Abstract
Objective
This study assessed T1ρ relaxation dispersion, measured by magnetic resonance imaging (MRI), as a tool to noninvasively evaluate cartilage material and biochemical properties. The specific objective was to answer two questions: (1) does cartilage initial elastic modulus (E0) correlate with T1ρ dispersion effects and (2) does collagen or proteoglycan content correlate with T1ρ dispersion effects?
Design
Cadaveric patellae with and without visible cartilage damage on conventional MR were included. T2 and T1ρ relaxation times at 500 and 1000 Hz spin-lock field amplitudes were measured. We estimated T1ρ dispersion effects by measuring T1ρ relaxation time at 500 and 1000 Hz and T2 relaxation time and using a new tool, the ratio T1ρ/T2. Cartilage initial elastic modulus, E0, was measured from initial response of mechanical indentation creep tests. Collagen and proteoglycan contents were measured at the indentation test sites; proteoglycan content was measured by their covalently linked sulfated glycosaminoglycans (sGAG). Pearson correlation coefficients were determined, taking into account the clustering of multiple samples within a single patella specimen.
Results
Cartilage initial elastic modulus, E0, increased with decreasing values of T1ρ/T2 measurements at both 500 Hz (P = 0.034) and 1000 Hz (P = 0.022). 1/T1ρ relaxation time (500 Hz) increased with increasing sGAG content (P = 0.041).
Conclusions
T1ρ/T2 ratio, a new tool, and cartilage initial elastic modulus are both measures of water–protein interactions, are dependent on the cartilage structure, and were correlated in this study.
Keywords: T1ρ dispersion, articular cartilage, initial elastic modulus, proteoglycan, collagen
Introduction
Quantification of material properties and macromolecular content of articular cartilage is important in understanding joint health and disease.1 A method for noninvasive assessment of cartilage material properties and macromolecular content would be valuable to evaluate disease modifying treatment strategies for osteoarthritis and to diagnose osteoarthritis prior to extensive cartilage damage.2 Magnetic resonance imaging (MRI) is one noninvasive method that could be used to determine cartilage material properties and thus evaluate cartilage health.
Cartilage modulus is a measure of normalized cartilage deformation for a given mechanical stress and may characterize cartilage health.3 Cartilage moduli have been correlated with cartilage macromolecular content, that is, proteoglycan and collagen.4,5 Cartilage macromolecular content has also been shown to be correlated with MRI measurements, including delayed gadolinium-enhanced MRI of cartilage (dGEMRIC),6 T1ρ,7 T2,8 sodium,9 glycosaminoglycan chemical exchange-dependent saturation transfer (gagCEST),10 diffusion,11 and quantitative magnetization transfer.12 Thus, MRI measurements are likely to correlate with cartilage modulus. Previous studies in human cartilage relating MRI measurements with cartilage modulus, however, report inconsistent results. While some studies have reported a correlation of dGEMRIC index and T2 relaxation time with dynamic and equilibrium elastic moduli,13-15 other studies have reported no relationships16 or mixed relationships.17,18 T1ρ relaxation correlated with proteoglycan content at 500 Hz spin-lock field amplitude,19,20 was visually related to proteoglycan content at 1000 Hz spin-lock field amplitude,21 and was related to changes in collagen orientation.22,23 One study found a relationship between T1ρ relaxation at 500 Hz with phase angle, but not with other material properties.24 The relationship between T1ρ relaxation and elastic modulus has yet to be established for human cartilage.
T1ρ dispersion is an interesting property of T1ρ that may be related to cartilage macromolecular content7 and thus, cartilage elastic modulus. T1ρ dispersion is characterized by an increase in T1ρ relaxation time with increasing spin-lock field amplitude.25-28 Previous studies quantified T1ρ relaxation dispersion by determining the slope of the dispersion curve,29,30 which varied for several biochemically distinct tissues.29 Another study used a power law model to quantify T1ρ relaxation dispersion.25 In bovine nasal cartilage, native and trypsin degraded (to decrease macromolecular content) samples had different T1ρ relaxation dispersion curve shapes over 0 to 6000 Hz spin-lock field amplitudes.23 Based on this relationship between cartilage macromolecules and T1ρ dispersion, we reasoned that cartilage elastic modulus may be related to T1ρ dispersion effects.
This study assessed the potential of T1ρ dispersion as a noninvasive method to evaluate changes in cartilage leading to degeneration. The specific objectives were to answer two questions: (1) does initial elastic modulus correlate with T1ρ dispersion effects and (2) does proteoglycan or collagen content correlate with T1ρ dispersion effects?
Methods
We took the following steps: specimen acquisition and preparation, MRI, mechanical testing, biochemistry, and statistical analysis. In agreement with previous studies,13,15,24 only the upper region of cartilage, the region most affected by the indentation test, was included in our analysis. Specific determination of the region of interest is discussed in the Magnetic Resonance Imaging and Biochemical Measurements subsections.
Specimens
We examined 17 cadaveric patellae from 10 males and 7 females ranging in age from 20 to 90 years (median age 57 years). Seven cadavers contributed 1 patella and 5 cadavers contributed both patellae to this study. Fresh-frozen cadaveric knee joints (mid-femur to mid-tibia) were acquired from the National Disease Research Interchange (Philadelphia, PA), Anatomy Gifts Registry (Glen Burnie, MD), and the University of California San Francisco Willed Body Program (San Francisco, CA). Healthy patellae and those with varied degrees of degeneration were included in this study (photograph of representative patella, Fig. 1). Any patella regions with full-thickness defects were excluded.
Figure 1.
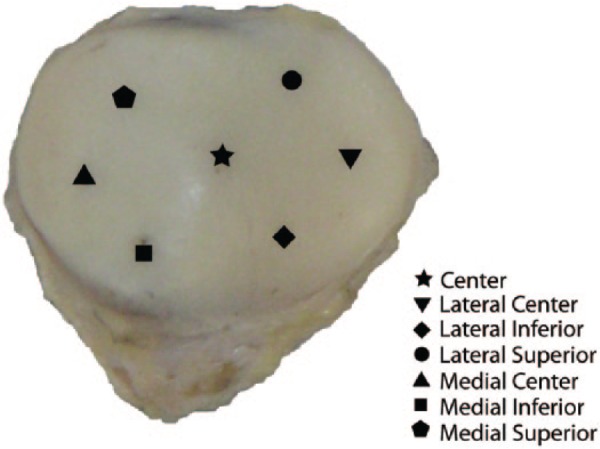
Photograph of a representative patella and illustration of the 7 regions. Figure adapted from Keenan et al. (2011).19
Specimen Preparation
The patella was dissected from the knee joint, and the anterior patella bone was removed with a band saw, leaving the intact cartilage surface attached to a layer of subchondral bone. The subchondral bone was bonded to an acrylic plate using ethyl-2-cyanoacrylate adhesive (Krazy Glue, New York, NY) as described by Keenan et al.,19 and then MRI was done. Between imaging and mechanical testing, the specimens were stored on their plates surrounded by gauze soaked in phosphate-buffered saline plus protease inhibitors (0.005 M benzamidine–HCl, 0.01 M N-ethylaleimide, 0.001 M phenylmethylsulfonylfluoride, 0.005 M disodium ethylenediamine tetra-acetic acid; Sigma Aldrich, St. Louis, MO)31 at −20 °C. Specimens were brought to room temperature prior to imaging and mechanical testing; biochemical measurements immediately followed the mechanical testing.
Magnetic Resonance Imaging
MRI at 3 T was performed using a GE HDx system (GE Healthcare, Milwaukee, WI) with a transmit/receive quadrature wrist coil (Mayo Clinical Medical Devices, Rochester, MN). The plate-mounted patella specimen was placed in a secondary container, which was filled with phosphate-buffered saline plus protease inhibitors. The patella was oriented with the normal to the most prominent point of the subchondral bone surface perpendicular to B0; the image plane was also oriented perpendicular to B0.19 A multislice, multiecho spiral 2-dimensional sequence was used to acquire T1ρ32 (spin-locking field amplitudes 500 and 1000 Hz) images with 3.0 mm slice thickness, 0 mm slice spacing, 10 cm field of view, 0.3 mm in-plane pixel size, 2-second repetition time, 6 ms echo time, and 5 spin-lock times: 0, 14, 29, 59, and 118 ms. T2 images were acquired with the same parameters as T1ρ images and echo times: 6, 20, 35, 65, and 124 ms. The total acquisition time for the independently acquired T1ρ 500 Hz, T1ρ 1000 Hz and T2 scans was approximately 21 minutes. A 3-dimensional spoiled gradient echo (SPGR) sequence with 3.0 mm slice thickness, 0 mm slice spacing, 10 cm field of view, 0.3 mm in-plane pixel size, repetition time/echo time 13.5/2.5 ms and 30° flip angle was obtained for anatomic reference and modified Noyes scoring.33
Mechanical Testing
Creep indentation tests were performed on a mechanical test system, as described by Keenan et al.34 Locations on the surface of each patella underwent indentation creep tests while submerged in a bath of phosphate-buffered saline plus protease inhibitors.35 The initial elastic modulus was estimated from the experimental data at an initial time, t0, of 0.15 seconds using the elastic solution for the initial modulus given by Hayes et al.36:
In Equation (1), P0 and d0 are the applied load (0.35 N) and resulting displacement of the creep test, respectively; ν0 is the initial Poisson’s ratio; r is the indenter radius (1 mm); and κ is a dimensionless number dependent on the indenter radius, indentation displacement, cartilage thickness and Poisson’s ratio.36 Based on the assumption of near-incompressibility,37 an appropriate value was assigned to the initial Poisson’s ratio, ν0 = 0.47 for all specimens.38 A needle-probe method was used to measure the cartilage thickness,39 and a small drop of India ink was applied at the location of the needle-probe thickness measurement.
Biochemical Measurements
Full-thickness cartilage cylindrical samples (3 mm diameter) were removed from locations that were both within the MR image slice and close to the indentation test sites, with care taken to avoid the site of needle-probe thickness measurement. The indentation test and needle-probe thickness measurements may breakdown the tissue and alter the cartilage properties; thus, a nearby site (within 2 mm) was selected for the biochemistry.4 A 3 mm × 3 mm grid, corresponding to the MRI slice thickness, was placed across the cartilage sample; biochemistry location was identified in the MR images based on its location on the grid. Cartilage samples were cut in half along a line drawn halfway between the articular surface and cartilage/bone interface of the cartilage sample, and only the upper region, which contributes more to the indentation test,13,15,24 was analyzed in this study. Each sample was weighed to obtain wet weight. Each sample was dried at 50 °C for 12 hours, then digested in 1 mL papain solution overnight in a water bath at 63 °C, and stored at 4 °C. Proteoglycan content was measured by their covalently linked sulfated glycosaminoglycans (sGAG). Total sGAG content was quantified using the dimethylmethylene blue assay, which measures sGAG content using chondroitin sulfate as a standard.40 Collagen content was determined from hydroxyproline content; the papain-digested samples were acid hydrolyzed, and hydroxyproline was measured using the chloramine-T/Ehrlich’s reagent assay.41 sGAG and collagen values are reported as a percentage of the wet weight of each 3 mm diameter sample.
T1ρ Ratio
We aim to measure T1ρ dispersion effects with a simple model, using clinically feasible measurements. Thus, we estimated T1ρ dispersion effects with a ratio, determining T1ρ dispersion as a function of the baseline T2 relaxation time: T1ρ/T2. We measured T2 relaxation and T1ρ relaxation at two spin-lock frequencies: 500 and 1000 Hz; a T2 measurement is equivalent to a T1ρ measurement at spin-lock field amplitude of 0 Hz.42
In a liquid, an unstructured environment with fast dipolar interactions, the T1ρ value will approach the T2 value, and the ratio T1ρ/T2 will approach 1.43-46 In a more structured environment, T1ρ will increase compared to T2, and the ratio T1ρ/T2 will be greater than 1. Thus, the ratio, T1ρ/T2, is a measure of the complexity of the local environment of the spins.
Data Analysis and Statistics
Seven regions across the surface of each patella were examined (Fig. 1): center, lateral center, lateral inferior, lateral superior, medial center, medial inferior, and medial superior.19 To assess cartilage damage, an experienced radiologist performed modified Noyes scoring33 of all patellae at each of the seven regions using the 3-dimensional SPGR images and the first echo of T2 images. Scores were assigned based on cartilage appearance as follows: 0, normal appearance; 1, increased signal intensity on the T2 images; 2A, superficial partial thickness defect; 2B, deep partial thickness defect; and 3, full-thickness lesion. Analyses were performed on the entire data set and on subsets determined by the Noyes score.
MRI regions of interest (ROI) were determined at the locations corresponding to the biochemical measurements. In the plane of the 3.0 mm MRI slice, the ROI was approximately 3.0 mm wide and a minimum of 0.6 mm (2 pixels) tall; the height of the ROI varied with specimen thickness. The upper region was determined by drawing a line halfway between articular surface and cartilage/bone interface across the 3 mm ROI width; the upper and lower bounds of the ROI followed the cartilage morphology. To limit partial volume effects, the pixel at the apparent cartilage surface was not included in the ROI, as it could include the cartilage and phosphate-buffered saline plus protease inhibitors interface. Sample locations were excluded due to imaging artifacts, full-thickness defect or thin cartilage (fewer than 4 pixels through the depth of the cartilage), and potential magic angle effect.47 To reduce interference from the magic angle effect, all patella were viewed in the sagittal plane, the plane parallel to B0. Gründer et al.48 and Xia et al.47 both showed that if there was a large curvature of the subchondral bone surface, resulting in an orientation angle greater than 10° from perpendicular to B0, the magic angle effect could alter the relaxation value; thus, the sample was excluded.
A total of 119 cartilage samples were examined; data from 79 locations were included in the analysis. Creep tests could not be performed at 13 locations because of excessive damage to the cartilage surface; thus, there was no estimate of initial elastic modulus at these locations. Twenty-one sample locations were excluded due to an image artifact (e.g., bubble), full-thickness defect or thin cartilage (3 of these sample locations were also locations where the creep indentation test could not be performed and thus already excluded from the analysis). Nine sample locations were excluded because of potential magic angle interference.
Average T1ρ and T2 relaxation times were measured for each ROI at each spin-lock field amplitude using OsiriX,49 and T1ρ/T2 was determined. T1ρ and T2 relaxation times were computed pixel-by-pixel and then averaged over the ROI. When determining T2 relaxation, the fifth echo was not used because the signal in the cartilage was not sufficiently different from the noise.
To assess relationships between T1ρ/T2 and initial elastic modulus, within-clusters Pearson correlation coefficients were determined; this method takes into account the clustering of multiple samples within a single patella specimen and within a donor.50 The data were divided into two subsets based on Noyes score33. The first subset (n = 58) consisted of locations with a Noyes score of 0 (no visible cartilage damage based on conventional SPGR MRI). The second subset consisted of locations with visible damage on MRI, with Noyes scores of 1 (n = 10), 2A (n = 10), 2B (n = 1), or 3 (n = 0). There were insufficient data (two or fewer samples per specimen) in the subset of nonzero Noyes scores to further subdivide that subset, and we could not complete a within-clusters Pearson correlation analysis. Statistical analyses were performed using Stata Release 12 (StataCorp LP, College Station, TX). A P value less than 0.05 was considered statistically significant.
Results
Initial elastic modulus increased with decreasing values of T1ρ/T2 ratio (Table 1, top right corner). Pearson correlation coefficients were larger for the subset of samples with no visible cartilage damage on conventional MR compared with the entire data set (Table 1). In all cases, there were moderate relationships between initial elastic modulus and T1ρ/T2 (Fig. 2). sGAG and collagen contents were not related to T1ρ/T2 (Table 1).
Table 1.
Pearson Correlation Coefficients Between Initial Elastic Modulus (MPa), sGAG Content (% Wet Weight), Collagen Content (% Wet Weight), and 1/T2, 1/T1ρ, T1ρ/T2.
| Relaxation (ms−1) |
T1ρ/T2 |
|||||
|---|---|---|---|---|---|---|
| Group (n) | 1/T2 | 1/T1ρ 500 Hz | 1/T1ρ 1000 Hz | T1ρ(500 Hz)/T2 | T1ρ(1000 Hz)/T2 | |
| E0 (MPa) | Noyes = 0 (58) | −0.09 (0.553) | 0.16 (0.320) | 0.15 (0.349) | –0.37 (0.016) | –0.32 (0.034) |
| All (79) | −0.09 (0.490) | 0.12 (0.340) | 0.17 (0.194) | –0.27 (0.034) | –0.29 (0.021) | |
| sGAG content (% wet weight) | Noyes = 0 (58) | 0.31 (0.044) | 0.34 (0.024) | 0.28 (0.072) | −0.01 (0.945) | 0.08 (0.630) |
| All (79) | 0.14 (0.273) | 0.26 (0.041) | 0.22 (0.082) | −0.14 (0.272) | −0.09 (0.490) | |
| Collagen content (% wet weight) | Noyes = 0 (58) | 0.07 (0.678) | 0.0 (0.992) | −0.01 (0.946) | −0.12 (0.466) | −0.10 (0.525) |
| All (79) | −0.05 (0.678) | −0.18 (0.166) | −0.20 (0.119) | −0.16 (0.204) | −0.15 (0.253) | |
Note: The correlation coefficient (R) is given with the P value in parentheses. A P value less than 0.05 was considered significant, and those results are given in boldface. sGAG = sulfated glycosaminoglycan.
Figure 2.
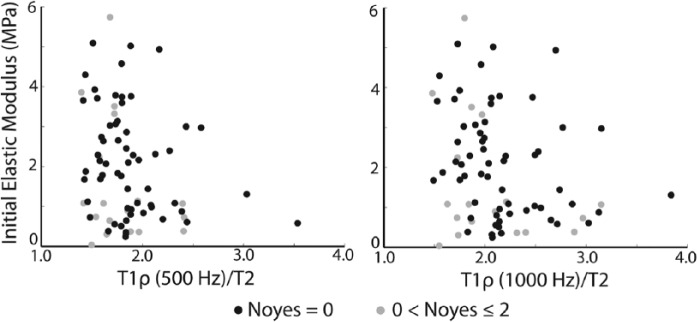
Plot of initial elastic modulus and T1ρ (500 Hz)/T2 (left) and T1ρ (1000 Hz)/T2 (right) data.
T1ρ dispersion curves from one patella sample are shown (Fig. 3); data from a single patella that is representative of the entire data set is shown for clarity. The T1ρ/T2 ratio for this specimen increased with increasing spin-lock frequency, as expected (Table 2). In the subset of samples with no visible cartilage damage on conventional MR, T1ρ/T2 was smaller than in the entire data set. Thus, while there was T1ρ dispersion, T1ρ was not much greater than T2 (Table 3). 1/T2 and 1/T1ρ relaxation times were similar between the entire data set and the subset of samples with no visible cartilage damage on conventional MR.
Figure 3.
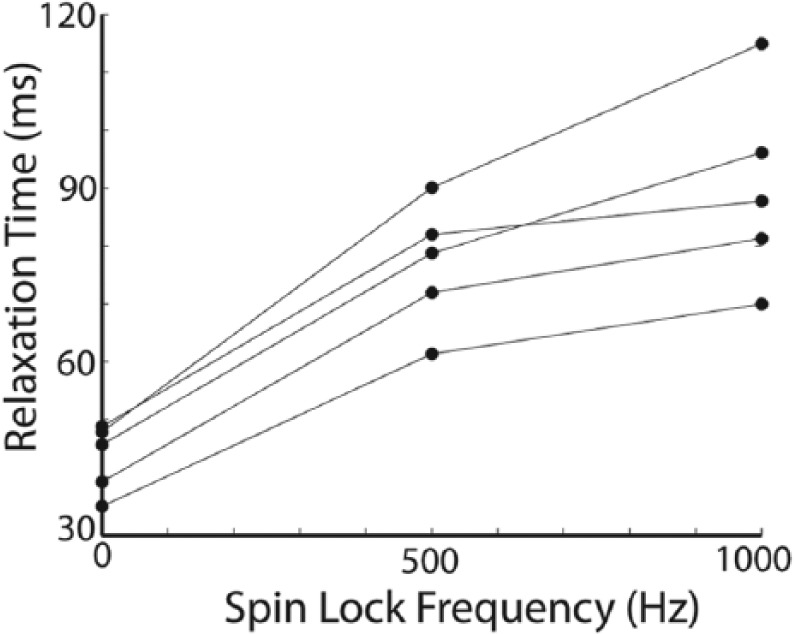
The dispersion curves from one patella sample are shown; T1ρ relaxation time increases with increasing spin-lock frequency. T2 relaxation time, equivalent to T1ρ (0 Hz), is given on the y-axis where spin-lock frequency is 0 Hz. T1ρ/T2 ratios for this patella sample are given in Table 2.
Table 2.
T1ρ/T2 Ratios for One Specimen at Spin-Lock Field Amplitudes 0, 500, and 1000 Hz (T1ρ Dispersion Plot for This Specimen Is Shown in Fig. 3).
| T1ρ/T2 | Spin-Lock = 0 Hz | Spin-Lock = 500 Hz | Spin-Lock = 1000 Hz | Noyes Score |
|---|---|---|---|---|
| Sample 1 | 1.00 | 1.72 | 2.11 | 0 |
| Sample 2 | 1.00 | 1.88 | 2.40 | 2 |
| Sample 3 | 1.00 | 1.83 | 2.07 | 0 |
| Sample 4 | 1.00 | 1.68 | 1.80 | 1 |
| Sample 5 | 1.00 | 1.75 | 2.00 | 0 |
Table 3.
Mean ± Standard Deviation Values for Cartilage Measurements.
| Parameter | All Samples (n = 79) | Noyes = 0 (n = 58) |
|---|---|---|
| E0 (MPa) | 1.99 ± 1.40 | 2.15 ± 1.36 |
| sGAG (% wet weight) | 1.66 ± 1.07 | 1.75 ± 1.05 |
| Collagen (% wet weight) | 1.74 ± 0.38 | 1.78 ± 0.42 |
| 1/T2 (s−1) | 26.97 ± 7.55 | 28.20 ± 7.62 |
| 1/T1ρ 500 Hz (s−1) | 14.56 ± 3.39 | 14.91 ± 3.30 |
| 1/T1ρ 1000 Hz (s−1) | 12.69 ± 2.86 | 12.91 ± 2.61 |
| T1ρ (500 Hz)/T2 | 1.86 ± 0.37 | 1.88 ± 0.38 |
| T1ρ(1000 Hz)/T2 | 2.14 ± 0.46 | 2.17 ± 0.45 |
Note: Results are presented for all samples and the subset of samples with no visible cartilage damage on conventional magnetic resonance (Noyes = 0).
Representative 1/T2 and 1/T1ρ relaxation time maps (Fig. 4B-D) and T1ρ/T2 maps (Fig. 4E and F) for a single axial slice from one patella specimen are shown.
Figure 4.
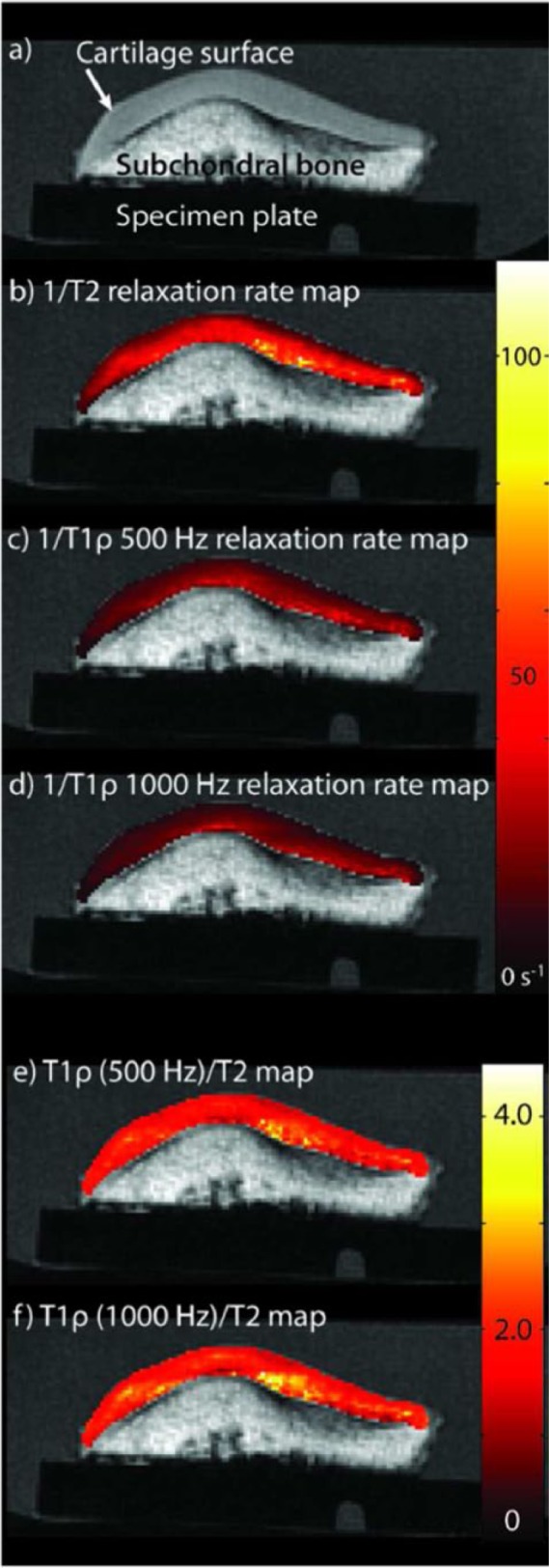
An axial spoiled gradient echo (SPGR) image from one patella specimen is shown (A); the main magnetic field, B0, is perpendicular to the image plane. Representative 1/T2 (B) and 1/T1ρ relaxation time maps for 500 Hz (C), and 1000 Hz (D) are shown. Representative T1ρ/T2 maps are shown for T1ρ 500 Hz (E) and T1ρ 1000 Hz (F).
There was no relationship between 1/T2, 1/T1ρ relaxation time (at either spin-lock field amplitude) and initial elastic modulus, and the relationships between 1/T2, 1/T1ρ relaxation time (at either spin-lock field amplitude) and macromolecular content were inconsistent. 1/T2 relaxation time and 1/T1ρ relaxation times at spin-lock field amplitudes 500 and 1000 Hz were related to initial elastic modulus neither in the entire data set nor in the subset of samples with no visible cartilage damage on conventional MR (Table 1). 1/T2 relaxation time increased with increasing sGAG content in the subset of samples with no visible damage on conventional MR (Table 1). 1/T1ρ relaxation time at spin-lock field amplitude 500 Hz increased with increasing sGAG content in the entire data set and in the subset of samples with no visible damage on conventional MR (Table 1). 1/T1ρ relaxation time at spin-lock field amplitude 1000 Hz had a similar relationship trend with sGAG content, though not statistically significant (Table 1). 1/T2 and 1/T1ρ relaxation times (at either spin-lock field amplitude) were not related to collagen content (Table 1).
Initial elastic modulus increased with increasing sGAG content in the entire data set (Table 4 and Fig. 5). Initial elastic modulus was neither related to collagen content in the entire data set nor to sGAG or collagen contents in the subset of samples with no visible damage on conventional MR (Table 4).
Table 4.
Pearson Correlation Coefficients Between Initial Elastic Modulus (MPa) and sGAG, Collagen Content (% Wet Weight).
| Group (n) | sGAG content | Collagen Content | |
|---|---|---|---|
| E0 | Noyes = 0 (58) | 0.25 (0.105) | 0.22 (0.153) |
| All (79) | 0.36 (0.004) | 0.01 (0.320) |
Note: The correlation coefficient (R) is given with the P value in parentheses. A P value less than 0.05 was considered significant, and those results are given in boldface. sGAG = sulfated glycosaminoglycan.
Figure 5.
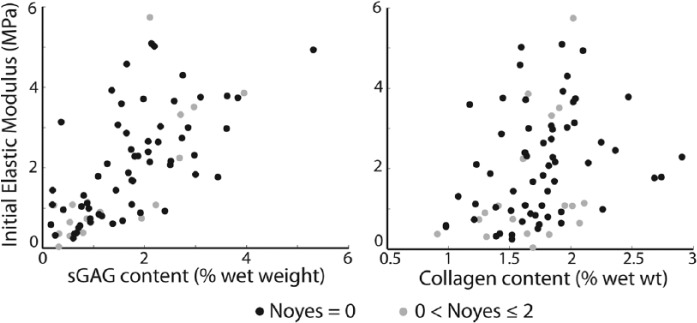
Plot of initial elastic modulus and sulfated glycosaminoglycan (sGAG) content data (left) and collagen content data (right).
Discussion
In this in vitro study of human patellar cartilage, we found that T1ρ/T2, an estimate of T1ρ dispersion effects, may be able to noninvasively evaluate changes in cartilage initial elastic modulus. Initial elastic modulus was related to T1ρ/T2 in the entire data set and in the subset of samples with no MR-visible cartilage damage. This finding is interesting in light of prior MR work that has related T1ρ to proteoglycan in cartilage19-21 and T2 to collagen orientation and structure.8,47 T1ρ/T2 may have a complex relationship with cartilage macromolecules, explaining the relationship between T1ρ/T2 and initial elastic modulus.
The T1ρ/T2 ratio is not sensitive to a single macromolecular effect; rather it is responsive to the bulk cartilage properties, including molecular-level dynamics—the collagen/sGAG molecules themselves, the way they interact with other molecules and with water. Similarly, cartilage initial elastic modulus, while correlated with sGAG content, is also dependent on the interactions between water, proteoglycans and collagen structure. Thus, T1ρ/T2 and initial elastic modulus were correlated in this study.
Based on the correlations between initial elastic modulus and sGAG content and 1/T1ρ and sGAG content, we expected 1/T1ρ relaxation time to correlate with initial elastic modulus. In this study, 1/T2 and 1/T1ρ (500 and 1000 Hz) relaxation times were not correlated to initial elastic modulus. This might be due to the challenges of cadaveric cartilage. Previous results relating MRI measurements to cartilage moduli were varied, especially in the patella and when using cadaveric cartilage.14,16-18,24 In agreement with previous results, cartilage elastic modulus increased with increasing sGAG content. Aggregate modulus and GAG content were significantly correlated in the patella (P < 0.001),4 while collagen has (P < 0.1)5 and has not4 been related to cartilage creep moduli in cadaveric cartilage.
In this study, the relationship between 1/T1ρ relaxation time and sGAG content varied with spin-lock field amplitude. Previous studies in human cartilage also varied: one study found 1/T1ρ relaxation time at 1000 Hz is influenced by tissue hydration and molecular-level dynamics in addition to GAG content and collagen orientation,21 one study found a correlation with sGAG content, but not collagen content at 500 Hz,20 and another study found no relationship with sGAG content at 500 Hz.19 In previous studies, T2 relaxation was51 and was not19,20 related to proteoglycan content, which could be a result of the cartilage hydration or complex molecular dynamics between sGAG, water and collagen. In this study, 1/T2 relaxation time was related to sGAG content only in the subset of samples with no visible damage on conventional MR. Once damage is visible on conventional MR, there are likely significant changes to the collagen matrix and significant loss of sGAG; thus, a comparison between sGAG content and 1/T2 relaxation time is less meaningful. T2 relaxation depends on collagen structure,52 but as reported previously, collagen content, measured biochemically, was not associated with T2 relaxation.53 In the future, polarized light microscopy can be used to better relate cartilage structure to MRI parameters.47
The initiation and progression of osteoarthritis is not well understood, and the evaluation of cartilage modulus and macromolecular content prior to and during the degenerative process may lead to new understandings of osteoarthritis etiology. We evaluated cartilage without and with some visible damage on conventional MR, but we did not include specimens with full-thickness defects, that is, with advanced cartilage degeneration. Additional data from samples with some MR-visible damage is needed to understand differences between these subsets. In the future, histology can be used to evaluate non–MR-visible changes and provide additional information.
The Pearson correlation coefficients relating T1ρ/T2 to elastic modulus and 1/T2, 1/T1ρ relaxation time to cartilage macromolecules were weak to moderate; thus, these relationships may be difficult to apply in individual patients. Previous cadaveric studies have faced similar challenges with moderate correlation coefficients.14 Correlation coefficients and clinical significance may improve if the entire knee joint is studied rather than focusing on the patella alone.4,17 In addition to using the entire knee joint, correlation coefficients could improve if more samples were included or if a wider range of cartilage is included; because of our mechanical test, we were limited to cartilage with an intact surface and a thickness greater than 1 mm. The majority of samples used in this study did not have visible cartilage damage on conventional MR, and there were no samples with full-thickness defects. The results of this study would therefore not necessarily apply to advanced osteoarthritis. Our results are primarily applicable to the early stages of cartilage degeneration. Between MR measurements and mechanical testing, the patella sample was frozen for no more than nine months, and this freeze/thaw cycle could have altered the cartilage properties, specifically the macromolecular content.54 Previous studies show that freeze/thaw cycles do54 and do not22 change the MR properties. Cartilage biochemistry may be affected by the indentation test. However, cartilage initial elastic modulus was strongly related to sGAG content in agreement with previous work5; this result is a validation of our experimental methods.
In summary, the method for estimation of T1ρ dispersion is interesting and warrants further research. T1ρ/T2 has potential to noninvasively determine cartilage modulus, especially at 500 Hz. Specific absorption rate constraints prohibit in vivo imaging at 1000 Hz,55 but advances in T1ρ imaging techniques may be able to overcome the specific absorption rate issues56 and allow application of T1ρ(1000 Hz)/T2 in vivo. T1ρ/T2 has clinical potential to advance the understanding of osteoarthritis and other cartilage diseases by noninvasively measuring changes in cartilage initial elastic modulus in vivo prior to advanced cartilage damage.
Footnotes
Acknowledgments and Funding: The authors wish to acknowledge Derek Lindsey (Department of Veterans Affairs, Rehabilitation R&D Musculoskeletal Research Laboratory, Palo Alto, CA) and Dr. Weitian Chen (Global Applied Science Laboratory, Menlo Park, CA) for technical support. This work was completed at Stanford University. The authors acknowledge financial support from the following sources: NIH EB002524; NIH EB005790; GE Healthcare; Bio-X Fellowship; Department of Veterans Affairs, Rehabilitation R&D Service Grant No. A2592R; and equipment resources from GE Healthcare.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs. Gold and Pauly receive research support from GE Healthcare. The other authors have no conflicts of interest to disclose.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Omoumi P, Teixeira P, Delgado G, Chung CB. Imaging of lower limb cartilage. Top Magn Reson Imaging. 2009;20:189-201. [DOI] [PubMed] [Google Scholar]
- 2. Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayes WC, Mockros LF. Viscoelastic properties of human articular cartilage. J Appl Physiol. 1971;31:562-8. [DOI] [PubMed] [Google Scholar]
- 4. Froimson MI, Ratcliffe A, Gardner TR, Mow VC. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage 1997;5:377-86. [DOI] [PubMed] [Google Scholar]
- 5. Kempson GE, Muir H, Swanson SA, Freeman MA. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970;215:70-7. [DOI] [PubMed] [Google Scholar]
- 6. Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857-65. [DOI] [PubMed] [Google Scholar]
- 7. Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1ρ-relaxation in articular cartilage: effects of enzymatic degradation. Magn Resonan Med. 1997;38:863-67. [DOI] [PubMed] [Google Scholar]
- 8. Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med. 2000;43:676-81. [DOI] [PubMed] [Google Scholar]
- 9. Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci U S A. 2008;105:2266-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11:465-78. [DOI] [PubMed] [Google Scholar]
- 12. Stikov N, Keenan KE, Pauly J, Smith R, Dougherty R, Gold GE. Cross-relaxation imaging of human articular cartilage. Magn Reson Med. 2011;66:725-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baldassarri M, Goodwin JS, Farley ML, Bierbaum BE, Goldring SR, Goldring MB, et al. Relationship between cartilage stiffness and dGEMRIC index: correlation and prediction. J Orthop Res. 2007;25:904-12. [DOI] [PubMed] [Google Scholar]
- 14. Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: relationships with tissue mechanical properties. J Orthop Res. 2006;24:366-74. [DOI] [PubMed] [Google Scholar]
- 15. Samosky JT, Burstein D, Eric Grimson W, Howe R, Martin S, Gray ML. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res 2005;23:93-101. [DOI] [PubMed] [Google Scholar]
- 16. Nissi MJ, Rieppo J, Toyras J, Laasanen MS, Kiviranta I, Nieminen MT, et al. Estimation of mechanical properties of articular cartilage with MRI—dGEMRIC, T-2 and T-1 imaging in different species with variable stages of maturation. Osteoarthritis Cartilage. 2007;15:1141-8. [DOI] [PubMed] [Google Scholar]
- 17. Kurkijarvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, Nieminen MT. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med 2004;52:41-6. [DOI] [PubMed] [Google Scholar]
- 18. Lammentausta E, Kiviranta P, Töyräs J, Hyttinen MM, Kiviranta I, Nieminen MT, et al. Quantitative MRI of parallel changes of articular cartilage and underlying trabecular bone in degeneration. Osteoarthritis Cartilage 2007;15:1149-57. [DOI] [PubMed] [Google Scholar]
- 19. Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T(1ρ) and T(2) in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1ρ MRI in articular cartilage systems. Magn Reson Med. 2004;51:503-9. [DOI] [PubMed] [Google Scholar]
- 22. Mlynarik V, Szomolanyi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169:300-7. [DOI] [PubMed] [Google Scholar]
- 23. Wang N, Xia Y. Orientational dependent sensitivities of T(2) and T (1ρ) towards trypsin degradation and Gd-DTPA (2-) presence in bovine nasal cartilage. MAGMA. 2012;25:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang SY, Souza RB, Ries M, Hansma PK, Alliston T, Li X. Local tissue properties of human osteoarthritic cartilage correlate with magnetic resonance T(1)ρ relaxation times. J Orthop Res. 2011;29:1312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen EL, Kim RJ. Magnetic resonance water proton relaxation in protein solutions and tissue: T(1ρ) dispersion characterization. PLoS One. 2010;5:e8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rommel E, Kimmich R. Volume-selective determination of the spin-lattice relaxation time in the rotating frame T1ρ, and T1ρ imaging. Magn Reson Med. 1989;12:209-18. [DOI] [PubMed] [Google Scholar]
- 27. Rommel E, Kimmich R. T1ρ dispersion imaging and volume-selective T1ρ dispersion weighted NMR spectroscopy. Magn Reson Med. 1989;12:390-9. [DOI] [PubMed] [Google Scholar]
- 28. Sepponen RE, Pohjonen JA, Sipponen JT, Tanttu JI. A method for T1ρ imaging. J Comput Assist Tomogr. 1985;9:1007-11. [DOI] [PubMed] [Google Scholar]
- 29. Koskinen SK, Niemi PT, Kajander SA, Komu ME. T1ρ dispersion profile of rat tissues in vitro at very low locking fields. Magn Reson Imaging. 2006;24:295-9. [DOI] [PubMed] [Google Scholar]
- 30. Niemi PT, Komu ME, Koskinen SK. Tissue specificity of low-field-strength magnetization transfer contrast imaging. J Magn Reson Imaging. 1992;2:197-201. [DOI] [PubMed] [Google Scholar]
- 31. Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619-36. [DOI] [PubMed] [Google Scholar]
- 32. Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929-36. [DOI] [PubMed] [Google Scholar]
- 33. Kijowski R, Blankenbaker DG, Klaers JL, Shinki K, De Smet AA, Block WF. Vastly undersampled isotropic projection steady-state free precession imaging of the knee: diagnostic performance compared with conventional MR. Radiology. 2009;251:185-94. [DOI] [PubMed] [Google Scholar]
- 34. Keenan KE, Kourtis LC, Besier TF, Lindsey DP, Gold GE, Delp SL, et al. New resource for the computation of cartilage biphasic material properties with the interpolant response surface method. Comput Methods in Biomech Biomed Eng. 2009;12:415-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keenan KE, Pal S, Lindsey DP, Besier TF, Beaupre GS. A viscoelastic constitutive model can accurately represent entire creep indentation tests of human patella cartilage. J Appl Biomech. 2013;29:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayes W, Keer L, Herrmann G, Mockros L. Mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541-51. [DOI] [PubMed] [Google Scholar]
- 37. Mak AF, Lai WM, Mow VC. Biphasic indentation of articular-cartilage. 1. Theoretical-analysis. J Biomech. 1987;20:703-14. [DOI] [PubMed] [Google Scholar]
- 38. Carter DR, Orr TE, Fyhrie DP, Schurman DJ. Influences of mechanical-stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res. 1987;(219):237-250. [PubMed] [Google Scholar]
- 39. Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330-40. [DOI] [PubMed] [Google Scholar]
- 40. Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247-8. [DOI] [PubMed] [Google Scholar]
- 41. Woessner M. Determination of hydroxyproline content in connective tissues. In: Hall DA, editor. The methodology of connective tissue research. Oxford, England: Joynson-Bruvvers; 1976. p. 227-34. [Google Scholar]
- 42. Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med. 2004;52:1103-9. [DOI] [PubMed] [Google Scholar]
- 43. Jones G. Spin-lattice relaxation in the rotating frame: weak-collision regime. Phys Rev. 1966;148:332-5. [Google Scholar]
- 44. Solomon I. Rotary spin echoes. Phys Rev Lett. 1959;2:301-2. [Google Scholar]
- 45. Torrey HC. Transient nutations in nuclear magnetic resonance. Phys Rev. 1949;76:1059-68. [Google Scholar]
- 46. Andronesi OC, Mintzopoulos D, Righi V, Psychogios N, Kewarwani M, He J, et al. Combined off-resonance imaging and T2 relaxation in the rotating frame for positive contrast MR imaging of infection in the murine burn model. J Magn Reson Imaging. 2010;32:1172-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393-406. [DOI] [PubMed] [Google Scholar]
- 48. Gründer W, Wagner M, Werner A. MR-microscopic visualization of anisotropic internal cartilage structures using the magic angle technique. Magn Reson Med. 1998;39:376-82. [DOI] [PubMed] [Google Scholar]
- 49. Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bland JM, Altman DG. Calculating correlation coefficients with repeated observations. Part 1—correlation within subjects. BMJ. 1995;310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, et al. T(1ρ) and T(2) mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35:147-55. [DOI] [PubMed] [Google Scholar]
- 52. Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602-21. [DOI] [PubMed] [Google Scholar]
- 53. Nissi MJ, Toyras J, Laasanen MS, Rieppo J, Saarakkala S, Lappalainen R, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557-64. [DOI] [PubMed] [Google Scholar]
- 54. Fishbein KW, Canuto HC, Bajaj P, Camacho NP, Spencer RG. Optimal methods for the preservation of cartilage samples in MRI and correlative biochemical studies. Magn Reson Med. 2007;57:866-73. [DOI] [PubMed] [Google Scholar]
- 55. Witschey WR, Borthakur A, Fenty M, Kneeland BJ, Lonner JH, McArdle EL, et al. T1ρ MRI quantification of arthroscopically confirmed cartilage degeneration. Magn Reson Med. 2010;63:1376-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wheaton AJ, Borthakur A, Corbo M, Charagundla SR, Reddy R. Method for reduced SAR T1ρ-weighted MRI. Magn Reson Med. 2004;51:1096-102. [DOI] [PubMed] [Google Scholar]


