,28,44,45 we demonstrated that the 1-step technique with MSC implantation provided significant improvement in functional scores and hyaline-like cartilage repair for full-thickness chondral lesions, at medium-term follow-up. Patients treated with BMAC showed significant higher improvement than MACI patients only in IKDC subjective score. However, regression analysis showed a statistically significant benefit of BMAC patients throughout the entire postoperative period for IKDC subjective, symptoms, SRA, and VAS scores. Similarly, Nejadnik et al.34 with first-generation ACI and BMAC treatment reported greater but nonsignificant improvement in the MSCs group at 2-year follow-up, with the exception of physical role functioning, in which MSCs group scored significantly higher.
Age was not a statistically significant prognostic factor in current study groups. Although MACI procedure is suggested and preserved for younger patients1,46-48; other studies have reported good clinical outcome in older patients.41,47,49 On the other hand, some studies reported a decrease in number and chondrogenic potency of MSCs with increased age with decreased capacity of differentiation, proliferation, attachment, or self-renewal capacity.50,51 However, other authors52,53 stated that the number of precursors and chondrogenic or osteogenic potential of human adult MSCs are independent of age or osteoarthritis, while previous clinical studies also reported that age did not affect the medium-term outcome after treatment with bone marrow-derived MSCs.28,45 Lesion size and number were important predictors of outcome in both groups. Reported better results in the subgroup of patients with smaller and single lesions in both groups correspond to previous reports1,28; however, literature lacks evidence of outcome results after MACI for such big lesions.
Histological examination of the performed biopsies revealed regeneration of new tissue with hyaline-like cartilage features, including presence of a noticeable proteoglycan component around chondrons in both groups. The biopsies showed good organization of proteoglycans and collagen in the extracellular matrix, an intact superficial zone, and a not well-defined tidemark, suggesting that maturation of the neotissue was still underway (Table 8). Histological features of biopsy specimens taken at about 6 months from implantation demonstrated immature cartilage tissue, suggesting that the repair tissue was still undergoing remodeling. The observed level of maturity seems higher in the BMAC group than that obtained with MACI at this time point, as in previous reports.46,54
MRI evaluation showed complete filling of the defect in both groups with no signs of hypertrophy in either group. Although MRI findings were not statistically analyzed and correlated to the clinical scores, there was a compatibility of the positive MRI findings and the good functional outcomes, which implies good efficacy of both procedures similarly to previous reports.1,27,28,48
MACI procedure has shown advantages over first-generation ACI in terms of easy handling and application of graft material via minimally invasive or arthroscopic techniques, shortened surgical time, avoiding the need for periosteal tissue harvest and periosteal hypertrophy and considerably lower failure rate (2.5%), compared with 5% to 13% failure rate reported with first-generation ACI.1,7,9 However, MACI still remains a 2-surgery technique and is an expensive solution.1,21
Increasing interest in MSCs research is because of the widespread availability of MSCs and the possibility to isolate them from various sites, making them easy targets for harvesting.14,15 The role of MSCs in cartilage repair has been investigated in several in vitro and animal studies.16-19,25,55 Ochi et al.55 observed that the injection of cultured MSCs combined with microfracture could accelerate the regeneration of cartilage in a rat model. An equine study showed enhanced chondrogenesis and improved cartilage healing after arthroscopic implantation with MSCs.19 However, a rapid loss of implanted cells and deterioration in cartilage quality were observed. Grigolo et al.25 transplanted a hyaluronan scaffold seeded with in vitro expanded bone marrow–derived MSCs in chondral lesions of the knee in a rabbit model and reported better quality of the regenerated tissue between the implants with scaffolds carrying MSCs compared to the scaffold alone or nontreated lesions in the control group at 6 months. Hui et al.56 compared MSCs transplants to cultured chondrocytes, osteochondral autograft and periosteal grafts in animal models of osteochondritis dissecans. Based on histological and biomechanical evaluation, the authors found the stem cells transplants to be comparable to cultured chondrocytes and superior to periosteum and osteochondral autograft in their ability to repair chondral defects. Wakitani et al.20 first described the use of expanded bone marrow derived MSCs to repair cartilage defects in osteoarthritic knees in a clinical study: they concluded that MSCs were capable of regenerating a repair tissue.
BMAC implantation is a single-step bedside technique, with no need for culture, thereby avoiding the expenditure for an extra procedure to retrieve chondral biopsy decreasing the total costs of the procedure and donor site morbidity. The use of a point-of-care device provides a sufficient concentration of total nucleated cells and platelets included in the aspirate that further contribute to the stimulation of progenitor cells acting as a chemoattractant.57 Furthermore, the finding that bone marrow–derived MSCs have a better proliferation rate than chondrocytes and that MSCs transplantation has comparable outcomes to ACI, portends positive trends for their use in cartilage surgery.34 The utilized HYAFF 11 scaffold provides multiple advantages, by providing a suitable environment to maintain cell in situ into the defect, supports adhesion, migration and proliferation of MSCs, as well as the synthesis and delivery of extracellular matrix components under static culture conditions.11,12,22-26
Both MACI and MSCs implantation techniques have been proven viable and effective for the treatments of large patellofemoral chondral defects at medium-term follow-up. MSCs implantation is a new technique with potential advantages, including a single-step surgery, no need of cartilage biopsy and cells cultivation, thus reducing the total cost. However, a larger number of patients and randomized comparative trials with longer follow-up are essential to establish conclusively about the comparative efficacy of these procedures in the treatment of patellofemoral lesions.
Footnotes
Authors’ Note: The investigation was performed at Orthopaedic Arthroscopy Surgery International, Milan, Italy.
Acknowledgments and Funding: The authors thank Mr Arvind Kavishwar, a professional statistician, for statistical analysis of this study. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Anika (Therapeutics, Srl Abano Terme, Italy) has provided a partial grant.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37(6):1083-92. [DOI] [PubMed] [Google Scholar]
- 2. Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42(3):626-34. [DOI] [PubMed] [Google Scholar]
- 3. Saleh KJ, Arendt EA, Eldridge J, Fulkerson JP, Minas T, Mulhall KJ. Operative treatment of patellofemoral arthritis. J Bone Joint Surg Am. 2005;87(3):659-71. [DOI] [PubMed] [Google Scholar]
- 4. Strauss EJ, Galos DK. The evaluation and management of cartilage lesions affecting the patellofemoral joint. Curr Rev Musculoskelet Med. 2013;6(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 6. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124. [DOI] [PubMed] [Google Scholar]
- 7. Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr, Erggelet C, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35(6):915-92. [DOI] [PubMed] [Google Scholar]
- 8. Brittberg M. The history of the treatment of cartilage injuries. In: Shetty AA, Kim SJ, Nakamura N, Brittberg M, editors. Techniques in cartilage repair surgery. Berlin, Germany: Springer-Verlag; 2014. p. 10-11. [Google Scholar]
- 9. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based status of second and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29(11):1872-8. [DOI] [PubMed] [Google Scholar]
- 10. Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19(23):2101-27. [DOI] [PubMed] [Google Scholar]
- 11. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;(435):96-105. [DOI] [PubMed] [Google Scholar]
- 12. Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39(12):2549-57. [DOI] [PubMed] [Google Scholar]
- 13. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI): 5-year follow-up. Knee. 2006;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 14. Caplan AI. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;1(1):6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huselstein C, Li Y, He X. Mesenchymal stem cells for cartilage engineering. Biomed Mater Eng. 2012;22:69-80. [DOI] [PubMed] [Google Scholar]
- 17. Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells 2004;22:1152-67. [DOI] [PubMed] [Google Scholar]
- 18. Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913-25. [DOI] [PubMed] [Google Scholar]
- 19. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. [DOI] [PubMed] [Google Scholar]
- 20. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199-206. [DOI] [PubMed] [Google Scholar]
- 21. Gobbi A, Karnatzikos G, Nakamura N, Mahajan V. Next generation cartilage solutions. In: Doral MN. editor. Sports injuries: prevention, diagnosis, treatment and rehabilitation. Berlin, Germany: Springer-Verlag; 2012. p. 739-49. [Google Scholar]
- 22. Pasquinelli G, Orrico C, Foroni L, Bonafè F, Carboni M, Guarnieri C, et al. Mesenchymal stem cell interaction with a non-woven hyaluronan- based scaffold suitable for tissue repair. J Anat. 2008;213(5):520-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lisignoli G, Cristino S, Piacentini A, Zini N, Noël D, Jorgensen C, et al. Chondrogenic differentiation of murine and human mesenchymal stromal cells in a hyaluronic acid scaffold: differences in gene expression and cell morphology. J Biomed Mater Res A. 2006;77(3):497-506. [DOI] [PubMed] [Google Scholar]
- 24. Facchini A, Lisignoli G, Cristino S, Roseti L, De Franceschi L, Marconi E, et al. Human chondrocytes and mesenchymal stem cells grown onto engineered scaffold. Biorheology. 2006;43(3-4):471-80. [PubMed] [Google Scholar]
- 25. Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647-58. [DOI] [PubMed] [Google Scholar]
- 26. Cavallo C, Desando G, Columbaro M, Ferrari A, Zini N, Facchini A, et al. Chondrogenic differentiation of bone marrow concentrate grown onto a hyaluronan scaffold: rationale for its use in the treatment of cartilage lesions. J Biomed Mater Res A. 2013;101(6):1559-70. [DOI] [PubMed] [Google Scholar]
- 27. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full thickness knee cartilage lesions: results at 2 year follow up. Cartilage. 2011;2(3):286-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648-57. [DOI] [PubMed] [Google Scholar]
- 29. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-13. [DOI] [PubMed] [Google Scholar]
- 30. Wright RW. Knee injury outcomes measures. J Am Acad Orthop Surg. 2009;17(1):31-9. [DOI] [PubMed] [Google Scholar]
- 31. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 32. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-9. [PubMed] [Google Scholar]
- 33. Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee: a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12:209-16. [DOI] [PubMed] [Google Scholar]
- 34. Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110-6. [DOI] [PubMed] [Google Scholar]
- 35. Takeda H, Nakagawa T, Nakamura K, Engebretsen L. Prevention and management of knee osteoarthritis and knee cartilage injury in sports. Br J Sports Med. 2011;45(4):304-9. [DOI] [PubMed] [Google Scholar]
- 36. Harris JD, McNeilan R, Siston RA, Flanigan DC. Survival and clinical outcome of isolated high tibial osteotomy and combined biological knee reconstruction. Knee. 2013;20(3):154-61. [DOI] [PubMed] [Google Scholar]
- 37. Bauer S, Khan RJ, Ebert JR, Robertson WB, Breidahl W, Ackland TR, et al. Knee joint preservation with combined neutralising high tibial osteotomy (HTO) and matrix-induced autologous chondrocyte implantation (MACI) in younger patients with medial knee osteoarthritis: a case series with prospective clinical and MRI follow-up over 5 years. Knee. 2012;19(4):431-9. [DOI] [PubMed] [Google Scholar]
- 38. Kramer DE, Kocher MS. Management of patellar and trochlear chondral injuries. Oper Tech Orthop. 2007;17(4):234-43. [Google Scholar]
- 39. Filardo G, Kon E, Di Martino A, Patella S, Altadonna G, Balboni F, et al. Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1704-13. [DOI] [PubMed] [Google Scholar]
- 40. Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, et al. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40(10):2273-80. [DOI] [PubMed] [Google Scholar]
- 41. Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668-75. [DOI] [PubMed] [Google Scholar]
- 42. Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng 2006;12(7):1787-98. [DOI] [PubMed] [Google Scholar]
- 43. Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte transplantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res 2010;468:147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buda R, Vannini F, Cavallo M, Baldassarri M, Luciani D, Mazzotti A, et al. One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone-marrow-derived cells: three years results. Musculoskelet Surg. 2013;97(2):145-51. 10.1007/s12306-013-0242-7. [DOI] [PubMed] [Google Scholar]
- 45. Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, et al. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013;41(3):511-8. [DOI] [PubMed] [Google Scholar]
- 46. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192. [DOI] [PubMed] [Google Scholar]
- 47. Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, et al. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88(1):61-4. [DOI] [PubMed] [Google Scholar]
- 48. Filardo G, Kon E, Di Martino A, Iacono F, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am J Sports Med. 2011;39(10):2153-60. 10.1177/03635465-11415658. [DOI] [PubMed] [Google Scholar]
- 49. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36(12):2336-44. [DOI] [PubMed] [Google Scholar]
- 50. Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314(9):1937-44. [DOI] [PubMed] [Google Scholar]
- 51. Chambers SM, Goodell MA. Hematopoietic stem cell aging: wrinkles in stem cell potential. Stem Cell Rev. 2007;3(3):201-11. [DOI] [PubMed] [Google Scholar]
- 52. Oreffo RO, Bennett A, Carr AJ, Triffitt JT. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol. 1998;27(6):415-24. [DOI] [PubMed] [Google Scholar]
- 53. Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Bühring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25(12):3244-51. [DOI] [PubMed] [Google Scholar]
- 54. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 55. Ochi M, Adachi N, Nobuto H, Yanada S, Ito Y, Agung M. Articular cartilage repair using tissue engineering technique: novel approach with minimally invasive procedure. Artif Organs. 2004;28(1):28-32. [DOI] [PubMed] [Google Scholar]
- 56. Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatr Orthop. 2004;24(4):427-33. [DOI] [PubMed] [Google Scholar]
- 57. Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, et al. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16(10):1059-69. [PubMed] [Google Scholar]