Abstract
Objective:
Intra-articular injection of local anesthetic and/or corticosteroid is an adjunct treatment for arthritic and inflammatory orthopedic conditions. Despite potential benefits, there is growing concern that these medications may cause significant morbidity, including potential toxicity to intra-articular chondrocytes and synoviocytes.
Design:
Twenty dogs underwent intra-articular injection of the shoulder joint using ultrasound guidance, with the following injectates (n = 5 each): negative control (saline), methylprednisolone/1.0% lidocaine, triamcinolone/1.0% lidocaine, and triamcinolone/0.0625% bupivacaine. The dogs were euthanized 24 hours postinjection for reasons unrelated to this study. Synovium/cartilage explants were harvested under sterile conditions and assessed immediately or cultured for 7 days. Synoviocyte and chondrocyte viability was determined on day 1 and day 7 using Calcien AM and Sytox Blue live/dead fluorescent stains, and cell metabolism determined on day 2 using the alamar blue additive test. Results were compared statistically.
Results:
On day 1 synovium exposed to 1%L/M demonstrated a significant decrease in cell metabolism (P = 0.0107) and subjective synoviocyte viability scores (P = 0.013) compared with the negative control. Cartilage exposed to 1%L/M demonstrated decreased chondrocyte viability and cell metabolism versus all other groups, although not significantly. After 7 days of culture, cartilage viable cell density in the 1%L/M group was significantly (P ≤ 0.001) lower than the negative control. Subjective synoviocyte viability scores was significantly lower in the 1%L/M (P = 0.013), 1%L/T (P ≤ 0.001), and 0.0625%B/T groups (P = 0.006) compared with the negative control.
Conclusions:
This study suggests potential negative effects of combination local anesthetic/corticosteroid on intra-articular cell viability and cell metabolism. Further study is needed before determining definitive clinical recommendations.
Keywords: local anesthetic, cartilage, synovium, corticosteroid
Introduction
Intra-articular injections are commonly used for diagnostic and therapeutic purposes in orthopedics. Injections of local anesthetics into the joint are used in the ambulatory setting to determine and distinguish sources of pain, and they are also used perioperatively for pain control.1 Combinations of local anesthetic and corticosteroid are often used to treat symptoms related to inflammatory and degenerative arthritis and have been reported to improve pain scores and reduce systemic effects of enteral and parenteral narcotics.2 Despite their widespread use, there is growing concern regarding the long-term effects of these agents on articular tissue.3,4 The in vitro toxicity of local anesthetics and corticosteroids on chondrocytes following a single exposure has been demonstrated in several studies,3,5-14 and the detrimental clinical effects of chondrolysis associated with continuous infusion of bupivacaine on articular cartilage of the shoulder6,7 have been well documented. To the authors’ knowledge, the in vivo effects of combinations of local anesthetic and corticosteroid following single intra-articular injection have not been reported in the peer-reviewed literature.
The joint is now considered by many to be an organ wherein all intra-articular tissue contributes to joint health, disease pathogenesis, and disease progression. Because the synovium contributes to nociceptive, inflammatory, and degradative responses, it is vital that the effects of intra-articular injections are studied not only on articular cartilage but synovium as well. Pilot in vitro studies at our institution have evaluated the effects of local anesthetics and corticosteroids on synovial and articular cartilage explants after a single exposure. Those screening studies have indicated that clinically relevant doses of lidocaine, bupivacaine, betamethasone, or methylprednisolone were significantly toxic to intact cartilage and synovium after a single exposure, whereas triamcinolone and low-dose bupivacaine demonstrated minimal to no detrimental effects on cellular viability and metabolism. After examining these compounds in vitro, the purpose of this study was to employ a translational animal model to investigate the in vivo effects of selected combinations of local anesthetics and corticosteroids on articular tissues, using a single injection of clinically relevant doses into canine shoulder joints. We hypothesized that the detrimental effects of these agents observed on in vitro monolayer or explant cultures would not be replicated in vivo and that clinically relevant doses would demonstrate minimal toxicity to the articular tissues.
Methods and Materials
Animal Model
With Institutional Animal Care and Use Committee (IACUC) approval, adult purpose-bred dogs (n = 20, mean weight 28.6 kg) were sedated using medetomidine (0.04 mg/kg) and prepared for aseptic injection of the right shoulder joint. Each dog (i.e., shoulder) was randomly assigned to receive one of four different injectates (n = 5 per group):
Control = 5 mL of 0.9% saline
L/M = 4 mL of 1% lidocaine (pH 6.5) + 1 mL of 40 mg/mL methylprednisolone (pH 6.5)
L/T = 4 mL of 1% lidocaine + 1 mL of 40 mg/mL triamcinolone (pH 7.0)
B/T = 4 mL of 0.0625% bupivacaine (pH 6.5) + 1 mL of 40 mg/mL triamcinolone
The dosage of 40 mg corticosteroid was selected as it is the recommended dose for the human wrist that would be similar in size/volume to the canine shoulder. Additionally, the recommended clinical veterinary dose for the canine shoulder is 30 to 40 mg. For each treatment, a 1.5-inch 22-gauge needle was inserted into the joint (verified by aspiration of 0.2-0.3 mL of synovial fluid), and then used to deliver the entire volume of the respective injectate into the joint. The dogs were recovered from sedation and were assessed by veterinarians for pain and function on recovery and every 8 hours of the 24-hour postinjection study period. Dogs were allowed full activity in their runs and then euthanatized 24 hours after injection as part of another IACUC-approved study. Synovial fluid was not aspirated at sacrifice. The injected shoulder joints were aseptically disarticulated to harvest joint capsule and full-thickness articular cartilage sections from the humeral head. Harvested tissues were placed in sterile closed containers filled with tissue culture media and transported to the laboratory either for immediate assessment (day 1) or processing for tissue culture.
Tissue Culture
Four-millimeter-diameter explants were aseptically prepared from the harvested cartilage and synovial tissues using a dermal biopsy punch (Fray Products, Buffalo, NY). Each explant was placed in a separate well of 24-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) containing Dulbecco’s modified Eagle’s medium with high glucose (Gibco, Invitrogen, Carlsbad, CA) supplemented with 1% ITS, penicillin, streptomycin, amphotericin B, L-ascorbic acid, L-glutamine, and nonessential amino acids. Explants were cultured in dedicated incubators at 37°C with 5% CO2 at 95% humidity.
Toxicity Assessments
Cartilage and synovial tissues were assessed immediately after harvest (day 1) and on day 7 of culture. Chondrocyte and synoviocyte viability was determined using a live-dead cell assay (Invitrogen, Carlsbad, CA). Tissues were incubated with Calcein AM (live cell stain) and Sytox Blue (dead cell stain) using manufacturer’s instructions, and then assessed by fluorescent microscopy to determine the number of live and dead cells in each section. Images of each cartilage section were captured using commercially available software (Microsuite, Olympus); live and dead cells were counted using a custom in-house validated cell counting program (Figs. 1 and 2). The area of the cartilage images was determined using Microsuite, and chondrocyte viable cell density (VCD) was reported as the number of live cells divided by the area of the tissue (µm). For the synovial explants, subjective assessment of viability was performed by five investigators blinded to treatment. Each synovial tissue explant was given a score ranging from 0 (0% viability) to 5 (100% viability). The scores from all observers were averaged to give a mean synoviocyte subjective viability score (SVS) for each explant.
Figure 1.
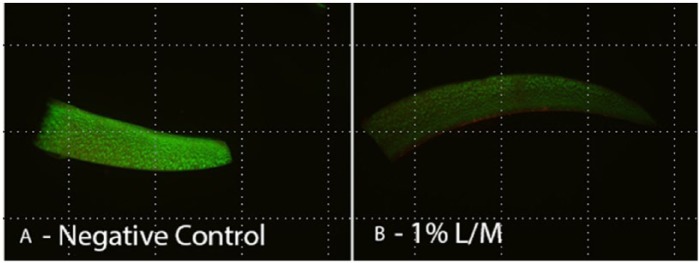
View of chondrocyte viability with (A) negative control and (B) at 1% L/M.
Figure 2.
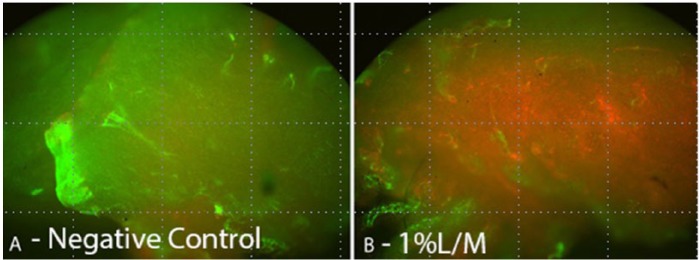
View of synoviocyte viability with (A) negative control and (B) at 1%L/M.
Tissue metabolism was assessed quantitatively using resazurin (Sigma Aldrich, St Louis, MO) fluorescent metabolic assay. Resazurin is converted to a fluorescent compound, resorufin, by metabolically active cells. The degree of fluorescence detected in the media provides a quantitative measure of the number of viable cells in tissue.15 For testing, 100 µL of resazurin reagent was added to the media of each tissue section and incubated overnight at 37°C. Fluorescence was measured (excitation, 530 nm; emission, 590 nm) the following day using a Synergy HT plate reader (BioTek, Winooksi, VT).
Statistical Analysis
Cartilage VCD, synoviocyte SVS, and metabolic assay fluorescence data were assessed for statistically significant differences among groups at each time point using one-way ANOVA with Tukey post hoc analyses. Significance was set at P < 0.05.
Results
Animals
All injections were accomplished without complication. All dogs recovered well from sedation and showed no lameness or functional impairment in any limb for the 24-hour duration of the study.
Cartilage
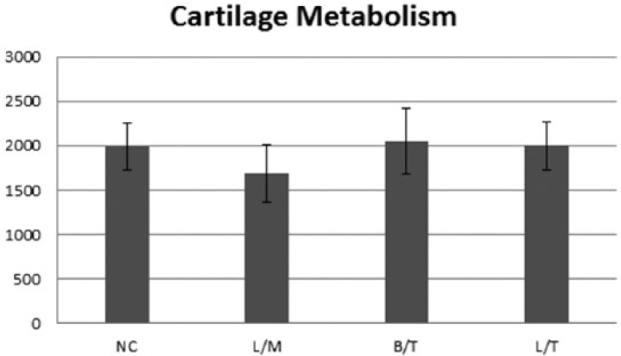
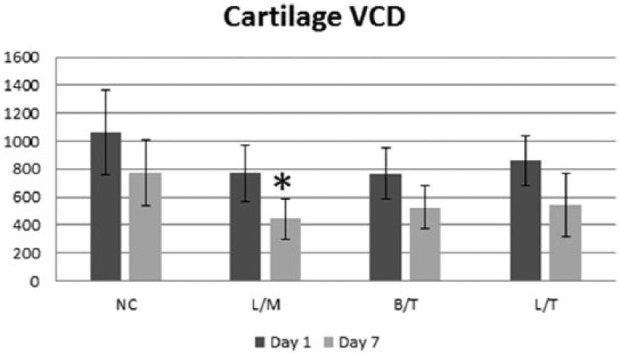
Figures 3 and 4 show that at day 1 (24 hours after intra-articular injection) all treatment groups had lower cartilage VCD compared with the negative control; however, no statistically significant differences among groups were noted (Fig. 3). After 7 days of culture, all groups had a lower VCD compared with the day 1 samples, but the decrease from day 1 to day 7 was only significant for the L/T group (P = 0.039). However, on day 7 the VCD in the L/M treatment group was the only group that was significantly (P = 0.028) lower than the negative control (Fig. 4).
Figure 3.
Day 1 (24 hours after intra-articular injection) %CV (A) and fluorescence levels (B) for cartilage explants in each treatment group. No statistically significant differences among groups were noted.
Figure 4.
Day 7 %CV (A) and fluorescence levels (B) for cartilage explants in each treatment group. All groups exposed to local anesthetic-steroid treatments had significantly (*) lower %CV compared with control, whereas only the 1% L/M group had a significantly (+) lower fluorescence level than control.
Synovium
Synovium exposed to L/M showed significantly (P < 0.021) lower fluorescence levels compared with control at day 1 (Fig. 5). The synovial tissue SVS for the L/M group was significantly (P = 0.013) lower than the control on day 1 (Fig. 6). Additionally, the decrease in the synovial tissue SVS for the L/T group compared with the control approached significance (P = 0.061). There was no significant (P = 0.289) difference between the B/T and control on day 1. On day 7, the L/M, L/T, and B/T groups all had significantly lower synovial tissue SVS compared with the control (P ≤ 0.006). However, there was no statistically significant difference in the synovial tissue SVS among treatment groups for day 1 or day 7.
Figure 5.
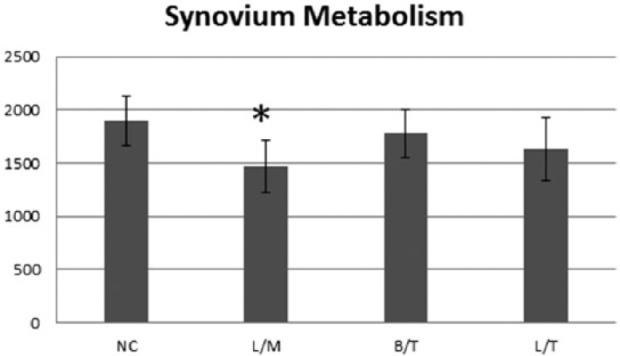
Day 1 (24 hours after intra-articular injection) synoviocyte viability score (A) and fluorescence levels (B) for synovial explants in each treatment group. Synovium exposed to 1% L/M and 1% L/T showed significantly (#) lower fluorescence levels compared with control.
Figure 6.
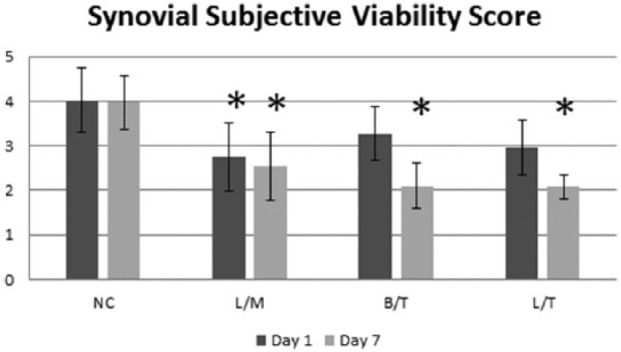
Day 7 synoviocyte viability score (A) and fluorescence levels (B) for synovial explants in each treatment group. No statistically significant differences were noted among groups.
Discussion
In this in vivo translational animal model study, a single intra-articular injection of clinically relevant doses of a combination of local anesthetic and corticosteroid was found to have detrimental effects on articular cartilage and synovium 24 hours after injection and for up to 7 days in culture. Compared with saline-injection controls, all local anesthetic-corticosteroid treatment groups were associated with significantly lower chondrocyte viability at 7 days. On day 1 following injection, 1% lidocaine-methylprednisolone and 1% lidocaine-triamcinalone were associated with significantly lower synoviocyte metabolism; 1% lidocaine-methylprednisolone was also associated with negative effects on chondrocyte metabolism at day 7 of culture. Low-dose (0.0625%) bupivacaine-triamcinalone was not associated with detrimental effects on chondrocyte or synoviocyte metabolism or synoviocyte viability at any time point in this study.
To the authors’ knowledge, this is the first in vivo study to substantiate the concern regarding chondrocyte and synoviocyte toxicity from a single intra-articular injection of clinically used local anesthetic-corticosteroid combinations. Several in vitro studies have been reported that have demonstrated chondrotoxic properties of local anesthetic agents. A 2006 study by Chu et al. found that even brief exposure of 0.5% bupivacaine to bovine chondrocytes, mimicking a single intra-articular injection, may cause a decrease in cell viability.13 Further work by the group found that exposure of bovine and human chondrocytes to 0.25% and 0.5% bupivacaine resulted in cytotoxic effects, whereas exposure to 0.125% bupivacaine resulted in no significant loss of cell viability compared with control12; the chondrotoxicity of bupivacaine alone was further validated in an in vivo rat study with 6-month follow-up.8
In a small-scale study conducted in 2011 by Syed et al., triamcinolone alone and in combination with bupivacaine demonstrated significant chondrotoxicity when tested against human monolayer cell cultures and articular plugs.16 Dragoo et al. found, in a study using similar samples, that lidocaine also exhibited significant chondrotoxic effects.5 Anz et al. examined the effects of bupivacaine and morphine on cartilage-synovium co-cultures, and they found a near 100% loss of tissue viability on exposure of 0.5% bupivacaine to the culture, with no gross chondrotoxicity of samples exposed to morphine.10 Farkas et al. observed that glucocorticoids may enhance the toxicity of local anesthetics when used in conjunction, on exposure to in vitro human chondrocyte cell cultures.3
Baker et al., in 2011, found that the introduction of magnesium as a local anesthetic helped reduce the toxic effect to the articular chondrocyte,9 and Piper and Kim have suggested that 0.5% ropivacaine may be a less chondrotoxic alternative to bupivacaine, based on the results of a 2008 in vitro study using human articular cartilage explants.17
In a canine explant culture model performed in our laboratory to screen individual products for determination of combinations for in vivo study, 1.0% lidocaine, 0.5% lidocaine, 0.25% bupivacaine, 0.125% bupivacaine, betamethasone, and methylprednisolone were associated with severe cell death and decreased cell metabolism within 24 hours of exposure compared with controls. In that study, even low-dose (0.0625%) bupivacaine demonstrated approximately 50% loss in cell viability despite a lack of significant changes in cell metabolism. Only triamcinolone was associated with no significant changes in cell viability or metabolism compared with control throughout the culture of the tissue.
This translational animal model study was designed to assess the most clinically relevant combinations from those screened in the in vitro study with respect to initial effects on chondrocyte and synoviocyte viability and metabolism. Dosage and volume of intra-articular injectate was chosen to directly correspond to those used in human patients, as well as current standard of care in veterinary medicine. Determining the short-term effects on the predominant cells present in all joints was felt to be the first step in addressing the safety of these local anesthetic-corticosteroid combinations. In general, physicians often utilize combination injections in clinical practice. The local anesthetic provides initial pain relief and allows the practitioner to differentiate the injected joint as a major pain generator or not, whereas the corticosteroid is included to decrease inflammation so that the patient may rehabilitate effectively. Based on data from the screening study, number of dogs available, and a prestudy power analysis, three local anesthetic-corticosteroid combinations were chosen for in vivo study: 1%L/M as the “worst case scenario,” 0.0625%B/T as the “best case scenario,” and 1%L/T as “mismatched” group to help determine whether local anesthetic or corticosteroid is most influential in terms of in vivo effects. The results from this translational animal model show that, of the pharmaceutical agents studied, only those combinations including lidocaine demonstrated a statistically significant deleterious effect on cell metabolism. The lidocaine-methylprednisolone group and the “mismatched” lidocaine-triamcinolone group behaved similarly, suggesting that 1% lidocaine may be the main culprit resulting in chondrotoxicity and synoviocyte toxicity after a single exposure. However, prior to testing each treatment alone and in combination, it is difficult to draw definitive conclusions regarding the synergistic toxicity of these agents in vivo.
Based on in vitro and in vivo data, the toxic effects of 1% lidocaine on intra-articular structures are, at the very least, concerning. As 1% lidocaine is short acting, and has no lasting analgesic effect within the joint, the utility of this agent for intra-articular injection is called into question. In our practices, we have discontinued all use of intra-articular lidocaine and recommend use of low-dose bupivacaine (0.0625%) for diagnostic purposes only when intra-articular local anesthetic is absolutely clinically necessary. Until further research can clarify the long-term safety of these or other local anesthetics on healthy and diseases articular cartilage and synovium, it seems clinically prudent to use only the least detrimental agent and as sparingly as possible.
The in vitro and in vivo data suggest more potential toxicity associated with use of methylprednisolone compared with triamcinolone. Historically, data regarding intra-articular triamcinolone injections have shown mixed results. Raynauld et al. reported in a randomized double-blind placebo controlled trial that intra-articular injections of triamcinolone alone have no long-term clinical effects on patients knees.18 However, other studies have reported that triamcinolone has chondrotoxic properties, mainly when used as a combination agent, based on histologic assessments.19-22 Our in vitro and in vivo studies demonstrate significant toxicity of methylprednisolone on healthy articular cartilage and synovium. Based on the current studies, we recommend triamcinolone for intra-articular corticosteroid injections when indicated, and we have discontinued using local anesthetic-corticosteroid combination injections in the clinical setting until further research demonstrates long-term safety and efficacy of these or other agents.
The mechanisms of toxicity associated with these agents are as yet unclear. Potential explanations include an incompatibility between synovial fluid and anesthetic rather than any direct toxicity of the anesthetics themselves,11 or possibly mitochondrial dysfunction stemming from mtDNA damage, leading to chondrocyte apoptosis.23 The effect of low pH in epinephrine-containing local anesthetic products has also been proposed as another possible factor contributing to their toxicity, based on a 2010 study comparing human chondrocyte cultures exposed to local anesthetics with and without epinephrine, as well as culture media titrated to particular pH levels; those cultures of low pH (4.0-5.5) were found to have higher losses of cell viability.24 Although pH of synovial fluid or culture media was not measured in this study, it seems unlikely that the effects of pH played a major role in chondrotoxicity and synoviotoxicity seen in this study based on the pH of the injectates and the use of only a single injection of each. In previous investigation, acidic levels of 5.5 or below were associated with higher loss of cell viability. All of the agents used in this study have pH 6.5 to 7.0. However, as the joint is mildy alkalotic at baseline, it is possible that relative changes in pH may still be responsible for cell toxicity. This should be examined in future studies aimed at determining mechanisms of action for toxicity in these combination products.
Limitations of this study must be considered. Financial and ethical limitations influenced the experimental design and prohibited the use of more dogs for this study. Treatment groups of local anesthetic-corticosteroid pairs were chosen based on our in vitro pilot data evaluating each substance alone, prior peer-reviewed literature, prestudy power analysis, and the common clinical practice pattern of combination injection. Future in vivo study should investigate these and other substances individually to control for variables that may have influenced our results. In our study, saline injection was used as negative control, as the contralateral shoulder was unavailable and being utilized for a different study. While it can be argued that saline injection is not equivalent to an untouched normal shoulder, our results in the saline group consistently demonstrated high viability and metabolic function for all samples at all time points. This profile compares favorably with results from historical controls using normal canine cartilage and synovium. When financial and/or ethical considerations limit use of research animals, placebo or sham controls are preferred over unaltered or normal controls and are considered adequate for hypotheses testing. In fact, placebo or sham controls (e.g., saline injection as in the present study) are required by most regulatory bodies, whereas unaltered, normal controls are not. Regarding the potential issue involving the use of the canines for multiple simultaneous studies, it is noted that this was IACUC approved and addresses the National Institutes of Health mandate of “Reduce, Refine, and/or Replace.” Dogs did not experience lameness or dysfunction in the 24 hours of study duration, and the other study involving the contralateral shoulder joint did not involve any systemic treatments. As such, we do not think this in any way affected our study results.
Other limitations of experimental design include the use of normal canine shoulder model with only two outcome measures at two short-term time points. While only two outcome measures at two short-term time points were included, the significant effects noted on both chondrocytes and synoviocytes in terms of both viability and function suggest that longer term, clinically significant toxicity is possible and should be investigated prior to recommending use of the combinations shown to be toxic in this study. While the canine shoulder closely mimics the human shoulder in terms of physiology, pathology, and clinical treatments, including intra-articular injections, important differences do exist that may influence short- and long-term responses to local anesthetics and corticosteroids. Furthermore, the injected joints were documented to be normal, healthy joints, and therefore may not fully represent the biologic environment or responses of a pathologic joint following intra-articular injection. However, the authors would suggest that the use of normal joints for this initial study provides stronger impact with respect to safety concerns for use of the agents that were shown to be toxic in this study. Nonetheless, these results should be replicated in a model of joint inflammation before definitive treatment recommendations can be made.
In conclusion, this translational animal model study is the first to demonstrate significant in vivo toxicity of local anesthetic-corticosteroid combinations to normal canine articular cartilage and synovium following a single intra-articular injection. The combination of 1% lidocaine-methylprednisolone was associated with the most adverse effects on articular tissues, whereas 0.0625% bupivacaine-triamcinalone was associated with minimal to no detrimental effects on chondrocyte and synoviocyte viability and metabolism when compared with saline. Our in vitro and in vivo data have resulted in changes to our clinical practices such that we use and recommend low-dose bupivacaine (0.0625%) for diagnostic purposes only when intra-articular local anesthetic is absolutely clinically necessary and triamcinolone for intra-articular corticosteroid injections when indicated. The authors have discontinued using local anesthetic-corticosteroid combination intra-articular injections until further research demonstrates long-term safety and efficacy of these or other agents.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The University of Missouri Animal Care and Use Committee granted authors approval to conduct this study (ACUC #7469 and ACUC #7332).
References
- 1. Allcock P. Diagnostic value of intra-articular anaesthetic in primary osteoarthritis of the hip. J Bone Joint Surg Br. 1998;80:934. [PubMed] [Google Scholar]
- 2. Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88:959-63. [DOI] [PubMed] [Google Scholar]
- 3. Farkas B, Kvell K, Czömpöly T, Illés T, Bárdos T. Increased chondrocyte death after steroid and local anesthetic combination. Clin Orthop Relat Res. 2010;468:3112-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1628-34. [DOI] [PubMed] [Google Scholar]
- 5. Dragoo JL, Braun HJ, Kim HJ, Phan HD, Golish SR. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 2012;40:794-9. [DOI] [PubMed] [Google Scholar]
- 6. Gomoll AH, Yanke AB, Kang RW, Chubinskaya S, Williams JM, Bach BR, et al. Long-term effects of bupivacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37:72-7. [DOI] [PubMed] [Google Scholar]
- 7. Gomoll AH, Kang RW, Williams JM, Bach BR, Cole BJ. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813-9. [DOI] [PubMed] [Google Scholar]
- 8. Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker JF, Byrne DP, Walsh PM, Mulhall KJ. Human chondrocyte viability after treatment with local anesthetic and/or magnesium: results from an in vitro study. Arthroscopy. 2011;27:213-7. [DOI] [PubMed] [Google Scholar]
- 10. Anz A, Smith MJ, Stoker A, Linville C, Markway H, Branson K, et al. The effect of bupivacaine and morphine in a coculture model of diarthrodial joints. Arthroscopy. 2009;25:225-31. [DOI] [PubMed] [Google Scholar]
- 11. Bogatch MT, Ferachi DG, Kyle B, Popinchalk S, Howell MH, Ge D, et al. Is chemical incompatibility responsible for chondrocyte death induced by local anesthetics? Am J Sports Med. 2010;38:520-6. [DOI] [PubMed] [Google Scholar]
- 12. Chu CR, Izzo NK, Coyle CH, Papas NE, Logar A. The in vitro effects of bupivacaine on articular chondrocytes. J Bone Joint Surg Br. 2008;90:814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu CR, Izzo NK, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693-9. [DOI] [PubMed] [Google Scholar]
- 14. Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39:2245-53. [DOI] [PubMed] [Google Scholar]
- 15. Perrot S, Dutertre-Catella H, Martin C, Rat P, Warnet JM. Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci. 2003;72:122-9. [DOI] [PubMed] [Google Scholar]
- 16. Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469:2941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91. [DOI] [PubMed] [Google Scholar]
- 18. Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370-7. [DOI] [PubMed] [Google Scholar]
- 19. Céleste C, Ionescu M, Robin Poole A, Laverty S. Repeated intraarticular injections of triamcinolone acetonide alter cartilage matrix metabolism measured by biomarkers in synovial fluid. J Orthop Res. 2005;23:602-10. [DOI] [PubMed] [Google Scholar]
- 20. Fubini SL, Todhunter RJ, Burton-Wurster N, Vernier-Singer M, MacLeod JN. Corticosteroids alter the differentiated phenotype of articular chondrocytes. J Orthop Res. 2001;19:688-95. [DOI] [PubMed] [Google Scholar]
- 21. Papacrhistou G, Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132-4. [DOI] [PubMed] [Google Scholar]
- 22. Robion FC, Doizé B, Bouré L, Marcoux M, Ionescu M, Reiner A, et al. Use of synovial fluid markers of cartilage synthesis and turnover to study effects of repeated intra-articular administration of methylprednisolone acetate on articular cartilage in vivo. J Orthop Res. 2001;19:250-8. [DOI] [PubMed] [Google Scholar]
- 23. Grishko V, Xu M, Wilson G, Pearsall AW., 4th Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18. [DOI] [PubMed] [Google Scholar]
- 24. Dragoo JL, Korotkova T, Kim HJ, Jagadish A. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38:1154-1159. [DOI] [PubMed] [Google Scholar]


