Abstract
Background
Cerebellar granule cell precursors are specifically generated within the hindbrain segment, rhombomere 1, which is bounded rostrally by the midbrain/hindbrain isthmus and caudally by the boundary of the Hoxa2 expression domain. While graded signals from the isthmus have a demonstrable patterning role within this region, the significance of segmental identity for neuronal specification within rhombomere 1 is unexplored. We examined the response of granule cell precursors to the overexpression of Hoxa2, which normally determines patterns of development specific to the hindbrain. How much does the development of the cerebellum, a midbrain/hindbrain structure, reflect its neuromeric origin as a hindbrain segment?
Results
We show that a Gbx2-positive, Otx2-/Hoxa2-negative territory corresponding to rhombomere 1 forms prior to an identifiable isthmic organiser. Early global overexpression of Hoxa2 at embryonic day 0 has no effect on the expression of isthmic signalling molecules or the allocation of rhombomere 1 territory, but selectively results in the loss of granule cell markers at embryonic day 6 and the depletion of cell bodies from the external granule cell layer. By comparison the trochlear nucleus and locus coeruleus form normally in ventral rhombomere 1 under these conditions. Microsurgery, coupled with electroporation, to target Hoxa2 overexpression to rhombic lip precursors, reveals a profound, autonomous respecification of migration. Rhombic lip derivatives, normally destined to occupy the external granule cell layer, violate the cerebellar boundary to form a ventrolateral nucleus in a position comparable to that occupied by rhombic lip derived neurons in rhombomere 2.
Conclusions
Different overexpression strategies reveal that the recognition of migration cues by granule cell precursors is dependent on their identity as rhombomere 1 derivatives. Segmental patterning cues operate autonomously within the rhombic lip precursor pool. By contrast, a subset of coextensive nuclei is refractory to ectopic Hoxa2 and is presumably induced solely by isthmic organiser activity. Thus, graded (isthmic) and segmental mechanisms may operate exclusively of one another in the specification of different neuronal populations within rhombomere 1. The early designation of an Otx2-negative, Hoxa2-negative region, prior to the appearance of the isthmic organiser, is a key initial step in the specification of the cerebellum.
Background
Subdivision along the rostrocaudal axis of the developing central nervous system assigns regional identity to neuronal precursor pools, influencing the fate of their progeny and generating structural diversity [1]. Strategies for conveying fine-grain positional information vary along the length of the axis. For example, the hindbrain is a transiently rhombomeric structure organised by segmentally expressed Hox genes. By contrast, the development of the midbrain and forebrain, which lie within the Otx expression domain, is regulated by signalling centres generating graded patterning cues. At the transition point, where the hindbrain system of segmentation gives way to regional patterning by local gradients in the midbrain, the most anterior hindbrain segment, rhombomere 1 (r1), expresses neither Hox or Otx genes and displays aspects of both patterning systems. A number of distinct neuronal populations are generated in r1, including cerebellar granule cells [2], the most populous neuron in the vertebrate CNS. How graded and segmental cues interact in specifying r1 precursors is key to understanding the origins of the cerebellum, a major structure in the higher vertebrate brain.
A number of studies have examined different facets of r1 specification. The rostral boundary of r1 comprises the midbrain/hindbrain isthmus, an important signalling centre in the development of the midbrain and r1. Loss of isthmic signalling severely disrupts both midbrain and cerebellar development [3-5], while application of FGF8, the putative isthmic signalling molecule, suggests a direct involvement of the isthmic organiser in cerebellar induction [6,7]. Moreover, the trochlear motor nucleus and the locus coeruleus, the latter of which is rostrocaudally coextensive with granule cell production, are born within r1 and are induced by isthmic FGF [5,8,9].
In addition to its isthmic identity, r1 also displays properties of a hindbrain segment. Rhombomeres 1 to 3 are defined by the expression of Gbx2: within this domain, r1 differs from the more caudal segments by the absence of Hox gene expression. In mice, a null mutation in Hoxa2, the only Hox gene expressed in adjacent r2, results in the caudal expansion of the cerebellum [10] and rostralisation of the second branchial arch to a first arch identity [11,12]. This suggests, in the absence of any changes in isthmic signalling, that suppressing Hox gene expression in the hindbrain is sufficient to confer an r1 identity, as experimentally confirmed in zebrafish [13]. Correspondingly, in chick, overexpression of Hoxa2 in r1 induces ectopic r2-like motor neurons [14], while in the first branchial arch, neural crest adopts second branchial arch characteristics [15,16]. These apparent transformations support a model that r1, as r2, is a homeotic iteration within the meristic series that comprises the rhombencephalon.
To examine isthmic and segmental cues in cerebellar development, we have used a combination of retroviral and microsurgical techniques to assess patterning in r1, in particular with respect to the production of granule cell precursors from the rhombic lip. This population undergoes two distinct phases of migration, the first giving rise to the external granule cell layer (EGL), which covers the pial surface of the cerebellum, and a second in which granule cells migrate radially inwards to form the granule cell layer. We find that while nuclei whose induction is dependent on isthmic signalling remain unchanged, the formation of the external granule cell layer is inhibited by ectopic Hoxa2. This suggests that both segmental and isthmic-derived patterning mechanisms may operate independently within r1. Furthermore, microsurgically targeted misexpression of Hoxa2 in the rhombic lip reveals that the loss of granule cell from the cerebellum can be explained by a dramatic re-specification of tangential migration. A complementary grafting strategy demonstrates that these changes in the patterning of migration occur autonomously at the rhombic lip.
Results
Overexpression of Hoxa2 does not affect r1 allocation or isthmic identity
Previous fate-maps have demonstrated that at embryonic day (E) 2 (stage [st.]10), r1 is defined by the boundaries of the expression domains of, rostrally, Otx2 [17] and, caudally, Hoxa2 [2]. By such criteria, in situ hybridisation establishes that a Gbx2-positive territory corresponding to rhombomere 1 is apparent from the earliest onset of Otx2 and Hoxa2 expression (Fig. 1A). By st.9-, the caudal boundary of Otx2 expression defines the midbrain/hindbrain boundary, or isthmus (mhb; Fig. 1B), an important signalling centre that expresses a number of molecules involved in patterning adjacent territory, including FGF8, Engrailed and Wnt1 [4].
Figure 1.
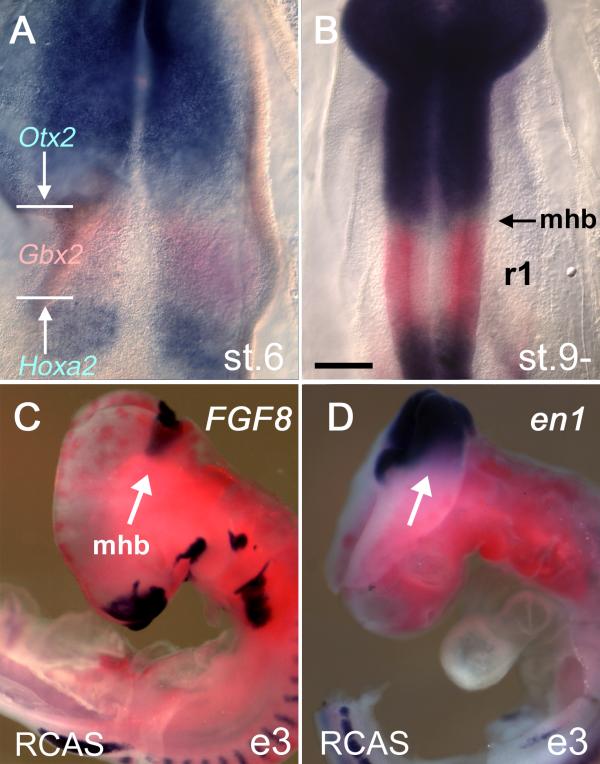
Establishment of rhombomere 1: Isthmic gene expression is refractory to Hoxa2 overexpression (A) At stage [st.] 6, Otx2 and Hoxa2 expression (both in blue) are separated by a Gbx2-positve domain (red) that defines rhombomere 1 (r1). The domain of Gbx2 expression encompasses r1–3. (B) At st.9-, the boundaries of the Otx2 and Hoxa2 expression domains define the segmental boundaries of r1 as determined by fate-map experiments [2, 17]. (C) Viral overexpression of RCASBP(B)Hoxa2 (red) at embryonic day (E) 0 fails to suppress the activation of either FGF8 (blue) or (D) en1 at E3 (e3). Scale bar (A,B) = 200 μm. mhb – midbrain/hindbrain isthmus.
Given that the isthmus has an important role in patterning the midbrain and maintaining r1 identity, we first assessed the effect of global Hoxa2 overexpression on key isthmic genes. The RCASBP(B)Hoxa2 retrovirus was injected into E0 chick eggs. The expression of FGF8 (Fig. 1C), Engrailed (en)1 (Fig. 1D) at E3 and Wnt1 at E6 (data not shown) were examined at the midbrain/hindbrain boundary. The gross morphology of the neural tube in these embryos was normal and r1 territory formed appropriately. Isthmic genes were expressed in domains encompassing the isthmus that were indistinguishable from controls (injected with RCANBP(B)Hoxa2; data not shown). This suggests that the signalling properties of the isthmus are unaffected by ectopic Hoxa2 at the isthmus.
Having determined that isthmic function was likely to be unimpaired, we chose to examine the effect of ectopic Hoxa2 on the development of three definitive r1 neuronal populations: cerebellar granule cell precursors, trochlear motor neurons and the locus coeruleus. Of these, only the origin of cerebellar granule cells has been microsurgically fate-mapped to r1 in chick [2]. Trochlear motor neurons and the catecholaminergic interneurons of the locus coeruleus can be allocated to r1 by the expression of their associated characteristic neuronal markers in relation to the molecular r1 boundaries defined above.
Hoxa2 overexpression in r1 leads to a cell-depleted EGL
RCASBP(B)Hoxa2 retrovirus was injected into the neural tube of st.10 (E2) embryos and neuronal phenotype assessed by in situ hybridisation, as flatmounts at E6 (Fig. 2A) or in transverse section at E9. At both ages, widespread infection with RCASBP(B)Hoxa2 led to a variable reduction in the size of the cerebellum, which is derived from dorsal r1 and becomes distinguishable at E5.
Figure 2.
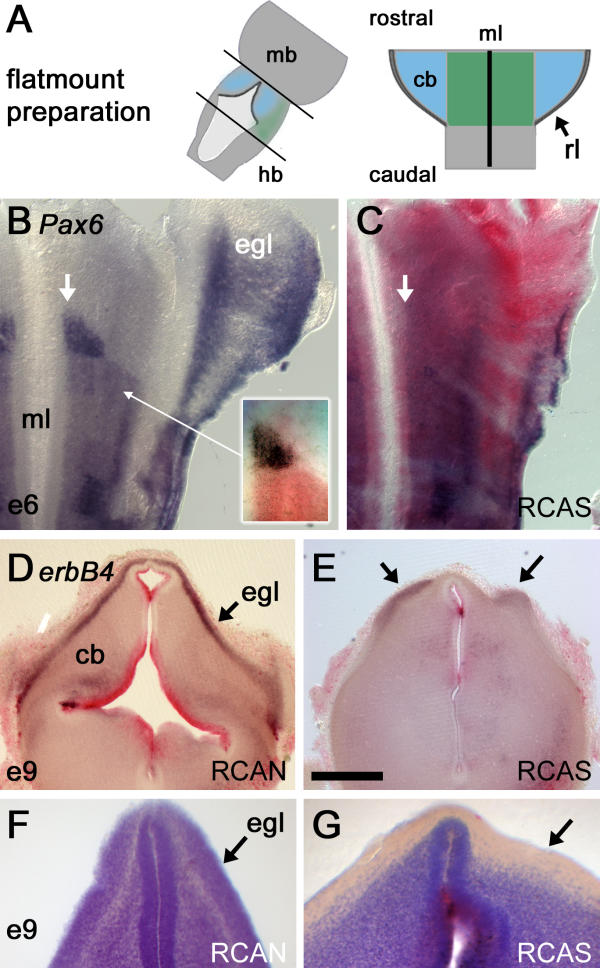
Global overexpression of Hoxa2 depletes rhombomere 1 of granule cell precursors (A) Flatmounts of brains up to embryonic day (E) 6 were prepared by opening the neural tube along the dorsal midline: cb – cerebellum, mb – midbrain, hb – hindbrain, rl – rhombic lip, ml – ventral midline. (B) The expression of Pax6 (blue) at E6 in one half of the anterior hindbrain viewed as a flatmount (rostral top, mediolateral axis runs left to right). Granule cell precursors born at the rhombic lip express Pax6 as they migrate over dorsal rhombomere 1 (r1) to condense as the external granule cell layer (EGL [egl]). Pax6 is also expressed in a ventral wedge of r1 precursors (arrow) abutting the anterior r2 boundary and ventral midline. The relationship of this Pax6 positive precursor pool (blue) to the anterior boundary of Hoxa2 (red) is shown inset. (C) Overexpression of Hoxa2 (red) leads to a loss of Pax6 in the EGL and prominent ventral wedge (arrow). This is replaced by a weaker, broadened, ventral Pax6 domain similar to that in the hindbrain. (D) At E9, in transverse section through a control embryo, the neuregulin receptor erbB4 labels the EGL. Red label dorsally reflects high levels of expression of the control virus, RCANBP(B)Hoxa2 (red), within the ventricular zone of the cerebellum (cb). (E) Injection of the active RCASBP(B)Hoxa2 virus leads to a reduction in erbB4. Patches of expression (arrows) correspond to residual EGL overlying a locally thickened cerebellum. (F) Cresyl violet stains in control and (G) RCASBP(B)Hoxa2-infected cerebella reveal an absence of cell bodies within a superficial, subpial layer (arrows), normally occupied by the EGL, following Hoxa2 overexpression. Scale bar (D,E) = 500 μm.
Cerebellar granule cell precursors are generated at the rhombic lip (E6 onwards), which comprises the interface between the neural tube and non-neural roofplate of the fourth ventricle. During their characteristic ventrolateral tangential migration to form the EGL, granule cell precursors express a number of distinct molecular markers, including a paired-homeodomain transcription factor, Pax6 [18], and the cell surface receptor, erbB4 [19]. At E6, Pax6 labels cells condensing as the EGL in dorsal r1 (Fig. 2B). This dorsal expression is lost following Hoxa2 overexpression (Fig. 2C), along with a small domain of ventricular Pax6 expression (Figs 2B,2C, white arrows), just anterior to the normal limit of Hoxa2 expression (Fig. 2B, inset). Loss of dorsal Pax6 suggests that rhombic lip derivatives either fail to be generated or that they down-regulate Pax6 during their tangential migration. At E9, the EGL is strongly positive for erbB4, as illustrated in an embryo infected with the control virus RCANBP(B)Hoxa2 (Fig. 2D). Following overexpression of Hoxa2, erbB4 becomes restricted to small islands of superficial cerebellum (Fig. 2E, arrows) overlying a locally thickened cerebellum. As with Pax6, no ectopic expression of erbB4 was observed that might account for an apparent loss of granule cells. The depletion of this cell type was confirmed by cresyl violet staining of control RCAN- (Fig. 2F) and RCAS-infected (Fig. 2G) cerebellum. Strikingly, Hoxa2 overexpression at E2 results in a cerebellum displaying a clearly demarcated external cerebellar layer at E9 that is nevertheless devoid of cell bodies.
Hoxa2 overexpression does not affect trochlear nucleus or locus coeruleus formation
The structure of the trochlear nucleus and locus coeruleus was assessed by the expression of Phox2a (Fig. 3A), a characteristic marker of both neuronal pools within r1 [20]. The locus coeruleus is generated dorsal to the trochlear motor nucleus (Fig. 3B iv), which lies just caudal to isthmus and the oculomotor nucleus (Fig. 3B iii) of the midbrain (Fig. 3B). In transverse section the locus coeruleus can be seen to condense adjacent to the ventricular layer in ventral r1, while dorsally Phox2a expression is excluded from the presumptive cerebellum (Fig. 3C). Within the hindbrain, caudal of the r1/2 boundary, Phox2a expression is organised into contiguous lateralised columns (Fig. 3A). Global overexpression of Hoxa2 has no effect on the size or position of either the trochlear motor nucleus or the locus coeruleus (Fig. 3D). Curiously, the level of Phox2a transcripts appears higher in trochlear motor axons following viral infection.
Figure 3.
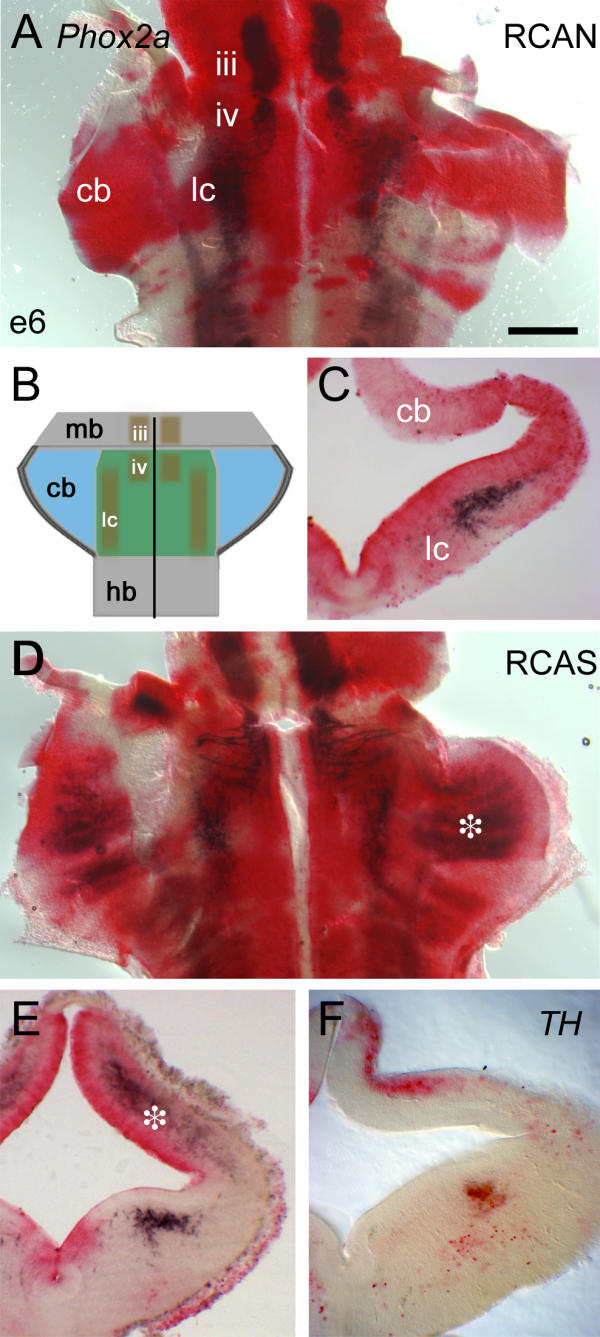
Locus coeruleus and trochlear nucleus formation is normal following global Hoxa2 overexpression (A) Flatmounted control embryo at embryonic day (E) 6, infected with RCANBP(B)Hoxa2 virus (red); Phox2a expression (black) marks the oculomotor nucleus (iii) in the midbrain, and trochlear motor nucleus (iv) and locus coeruleus (lc) in rhombomere 1 (r1). There is no Phox2a expression within the cerebellar anlage (cb). (B) Schematic diagram showing the arrangement of Phox2a-positive nuclei within r1, as shown in (A). (C) This lack of dorsal expression is confirmed in transverse section, while the locus coeruleus is clearly identified by Phox2a. (D) Hoxa2 up-regulation with RCASBP(B)Hoxa2 (red) leads to ectopic Phox2a expression in the dorsal r1/cerebellum (asterisk). (E) In a transverse section slightly caudal to that in C, ectopic expression of Phox2a is confined to the deeper cerebellar layers (asterisk), while ventral expression of Phox2a in the locus coeruleus is unaltered. (F) Hoxa2 up-regulation does not result in increased tyrosine hydroxylase (TH) staining within dorsal r1. Scale bar (A,D) = 400 μm. mb – midbrain, hb – hindbrain.
By contrast, in dorsal r1, Phox2a expression was induced throughout the presumptive cerebellum excluding the rhombic lip (Fig. 3D, asterisk). In transverse section, ectopic Phox2a expression in the cerebellum lies within the mantle, outside the cell-depleted EGL (Fig. 3E, asterisk). Dorsal r1 is the origin of ventrally migrating locus coeruleus neuroblasts, raising the possibility that Hoxa2 may have generated an ectopic locus coeruleus population within dorsal r1. However, in situ hybridisation for the enzyme tyrosine hydroxylase (TH), which is expressed at the earliest stages of locus coeruleus migration [21], demonstrates that ectopic Phox2a-positive cells are not catecholaminergic: in RCAS infected embryos, TH is expressed only within the ventral pool of locus coeruleus neurons (Fig. 3F).
Hoxa2 overexpression disrupts the migration of rhombic lip derivatives in r1
The loss of granule cell markers and up-regulation of Phox2a in deeper cerebellar layers raise the possibility that the migration and/or fate of rhombic lip derivatives are specifically affected by ectopic Hoxa2. Correspondingly, a previous lineage study hypothesised that the locus coeruleus is derived from the EGL [22], which might favour an argument that Hoxa2 transfection biases production of rhombic lip derivatives towards a deeper-migrating, Phox2a-positive derivative.
To assess the fate and movement of r1 rhombic lip derivatives expressing Hoxa2, we devised a microsurgical chimaera strategy whereby gene overexpression could be targeted precisely to the rhombic lip precursor pool [23]. RCASBP(B)Hoxa2 plasmid was electroporated into donor quail tissue at E2. Dorsal r1, which maps to the cerebellar rhombic lip in later embryos [2,23], was then transplanted unilaterally into dorsal r1 of a host chick embryo at E2. At E6, the fate of quail cells derived from the rhombic lip was logged using a quail specific perinuclear marker, Q¢PN, and double in situ hybridisation for both Hoxa2 and Phox2a (Fig. 4A).
Figure 4.
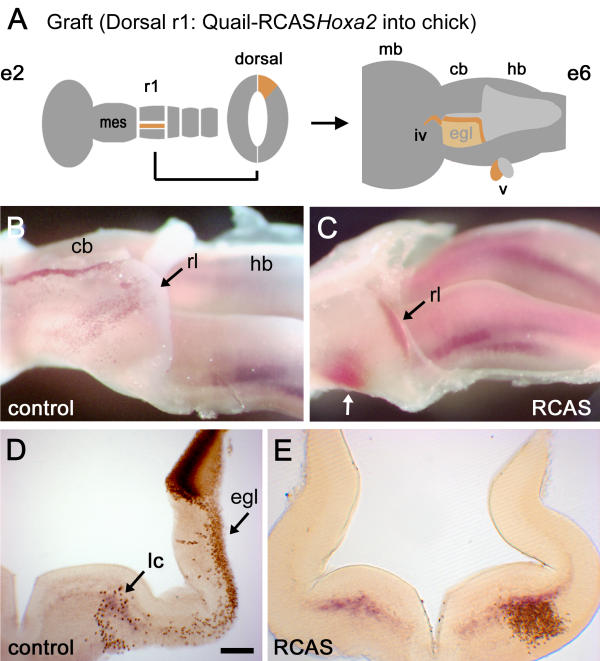
Targeted Hoxa2 overexpression respecifies the migration of rhombic lip derivatives (A) Schematic diagram showing the location of donor tissue grafted from Hoxa2 electroporated quail embryos at embryonic day (E) 2 (e2; left). A graft of dorsal rhombomere 1 (r1) maps to the cerebellar rhombic lip and roofplate at E6 (e6; right) and contributes to both the external granule cell layer (EGL) and neural crest derivatives in the trochlear (iv) and trigeminal (v) nerves [2]. (B) Lateral view of a wholemount dissection of the cerebellum (cb) and hindbrain (hb) of a control E6 chimaeric embryo stained with a quail-specific antibody, Q¢PN (brown), and stained for Phox2a (purple). The rostrocaudal axis runs left to right. A stream of ventrally migrating quail cells originating from caudal rhombic lip (rl) lies over the presumptive cerebellum (point of origin indicated by a black arrow). A thin strip of quail cells also lies in the roofplate separating the bilateral anlage. (C) Migration patterns are substantially altered when grafted donor cells (black arrow) are electroporated with RCASBP(B)Hoxa2. Rhombic lip derivatives fail to condense in the EGL but rather migrate into a single ventrolateral nucleus (white arrow), which expresses high levels of ectopic Hoxa2 (red). (D) Transverse section through the cerebellum of a control chimaera showing the donor progenitors in the dorsal neural tube. The majority of quail cell derivatives lie within the EGL (egl), with a scattering of cells ventrally overlapping the Phoxa2 expression in the locus coeruleus (light blue). (E) By contrast, the majority of cells from grafts electroporated with Hoxa2 condense ventrally (where quail cell nuclear diaminobenzidine label obscures the red in situ product marking Hoxa2). Scale bar (D,E) = 200 μm. mb – midbrain; mes – mesencephalon.
Figure 4B shows a wholemount cerebellum from a control (uninfected) quail–chick chimaera at E6. Darkly stained graft-derived precursors occupy the narrow insertion of roofplate at the midline of the cerebellum. Where quail cells overlap the rhombic lip (more caudally), migrating EGL precursors are distributed across the surface of the cerebellum (black arrow). In Fig. 4C, the distribution of rhombic lip derivatives is profoundly changed when quail tissue electroporated with Hoxa2 is used as donor tissue (n = 6/7). No quail cells are seen in the presumptive cerebellum (dorsal r1). Instead, rhombic lip derivatives expressing high levels of Hoxa2 condense in ventrolateral r1 (white arrow).
The effect of exclusively targeting Hoxa2 to the rhombic lip is seen more clearly in transverse section. In control chimaeras, only a small population migrates into ventrolateral r1 (Fig. 4D), while the majority of migrating cells condense in the EGL. A few scattered cells lie within the pool of Phox2a-positive, presumptive locus coeruleus neurons. When Hoxa2-grafted r1 quail cells express Hoxa2, they condense in a dense cluster within ventrolateral r1 (Fig. 4E). The boundaries of their distribution are the same as the relatively small ventrolateral contribution in normal embryos. Despite the expanded rhombic lip contribution to ventral r1, the expression of Phox2a on control and operated sides of the brain is equivalent, suggesting that locus coeruleus formation is independent of changes in the population derived from the rhombic lip (Fig. 4E). Furthermore, while RCASBP(B)Hoxa2 induces a profound change in the migration path, transfected rhombic lip derivatives are unlikely to account for the Phox2a-expressing neurons in the cerebellum following a global virus transfection.
Effects of Hoxa2 on neuronal migration are rhombic-lip-autonomous
The migratory behaviour of r1 rhombic lip cells can be altered by targeting Hoxa2 overexpression to the precursor pool, suggesting that trajectory of its derivatives is at least partially determined by signals autonomous to the precursor pool. However, the environment through which cells migrate may also influence migration and condensation patterns. In particular, Hoxa2 expression outside the rhombic lip may cause an accumulation of Phox2a-positive rhombic lip derivatives in dorsal r1 through a non-autonomous mechanism. To assess this possibility, normal quail cells were grafted orthotopically into dorsal r1 in chick embryos that had been electroporated with RCASBP(B)Hoxa2 (n = 6). Figure 5A,5B,5C contrast, respectively, the contribution of rhombic lip cells to the EGL and ventrolateral r1 in three different combinations: control chimaeras, Hoxa2-transfected quail rhombic lip derivatives in a normal background, and normal quail derivatives in a Hoxa2-transfected background. While the EGL is depleted and a ventral component of the migration stream enhanced when r1 rhombic lip expresses Hoxa2 (Fig. 5B), normal quail cells show equivalent migration on either a normal (Fig. 5A) or a Hoxa2-transfected background (Fig. 5C). This confirms that the regionally determined responses to migratory cues are determined autonomously within the rhombic lip.
Figure 5.
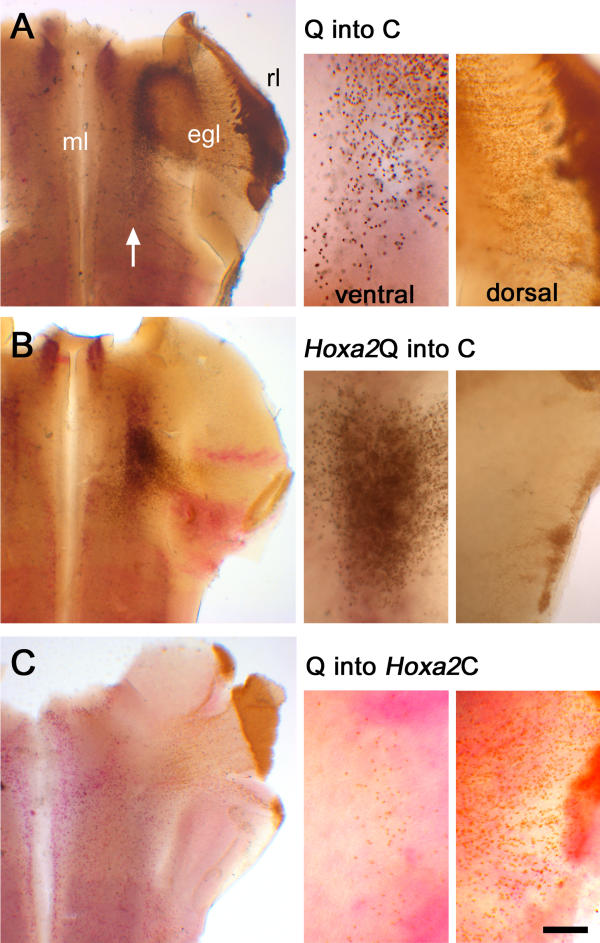
Effects on tangential migration of ectopic Hoxa2 are rhombic-lip-autonomous A comparison of E6 quail-chick chimaeras with different combinations of targeted Hoxa2 overexpression (red), in which the relative proportions of dorsal and ventral rhombic lip derivatives are compared (right). (A) Uninfected quail (Q) donor tissue in a normal chick (C) host (Q into C) gives rise to EGL cells and a small number of neurons in ventral r1 (white arrow). (B) Quail cells expressing Hoxa2 in a normal host (Q-Hoxa2) bypass the EGL and condense ventrally. (C) Normal quail cells migrate in a host expressing ectopic Hoxa2 (Q into Hoxa2 C) migrate as controls. Scale bar for high-power insets = 100 μm. egl – external granule cell layer; ml – midline.
Is the transformation of r1 rhombic lip by Hoxa2 homeotic?
Previous studies have shown that the overexpression of vertebrate Hox genes in the hindbrain consistently results in transformations that can be described as homeotic, in that they recapitulate segmental characteristics of specific rhombomeres. Hoxa2 chiefly regulates the identity of neurons in r2: thus, r1 rhombic lip cells transfected with Hoxa2 might be expected to behave as r2 rhombic lip derivatives. In the absence of appropriate molecular markers for specific r2 populations, we used a grafting strategy to test this hypothesis.
We first assessed the normal migration path of r2 rhombic lip derivatives by grafting a narrow strip of dorsal quail r2 into dorsal chick r2 at E2, in ovo. In a significant fraction of the resultant chimaeras, analysed at E6 or E7, quail cells were found in the neural crest alone (n = 8/18), demonstrating that this grafting strategy effectively targeted the most dorsal cells of the neural tube [2]. In the remainder of the chimaeras, the grafted tissue was sufficiently large to generate migratory dorsal derivatives within the neural tube that condensed in a single ventrolateral nucleus, as shown in flatmounted (Fig. 6A) and transversely sectioned (Fig. 6B) embryos. These derivatives were assumed to constitute the r2 rhombic lip derived population.
Figure 6.
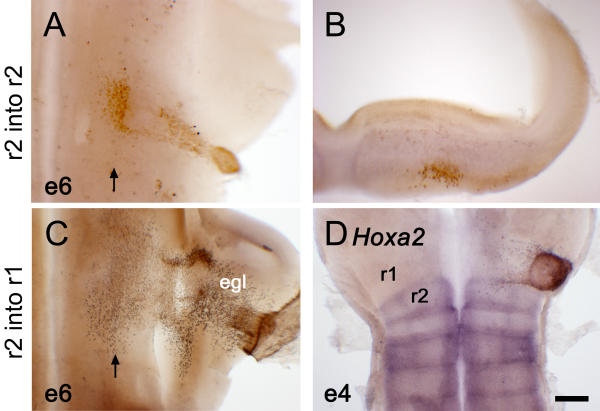
Derivatives of r2 dorsal neural tube have a characteristic migration pattern correlated to maintenance of endogenous Hoxa2 expression (A) Flatmount of a fate-map chimaera at embryonic day (E) 6 (e6), in which quail r2 was orthotopically grafted into rhombomere 2 (r2) of a chick host. At E6, in flatmount, quail derivatives (brown) condense primarily in a single ventrolateral nucleus. (B) In transverse section, r2 migrants can be seen to settle in a subpial location similar to that of ventral derivatives in r1. (C) When transplanted into r1, quail r2 cells contribute to the EGL while maintaining a sparse ventral contribution. (D) When analysed by in situ hybridisation at E4 (e4), r2 grafted into r1 has lost its endogenous Hoxa2 expression (blue). Scale bar = 100 μm.
We then grafted equivalent dorsal quail r2 heterotopically into r1, to directly compare the behaviour of r2 rhombic lip derivatives with that of r1 derivatives infected with Hoxa2 (Figs. 4, 5). In contrast to orthotopically grafted tissue, the distribution of r2 quail cells within r1, analysed at E6 (n = 6), more closely resembled that of normal r1 rhombic lip derivatives. Rhombic lip from r2 contributed to both the EGL and a ventrolateral nucleus (Fig. 6C). The difference in behaviour between r2 dorsal derivative within r2 (Fig. 6A) and r1 (Fig. 6B) can be attributed to the respecification of r2 precursors grafted into r1. In situ analysis of heterotopically grafted r2 demonstrates that, as early as E4 (n = 7), endogenous Hoxa2 expression is down-regulated in donor quail cells (Fig. 6D), presumably by signals from the isthmus [24,25]. Thus, it is impossible within r1 to directly compare the migration path of rhombic lip derivatives from Hoxa2-infected r1 with those from r2 (Fig. 6A,6B). However, Hoxa2 overexpression within the cerebellar rhombic lip results in a rhombic lip migration path reminiscent of that in r2 (Fig. 6A,6B), supporting a conclusion that the transformation is homeotic. Furthermore, the maintenance of an r2-specific migration pattern is correlated with the maintenance of endogenous Hoxa2 expression.
Discussion
We have used a combination of viral overexpression and electroporation coupled with grafting to assess the effects of ectopic Hoxa2 on selected features of r1. Viral infection allowed for the study of early-onset gene transfection on patterning of anterior hindbrain. Nonspecific effects of RCAS infection, reported for electroporated embryos [26], were controlled for by injections RCAN virus, which lacks a splice acceptor and thus produces mRNA but no protein. At later stages, a combination of electroporation into quail embryos and microsurgery allowed us to target overexpression to specific precursor pools. The results of these different technical approaches revealed a clear division within r1 development between precursors that are sensitive and those that are refractory to Hoxa2 overexpression (Fig. 7). These observations were consistent for different degrees of transfection, suggesting that our results are unlikely to reflect a differential response by r1 precursors to varying Hox gene dosage. Rather, we interpret this observation as reflecting divergent patterning strategies within r1 for different neuronal groups, as revealed by an all-or-nothing sensitivity to ectopic Hoxa2.
Figure 7.
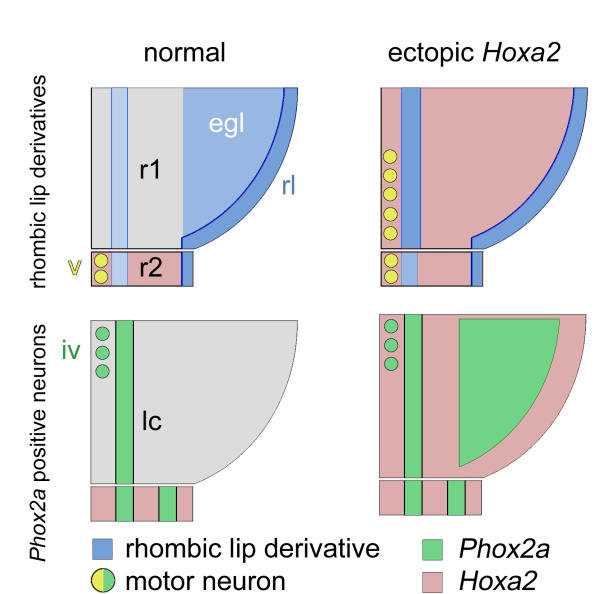
Summary of the effects of Hoxa2 overexpression on r1 derivatives The arrangement of rhombic lip derivatives in rhombomere 1 (r1) (top) and Phox2a-positive neurons (bottom) shown on a schematised flatmount of a half neural tube from normal (left) and Hoxa2-infected (right) embryos. (Top) Hoxa2 overexpression in r1 results in the loss of the EGL and an expansion of ventral rhombic lip derivatives. Ectopic trigeminal-like motor neurons are generated in caudal r1 [14]. (Bottom) The trochlear nucleus and locus coeruleus form normally when Hoxa2 is overexpressed; however, dorsal r1 expresses high levels of ectopic Phox2a. The cells of this latter population are neither TH-positive locus coeruleus precursors that have stalled during migration nor rhombic lip derivatives that have altered their fate. egl – external granule cell layer; rl – rhombic lip.
Features of r1 that are unaffected by ectopic Hoxa2
We found that the initial allocation of r1 territory, the expression of isthmic signalling genes FGF8, Wnt1 and en1, the formation of the trochlear nucleus and the establishment of the locus coeruleus were unaffected by ectopic Hoxa2 expression within r1. By using retrovirus injection into pregastrula embryos, overexpression was induced before the establishment of either an Otx2-, Hoxa2-negative domain (at st.6) or isthmic organiser (st.8; [27]). Thus, ectopic Hoxa2 can be presumed to have no influence on the inductive interactions between midbrain (Otx2-positive) and hindbrain (Gbx2-positive) territory that establish isthmic signalling [3,4]. While FGF8 can repress Hoxa2 in the hindbrain (Fig. 6B; [24,25]), the converse is not found at the isthmus.
Isthmic signalling plays a role in regulating the development of r1 [3,6,8,24] where FGF8 is sufficient for the induction the trochlear nucleus [8] and necessary for locus coeruleus specification [9]. These processes, which are overtly linked to isthmic activity, are refractory to experimental Hoxa2 up-regulation, suggesting that a complex of isthmic induction pathways in r1 is independent of a set of patterning processes that rely on segmental identity cues.
Structures affected by Hoxa2 overexpression
By contrast, various aspects of r1 specification and patterning are profoundly influenced by ectopic Hoxa2. In a previous study, RCASBP(B)Hoxa2 was shown to induce an r2-specific motor neuron phenotype within ventral r1 [14], caudal to the trochlear nucleus, in a region normally devoid of any motor efferents. Ventral progenitors were thus presumed to undergo a homeotic transformation to a more caudal fate (Fig. 7).
Within dorsal r1, ectopic Hoxa2 results in a smaller cerebellum, consistent with the loss of the EGL due to the aberrant migration of granule cells to a ventrolateral target. Both the loss of specific granule cell markers and the induction of a pattern of nuclear condensation corresponding to that of r2 derivatives suggest that rhombic lip precursors have been caudalised in a homeotic manner. Furthermore, a combination of grafting and electroporation reveals that this effect is independent of changes to r1 as a whole. This suggests that segmental/rhombomeric identity programmes rhombic lip derivatives with region specific responses to generalised dorsoventral migration cues such as netrin [28,29] and slit [23].
This transformation of rhombic lip migration pattern is independent of the formation of a cell-free layer corresponding to the EGL (Fig. 2F,2G), in distinct contrast to mouse models of granule cell depletion, such as the MATH1 mutant, where the EGL fails to form [30]. This indicates that the transient presence of Hoxa2-positive rhombic lip derivatives migrating over the cerebellar anlage is apparently sufficient to trigger the allocation of space for an EGL, even if this layer is subsequently unoccupied.
Our observations cast doubt on lineage analyses implicating the rhombic lip as the origin of locus coeruleus neurons [22]. Despite dramatic alterations in migration and condensation patterns of rhombic lip derivatives, the locus coeruleus always forms correctly. Furthermore, Hoxa2 induced, ecoptic Phox2a-positive neurons, which seemed ideal candidates for the 'lost' EGL population, have an as yet indeterminate origin. Our results correspond with observations indicating that locus coeruleus neurons are born earlier than EGL precursors [21] from a Mash1-positive precursor pool [31], which lies ventral to the Math1-positive rhombic lip [32].
Finally, the fate of other neuronal types born within dorsal r1, including the remainder of the cerebellum [33], remains to be determined. Ectopic dorsal Phox2a is seen in globally infected embryos but not when Hoxa2 is targeted to the rhombic lip (Fig. 4E). This points to further transformations of cerebellar neurons when Hoxa2 is overexpressed throughout dorsal r1 that are not secondary to the loss of granule cells. However separating autonomous and non-autonomous effects of ectopic Hoxa2 in dorsal r1 will require an accurate fate-map of the origins of each cerebellar neuronal population. With the exception of granule cells, the dorsoventral origins of each cerebellar neuronal type have not yet been established.
Origins of the cerebellum: segmental identity versus organiser activity
While midbrain/hindbrain patterning has focused on the role of the isthmic organiser in regional patterning [5,24,25], Hoxa2 overexpression reveals a dependence on segmental identity for the specification of cerebellar granule cells. It thus seems likely that the establishment of a specific Otx2-, Hoxa2-negative region at st.6 (Fig. 1A) is an important step in the specification of the cerebellum. Consistent with this hypothesis, the loss of Hoxa2 expression in r2 leads to a caudal expansion of the cerebellum [10], presumably in the absence of any changes in isthmic signalling cues. These results suggest that cerebellar granule cell induction is independent of isthmic signalling per se. Our results agree with suggestions that the effects of experimental manipulation of FGF on cerebellar induction act through modulation of Otx2 [5,7], rather than a direct induction. Thus, the induction of ectopic cerebellar neurons by exogenous FGF application can be attributed to the down-regulation Otx2 in the midbrain [6,7] or Hoxa2 in the hindbrain [24], recapitulating the molecular characteristics of r1. Correspondingly, loss of FGF signalling in the zebrafish ace mutant leads to an expansion of Otx2 within r1 territory, suppressing granule cell induction [5].
The establishment of r1 is therefore necessary for cerebellar induction and, by molecular criteria, precedes that of any detectable isthmic organiser. Whether the absence of both Otx and Hox genes is sufficient to potentiate cerebellar development in the absence of isthmic signalling is unclear. Complete suppression of Hox gene function results in a hindbrain that adopts a 'default' r1 phenotype in zebrafish [13], but whether later dorsal development is 'cerebellar' has not been determined. As in ontogeny, the appearance of r1 precedes, phylogenetically, that of both the isthmic organiser [34] and the cerebellum [35]. The evolution of a cerebellum would therefore seem to require a modification of either extrinsic signalling cues or responses within r1, in addition to the simple allocation of r1 territory.
Conclusions
Our results suggest that rhombomere 1 displays patterning influences that are characteristic of both the isthmic organiser and the rhombomeric organisation of the hindbrain. Using Hoxa2 overexpression to repattern regional identity of r1, rhombic lip derivatives that give rise to granule cell precursors can be forced to undergo a radical reprogramming of their migration and fate. By contrast, isthmic signalling molecules and both the territorial extent and the establishment of a subset of r1 nuclei are refractory to segmental cues and thus are presumed to be patterned independently by the midbrain/hindbrain organiser. This supports an argument that granule cells are a product of the molecular identity of r1 and induced in an isthmus-independent manner.
Methods
Viral overexpression of Hoxa2
Chick eggs (Hi-Sex White) were windowed with scissors at either E0 (6 hours of incubation) or E2 (stage [st.] 8+ to 10-, [36]). The anterior neural tube was injected with the retrovirus encoding Hoxa2, RCASBP(B)Hoxa2 (108 to 109 virions/ml) To control for the nonspecific effects of viral infection, a different group of embryos were injected with the retrovirus, RCANBP(B)Hoxa2, which lacks a splice acceptor site immediately 5' of Hoxa2 and hence fails to produce functional Hoxa2 protein [14]. Eggs were sealed with tape and reincubated at 37°C for 4 to 7 days.
Electroporation and microsurgery
Donor quail embryos were windowed at E2 and plasmid containing the proviral sequences of RCASBP(B)Hoxa2 (1 mg/ml in water containing 0.0015% fast green) was injected into the neural tube at the level of r1/2. Cells were then electroporated (1–2 pulses, 200 milliseconds/10 V, square waveform) using electrodes placed ventral and dorsal to the donor r1. These and a control group of unelectroporated donor embryos were harvested and the anterior neural tube isolated in Tyrode's solution (137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 3.5 mM NaH2PO4, 0.1 mM MgCl2, 5.5 mM D-glucose). The dorsal part of r1 was dissected away, after treatment with dispase (Roche, 1 mg/ml in L15, 10 minutes), using flame-sharpened tungsten wire [37]. Tissue from both donor groups was orthotopically grafted into stage-matched host chick embryos, which were then sealed and reincubated for 4 or 5 days. In a complementary strategy, unelectroporated quail dorsal r1 was grafted into chick hosts that had been electroporated with RCASBP(B)Hoxa2. In a separate set of fate-mapping experiments, dorsal r2 from quail was grafted orthotopically into r2 or heterotopically into r1 of host chick embryos, which were reincubated for 2 to 5 days.
In situ hybridisation and immunohistochemistry
Virally infected, chimaeric embryos and controls were harvested at E6 in 4% paraformaldehyde in 0.01 M phosphate buffer (PFA) at 4°C. The cerebellum and hindbrain region were partially dissected and processed for in situ hybridisation as described elsewhere [38]. Plasmid templates from a number of sources were used to generate digoxygenin- (dig-) and FITC-labelled antisense riboprobes: Hoxa2 [39], Phox2a (from P Goridis, INSERM, Marseilles), erbB4 [19], Pax6 [40], TH [21], en1 [41], Wnt1 (Dr Anthomy Graham, King's College London, UK), FGF8 [27], Gbx2 [42] and Otx2 [17].
Quail–chick chimaeras were further stained using an antibody, Q¢PN (DSHB, Iowa University, Iowa, USA) to a quail-specific perinuclear marker and a peroxidase-conjugated secondary antibody. Embryonic brains were sectioned transversely at 50 μm on a vibratome, mounted on slides in glycerol/PBS (9:1) and photographed using an Axiocam mounted on a Zeiss Axiophot.
Authors' contributions
ME and LT contributed equally to studies of the overexpression of Hoxa2 and its analysis. EB characterised early gene expression in the midbrain and hindbrain. RJTW planned the study, performed the microsurgery and analysed the results. All the authors have read and approved the manuscript.
Acknowledgments
Acknowledgements
We thank Leigh Wilson and Jon Gilthorpe for their comments on the manuscript. The monoclonal antibody Q¢PN (B Carlson and J Carlson) was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Iowa City (USA), under contract N01-HD-7-3263 from the NICHD, Maryland, USA. This study was supported by the Medical Research Council (London, UK) and the Wellcome Trust (London, UK), of which RJTW was a Research Career Development Fellow.
Contributor Information
Mark Eddison, Email: eddison@itsa.ucsf.edu.
Leah Toole, Email: leahtomlin@hotmail.com.
Esther Bell, Email: esther.bell@kcl.ac.uk.
Richard JT Wingate, Email: richard.wingate@kcl.ac.uk.
References
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Wingate RJT, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain – hindbrain development. Trends Genet. 1996;12:15–20. doi: 10.1016/0168-9525(96)81383-7. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Brand M. The midbrain – hindbrain boundary organizer. Curr Opin Neurobiol. 2001;11:34–42. doi: 10.1016/S0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Jaszai J, Reifers F, Picker A, Langenberg T, Brand M. Isthmus-to-midbrain transformation in the absence of midbrain-hindbrain organizer activity. Development. 2003;130:6611–6623. doi: 10.1242/dev.00899. [DOI] [PubMed] [Google Scholar]
- Sato T, Araki I, Nakamura H. Inductive signal and tissue responsiveness defining the tectum and the cerebellum. Development . 2001;128:2461–2469. doi: 10.1242/dev.128.13.2461. [DOI] [PubMed] [Google Scholar]
- Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development . 1999;126:1189–1200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, Rosenthal A. Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999;24:555–566. doi: 10.1016/S0896-6273(00)81112-5. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Davenne M, Lumsden A, Chambon P, Rijli FM. Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning. Development. 1997;124:3693–3702. doi: 10.1242/dev.124.19.3693. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M, Mallo M, Zhang M, Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75:1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell . 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev Cell. 2002;3:723–733. doi: 10.1016/S1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Jungbluth S, Bell E, Lumsden A. Specification of distinct motor neuron identities by the singular activities of individual Hox genes. Development. 1999;126:2751–2758. doi: 10.1242/dev.126.12.2751. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127:5355–5365. doi: 10.1242/dev.127.24.5355. [DOI] [PubMed] [Google Scholar]
- Millet S, Bloch-Gallego E, Simeone A, Alvarado-Mallart RM. The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development. 1996;122:3785–3797. doi: 10.1242/dev.122.12.3785. [DOI] [PubMed] [Google Scholar]
- Engelkamp D, Rashbass P, Seawright A, van Heyningen V. Role of Pax6 in development of the cerebellar system. Development. 1999;126:3585–3596. doi: 10.1242/dev.126.16.3585. [DOI] [PubMed] [Google Scholar]
- Dixon M, Lumsden A. Distribution of neuregulin-1 (nrg1) and erbB4 transcripts in embryonic chick hindbrain. Mol Cell Neurosci . 1999;13:237–258. doi: 10.1006/mcne.1999.0749. [DOI] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron . 1997;18:411–423. doi: 10.1016/S0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Vogel-Höpker A, Rohrer H. The specification of noradrenergic locus coeruleus (LC) neurones depends on bone morphogenetic proteins (BMPs) Development. 2002;129:983–991. doi: 10.1242/dev.129.4.983. [DOI] [PubMed] [Google Scholar]
- Lin JC, Cai L, Cepko CL. The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain. J Neurosci. 2001;21:159–168. doi: 10.1523/JNEUROSCI.21-01-00159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilthorpe JD, Papantoniou EK, Chedotal A, Lumsden A, Wingate RJ. The migration of cerebellar rhombic lip derivatives. Development. 2002;129:4719–4728. doi: 10.1242/dev.129.20.4719. [DOI] [PubMed] [Google Scholar]
- Irving C, Mason I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development. 2000;127:177–186. doi: 10.1242/dev.127.1.177. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Ariza-McNaughton L, Krumlauf R. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science. 2002;295:1288–1291. doi: 10.1126/science.1064540. [DOI] [PubMed] [Google Scholar]
- Hermann PM, Logan CC. Electroporation of proviral RCAS DNA alters gene expression in the embryonic chick hindbrain. Biotechniques. 2003;35:942–949. doi: 10.2144/03355st01. [DOI] [PubMed] [Google Scholar]
- Shamim H, Mahmood R, Logan C, Doherty P, Lumsden A, Mason I. Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development. 1999;126:945–959. doi: 10.1242/dev.126.5.945. [DOI] [PubMed] [Google Scholar]
- Alcántara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- Yee KT, Simon HH, Tessier-Lavigne M, O'Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24:607–622. doi: 10.1016/S0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Human Molecular Genetics. 1996;5:1207–1216. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- Hallonet ME, Le Douarin NM. Tracing neuroepithelial cells of the mesencephalic and metencephalic alar plates during cerebellar ontogeny in quail-chick chimaeras. Eur J Neurosci. 1993;5:1145–1155. doi: 10.1111/j.1460-9568.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Knight RD. Origins of anteroposterior patterning and Hox gene regulation during chordate evolution. Philos Trans R Soc Lond B Biol Sci. 2001;356:1599–1613. doi: 10.1098/rstb.2001.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, eds . The Central Nervous System of Vertebrates. Berlin: Springer-Verlag; 1998. [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Wingate RJ, Lumsden A. Persistence of rhombomeric organisation in the postsegmental hindbrain. Development. 1996;122:2143–2152. doi: 10.1242/dev.122.7.2143. [DOI] [PubMed] [Google Scholar]
- Myat A, Henrique D, Ish-Horowicz D, Lewis J. A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev Biol. 1996;174:233–247. doi: 10.1006/dbio.1996.0069. [DOI] [PubMed] [Google Scholar]
- Prince V, Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development. 1994;120:911–923. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Logan C, Wizenmann A, Drescher U, Monschau B, Bonhoeffer F, Lumsden A. Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr Biol. 1996;6:1006–1014. doi: 10.1016/s0960-9822(02)00645-0. [DOI] [PubMed] [Google Scholar]
- Niss K, Leutz A. Expression of the homeobox gene GBX2 during chicken development. Mech Dev. 1998;76:151–155. doi: 10.1016/S0925-4773(98)00103-8. [DOI] [PubMed] [Google Scholar]


