Abstract
Objective:
The objective of this study was to assess the outcome of osteochondral allograft (OCA) transplantation as a salvage procedure after various cartilage repair surgeries.
Design:
One hundred sixty-four knees in 163 patients (mean age = 32.6 years; range = 11-59 years; 55% males) were treated with OCA transplantation after subchondral marrow stimulation (SMS), osteochondral autograft transplantation (OAT), and autologous chondrocyte implantation (ACI). The majority of previous procedures were isolated SMS in 145 knees (88.4%). Mean allograft size was 8.5 ± 7.9 cm2. The most common location was in femoral condyle. The number and type of reoperations on the operative knee were assessed. Failure of the OCA transplantation was defined as any reoperation resulting in removal of the allograft. Functional outcomes were evaluated.
Results:
Sixty-eight knees had reoperations after OCA transplantation. Thirty-one knees (18.9%) were classified as allograft failures. The median time to failure was 2.6 ± 6.8 years (range = 0.7-23.4 years). Survivorship of the graft was 82% at 10 years and 74.9% at 15 years. Patients whose grafts were still in situ had a mean of 8.5 ± 5.6 years of follow-up. Scores on all functional outcomes scales improved significantly from preoperatively to latest follow-up. Eighty-nine percent of OCA transplantation patients reported being “extremely satisfied” or “satisfied.”
Conclusion:
Despite the high reoperation rate, OCA transplantation is a successful salvage surgical treatment after cartilage repair procedures. This cohort showed improved survivorship and functional outcomes of OCA transplantation after SMS, ACI, and OAT.
Keywords: osteochondral allograft, previous cartilage repair, knee
Introduction
Chondral and osteochondral lesions are described in 34% to 62% of knee arthroscopies1-4 and can be present in up to 50% of asymptomatic athletes.5 Frequently found in young and highly active patients, a cartilage injury can cause major clinical disability.6 Cartilage injuries have shown low potential to regenerate. Although the process is not completely understood, cartilage lesions degenerate over time and may progress to osteoarthritis.7,8
Several cartilage surgeries have been described, such as autologous chondrocyte implantation (ACI), subchondral marrow stimulation (SMS), osteochondral autograft transplantation (OAT), and osteochondral allograft (OCA) transplantation 9,10 In case of a failed cartilage repair, successive cartilage repairs are performed according to several treatment algorithms that have been reported in the literature.11-15 Susceptive attempts of cartilage restoration can damage the subchondral bone. 30% to 50% of patients after an SMS procedure presented subchondral bone damage.16 Several attempts of prior treatment may also increase the lesion size. Recently, a major concern has been raised about successive procedures in cartilage repair, especially when the ACI is shown to fail three to five times more frequently when performed after previous cartilage repair procedures such as SMS and OAT.9,10,17
Large cartilage lesions can also be caused by other diseases such as osteochondritis dissecans (OCD), avascular necrosis (AVN), and trauma.12,18,19 These cartilage lesions with areas greater than 2 to 4 cm2 are associated with damaged subchondral bone and are considered an indication for an OCA transplantation.12,20-24 In such cases, the OCA procedure can immediately restore the structure of the cartilage by transplanting an equivalent-sized fragment of fresh allograft cartilage with supportive subchondral bone.
Although the OCA transplantation has shown consistent improvement in functional outcomes and reliable survivorship in the literature,13,19,25 no particular study focused on evaluating the influence of various previous cartilage repair procedures in the final outcome of OCA transplantation.
Material and Methods
From 1983 to 2011, data from more than 800 OCA procedures have been prospectively collected and entered into our institution’s institutional review board-approved outcomes database. Of these, 163 patients (164 knees) underwent cartilage repair surgery prior to the OCA transplantation and had a minimum follow-up of 2 years. Patients whose grafts were still in situ had a mean of 8.5 ± 5.6 years of follow-up. Patients with failed previous SMS, OAT, implantation of synthetic bone plugs, or ACI were included.
Patients were examined in the clinic to measure current pain levels, joint function, and satisfaction with the procedure. Patients unable to return to follow-up were contacted by telephone and mail. Telephone solely followed-up reoperation data. Clinical scores were obtained by mailed questionnaires.
All OCA transplantations were performed between 1983 and 2011 by two surgeons. The indications of these surgeries were focal osteochondral lesions with International Cartilage Repair Society (ICRS) grades 3 and 4, patients who had failed previous surgical treatment and/or wished to avoid prosthetic arthroplasty.
Osteochondritis dissecans and degenerative condral lesions accounted for 64% of the cases. The medial femoral condyle (44.8%) was the most common location of the cartilage injury (Table 1). SMS was the most common isolated previous procedure (88.4%). One or two prior procedures had been performed in 58% of the patients (Table 2).
Table 1.
Patient Demographic Information and Clinical Assessment.
| Measure | Mean ± Standard Deviation |
|---|---|
| Age, years | 32.6 ± 10.6 |
| Body mass index, kg/m2 | 26.1 ± 4.7 |
| Male/female | 90/74 |
| Median allograft size, cm2 | 6.8 ± 8.0 |
| n (%) | |
| Diagnosis | |
| Osteochondritis dissecans | 58 (35.6%) |
| Degenerative chondral lesion | 47 (28.8%) |
| Traumatic chondral injury | 34 (20.9%) |
| Osteoarthritis | 15 (9.2%) |
| Avascular necrosis | 7 (4.3%) |
| Tibial plateau fracture | 1 (0.6%) |
| Osteochondral fracture | 1 (0.6%) |
| Location of the graft | |
| Medial femoral condyle | 73 (44.8%) |
| Lateral femoral condyle | 27 (16.6%) |
| Trochlea | 15 (9.2%) |
| Patella | 5 (3.1%) |
| Lateral tibial plateau | 2 (1.2%) |
| More than one location | 41 (25.1%) |
| Number of grafts | |
| One allograft | 103 (63.2%) |
| Two allografts | 42 (25.8%) |
| Three allografts | 12 (7.4%) |
| Four allografts | 6 (3.7%) |
Table 2.
Frequency and Type of Previous Cartilage Repair.
| Procedure | n | Percentage |
|---|---|---|
| Method | ||
| Arthroscopic | 112 | 68.3 |
| Arthroscopic and open | 48 | 29.3 |
| Open | 4 | 2.4 |
| SMS | 145 | 88.4 |
| OAT and SMS | 5 | 3 |
| ACI and SMS | 5 | 3 |
| OAT | 3 | 1.8 |
| ACI and SMS and synthetic plugs | 1 | 0.6 |
| Periosteal implant | 1 | 0.6 |
| Number of previous surgeries | ||
| One surgery | 46 | 28 |
| Two surgeries | 49 | 29.9 |
| Three surgeries | 34 | 20.7 |
| Four surgeries | 9 | 5.5 |
| Five or more surgeries | 26 | 15 |
SMS = subchondral marrow stimulation; OAT = osteochondral autograft transplantation; ACI = autologous chondrocyte implantation.
Forty-five patients had concomitant procedures during OCA surgery. Concomitant surgeries included 24% hardware removal, 11% lateral retinacular release, 11% high tibial osteotomy, 3.6% distal femoral osteotomy, and 35% other procedures (arthroscopy, loose body removal, synovectomy, and anterior cruciate ligament reconstruction).
All patients had clinical evaluation preoperatively and postoperatively with the modified Merle d’Aubigné-Postel (18-point) scale,26 International Knee Documentation Committee (IKDC) subjective knee evaluation form,27 Knee injury and Osteoarthritis Outcome Score (KOOS), and the Knee Society function (KS-F) scale.28 The Merle d’Aubigné-Postel (18-point) scale was modified to the range of motion (ROM) feature with values relevant to the knee rather than the hip. This scale includes physical examination characteristic and includes a maximum of 6 points each for pain, knee ROM, and knee function for a total of 18 points. Patient satisfaction using a 5-point scale was recorded. Survivorship was based on the time to failure. Failure was considered any reoperation resulting in removal of the graft, such as allograft revision, and any form of arthroplasty and arthrodesis.
Surgical Technique
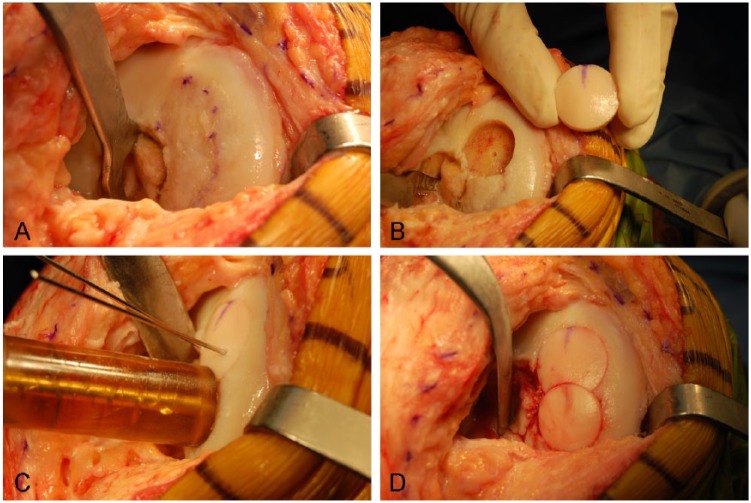
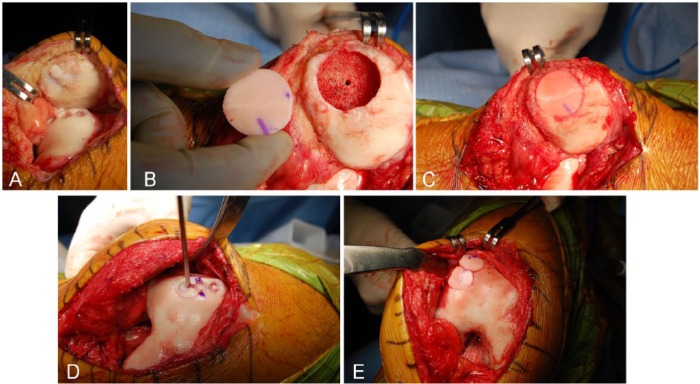
All surgeries were performed through a medial or lateral parapatellar arthrotomy. The size of the lesion was recorded and the nonviable tissue found was debrided to a depth necessary to achieve a homogeneous bleeding surface of subchondral bone, generally around 6 to 8 mm of depth.24 Then the lesion was prepared in a geometric format to a depth of 3 to 8 mm (Figs. 1A, B and 2A, B, D). For lesions up to 10 cm2, a dowel technique was used. For lesions larger than 10 cm2, a shell allograft technique was chosen, as described in previous reports.25,29 To decrease the immunogenicity of the graft, the immunogenic elements of marrow from the osseous surface was washed out with a pulsatile lavage. The graft was tailored into a shape matching the lesion and trial fittings were performed until a well-positioned graft was achieved. The grafts were fixed either by press-fit fixation (Figs. 1C, D and 2C, E) or with the use of bioabsorbable pins (Chondral Dart; Arthrex, Naples, FL, USA).
Figure 1.
Intraoperative photograph of dowell technique after a failed autologous chondrocyte implantation in the medial femoral condyle. (A) A medial arthrotomy is performed, and the failed ACI is identified. (B) The diameter of the defect is prepared for the first allograft. (C) Two divergent k-wires are used to hold the allograft in place and the “snowman” technique is performed. (D) The second plug of the allograft is tailored and fitted into the defect.
Figure 2.
Intraoperative photograph of a failed osteochondral autologous transplantation for the patella and trochlea; the donor area also failed to regenerate. (A) A medial arthrotomy is performed; the patella everted and the failed OAT is identified. (B) The diameter of the defect is measured and the patellar lesion is prepared for one plug. (C) The allograft is press-fit into the defect. (D) After the measurement of the defect, trochlear area is prepared for the transplantation of two plugs (“snowman” technique). (E) The allograft is inserted in the defect.
Postoperatively, our rehabilitation protocol was followed. Patients participated in full active ROM with no weightbearing for 8 to 12 weeks. After patellofemoral transplantation, patients were allowed passive ROM, and weightbearing as tolerated with the knee locked in extension for 3 to 4 weeks. Generally after 6 months, patients were allowed to participate in recreational and sports activities. All patients were followed clinically and radiographically until graft healing was achieved.
Statistical Analysis
Means and frequencies were calculated to describe patient demographics (age, sex, body mass index [BMI], number of previous surgeries on operated knee, diagnosis), surgical details (graft size, number of grafts, location of graft, concomitant procedures), and follow-up data (follow-up duration, number and type of further surgeries, patient satisfaction). Osteochondral allograft survivorship, with removal of the allograft as the endpoint, was calculated using the Kaplan–Meier method. Wilcoxon signed-rank tests were used to compare preoperative and postoperative changes on the modified Merle d’Aubigné-Postel (18-point), IKDC, KOOS, and KS-F scores. Comparison of OCA failures and nonfailures on select variables were performed using chi-square tests for categorical variables and independent samples t tests (or Mann–Whitney U-test when appropriate) for continuous variables. Statistical significance was set at P < 0.05. SPSS Version 13.0 was used for all analyses (IBM Corporation, Armonk, NY, USA)
Results
Sixty-eight knees (41.5%) had reoperations after OCA transplantation with 24 knees submitted for more than one surgery. Some reoperations were not related to graft removal or debridement (Table 3). Of the patients who had reoperation, 31 (45.6%) OCA transplants were considered failures. Of 164 knees, 18 (11%) failed knees were converted to total knee arthroplasty, 9 (5%) failed knees had an OCA revision, 2 (1.2%) failed knees were converted to unicompartmental knee arthroplasty, 1 (0.6%) failed knee was converted to patellofemoral arthroplasty, and 1 (0.6%) failed knee was converted to arthrodesis. The median time to failure was 2.6 ± 7.6 years (range = 0.7-23.4 years). Survivorship of the OCA transplantation was 87.8% at 5 years and 82% at 10 years (Fig. 3).
Table 3.
Frequency and Type of Reoperations After Allograft Surgery.
| Reoperations | Frequency |
|---|---|
| Arthroscopic debridement or loose body removal | 36 |
| Hardware removal | 9 |
| Meniscus repair or meniscectomy | 7 |
| High tibial osteotomy | 6 |
| Distal femoral osteotomy | 3 |
| Lateral retinacular release | 2 |
| Extensor mechanism realignment | 1 |
Figure 3.
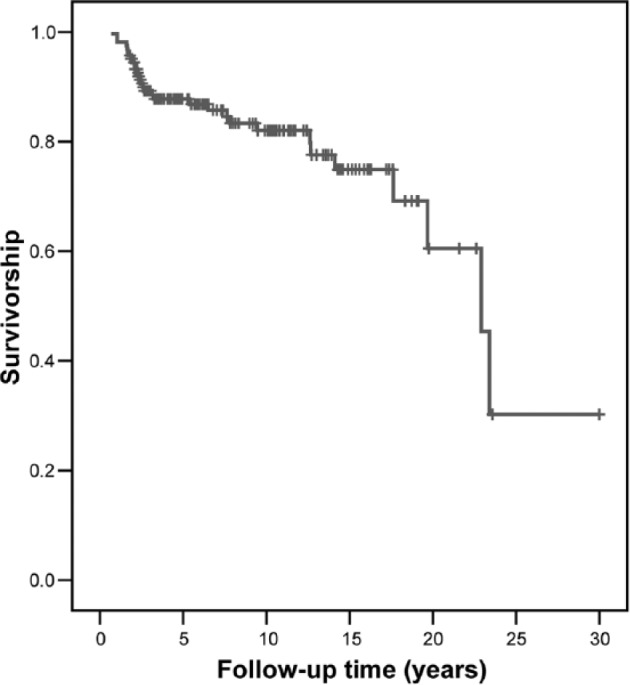
The graphic shows the overall graft survivorship and revision of the allograft or arthroplasty surgery is considered the end point. The survivorship of the OCA transplantation was 87.8% at 5 years, 82% at 10 years, and 74.9% at 15 years.
The mean follow-up time of the 133 (81%) patients whose grafts remained in situ was 8.5 ± 5.6 years. Improvement in pain and function was noted preoperatively to the latest follow-up in all functional scores used (Table 4). Eighty-nine percent of the patients were “extremely satisfied” or “satisfied” with the treatment.
Table 4.
Preoperative and Postoperative Comparison of Functional Outcomes.a
| Measure | Preoperative Mean or n (%) | Postoperative Mean or n (%) |
|---|---|---|
| Modified Merle d’Aubigné-Postel (18-point) scale | 12.6 | 16.0 |
| Excellent (18) | 1 (0.8%) | 30 (24%) |
| Good (15-17) | 25 (19%) | 73 (58%) |
| Fair (12-14) | 19 (46%) | 17 (13%) |
| Poor (<12) | 40 (30%) | 4 (3%) |
| IKDC pain | 5.9 | 2.9 |
| IKDC function | 3.1 | 7.1 |
| IKDC total | 37.6 | 72.6 |
| KS-F | 66.9 | 88.2 |
| KOOS Symptoms | 57.5 | 78.8 |
| KOOS Pain | 61.1 | 82.5 |
| KOOS ADL | 69.7 | 88.3 |
| KOOS Sport/Rec | 30.0 | 65.0 |
| KOOS QOL | 19.1 | 60.4 |
IKDC = International Knee Documentation Committee scale; KS-F = Knee Society function scale; KOOS = Knee injury and Osteoarthritis Outcome Score. KOOS: Function in daily living (ADL), Function in sport and recreation (Sport/Rec), and knee-related quality of life (QOL).
All P < 0.01.
The BMI and the number of previous surgeries were related to allograft failure. OCA failure patients had a significantly increased BMI and number of previous surgeries (Table 5) compared with OCA nonfailures patients. Other patient characteristics did not show significant differences.
Table 5.
Comparison of OCA Failures (n = 31) and Nonfailures (n = 133).
| Measure | Failures | Nonfailures | P Value |
|---|---|---|---|
| Age, years (mean) | 36.0 ± 10.0 | 31.2 ± 10.7 | 0.051 |
| Male (%) | 45.2 | 56.4 | 0.258 |
| Body mass index, kg/m2 (mean) | 28.4 ± 5.1 | 25.8 ± 4.7 | 0.033 |
| Number of previous surgeries (median) | 3.0 ± 2.7 | 2.0 ± 3.1 | 0.001a |
| Diagnosis (%) | 0.084 | ||
| DCL/OA | 51.6 | 34.8 | |
| Other | 48.4 | 65.2 | |
| Allograft size, cm2 (median) | 8.5 ± 15.5 | 6.3 ± 4.9 | 0.078a |
| Number of grafts (%) | 0.137 | ||
| One allograft | 51.6 | 65.9 | |
| Two or more allografts | 48.4 | 34.1 |
DCL = degenerative chondral lesion; OA = osteoarthritis.
Mann–Whitney U test.
Discussion
This study represents the largest cohort of patients undergoing OCA after failure of other cartilage repair procedures. Functional outcomes improved considerably from preoperatively to the latest follow-up with an excellent patient satisfaction rate. Although a high reoperation rate was found, only 18.9% of knees were related to failure of the transplant. Overall, survivorship of 82% at 10 years was observed.
Cartilage lesion treatment still represents a challenging issue for orthopaedic surgeons and becomes more unreliable when a first-line treatment fails. Microfracture and mosaicplasty, considered a first-line treatment for small and localized lesions (2-4 cm2),12 showed considerable improvement in clinical outcomes after surgery. For large defects (>2-4 cm2), microfracture is less optimal due to declining clinical outcomes after 2 years of follow-up and is likely related to unpredictable cartilage repair volume.30 Mosaicplasty has shown good results with lesions up to 4 cm2, but donor size limitation and donor morbidity are major concerns.31 ACI has been recommended for large lesions in the knee; however, controversial results after a failed cartilage repair surgery have been presented in the literature. Minas et al.9 presented a cohort of 321 patients treated with ACI after SMS showing a failure rate three times that of ACI primary treatment. Zaslav et al.32 showed conflicting findings in a multicenter trial including 126 patients who had ACI after a failed prior cartilage repair. Besides the higher reoperation rate reported (49%), Zaslav et al.32 showed improvement in outcomes regarding pain and function comparable with ACI as a primary treatment option. Nawaz et al.10 in a cohort of 1,000 patients with an average follow-up time of 6.2 years reported a failure rate five times higher in patients previously treated with cartilage repairs such as Pride drilling, microfracture, and mosaicplasty compared with ACI as a primary treatment.
Despite controversial reports, ACI seems to be influenced negatively by the damage of the subchondral bone and plate caused by prior cartilage repairs. The subsequent alterations of the subchondral bone after a cartilage repair, such as osseous overgrowth, cystic formation, and harder subchondral plate, may explain the higher failure rate.30,33 Osteochondral allograft transplantation has shown a different behavior than has been shown in ACI after a failed cartilage repair. The OCA technique removes the damaged subchondral bone to a depth necessary to achieve normal subchondral bone. The subchondral bone is excised and substituted by the donor bone, which makes the OCA technique resistant to subchondral bone abnormalities. Although the subchondral bone damage caused by previous cartilage repair surgeries could be totally replaced, extended lesions of the subchondral bone should be analyzed with care, as OCA failures are frequently related to the lack of integration of the subchondral bone.19,34
We performed a comparison of OCA failures and nonfailures on select variables in an attempt to describe variables associated with clinical failure of the OCA transplantation. The number of previous surgeries and the BMI were related to increased risk of failure (Table 5). No significant association was found with older patients (P = 0.051), but a larger sample may change this correlation. These data are partially in agreement with the literature showing a higher failure in patients with two or more previous surgeries, patients older than 30 years, uncorrected limb malalignment, diagnosis of osteoarthritis, and bipolar allografts.19,25,34 Hence, patients with BMI lower than 26 kg/m2 with at most 2 surgeries are the best candidates for OCA transplantation.
Historically, OCA transplantation is recommended as a salvage treatment option for traumatic or degenerative chondral lesions, osteochondritis dissecans, or any other osteochondral defect bigger than 2 to 4 cm2, particularly with bone loss larger than 6 mm of depth.12,15 When data of allograft studies were pooled together, the outcomes of OCA transplantation in the adult knees showed significant improvement in functional outcomes scores (IKDC, KOOS, Lysholm).25,35,36 By an average of 86%, the patients were either extremely or mostly satisfied.25,36-38 Survivorship of OCA transplantation varied from 76% to 91% at 10 years in studies with long-term follow-up.19,38 These reports included heterogeneous populations regarding previous cartilage repair. Our cohort is comparable with these reports.
This study showed 41.5% of patients underwent reoperation, but only 18.9% were considered failures. Our reoperation rate is comparable with other OCA studies25,34,36 and is expected in a young and active population at high risk for further injuries in the knee. Levy et al.25 evaluated 122 patients with 91% of patients with more than 10 years of follow-up after OCA of the femoral condyle who presented a reoperation rate of 47%, of which 24% were related to allograft failure. Other cartilage repairs also present a substantial reoperation rate, such as Zaslav et al.32 described a cohort of 154 patients who underwent ACI in the knee and showed a reoperation rate of 49%, of which 83% underwent an arthroscopy or manipulation.
Our study has some limitations. Radiography and magnetic resonance imaging were not routinely performed at final follow-up, so no radiographic criteria of failure could be assessed. However, we obtained radiographs of all patients until allograft incorporation was observed. We used the modified Merle d’Aubigné-Postel (18-point) scale, which is a system not validated in the knee. This scoring system has been used since 1983, when no validated outcome scores were available. This scoring system has a simple score for pain and function that allows an intrasample comparison within the cohort. The retrospective nature of the study also introduces potential bias.
This study demonstrated improvement in pain and function of the patients studied with satisfactory survivorship. Despite the high reoperation rate, OCA transplantation is considered a suitable option as a salvage technique for cartilage lesions of the knee.
Footnotes
Acknowledgments and Funding: G.M. was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (Ref: B.14.2.TBT.0.06.01-219-6040). G.C.G. was supported by Coordination for the Improvement of Higher Education Personnel (CAPES) of Brazil.
Declaration of Conflicting Interests: One or more of the authors has declared the following potential conflict of interest or source of funding: W.D.B. is a consultant for and has received research support from the Joint Restoration Foundation, a nonprofit tissue bank that receives, processes, and distributes osteochondral allografts
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 2. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 4. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-82. [DOI] [PubMed] [Google Scholar]
- 5. Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795-801. [DOI] [PubMed] [Google Scholar]
- 6. Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. [DOI] [PubMed] [Google Scholar]
- 7. Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337-42. [DOI] [PubMed] [Google Scholar]
- 8. Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85(Suppl 2):8-16. [DOI] [PubMed] [Google Scholar]
- 9. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. [DOI] [PubMed] [Google Scholar]
- 10. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96(10):824-30. [DOI] [PubMed] [Google Scholar]
- 11. de Windt TS, Vonk LA, Brittberg M, Sarris DBF. Treatment and prevention of (early) osteoarthritis using articular cartilage repair—fact or fiction? A systematic review. Cartilage. 2013;4(3):5S-12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomoll AH, Farr J, Gillogly SD, Kercher JS, Minas T. Surgical management of articular cartilage defects of the knee. Instr Course Lect. 2011;60:461-83. [PubMed] [Google Scholar]
- 13. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468(5):1269-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris JD, Brophy RH, Siston RA, Flanigan DC. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26(6):841-52. [DOI] [PubMed] [Google Scholar]
- 15. Moran CJ, Pascual-Garrido C, Chubinskaya S, Potter HG, Warren RF, Cole BJ, et al. Restoration of articular cartilage. J Bone Joint Surg Am. 2014;96(4):336-44. [DOI] [PubMed] [Google Scholar]
- 16. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 17. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325-31. [DOI] [PubMed] [Google Scholar]
- 18. Bugbee WD, Khanna G, Cavallo M, McCauley JC, Görtz S, Brage ME. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am. 2013;95(5):426-32. [DOI] [PubMed] [Google Scholar]
- 19. Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87. [DOI] [PubMed] [Google Scholar]
- 20. Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(Suppl 1):148S-55S. [DOI] [PubMed] [Google Scholar]
- 21. Gomoll AH, Filardo G, Almqvist FK, Bugbee WD, Jelic M, Monllau JC, et al. Surgical treatment for early osteoarthritis. Part II: allografts and concurrent procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):468-86. [DOI] [PubMed] [Google Scholar]
- 22. Kalson NS, Gikas PD, Briggs TW. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64(10):1444-52. [DOI] [PubMed] [Google Scholar]
- 23. Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466(4):952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-33. [DOI] [PubMed] [Google Scholar]
- 25. Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;(360):159-68. [PubMed] [Google Scholar]
- 27. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-13. [DOI] [PubMed] [Google Scholar]
- 28. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-4. [PubMed] [Google Scholar]
- 29. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635-40. [DOI] [PubMed] [Google Scholar]
- 30. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 31. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-508. [DOI] [PubMed] [Google Scholar]
- 32. Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42-55. [DOI] [PubMed] [Google Scholar]
- 33. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. [DOI] [PubMed] [Google Scholar]
- 34. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 35. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805-11. [DOI] [PubMed] [Google Scholar]
- 36. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411-20. [DOI] [PubMed] [Google Scholar]
- 37. Chahal J, Gross AE, Gross C, Mall N, Dwyer T, Chahal A, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-88. [DOI] [PubMed] [Google Scholar]
- 38. Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-14. [DOI] [PubMed] [Google Scholar]