Abstract
Aim:
To show the distribution and changes of causative agents of urinary tract infections in children and resistance rates by years and select the most appropriate antibiotics.
Material and Methods:
In this study, the Başkent University Alanya Research and Application Hospital automation system microbiology recording book was screened retrospectively. Growth of a single microorganism above 105 colonies (cfu/mL) was included in the assessment. Throughout the study, 10 691 urinary cultures were studies and growth was found in 392 (3.7%).
Results:
Three hundred and nine (78.8%) of the samples with growth belonged to girls. Growth was found in the neonatal period in 32 patients (8.2%). The most commonly isolated microorganism was Escherichia coli (E. coli) which was found in 68.4% of the patients. Klebsiella spp. were found with a rate of 12.0%; Enterobacter spp. were found with a rate of 10.7% and proteus spp. were found with a rate of 5.1%. Resistance to cefalotin (62.1%), trimethoprim-sulfamethoxasole (43.1%), amoxycillin-clavulanate (34.8%), ampicillin (30.4%), cefixim (26.3%) and nitrofurantoin (3.6%) was found in E. coli species. The antibiotic which had the highest resistance rate was ampicillin with a rate of 93.2% for klebsiella and 83.4% for enterobacter. Klebsiella spp. were the most commonly grown pathogens in newborns (40.6%). In a follow-up period of 5 years, the resistance of E. coli to amoxycillin-clavulanate regressed from 40.3% to 31.3%, while the resistance to trimethoprim-sulfamethoxasole (TMP-SMX) regressed from 45.6% to 34.7%.
Conclusions:
A high resistance against first-generation cephalosporins, ampicillin, amoxycillin-clavulanate and TMP-SMX which are the first-line antibiotics in childhood urinary tract infections was found. Carbapenem (meropenem, imipenem) resistance was not found in our center. Nitrofurantoin, aminoglycosides and cefixime can be recommended for empirical treatment in our hospital because of low resistance. Antibiotic treatment should be redecided according to in vitro antibiotic sensitivity results.
Keywords: Antibiotic resistance, children, urinary culture, urinary tract infections, newborn
Introduction
Urinary tract infection (UTI) is a common disease in children and it is observed in 3–5% of girls and in 1% of boys (1). While it is observed with a 5-fold higher rate in boys below the age of 5 years, after 5 years of age it is observed with a 10-fold higher rate in girls (2). Despite all advancements in diagnosis and treatment, UTI can recur frequently (3). It still continues to be a problem with its long-term complications including growth retardation, hypertension and renal failure (4, 5).
Amoxycillin, trimethoprim-sulfamethoxazole (TMP-SMX) and cephalosporins are generally used for urinary tract infection in children (6). Selection of appropriate emprical antibiotic initially will considerably decrease morbidity and mortality rates. We aimed to examine the distribution of the causative agents of UTI and resistance rates in our region and to ensure appropriate antibiotic selection by demonstrating changes in these.
Material and Methods
Patiens aged between 0 and 6 years who presented to Başkent University Research and Application Hospital Pediatrics Out-patient Clinic between April 2008 and March 2013 were included in the study. The hospital automation system, patient files and microbiology recording books were screened retrospectively. Patients with a history of recurrent UTI and known urinary tract anomaly were not included in the study. Midstream urine samples or clean urine samples collected into urine bags were kept in 5% sheep blood agar and eosin methylene blue medium at 37 degrees for 24 hours; more than 100 000 colonies (cfu/mL) and growth of a single microorganism was considered positive culture. The bacteriae grown were defined with traditional methods and antibiotic sensitivities were tested by disc diffusion method in accordance with “The Clinical and Laboratory Standards Institute” (CLSI) methods (7).
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Scien ces (SPSS ver. 11.0 Inc. Chicago, IL, USA) 11.0 package program and “Office Excel”. Verbal informed consent was obtained from the families who could be reached.
Results
In a 5-year follow-up period, 10 691 urine cultures were examined. Growth occured in 392 (3.7%) of the samples. 309 (78.8%) of the samples which had positive growth were taken from girls and 83 (21.2%) were taken from boys. Growth numbers by gender are shown in Table 1. The mean age of the patients was 54.19±47.91 (1–191) months. In the newborn period, growth occured in 32 (8.2%) patients. 123 (31.4%) of the subjects were younger than 12 months and 193 (49.2%) were younger than 48 months. 71 (57.7%) of the pediatric patients who were below the age of one year were female and 52 (42.3%) were male.
Table 1.
Distribution of the microorganisms by gender
| Male | Female | Total | |
|---|---|---|---|
| Escherichia coli | 44 (16.4%)† | 224 (83.6%) | 268 (68.4%) |
| Klebsiella spp. | 17 (36.1%) | 30 (63.9%) | 47 (12.0%) |
| Enterobacter spp. | 7 (16.6%) | 35 (83.4%) | 42 (10.7%) |
| Proteus spp. | 7 (35.0%) | 13 (65.0%) | 20 (5.1%) |
| Other* | 8 (53.3%) | 7 (46.7%) | 15 (3.8%) |
| Total | 83 (21.2%) | 309 (78.8%) | 392 (100%) |
Growth number, percentage of microorganism,
Staphylococcus epidermidis, Pseudomonas spp., Staphylococcus aureus, Group B strreptococci, Candida
E. coli was the most commonly grown organism which was found in 268 (68.4%) patients. Klebsiella spp. were grown in 47 (12.0%) patients, Enterobacter spp. were grown in 42 (10.7%) patients and Proteus spp. were grown in 20 (5.1%) patients. Staphylococcus epidermis and Pseudomonas spp. were grown in 6 patients each (1.5%). S. aureus, Group B streptococci and candida were grown in three different patients seperately. The number of microorganisms by years are shown in Table 2.
Table 2.
Distribution of microorganism growths by years
| 2008 | 2009 | 2010 | 2011 | 2012 | Total | |
|---|---|---|---|---|---|---|
| Escherichia coli | 59 (22.0%)† | 62 (23.1%) | 53 (19.8%) | 44 (16.4%) | 50 (18.7%) | 268 (100%) |
| Klebsiella spp. | 7 (14.9%) | 15 (31.9%) | 11 (23.4%) | 10 (21.3%) | 4 (8.5%) | 47 (100%) |
| Enterobacter spp. | 4 (9.5%) | 7 (16.7%) | 15 (35.7%) | 12 (28.6%) | 4 (9.5%) | 42 (100%) |
| Proteus spp. | 4 (20.0%) | 8 (40.0%) | 1 (5.0%) | 5 (25.0%) | 2 (10.0%) | 20 (100%) |
| Other* | 4 (26.7%) | 6 (40.0%) | 3 (20.0%) | 0 | 2 (13.3%) | 15 (100%) |
| Total | 78 (19.9%)‡ | 98 (25.0%) | 83 (21.2%) | 71 (18.1%) | 62 (15.8%) | 392 (100%) |
Growth number, percentage of microorganism,
Staphylococcus epidermidis, Pseudomonas spp., Staphylococcus aureus, Group B strreptococci, Candida,
annual total growth number, percentage in five years
Sensitivities of the microorganisms which were grown most commonly in urine cultures to various antibiotics are shown in Table 3 and Figure 1. E. coli was the most commonly grown microorganism excluding the neonatal period. It was resistant to cephalotin with a rate of 62.1%. The resistance rates for trimethoprim-sulfamethoxazole, amoxycillin clavulanate, ampicillin, cefixime and nitrofurantoin were found to be 43.1%, 34.8%, 30.4%, 26.3% and 3.6%, respectively. Klebsiella was found to be resistant to ampicillin with a rate of 93.2% and Enterobacter was found to be resistant to ampicillin with a rate of 83.4%. The resistance rate of Klebsiella spp. grown was found to be 34.1% for amoxycillin clavulanate, 17.8% for TMP-SMX, 14.7% for cefixime and 11.2% for nitrofurantoin. The resistance rate of Enterobacter was found to be 74% for cefixime, 53.9% for amoxycillin clavulanate, 20% for TMP-SMX and 5.6% for nitrofurantoin. Proteus was found to be resistant to nitrofurantoin with the higest rate (85.0%). The resistance rate of Proteus was found to be 46.7% for ampicillin, 35.3% for TMP-SMX, 17.7% for amoxycillin clavulanate and 5.6% for cefixime.
Table 3.
Sensitivity rates of gram negative microorganisms obtained in urine cultures against various antibiotics
| Escherichia coli 268 | Klebsiella spp. 47 | Enterobacter spp 42 | Proteus spp. 20 | |||||
|---|---|---|---|---|---|---|---|---|
| Amikacin | 242/250 | 96.8% | 45/45 | 100% | 12/12 | 100% | 18/18 | 100% |
| Amoxycilline-clavulanate | 165/253 | 65.2% | 29/44 | 65.9% | 6/13 | 46.1% | 14/17 | 82.3% |
| Ampicillin | 172/247 | 69.6% | 3/44 | 6.8% | 2/12 | 16.6% | 8/15 | 53.3% |
| Phosphamycine | 194/242 | 80.1% | 18/43 | 41.8% | 13/21 | 61.9% | 12/18 | 66.6% |
| Gentamycin | 220/253 | 86.9% | 43/45 | 95.5% | 33/35 | 94.2% | 18/19 | 94.7% |
| Imipenem | 251/251 | 100% | 41/42 | 97.6% | 13/13 | 100% | 19/20 | 95.0% |
| Meropenem | 254/254 | 100% | 45/46 | 97.8% | 13/13 | 100% | 20/20 | 100% |
| Nitrofurantoin | 244/253 | 96.4% | 40/45 | 88.8% | 34/36 | 94.4% | 3/20 | 15.0% |
| Norfloxacin | 227/251 | 90.4% | 42/43 | 97.6% | 24/32 | 75.0% | 19/19 | 100% |
| Piperacillin | 118/227 | 51.9% | 25/40 | 62.5% | 10/13 | 76.9% | 13/17 | 76.4% |
| Cefalotin | 90/237 | 37.9% | 27/42 | 64.2% | 5/16 | 31.2% | 11/15 | 73.3% |
| Cefoperazone | 177/229 | 77.2% | 33/41 | 80.4% | 9/13 | 69.2% | 15/16 | 93.7% |
| Cefazoline | 169/243 | 69.5% | 32/41 | 78.0% | 4/7 | 57.1% | 13/18 | 72.2% |
| Cefepime | 221/230 | 96.0% | 38/39 | 97.4% | 12/12 | 100% | 17/17 | 100% |
| Cefixime | 180/244 | 73.7% | 35/41 | 85.3% | 6/23 | 26.0% | 17/18 | 94.4% |
| Ceftazidime | 228/256 | 89.0% | 43/45 | 95.5% | 12/13 | 92.3% | 19/20 | 95.0% |
| Ceftriaxone | 215/259 | 83.0% | 43/46 | 93.4% | 10/13 | 76.9% | 19/20 | 95.0% |
| Cefuroxim | 188/236 | 79.6% | 32/44 | 72.7% | 4/10 | 40.0% | 16/18 | 88.8% |
| Ciprofloxacin | 232/256 | 90.6% | 45/46 | 97.8% | 31/37 | 83.7% | 20/20 | 100% |
| TMP-SMX | 147/258 | 56.9% | 37/45 | 82.2% | 12/15 | 80.0% | 11/17 | 64.7% |
calculated by number of sensitive microorganisms/number of samples studied.
TMP-SMX: trimethoptim-sulfamethoxazole
Figure 1.
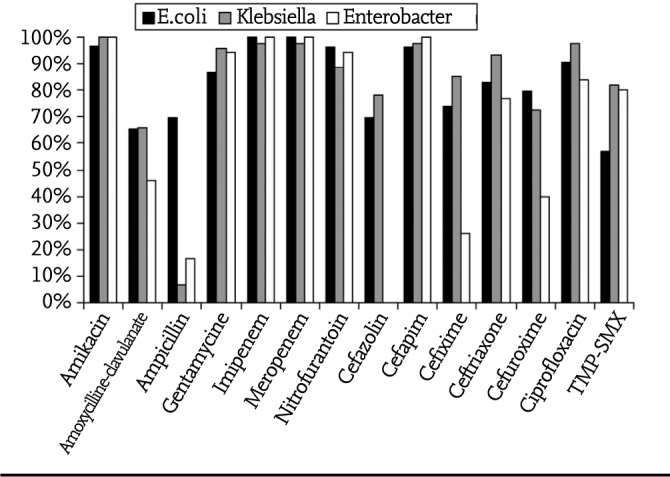
Antibiotic sensitivities of the three most common microorganims
Comparison of the resistances of E. coli spp. grown in 2008 and 2012 is summarized in Figure 2. While resistance to amoxycillin clavulanate reduced from 40.3% to 31.3%, resistance to TMP-SMX reduced from 45.6% to 34.7%. A reduction of 13.5%, 5.3%, 3.5% and 2.5% was observed in resistance to phosphomycin, amikacin, nitrofurantoin and cefixime, respectively. While ceftiraxon resistance remained the same, cefoperazone and cefalotin resistance increased.
Figure 2.
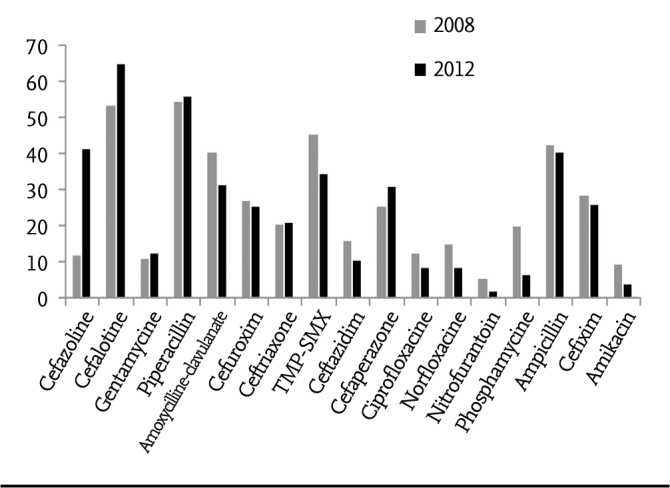
Comparison of E.coli resistances of some antibiotics in 2008–2012
Growth occured in 32 newborns 12 of whom were male and 20 of whom were female. The most commonly grown agents included Klebsiella spp. in 13 patients (40.6%, 8 boys, 5 girls), E. coli in 11 patients (34.4%, 8 girls, 3 boys) and Enterobacter in 6 patients (18.8%, 4 girls, 2 boys). Staphylococcus epidermis and Pseudomonas spp. were grown in two different male patients. The sensitivity rates of some of the antibiotics which are used frequently in the neonatal period are shown in Table 4. Ampicillin resistance was found with a rate of 100% for Enterobacter, 90,9% for Klebsiella and 44,4% for E. coli.
Table 4.
Sensitivity rates of the microorganisms grown in newborns against various antibiotics
| Escherichia coli 11 | Klebsiella spp. 13 | Enterobacter spp. 6 | ||||
|---|---|---|---|---|---|---|
| Ampicillin | 5/9 | 55.6*% | 1/11 | 9.1% | 0/5 | 0% |
| Amikacin | 10/11 | 90.9% | 12/12 | 100% | 5/6 | 83.3% |
| Amoxycilline-clavulanate | 10/10 | 100% | 8/12 | 66.7% | 2/5 | 40% |
| Gentamycine | 10/11 | 90.9% | 10/11 | 90.9% | 6/6 | 100% |
| Cefixim | 9/10 | 90% | 8/11 | 72.7% | 2/5 | 40% |
| Ceftriaxone | 9/10 | 90% | 11/12 | 91.7% | 5/5 | 100% |
| Ceftazidim | 10/10 | 100% | 11/12 | 91.7% | 5/5 | 100% |
calculated by number of sensitive microorganisms/number of samples studied
Discussion
Urinary tract infection is an important infectious disease which is observed commonly in children. UTI is responsible of 5–6% of the infections in children evaluated because of fever. Colonization of the periurethral region with enteric pathogens is the first step of UTI. Inflammatory response starts when these microorganisms gain pathogenic properties by way of virulance factors and the infection may progress up to the bladder and kidney (8). Vesicoureteral reflux, neuromuscular dysfunction, mixion disorder, constipation, bladder neck stenosis and presence of catheter facilitate development of UTI (9). Familial and genetic predispositions have also been reported (10).
Oral UTI treatment is as efficient as parenteral treatment (11). Hospitalization or parenteral treatment is indicated in infants younger than 2 months with toxic appearance who can not take fluid or medication by the oral route, in immune deficiencies and when there is social justification (8, 11). Empirical antibiotic which can be selected for oral administration include amoxycillin clavulanate, TMP-SMX, cefixime, sefpodoxim, cefprozil, cefuroxime axetil and cefalexin. Emprical parenteral treatment options include ceftriaxon, cefotaxim, ceftazidim, gentamycin, tobramycin and piperacillin (12). The total treatment time is 7–14 days (13). Although there are different approaches, urinary tract ultrasonography is consistently recommended after the first UTI (14). The risk of recurrence is higher in the first 6 months. Use of prophylactic antibiotic decreases recurrence in patients with recurrent UTI, but it has little contribution to the patient and the decision should be made considering the possibility of development of resistance (15).
78.8% of the patients who had a positive urine culture were female. Although UTI is observed with a higher rate in boys below the age of 1 year, it was found with a higher rate in girls at all age groups.
E. coli which is one of the gram negative microorganisms has been found as the most common causative agent in studies performed in our country. In our study, E. coli, Klebsiella and Enterobacter were the most commonly grown microorganisms. In the studies conducted in İzmir (16), Bursa (17), Tokat (18) and Ankara (19), the same three agents were in the first three orders. While Proteus spp. were in the fourth order in our study, İpek et al. (20) and Senel et al. (21) found the same agent in the second order. Güneş et al. (22) found coagulase negative staphylocicci to be in the second order, whereas eneterococci were the second most common microorganism in the study of Küçükbasmacı et al. (23).
While Klebsiella spp. were found to be resistant to amikacin with a rate of 100% in our study, they were found to be resistant to ampicillin with a rate of 93,2%. Aydemir (24) found an ampicillin resistance of 100% and an amikacin sensitivity of 94.3%. Resistance of Klebsiella spp. to empirical TMP-SMX was found to be 17.8%. This rate of resistance was found to be 47.8% in the study of Gündüz et al. (25), whereas it increased up to 67% in the study of Mir et al. (26). Güner et al. (27) found resistance rates similar to our study for all these three antibiotics (amikacin 1.1%, ampicillin 88.2%, TMPSMX 20%).
In our study, Enterobacter had a sensitivity of 100% against amikacin, imipenem, meropenem and cefepim. The resistance rate was found to be 83.4% for ampicillin which is frequently prescribed for oral administration, 74% for cefixim and 53.9% for amoxycillin clavulanate. While Salduz et al. (28) found ampicillin resistance to be 100%, amikacin resistance was found to be 39.25% in the study performed by Mir et al. (16).
Proteus species grown in our hospital did not develop resistance to amikacin, ciprofloxacin, norfloxacin, cefepim and meropenem. An ampicillin resistance of 46.7% and a TMPSMX resistance of 35.3% was found. Since Proteus spp. have intrinsic resistance to nitrofurantoin, this antibiotic is not used in treatment. In the study of Şahin et al. (29), ampicillin resistance was found to be 92.6% and TMP-SMX resistance was found to be 37.0%.
E. coli is the most common problem. While it has a sensitivity of 100% to imipenem and meropenem, it has a resistance of 62.1% to cefalotin and a resistance of 48.1% to piperacillin. The resistance rate was found to be 43.1% for TMP-SMX which is preferred for empirical treatment, 30.4% for ampicillin, 26.3% for cefixim and 34.8% for amoxycillin clavulanate. While Cebe et al. (30) found TMP-SMX resistance to be 43.6% and amoxycillin clavulanate resistance to be 28.6%, Motor et al. (31) found the same rates to be 61% and 65%, respectively. In our study, ceftriaxone resistance was found to be 17%, whereas Abuhandan et al. (32) calculated this resistance to be 39.5%. Üstün et al. (33) found no resistance to meropenem and amikacin. In our study, no resistance to meropenem was found and amikacin resistance was found with a rate of 3.2%.
When the resistances of some antibiotics in E. coli growhts in 2008 and 2012 were compared, it was pleasing that resistances to most antibiotics were reduced. However, cefazolin resistance increased from 32.2% to 41.6%, cefalotin resistance increased from 53.5% to 65% and cefaperazon resistance increased from 25.5% to 31.1%. It was observed that trimetoprim sulfametoxazole resistance was reduced from 45.6% to 34.6%, amoxycillin clavulanate resistance was reduced from 40.3% to 31.2% and nitrofurantoin resistance was reduced from 5.6% to 2.1%. Çetin et al. (34) found an increase in TMP-SMX resistance in their thre-year follow-up and observed a reduction in amoxycillin clavulanate resistance similar to our study. In the study performed by Erdoğan et al. (35) from our center covering the year of 2004, ampicillin resistance was found to be 71.7%, TMP-SMX resistance was found to be 54.3% and amoxycillin clavulanate resistance was found to be 43.5%. Amikacin and nitrofurantoin resistances are similar. Although ampicillin resistance reduced from 71.7% to 42.6% between 2004 and 2008, it regressed to 40.5% in a five-year follow-up period. The fact that enteric bacteria can produce large-spectrum beta lactamase which inactivates third and fourth generation cephalosporins is an important problem and this rate ranges between 1% and 10% in community-acquired UTI (35). When the two studies were compared, it was observed that cefixim resistance increased from 4.3% to 26.3% and ceftriaxon resistance increased form 4.3% to 17%.
In newborns, treatment is initiated after obtaining blood sample for culture. The first-line options in treatment include ampicillin, gentamycin and third generation cephalosporins (36). E. coli is resistant to ampicillin with a rate of 44.4% and the general ampicillin resistance in newborns has been found to be 76%. In newborns, amikacin and ceftriaxon sensitivity was found to be 93.1% and cefixim sensitivity was found to be 73%. In the study of Arıkan et al. (37), E. coli was the most common causative agent, the rate of renal damage was high in these patients and the authors recommended long-term follow-up for these patients. Some authors definetely forbid use of ceftriaxon in newborns (38).
Since this study was a retrospective study, reliable information related with clinical data and antibiotic use of the patients could not be reached. There were also patients with a possibility of asymptomatic bacteriuria. These are the limitations of our study. Conclusively, a high rate of resistance was found to first generation cephalosporins, ampicillin, amoxycillin clavulanate and TMP-SMX which are the first-line agents in childhood UTI. In our center, no resistance to carbapenem (meropenem, imipenem) was found. Nitrofurantoin, aminoglycosides and cefixime can be reccomended in emprical treatment in our hospital because of low resistance. Antibiotic treatment should be redecided according to the results of in vitro antibiotic sensitivity tests.
Footnotes
Ethics Committee Approval: Due to the retrospective design of the study, the ethics committee approval was waived.
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.Ç., E.B.; Design - B.Ç., N.Ü., H.K.; Supervision - B.Ç., H.E.; Funding - B.Ç., B.T.; Materials - B.Ç.; H.E.; Data Collection and/or Processing - B.Ç., B.T., H.K., N.Ü.; Analysis and/or Interpretation - B.Ç., N.Ü., H.K., B.T.; Literature Review - B.Ç., E.B.; Writer - B.Ç.; / Critical Review - B.Ç., N.Ü., B.T., H.K., E.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Söylemezoğlu O. Üriner sistem enfeksiyonları. In: Hasanoğlu E, Düşünsel R, Bideci A, editors. Temel pediatri. Ankara: Güneş Tıp Kitabevleri; 2010. pp. 929–35. [Google Scholar]
- 2.Berger E, Langlois V. Nephrology. In: Dipchand A, Friedman J, editors. The hospital for sick children handbook of pediatrics. 11th edt. Toronto: Saunders Elsevier; 2009. pp. 632–4. [Google Scholar]
- 3.Larcombe J. Urinary tract infection in children. BMJ. 1999;319:1173–5. doi: 10.1136/bmj.319.7218.1173. http://dx.doi.org/10.1136/bmj.319.7218.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirin A, Emre S, Alpay H, Nayir A, Bilge I, Tanman F. Etiology of chronic renal failure in Turkish children. Pediatr Nephrol. 1995;9:549–52. doi: 10.1007/BF00860926. http://dx.doi.org/10.1007/BF00860926. [DOI] [PubMed] [Google Scholar]
- 5.Elder JS. Urinary tract infections. In: Kliegman RM, Stanton BF, St Geme JW, editors. Nelson textbook of pediatrics. 19th edt. Philadelphia: Elsevier Saunders; 2011. pp. 1829–34. http://dx.doi.org/10.1016/B978-1-4377-0755-7.00532-7. [Google Scholar]
- 6.Özen M. İdrar yolu enfeksiyonları. In: Kara A, editor. Çocuk enfeksiyon hastalıkları klinik rehberi. İstanbul: Nobel Tıp Kitapevleri; 2008. pp. 102–7. [Google Scholar]
- 7.Clinical and laboratory standarts institute. Performance standards for antimicrobial testing. 2007. pp. M100–S16. 7th International. CLSI Document, [DOI] [PMC free article] [PubMed]
- 8.Tapısız A.Üriner enfeksiyonlarda antibiyotik kullanımı 8. Ulusal Çocuk Enfeksiyon Hastalıkları Kongresi,10–14Mayıs 2013Antalya, konuşma metinleri ve bildiri özetleri kitabı,37–41.
- 9.Wald ER. Cystitis and pyelonephritis. In: Feigin RD, Cherry JD, Demler-Harrison GJ, editors. Textbook of pediatric ınfectious diseases. 6th edt. Philadelphia: Saunders; 2009. pp. 554–69. [Google Scholar]
- 10.Zaffenollo M, Malerba G, Cataldi L, et al. Genetic risk for recurrent urinary tract infections in humans: A systematic review. J Biomed Biotechnol. 2010;2010:321082. doi: 10.1155/2010/321082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman TB. The new American Academy of Pediatrics urinary tract infection guideline. Pediatrics. 2011;128:572–5. doi: 10.1542/peds.2011-1818. http://dx.doi.org/10.1542/peds.2011-1818. [DOI] [PubMed] [Google Scholar]
- 12.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. http://dx.doi.org/10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 13.Paintsil E. Update on recent guidelines for the management of urinary tract infections in children: the shifting paradigm. Curr Opin Pediatr. 2013;25:88–94. doi: 10.1097/MOP.0b013e32835c14cc. http://dx.doi.org/10.1097/MOP.0b013e32835c14cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penesi M, L’Erario I, Travan L, Ventura A. Managing children under 36 months of age with febrile urinary tract infection: a new approach. Pediatr Nephrol. 2012;27:611–5. doi: 10.1007/s00467-011-2087-3. http://dx.doi.org/10.1007/s00467-011-2087-3. [DOI] [PubMed] [Google Scholar]
- 15.Williams K, South M. Pros and cons of antibiotics for preventing recurrent urinary tract infection. J Paediatr Child Health. 2013;49:75–7. doi: 10.1111/jpc.12070. http://dx.doi.org/10.1111/jpc.12070. [DOI] [PubMed] [Google Scholar]
- 16.Mir S, Erdoğan H, Güler S, Şengül GN, Koyu A, Aydemir Ş. Çocuk yaş grubu idrar yolu enfeksiyonlarında Ege Bölgesi antibiyotik direnci. Ege Tıp Dergisi. 2002;41:207–10. [Google Scholar]
- 17.Yılmaz E, Özakın C, Sınırtaş M, Gedikoğlu S. Uludağ Üniversitesi laboratuarında 1999–2002 yılları arasında idrar örneklerinde izole edilen mikroorganizmalar ve antibiyotik duyarlılıkları. İnfek Derg. 2005. pp. 91–6.
- 18.Yılmaz R, Karaaslan E, Özçetin M, et al. Çocuklarda idrar yolları enfeksiyonu etkenleri ve antibiyotik duyarlılıkları. J Contemp Med. 2012;2:17–21. [Google Scholar]
- 19.Yuksel S, Ozturk B, Kavaz A, et al. Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Int J Antimicrob Agents. 2006;28:413–6. doi: 10.1016/j.ijantimicag.2006.08.009. http://dx.doi.org/10.1016/j.ijantimicag.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.İpek IO, Bozaykut A, Arman DC, Sezer RG. Antimicrobial resistance patterns of uropathogens among children in Istanbul, Turkey. Southeast Asian J Trop Med Public Health. 2011;42:355–62. [PubMed] [Google Scholar]
- 21.Senel S, Karacan C, Erkek N, Göl N. A Single-center experience of antimicrobial resistance patterns in pediatric urinary tract infection. Med Princ Pract. 2010;19:359–63. doi: 10.1159/000316373. http://dx.doi.org/10.1159/000316373. [DOI] [PubMed] [Google Scholar]
- 22.Güneş H, Donma MM, Nalbantoğlu B. Namık Kemal Üniversitesi Araştırma ve Uygulama Hastanesi’ne başvuran çocuklarda idrar örneklerinden izole edilen etkenler ve antibiyotik direnç durumları. Cumhuriyet Med J. 2013;35:1–8. http://dx.doi.org/10.7197/1305-0028.1662. [Google Scholar]
- 23.Küçükbasmacı Ö, Çelik N. Çocuk hastaların idrar örneklerinden izole edilen bakteriler ve antibiyotiklere duyarlılıkları. Türk Mikrobiyol Cem Derg. 2009;39:40–3. [Google Scholar]
- 24.Aydemir C, Polat R, Eldeş N, et al. Çocukluk çağı üriner sistem sistem enfeksiyonlarında izole edilen patojenler ve antibiyotik direnci. Ulusal Çocuk Enfeksiyon Hastalıkları Kongresi Poster Bildiriler. J Pediatr Inf. 2007;1:93. [Google Scholar]
- 25.Gündüz T, Tosun S, Demirel MM, Pelin E. Çocuklarda idrar yolu enfeksiyonlarında antibiyotik direnci: Beş yıllık sonuçlar. Pam Med J. 2008;1:87–90. [Google Scholar]
- 26.Mir S, Dönmez O, Kabasakal C, Sönmez F, Cura A. Çocukluk çağı idrar yolu enfeksiyonlarında ilk tedavi seçeneği ne olmalıdır? Türk Nefrol Diyal Transplant Derg. 1997;2:149–53. [Google Scholar]
- 27.Güner ŞN, Göktürk B, Bayrakçı US, Baskın E. Çocuklarda idrar örneklerinden saptanan toplum kaynaklı gram negatif mikroorganizmaların dağılımı ve 2003–2010 yılları arasında antibiyotik direncindeki artışın değerlendirilmesi. Türk Ped Arş. 2012;47:107–3. http://dx.doi.org/10.4274/tpa.721. [Google Scholar]
- 28.Salduz ZİY, Yiğit Ö. Antibiotic Susceptibility of Bacteria Isolated From Children with Urinary Tract Infection. J Pediatr Inf. 2010;4:138–42. http://dx.doi.org/10.5152/ced.2010.28. [Google Scholar]
- 29.Şahin İ, Öksüz Ş, Kaya D, Şencan İ, Gülcan A. Çocuk yaş grubunda servis ve poliklinik kökenli üropatojen gram negatif çomakların antibiyotik duyarlılıkları. ANKEM Derg. 2004;18:101–4. [Google Scholar]
- 30.Cebe A, Ayvaz A, Yıldız N, Çetinkaya S. Sivas ilinde çocukluk çağı idrar yolu enfeksiyonlarında idrar kültür sonuçları: İlk tedavi seçimi nasıl olmalıdır? Van Tıp Dergisi. 2008;15:7–12. [Google Scholar]
- 31.Motor VK, Tutanç M, Arıca V, Arıca S, Ay B. Üropatojen Escherichia Coli suşlarının üriner sistem enfeksiyonlarının tedavisinde sık kullanılan antibakteriyel ajanlara duyarlılıkları. ANKEM Derg. 2010;24:198–201. [Google Scholar]
- 32.Abuhandan M, Güzel B, Oymak Y, Çiftçi H. Antibiotic sensitivity and resistance in children with urinary tract infection in Sanliurfa. Turkish Journol of Urology. 2013;39:106–10. doi: 10.5152/tud.2013.022. http://dx.doi.org/10.5152/tud.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Üstün C, Demir YS, Demir S, Demirören S, Kurtoğlu MG. Pediyatrik yaş grubu toplum kökenli üriner sistem enfeksiyonlarından izole edilen Escherichia coli ve Klebsiella spp. suşlarının in-vitro antibiyotik direnci. ANKEM Derg. 2009;23:155–60. [Google Scholar]
- 34.Çetin H, Öktem F, Örmeci AR, Yorgancıgil B, Yaylı G. Çocukluk çağı idrar yolu enfeksiyonlarında Escherichia coli ve antibiyotik direnci. S.D.Ü. Tıp Fak Derg. 2006;13:12–6. [Google Scholar]
- 35.Erdoğan H, Arslan H. Çocuklarda toplum kaynaklı üriner sistem infeksiyon etkenleri ve antibiyotik duyarlılıkları. Nobel Med. 2011;7:15–8. [Google Scholar]
- 36.Canter D. İdrar Yolu Enfeksiyonu. In: Çoban A, İnce Z, translators. Neonatoloji: Tedavi, girişimler, sık karşılaşılan sorunlar, hastalıklar ve ilaçlar. İstanbul: İstanbul Tıp Kitabevi; 2012. pp. 722–3. [Google Scholar]
- 37.Arıkan Fİ, Acar BÇ, Tıraş Ü, et al. Yenidoğan idrar yolu enfeksiyonları. Bakırköy Tıp Dergisi. 2009;5:109–12. [Google Scholar]
- 38.Cataldi L, Zaffenello M, Gnarra M, Fanos V. Urinary tract infection in the newborn and the infant: state of the art. J Matern Fetal Neonatal Med. 2010;23:90–3. doi: 10.3109/14767058.2010.513851. http://dx.doi.org/10.3109/14767058.2010.513851. [DOI] [PubMed] [Google Scholar]


