Abstract
Objective
The efficacy and safety of BST-CarGel®, a chitosan scaffold for cartilage repair was compared with microfracture alone at 1 year during a multicenter randomized controlled trial in the knee. This report was undertaken to investigate 5-year structural and clinical outcomes.
Design
The international randomized controlled trial enrolled 80 patients, aged 18 to 55 years, with grade III or IV focal lesions on the femoral condyles. Patients were randomized to receive BST-CarGel® treatment or microfracture alone, and followed standardized 12-week rehabilitation. Co-primary endpoints of repair tissue quantity and quality were evaluated by 3-dimensional MRI quantification of the degree of lesion filling (%) and T2 relaxation times. Secondary endpoints were clinical benefit measured with WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) questionnaires and safety. General estimating equations were used for longitudinal statistical analysis of repeated measures.
Results
Blinded MRI analysis demonstrated that BST-CarGel®-treated patients showed a significantly greater treatment effect for lesion filling (P = 0.017) over 5 years compared with microfracture alone. A significantly greater treatment effect for BST-CarGel® was also found for repair tissue T2 relaxation times (P = 0.026), which were closer to native cartilage compared to the microfracture group. BST-CarGel® and microfracture groups showed highly significant improvement at 5 years from pretreatment baseline for each WOMAC subscale (P < 0.0001), and there were no differences between the treatment groups. Safety was comparable for both groups.
Conclusions
BST-CarGel® was shown to be an effective mid-term cartilage repair treatment. At 5 years, BST-CarGel® treatment resulted in sustained and significantly superior repair tissue quantity and quality over microfracture alone. Clinical benefit following BST-CarGel® and microfracture treatment were highly significant over baseline levels.
Keywords: cartilage repair, chitosan, quantitative MRI, microfracture, scaffold
Introduction
The search for a solution to problematic articular cartilage lesions continues despite decades of orthopedic experience in the knee. None of the current repair procedures, which include bone marrow stimulation, cultured cell-based therapies, and grafting, have been studied sufficiently, particularly in the mid to long term (5-10 years), to fully understand which factors dictate longer term outcomes for this troublesome pathology.1-3 Nonetheless, the fundamental goal of any cartilage repair treatment is to avoid the progression to secondary osteoarthritis4,5 by achieving structural repair that is comparable to native hyaline cartilage, and thereby assuring long-term durability, joint function, and pain relief.
Microfracture (MFX), the de facto standard of care and the most commonly used first-line surgical treatment6,7 for small cartilage lesions, is the deliberate penetration of the subchondral bone below a cartilage lesion to elicit bleeding and a subsequent bone marrow–derived repair response.8,9 It has been widely purported that MFX results in a fibrocartilaginous repair tissue lacking hyaline articular structure6,8,10,11 and clinical benefit that is variable beyond 2 to 3 years.6,12,13 This inconsistency and suboptimal repair tissue quantity and quality may derive from the instability of the fibrin clot formed from marrow blood in the lesion,6,11,14-16 which shrinks and detaches postsurgery as a result of platelet-driven clot retraction.14,17-19
BST-CarGel® (Piramal Life Sciences, Bio-Orthopaedics Division) was therefore developed to stabilize the MFX-based blood clot by dispersing a soluble polymer scaffold containing chitosan throughout whole blood and implanting the mixture over marrow access holes in a cartilage lesion. Chitosan is an abundant glucosamine polysaccharide found in the exoskeleton of crustaceans and has many desirable biomaterial properties.20-22 BST-CarGel® is prepared as a cytocompatible liquid chitosan solution with physiological pH,23 which does not interfere with normal whole blood coagulation, but quantifiably reinforces the implanted clot by impeding its retraction.19 By physically maintaining critical blood factors above marrow access holes,14,19,24 BST-CarGel® has been shown to increase the quantity and the quality of repair cartilage14,19,25,26 as a result of specific modifications in the repair sequence compared with bone marrow stimulation alone.24,27,28
An international, multicenter randomized controlled trial (RCT) comparing BST-CarGel® treatment of symptomatic cartilage lesions of the femoral condyle to MFX alone was conducted.26 The primary efficacy analysis was based on the co-primary endpoint of repair cartilage quantity and quality at 1 year as measured by quantitative 3-dimensional MRI, and a secondary endpoint was clinical benefit measured with the Western Ontario and McMaster Osteoarthritis Index (WOMAC; visual analogue scale [VAS] version),29 questionnaire. The 1-year RCT was used as the basis for BST-CarGel® marketing approval in Canada and Europe and was continued under an extension protocol for long-term follow-up for 5 years posttreatment.
Materials and Methods
The full description of the methodology used in the original 1-year multicenter RCT (https://clinicaltrials.gov; #NCT00314236) has been reported previously,26 including patient eligibility criteria, descriptions of randomization, surgical treatment, and rehabilitation, as well as outcome measures, including details of the 3-dimensional quantitative MRI analyses and self-administered questionnaires. The same follow-up procedures and outcome methodologies were applied in the 5-year extension protocol (https://clinicaltrials.gov; #NCT012-46895) and performed in accordance with guidelines for Good Clinical Practice.
Study Design and Participants
The initial 1-year trial26 enrolled 80 patients at 26 clinical sites. Eligible male and female patients were 18 to 55 years old with a single, focal cartilage lesion on the femoral condyles and moderate knee pain (>4 on a 10 cm VAS). Patients were randomized (1:1) to receive BST-CarGel® treatment or MFX alone, and agreed to follow a 12-week standardized posttreatment rehabilitation program.
The trial was single-blind since the independent third party carrying out the analyses of primary endpoints was unaware of patient treatment. Investigators and patients were not blinded because of differences in incision size related to treatment. The extension protocol was originally designed to provide longer term follow-up at 2, 3, 4, and 5 years and followed identical outcome measures. All subjects who participated in the initial 1-year trial were asked to provide written informed consent prior to study activities to be part of this extension study, which was approved by the institutional review boards at each of the clinical sites prior to initiation of activities.
Outcome Measures
Primary Outcome
Repair tissue structure, defined as both the quantity and quality of new tissue, was assessed as the primary outcome. Standardized 1.5-T MRI scans were obtained for each patient with dedicated transmit–receive knee coils at prequalified and trained MRI clinics for the initial trial at pretreatment, 1 month posttreatment, and 1 year posttreatment, and for the extension study, at enrolment and 2, 3, 4, and/or 5 years depending on individual patient follow-up status. Customized high–spatial resolution pulse sequences specific for morphological or T2 relaxation time analyses of regions of interest were used. For morphological analyses of cartilage, cartilage lesions and bone, both coronal and sagittal 3-dimensional fat-suppressed spoiled gradient echo (SPGR), and sagittal 3-dimensional gradient echo (GRE) sequences were used. Sagittal fat-suppressed dual echo fast spin echo sequences were used for transverse relaxation time (T2) analyses. All blinded scans were sent to imaging core labs for centralized scan quality review and storage (VirtualScopics, Rochester, NY) and blinded quantitative analysis (Qmetrics Technologies, Rochester, NY) using validated techniques. The quantification of lesion and repair biomarkers used proprietary, semiautomated (radiologist-corrected) morphological segmentation with a programmed anatomical atlas for all knee bone and cartilage structures. A musculoskeletal radiologist with expertise in cartilage repair manually traced the lesion boundaries on the 1-month posttreatment scan, which provided the reference for co-registration with 1-, 3-, 4-, and 5-year scans. Debrided lesions quantified using 1-month posttreatment scans represented baseline values for lesion surface area and volume. The segmented 3-dimensional volume of new tissue at each time point was then evaluated for %Fill and T2 relaxation time. (Further details and illustrations in Stanish et al.26) A radiologist-selected region on the posterior medial femoral condyle in the same knee was analyzed for each patient as a native cartilage control.
Secondary and Tertiary Outcomes
Clinical benefit was evaluated as a secondary outcome at initiation, 2, 3, 4, and 5 years posttreatment using the WOMAC questionnaire consisting of 3 subscales: Pain, Stiffness, and Physical Function.
Safety was assessed through recording of all adverse events (AEs) up to 5 years posttreatment. The safety definitions used during this trial conformed to international regulatory norms for clinical trials investigating medical devices.
The tertiary endpoint was the Medical Outcomes Study 36-Item Short-Form Health Survey version 2 (SF-36),30 which includes 2 aggregate measures, the physical and mental components, derived from 8 subscales.
All questionnaires were provided to patients during on-site study visits or by mail as needed.
Statistical Analysis
Sample size determination for the 1-year trial was previously reported.26 All treated participants who enrolled in the extension study were included in the efficacy analyses, which were performed according to a preapproved statistical analysis plan. General estimating equations were used for longitudinal analysis of repeated correlated measures using baseline (1 month posttreatment) lesion volume as a prespecified covariate for lesion %Fill and T2 MRI parameters. The longitudinal models for change from baseline for WOMAC and SF-36 comparisons were adjusted for baseline values.
To account for potential enrolment bias, patients who did not enroll in the extension study were compared with those who enrolled, looking at baseline demographic variables of gender, smoking status, previous arthroscopies, activity level, age, body mass index (BMI), number of physiotherapy sessions after study treatment, and treated lesion size as well as 1-year MRI outcome variables of lesion %Fill and mean T2 MRI values. The bootstrap method was used to account for sample size differences.
Data were analyzed using the Statistical Analysis System software (version 9.3, SAS Institute, Cary, NC). All reported P values are 2-sided. P values of less than 0.05 were considered statistically significant.
Results
Enrollment and Baseline Characteristics of the Patients
Screening and enrollment for the initial 1-year trial took place from May 2006 to January 2009, and 1-year follow-up was concluded in February 2010. Screening and enrollment into the extension study took place from March 2011 to October 2013 and the 5-year follow-up was concluded in February 2014. The extension study suffered patient loss to follow-up at all planned time points due to several factors, including extremely protracted enrolment periods for both the initial 12 month trial and the extension study, compounded by financial bankruptcy of the original trial sponsor (BioSyntech Canada Inc.) and a period of transition for the current trial sponsor (Piramal Life Sciences, Bio-Orthopaedics Division). The delayed initiation of the extension study regretfully allowed all but 4 patients to surpass their 2-year follow-up time point, and many others to pass their 3-, 4-, and 5-year time points prior to enrolling into the extension study. Ultimately, a total of 67 (84%) of the initial 80 patients consented to participate in the extension study, but their enrolment was distributed across the 5-year study period, and data were available from only 4 patients (5%) at 2 years, 32 patients (40%) at 3 years, 47 (59%) at 4 years, and 60 (75%) at 5-year follow-up. Only 2 patients had complete data for 1, 2, 3, 4, and 5 years. Because of excessively small group sample sizes, analyses in this report are thus limited to patients with 5 year follow-up data, which for BST-CarGel® and MFX, respectively, included 33/41 and 26/39 for %Fill measurements, 29/41 and 22/39 for T2 relaxation times, and 33/41 and 26/39 for all 3 WOMAC subscales. A statistical comparison of baseline characteristics of those patients who did not enroll into the extension enrolment with those who did, found that enrolled patients had significantly higher BMIs, larger treated lesion areas, and attended more posttreatment physiotherapy sessions than those that did not enroll, but were similar for all other parameters.
Baseline demographic characteristics of the patients with 5-year data were generally similar between treatment groups (Table 1), with the exception that BST-CarGel® patients compared with MFX patients were significantly younger (34.3 ± 9.7 vs. 40.0 ± 10.0 years; P = 0.03), had significantly higher BMIs (27.6 ± 2.8 vs. 25.7 ± 2.9 kg/m2; P = 0.01), and had 68% larger (P = 0.08) baseline (postdebridement) lesion volumes as determined by quantitative MRI. The BST-CarGel® group also had a shorter median time to onset of symptoms than the MFX group (1.4 vs. 3 years; P = 0.047).
Table 1.
Baseline Characteristics of Patients with 5-Year Data.a
| Characteristic | BST-CarGel® (n = 34) | Microfracture (n = 26) | P Valueb |
|---|---|---|---|
| Age, years | 34.3 ± 9.7 | 40.1 ± 10.1 | 0.030 |
| Gender, n (%) | 0.395 | ||
| Male | 22 (64.7) | 14 (53.8) | |
| Female | 12 (35.3) | 12 (46.2) | |
| Body mass index, kg/m2 | 27.6 ± 2.7 | 25.7 ± 2.9 | 0.013 |
| Activity level, n (%) | 0.520 | ||
| High | 16 (47.1) | 15 (57.5) | |
| Medium | 16 (47.1) | 11 (42.3) | |
| Low | 2 (5.8) | 0 (0) | |
| Smoking status, n (%) | 0.939 | ||
| Never smoked | 20 (58.8) | 14 (53.9) | |
| Former smoker | 6 (17.7) | 5 (19.2) | |
| Current smoker | 8 (23.5) | 7 (26.9) | |
| Time since symptom onset, years (median, range) | 1.4 (0.1-19.6) | 3.0 (0.3-27.8) | 0.047 |
| VAS at screening, cm | 6.7 ± 1.3 | 6.9 ± 1.2 | 0.496 |
| WOMAC Subscale Scores (baseline)c,d | |||
| Pain | 21.2 ± 11.0 | 22.8 ± 8.7 | 0.544 |
| Stiffness | 10.2 ± 4.6 | 8.7 ± 4.3 | 0.224 |
| Function | 76.9 ± 38.7 | 73.3 ± 38.2 | 0.721 |
| Index lesione | |||
| Area, cm2 (max) | 2.41 ± 1.50 (6.77) | 2.08 ± 1.22 (4.46) | 0.373 |
| Volume including missing bone (cm3) | 0.99 ± 0.87 | 0.67 ± 0.47 | 0.076 |
| Rehabilitation | |||
| Sessions per patient over 12-week period | 29.0 ± 7.4 | 27.9 ± 7.4 | 0.588 |
WOMAC = Western Ontario McMaster Universities Osteoarthritis Index; VAS = visual analogue scale 10 cm in length.
Values are means ± standard deviations unless otherwise indicated.
P values were obtained using either 2-sample Student’s t test, Wilcoxon, Pearson’s chi-square test, or Fisher’s exact test.
WOMAC includes 3 subscales: Pain, Stiffness, Function in VAS format: 0-10 cm. Scores have maximum value of 50 for Pain, 20 for Stiffness, and 170 for Function.
n = 33 BST-CarGel®.
Lesion area and volume determined postdebridement using quantitative MRI at 1 month posttreatment.
Primary Outcomes
BST-CarGel®-treated patients showed a significantly greater treatment effect over the 5-year follow-up (P = 0.017) compared with MFX for lesion %Fill, resulting in 5-year %Fill levels of 93.79% ± 1.16% versus 86.96% ± 2.85% (least squares means ± standard error), respectively (see Fig. 1 and Table 2), which were almost identical to the %Fill found at 1 year (P = 0.011).
Figure 1.
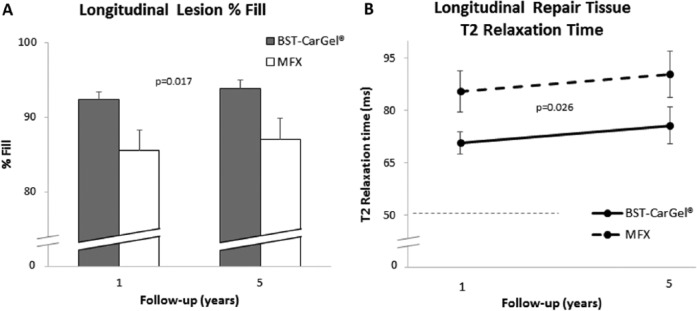
Longitudinal analysis of repeated measures using general estimating equations (GEE) for the quantity and quality of repair cartilage over 5 years posttreatment. Values represent least squares means adjusted for lesion volume and the standard error. (A) Lesion %Fill: Statistically significant improvement for the BST-CarGel® group over microfacture (MFX) (P = 0.017). Lesion %Fill is calculated from the ratio of new repair tissue volume to the baseline (postdebridement) cartilage lesion volume at 1-month posttreatment. (B) Repair tissue T2 relaxation times: Statistically significant improvement for the BST-CarGel® group over MFX (P = 0.026). Mean T2 is derived from the entire repair cartilage volume. Dashed line represents mean ipsilateral native cartilage T2 value.
Table 2.
Model-Based Primary, Secondary, and Tertiary Outcomes at 5 Years.a
| Variable | BST-CarGel® | Microfracture | P Valueb |
|---|---|---|---|
| Co-primary endpointsc | |||
| Degree of lesion Fill (%) | 0.017 | ||
| Year 1 (n) | 92.37 ± 1.04 (41) | 85.54 ± 2.74 (37) | |
| Year 5 (n) | 93.79 ± 1.16 (33) | 86.96 ± 2.85 (26) | |
| T2 relaxation time (ms) of repair cartilage | 0.026 | ||
| Year 1 (n)d | 70.78 ± 3.15 (39) | 85.51 ± 5.95 (33) | |
| Year 5 (n)d | 75.68 ± 5.25 (29) | 90.41 ± 6.56 (22) | |
| Secondary endpoint | |||
| WOMAC subscale (change from baseline)e | |||
| Pain | 0.474 | ||
| Year 1 (n) | −15.85 ± 1.24 (40) | −17.03 ± 0.95 (37) | |
| Year 5 (n) | −15.37 ± 1.47 (33) | −16.56 ± 1.19 (26) | |
| Stiffness | 0.236 | ||
| Year 1 (n) | −5.68 ± 0.69 (40) | −6.73 ± 0.52 (37) | |
| Year 5 (n) | −5.63 ± 0.72 (33) | −6.68 ± 0.58 (26) | |
| Physical function | 0.326 | ||
| Year 1 (n) | −55.03 ± 4.59 (40) | −60.62 ± 2.90 (36) | |
| Year 5 (n) | −56.52 ± 4.57 (33) | −62.10 ± 3.43 (26) | |
| Tertiary endpoint | |||
| SF-36 v2 score (change from baseline)f | |||
| Physical component | |||
| Year 1 (n) | 13.01 ± 1.43 (36) | 14.37 ± 1.35 (26) | 0.478 |
| Year 5 (n) | 13.12 ± 1.63 (34) | 14.48 ± 1.42 (27) | |
| Mental component | |||
| Year 1 (n) | 3.38 ± 1.42 (36) | 0.49 ± 1.42 (26) | 0.125 |
| Year 5 (n) | 2.72 ± 1.30 (34) | −0.17 ± 1.76 (27) | |
T2 = transverse relaxation time; WOMAC = Western Ontario McMaster Universities Osteoarthritis Index; SF-36 v2 = Short Form–36 version 2.
Values are multivariable adjusted estimates and are presented as least squares means ± standard errors.
P values were obtained using general estimating equations (GEE) for longitudinal analysis of repeated correlated measures.
Least squares means were adjusted for baseline defect volume.
Because of aberrant data points (relaxation times >200 ms), 2 patients in the BST-CarGel® group and 4 patients in the microfracture (MFX) group were removed from the year 1 data, and 3 patients in the BST-CarGel® group and 3 patients in the MFX group were removed from the year 5 data.
WOMAC includes 3 subscales: Pain, Stiffness, and Function in visual analogue scale format: 0-10 cm. Least squares means are adjusted for baseline. Scores have maximum change of 50 for Pain, 20 for Stiffness, and 170 for Function. Lower negative scores indicate better results.
SF-36 v2 includes 2 aggregate measures—Physical and Mental components—derived from 8 subscales. Least squares means are adjusted for baseline. Higher positive scores indicate better results.
A significantly greater treatment effect over the 5-year follow-up period (P = 0.026) was also observed for repair tissue T2 values for the BST-CarGel® group compared with the MFX group (see Fig. 1 and Table 2). This is consistent with the significant difference found at 1 year (P = 0.033).26 T2 values at 5 years were 75.7 ± 3.2 and 90.4 ± 6.6 ms (least squares means ± standard error) for BST-CarGel® and MFX groups, respectively, and T2 values in the BST-CarGel® group were always lower and closer to the ipsilateral native cartilage T2 value of 51.5 ± 7.5 ms (means ± standard deviation; n = 59) measured in the 5-year patients.
Secondary and Tertiary Outcomes
BST-CarGel® and MFX groups both showed significant improvement from pretreatment baseline at 5 years for all 3 WOMAC subscales (P < 0.0001) (see Fig. 2). There were no differences between the treatment groups over the 1- to 5-year period for the WOMAC subscales of Pain (P = 0.47), Stiffness (P = 0.24), or Function (P = 0.33) (see Fig. 3 and Table 2).
Figure 2.
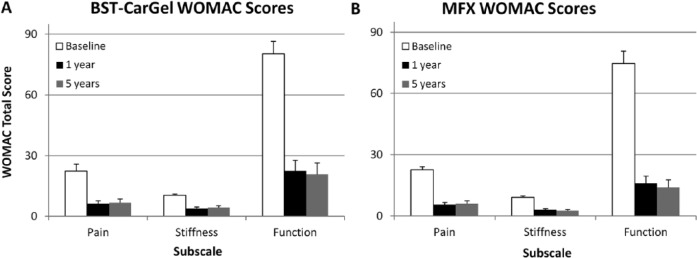
Improvement in clinical outcomes following (A) BST-CarGel® and (B) microfracture (MFX) treatments assessed by Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) (visual analogue scale) scores. Change from baseline was statistically significant for all subscales (P < 0.0001) at 1 and 5 years for BST-CarGel® and MFX groups. Values represent means and standard errors. Lower numbers represent better outcomes.
Figure 3.
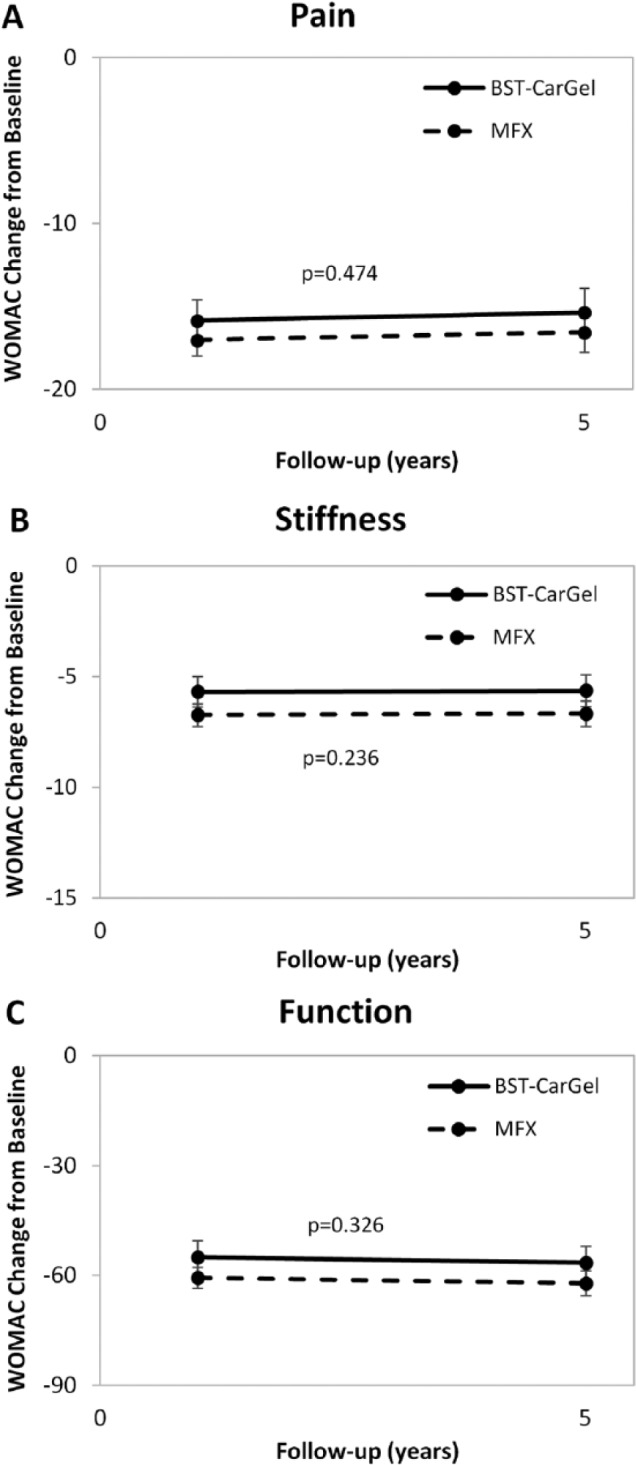
Longitudinal analysis of repeated measures using general estimating equations (GEE) for Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) scoring over 5 years posttreatment. Values represent mean change from baseline adjusted for baseline and standard errors. Similar improvements for both BST-CarGel® and microfracture (MFX) groups were observed for subscales (A) Pain (P = 0.474), (B) Stiffness (P = 0.236), and (C) Function (P = 0.326).
For the assessment of quality of life, the improvement of the physical and mental components of the SF-36 following BST-CarGel® treatment was maintained over 5 years and there were no statistically significant differences between the BST-CarGel® and MFX groups from 1 to 5 years (Table 2). However, MFX patients demonstrated worse mental component scores that fell below baseline at 5 years compared with BST-CarGel® patients (P = 0.125).
Safety
Overall, both trial treatments were well tolerated and the safety profiles were considered comparable. During the 5-year follow-up period, 54 AEs were reported in 31 individual patients, 13 (19.4%) BST-CarGel® patients and 18 (26.9%) MFX patients. Most AEs (>90%) were considered mild to moderate in severity. The most frequently observed AE in both groups was knee pain (11% vs. 17% for BST-CarGel® and MFX, respectively). For the BST-CarGel® group, 2 unanticipated procedure-related AEs occurred for 1 patient (1.5%) and 1 anticipated AE was reported by 1 patient (1.5%), all that were mild to moderate in severity and ongoing at the 5-year follow-up. For the MFX group, 2 anticipated procedure-related AE occurred for 2 patients (3%) which were mild in severity and ongoing at the 5-year follow-up. No unanticipated AEs were reported for the MFX group. For the BST-CarGel® group, 2 unanticipated device-related AEs occurred for 1 patient (1.5%) that were mild to moderate in severity and ongoing at the 5-year follow-up. There were no anticipated device-related AEs for the BST-CarGel® group. No anticipated or unanticipated device-related AEs were reported for the MFX group. One (1) serious AE (SAE) was reported by 1 subject in the MFX group, which was moderate in severity and not related in any way to the study treatment or index knee but required surgery and radiotherapy. Limited information was released by the patient except that the SAE was ongoing at the time of the 5-year follow-up period. No patient in either treatment group was discontinued from the study because of an AE, SAE, or incident. There were no deaths over the 5-year period of the study.
Discussion
This study has demonstrated that the more voluminous, higher quality repair cartilage found at 1 year26 following BST-CarGel® treatment of isolated full-thickness lesions was sustained over 5 years, supporting the hypothesis that BST-CarGel® increases the consistency of cartilage repair and enhances long-term structural superiority compared with MFX. Improvements in pain, stiffness, and function were highly significant over baseline (P < 0.0001) for the 5-year follow-up for both treatment groups, and BST-CarGel® use demonstrated a safety profile equivalent to MFX.
The baseline characteristics for the patients included in this 5 year analysis were generally well-balanced, except for a few notable exceptions: (1) The BST-CarGel® patient population had higher baseline BMIs (P = 0.013) and larger total lesion volumes (P = 0.076) than the MFX and (2) The BST-CarGel® group was significantly younger (P = 0.03) and had a shorter median time from onset of symptoms (P = 0.047) than the MFX group. Higher baseline BMIs and larger lesion volumes could each be considered biases against BST-CarGel® outcomes, while lower ages and shorter time from onset of symptoms could be advantages for BST-CarGel®. None of the characteristics were found to be significant covariates leading to bias during sensitivity analyses, despite reports that clinical outcomes after microfracture are age dependent.31 As well, the question of chronicity (or the time from onset of symptoms to surgery) and MFX repair is still under discussion in the literature, with some reporting improved outcomes with lesions less than 1 year old,6,32 and others suggesting no effects of chronicity.33 MFX performed equally as well with early compared to old lesions in a trial where characterized chondrocyte implantation resulted in better outcomes for fresher lesions (i.e., time from onset of symptoms <3 years).34 Similarly, the present study found that the MFX group outcomes were not sensitive to time to onset for all 3 WOMAC subscales and lesion %Fill, although T2 relaxation time was worse for the MFX group when time to onset of symptoms was ≥3 years (statistical interaction P = 0.008). Adjusting for time to onset with a 3-year cutoff in the general estimating equations model in this study, T2 outcomes remained unchanged and the BST-CarGel group maintained its superiority over MFX, albeit more significantly (P = 0.009). Markedly, neither the structural nor the clinical outcomes for the BST-CarGel® group were affected by time from onset of symptoms (≥3, <3 years), likely because BST-CarGel® asserts its effects down into the underlying bone to a substantially greater extent than MFX thereby enhancing early marrow-derived repair processes.24,27
Unique to this trial was the new level of evidence brought by the use of validated 3-dimensional quantitative MRI, which assessed the structural outcomes of repair tissue quantity and quality over 5 years with a high level of standardization and precision not previously achieved in a Good Clinical Practice–compliant RCT for cartilage repair. The superior quantity of new repair cartilage in BST-CarGel® lesions at 5 years (93.79%) was sustained from that found for BST-CarGel® over MFX (P = 0.011) at 1 year.26 A sufficient degree of lesion filling is critical in that any joint resurfacing should aim to reestablish mechanical homeostasis and anatomical shape with an integrated surface, particularly since chondrocyte-mediated biosynthesis, remodeling, and either tissue repair or degradation over time is dependent on the mechanical loading conditions of cartilage.35 Normal surface morphology and joint articulation would be expected to improve biomechanical conditions and inhibit the degeneration of lesional and perilesional tissues and slow the progression of secondary osteoarthritis.4,5
Quantitative MRI also demonstrated higher quality repair tissue by T2 for the BST-CarGel® group over 5 years in this study. T2 (or transverse) relaxation time is well known to be sensitive to, and highly dependent on, the extracellular cartilage matrix and particularly the collagen network structure, orientation, as well as macromolecular concentration, and tissue hydration.36-40 Since the quality of articular tissue can be assessed by the closeness of measured T2 values to that found in normal articular cartilage for the same joint compartment,41 the significantly different and lower BST-CarGel® mean T2 values at both 1 and 5 years compared with that of the MFX group indicates an improved level of tissue quality sustained over time. This interpretation for T2 MRI and its relationship to collagen organization is substantiated by a previous statistical correlation between T2 and polarized light microscopy scoring of 38 repair tissue biopsies retrieved at 1 year posttreatment in this same study.42 This scoring found significantly better zonal organization and collagen characteristics for the BST-CarGel® biopsies over MFX biopsies.43,44 Improved collagen content and zonal organization are necessary components for long-term durability of repair cartilage since collagen breakdown is considered to be a critical step in the progression of osteoarthritis.45,46
Improvements in pain, stiffness, and function were highly significant over baseline (P < 0.0001) for the 5-year follow-up for both treatment groups. The equivalent clinical improvement between groups was an expected finding for 2 predictable reasons. First, the study was not powered for a clinical benefit endpoint. Second, optimal MFX surgical technique was strictly obeyed in both groups. The equivalent clinical improvements found in this trial add to accumulating evidence that when performed properly,9 microfracture can effectively improve clinical pain and function for the mid- to long-term despite the widely purported clinical outcome expectancy of 2 to 3 years linked to a mechanically deficient fibrocartilaginous repair tissue and excessive intralesional bony overgrowth.6,13,32,47 The clinical equivalency of MFX to more “advanced therapies” has been convincingly demonstrated by the present RCT and other studies at 1 year posttreatment,26,48,49 at 5 years,34,50 and even up to 11 years.33,51 Furthermore, Shive et. al 52 showed for the first time with standardized, 3-dimensional quantitative MRI that very minimal bony overgrowth occurs with MFX-based repair. When it did occur, it was linked to a predisposing factor (e.g., preexisting bone abnormalities, prior surgeries) and not to MFX, per se. A possible explanation of the reported short-term success MFX could be the fact that orthopedic surgeons practicing MFX are often not performing critical steps of the technique as published originally by Steadman et al.,9 and may not be aware of potential factors influencing outcomes such as BMI, age, and rehabilitation. In a survey of practicing orthopedic surgeons, only 69% of 131 surgeons indicated that prior to creating holes, they remove the calcified cartilage layer,53 despite the fact that this is likely the most critical MFX procedural step.54 This suggests that technique alone may be the single most determining factor for the success or failure of MFX with regard to clinical outcomes.
To further understand the equivalence found between the WOMAC subscales for each group at 5 years, post hoc sensitivity analysis was carried out to determine if specific patient characteristics or the structural variables of %Fill or T2 contributed to 5-year clinical outcomes. No single factor was identified that correlated with WOMAC pain, stiffness, and function. Ultimately, the determination of what factors are predictive of clinical outcome following cartilage repair will be multivariate, considering the numerous patient-specific and cartilage lesion–specific variables. Patient age,31,50,55,56 BMI,32,57 time from onset of symptoms,34,56 gender, lesion size, and location are all relevant,58 although other procedure-based parameters such as technical aspects of the surgical treatment and postoperative rehabilitation58,59 could also play critical roles. Consequently, the correlation between repair tissue structure and clinical outcomes has been elusive. Several studies have reported relationships between structural assessments and long-term clinical outcomes, but these studies suffer from small sample sizes, statistical rigor, and subjective scoring of both the structural and the clinical components.50,56,60,61 A recent cohort study following MACI treatment used multivariate models to assess predictive factors, including a subjective MRI composite score, but no correlation with KOOS (Knee injury and Osteoarthritis Outcome Score) sports/recreation or SF-36 was identified.58 Reviews of the topic agree that there are major inconsistencies between MRI structural outcomes, repair procedures, and their resulting clinical benefits,62,63 and that analysis of longer term studies are complicated by the potential for progression of degenerative changes in the knee or new unrelated knee pathology. Previous studies have been limited to subjective semiquantitative MRI analysis using MOCART64 or Henderson scores65 and none used quantitative 3-dimensional analysis for structural assessments as described in this study.
A critical objective of BST-CarGel® treatment is to reduce pain and symptoms over long term and thus offer the patient a return to normal lifestyle, which can only be assessed through patient-reported instruments. There is currently no consensus regarding the optimal patient-based instruments for outcome assessments in cartilage repair. Some have been validated but are insensitive and incomplete.66 This trial, using the WOMAC tool, failed to demonstrate clinical differences between the 2 groups and although it is validated and designed for patients with osteoarthritis, the 3 WOMAC constructs for pain, stiffness, and function may not be responsive enough to measure important changes between these groups. Clearly, more and longer studies are required both with structural tools and patient-reported clinical measures that are specific enough to detect improvements following cartilage repair before the relationship between clinical and structural outcomes will emerge. Thus, with clinical benefit being shown at acceptable levels for most cartilage repair therapies33,34,67,68 and for significantly long follow-up periods, superiority of one therapy or technique falls to the structure of the replaced or regenerated cartilage within the lesion, such as was found in this trial. It can be easily argued that a sufficient quantity of repair cartilage with hyaline features enables appropriate articulation, biomechanical loading, and tissue metabolism, which would be necessary for long-term durability and function. Furthermore, repair tissue structure represents a reliable clinical trial endpoint since hyaline cartilage has an exquisite structure characterized by hallmark features, including collagen content and zonal organization, glycosaminoglycan, and cell population,69 which can be easily discriminated by highly accurate quantitative measures sensitive to early changes in cartilage structure under reasonable clinical trial time frames.
The major limitation of this study was the number of patients lost to follow up (25%). As described, this was related to several factors, including financial bankruptcy, long time frames, and patient trial fatigue, although our trial was similar to others who reported 5-year results from 77%50 and 79%34 of trial patients. Furthermore, the trial outcomes reported here at 5 years are likely conservative estimates since 2 negative prognosticators, higher BMIs and larger lesions, were found in the enrolled patients compared with those who did not enroll in the extension study, although neither were found to be significant statistical covariates.
In summary, it has been shown that patients treated with BST-CarGel® demonstrate significant structural superiority of repair tissue quantity and quality over MFX for a period of 5 years posttreatment. The clinical benefit of BST-CarGel® is highly significant over baseline levels of pain, stiffness, and function, illustrating that BST-CarGel® is a safe and effective treatment for symptomatic full-thickness cartilage lesions.
Acknowledgments
The extension study was conducted by Piramal Life Sciences, Bio-Orthopaedics Division. The critical efforts of the BST-CarGel® Clinical Study Group, including clinical site investigators and sub-investigators and their research coordinators who tirelessly contributed to the study success are warmly acknowledged. The BST-CarGel® Clinical Trial Group investigators and sub-investigators were (in Canada) William Stanish (Halifax), Nicholas Mohtadi (Calgary), Peter MacDonald (Winnipeg), Robert McCormack, Jordan Leith, Patrick Chin, and Mike Gilbart (Vancouver), Stéphane Pelet, Réjean Cloutier, Jean Lamontagne, and Sylvain Belzile (Quebec City), Don Johnson and Allan Liew (Ottawa), Paul Marks (Toronto), Michel Malo, Julio Fernandes, Pierre Ranger, Jacques Desnoyers, Patrick Lavigne, and Sébastien Guimond Simard (Montreal), Paul Zalzal and Tim Deakon (Oakville), and Frank Smith (Hamilton); (in Spain) Francisco Forriol, Felipe Lopez-Oliva, Gloria Lopez, Manuel Leyes, Javier Vaquero, Diego Garcia, Santiago Bello, Alonso Moreno, and Patricia Villanueva (Madrid), Francisco Macule (Barcelona), and Antonio Maestro Fernandez (Gijon); and (in South Korea) Myung Chul Lee, Sang-Hoon Lee, and Kyoung Ho Yoon (Seoul). MRI site qualification and management was carried out by VirtualScopics (Rochester, NY), and MRI quantitative analyses were performed by Qmetrics Technologies (Rochester, NY).
Footnotes
Authors’ Note: This investigation was performed at 26 clinical sites in Canada, Spain, and South Korea (complete list is given in the Acknowledgments section).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: One or more of the authors received payments, either directly or indirectly (i.e. via their institution), from a third party in support of an aspect of this work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Piramal Healthcare (Canada) Ltd. was the sole funding source and was responsible for study design, data interpretation, and manuscript publication. Data collection and blinded analyses were conducted by third parties as described herein.
Ethical Approval: The extension study protocol (https://clinicaltrials.gov; #NCT012-46895) was approved by each institutional review board prior to initiating study activities.
References
- 1. Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-9. [DOI] [PubMed] [Google Scholar]
- 2. Benthien JP, Schwaninger M, Behrens P. We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2011;19:543-52. [DOI] [PubMed] [Google Scholar]
- 3. Worthen J, Waterman BR, Davidson PA, Lubowitz JH. Limitations and sources of bias in clinical knee cartilage research. Arthroscopy. 2012;28:1315-25. [DOI] [PubMed] [Google Scholar]
- 4. Buckwalter JA, Mankin HJ. Instructional Course Lectures, The American Academy of Orthopaedic Surgeons—Articular Cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am 1997;79:612-32. [Google Scholar]
- 5. Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337-42. [DOI] [PubMed] [Google Scholar]
- 6. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 7. U.S. Food and Drug Administration. Guidance for industry. Preparation of IDEs and INDs for products intended to repair or replace knee cartilage. Silver Spring, MD: U.S. Food and Drug Administration. [Google Scholar]
- 8. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460-6. [PubMed] [Google Scholar]
- 9. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-6. [PubMed] [Google Scholar]
- 10. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. [DOI] [PubMed] [Google Scholar]
- 11. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;(407):215-27. [DOI] [PubMed] [Google Scholar]
- 12. Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:834-42. [DOI] [PubMed] [Google Scholar]
- 13. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee, Osteoarthritis Cartilage. 2006;14:1119-25. [DOI] [PubMed] [Google Scholar]
- 14. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671-86. [DOI] [PubMed] [Google Scholar]
- 15. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213-21. [DOI] [PubMed] [Google Scholar]
- 16. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-37. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532-53. [DOI] [PubMed] [Google Scholar]
- 18. Johnson LL. Characteristics of the immediate postarthroscopic blood clot formation in the knee joint. Arthroscopy. 1991;7:14-23. [DOI] [PubMed] [Google Scholar]
- 19. Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78-89. [DOI] [PubMed] [Google Scholar]
- 20. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017-84. [DOI] [PubMed] [Google Scholar]
- 21. Muzzarelli RAA, Muzzarelli C. Chitosan chemistry: relevance to the biomedical sciences. Adv Polym Sci. 2005;186:151-209. [Google Scholar]
- 22. Shigemasa Y, Minami S. Applications of chitin and chitosan for biomaterials. Biotechnol Genet Eng Rev 1996;13:383-420. [DOI] [PubMed] [Google Scholar]
- 23. Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155-61. [DOI] [PubMed] [Google Scholar]
- 24. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316-27. [DOI] [PubMed] [Google Scholar]
- 25. Shive M, Hoemann C, Restrepo A, Hurtig M, Duval N, Ranger P, et al. BST-CarGel®: in situ chondroinduction for cartilage repair. Oper Tech Orthop. 2006;16:271-8. [Google Scholar]
- 26. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel® treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-50. [DOI] [PubMed] [Google Scholar]
- 27. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Temporal and spatial modulation of chondrogenic foci in subchondral microdrill holes by chitosan-glycerol phosphate/blood implants, Osteoarthritis Cartilage 2011;19:136-144. [DOI] [PubMed] [Google Scholar]
- 28. Buschmann MD, Hoemann CD, Hurtig M, Shive MS. Cartilage repair with chitosan/glycerol-phosphate stabilized blood clots. In: Williams RJ, editor. Cartilage repairstrategies. Totowa, NJ: Humana Press; 2007. p. 85-104. [Google Scholar]
- 29. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 30. Ware JE., Jr. SF-36 health survey update. Spine. 2000;25:3130-9. [DOI] [PubMed] [Google Scholar]
- 31. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180-6. [DOI] [PubMed] [Google Scholar]
- 32. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. [DOI] [PubMed] [Google Scholar]
- 33. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-84. [DOI] [PubMed] [Google Scholar]
- 34. Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566-74. [DOI] [PubMed] [Google Scholar]
- 35. Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691-713. [DOI] [PubMed] [Google Scholar]
- 36. Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487-93. [DOI] [PubMed] [Google Scholar]
- 37. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355-68. [DOI] [PubMed] [Google Scholar]
- 38. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 39. Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med. 2002;48:460-9. [DOI] [PubMed] [Google Scholar]
- 40. Trattnig S, Mamisch TC, Welsch GH, Glaser C, Szomolanyi P, Gebetsroither S, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42:442-8. [DOI] [PubMed] [Google Scholar]
- 41. Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gabler C, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome—preliminary results. Osteoarthritis Cartilage. 2008;16:903-8. [DOI] [PubMed] [Google Scholar]
- 42. Shive M, Buschmann MB, Yaroshinsky A, Tamez-Pena J, Schreyer E, Totterman S, et al. T2 MRI of repair cartilage reflects both tissue quality and quantity. Paper presented at: Fifth Annual Osteoarthritis Imaging Workshop; June 8-11, 2011; Salzburg, Austria. [Google Scholar]
- 43. Changoor A, Nelea M, Methot S, Tran-Khanh N, Chevrier A, Restrepo A, et al. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage. 2011;19:1458-68. [DOI] [PubMed] [Google Scholar]
- 44. Changoor A, Tran-Khanh N, Methot S, Garon M, Hurtig MB, Shive MS, et al. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthritis Cartilage. 2011;19:126-35. [DOI] [PubMed] [Google Scholar]
- 45. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu JP, Kirk TB, Zheng MH. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. J Orthop Surg Res. 2008;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384-94. [DOI] [PubMed] [Google Scholar]
- 48. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surgery Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 49. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 50. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 51. Ulstein S, Aroen A, Rotterud JH, Loken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22:1207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shive MS, Restrepo A, Totterman S, Tamez-Pena J, Schreyer E, Steinwachs M, et al. Quantitative 3D MRI reveals limited intra-lesional bony overgrowth at 1 year after microfracture-based cartilage repair. Osteoarthritis Cartilage. 2014;22:800-4. [DOI] [PubMed] [Google Scholar]
- 53. Theodoropoulos J, Dwyer T, Whelan D, Marks P, Hurtig M, Sharma P. Microfracture for knee chondral defects: a survey of surgical practice among Canadian orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20:2430-7. [DOI] [PubMed] [Google Scholar]
- 54. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34:1824-31. [DOI] [PubMed] [Google Scholar]
- 55. de Windt TS, Bekkers JE, Creemers LB, Dhert WJ, Saris DB. Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am J Sports Med. 2009;37(Suppl 1):58S-62S. [DOI] [PubMed] [Google Scholar]
- 56. Krishnan SP, Skinner JA, Jagiello J. Durability of cartilage repair—does histology matter? J Bone Joint Surg Br 2006;90:323-4. [Google Scholar]
- 57. Jaiswal PK, Bentley G, Carrington RW, Skinner JA, Briggs TW. The adverse effect of elevated body mass index on outcome after autologous chondrocyte implantation. J Bone Joint Surg Br. 2012;94:1377-81. [DOI] [PubMed] [Google Scholar]
- 58. Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. Am J Sports Med. 2013;41:1245-54. [DOI] [PubMed] [Google Scholar]
- 59. Reinold MM, Wilk KE, Macrina LC, Dugas JR, Cain EL. Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports Phys Ther. 2006;36:774-94. [DOI] [PubMed] [Google Scholar]
- 60. Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther. 2008;10:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S-19S. [DOI] [PubMed] [Google Scholar]
- 62. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41:1426-34. [DOI] [PubMed] [Google Scholar]
- 63. de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41:1695-702. [DOI] [PubMed] [Google Scholar]
- 64. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 65. Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85:1060-6. [DOI] [PubMed] [Google Scholar]
- 66. Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36:1695-704. [DOI] [PubMed] [Google Scholar]
- 67. Hangody L, Duska Z, Karpati Z. Autologous osteochondral mosaicplasty. Tech Knee Surg. 2002;1:13-22. [Google Scholar]
- 68. Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37:42-55. [DOI] [PubMed] [Google Scholar]
- 69. Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;(391 Suppl):S26-33. [DOI] [PubMed] [Google Scholar]


