Abstract
Gonadotropins which are high in the middle of the fetal life are measured to be considerably low in the cord blood and estrogen is found to be high in the cord blood. Gonodotropins are supressed by estrogen. After delivery, the hypothalamo-pituitary-gonadal axis is activated when estrogen is eliminated and a hormone profile which reaches pubertal levels is established. These changes are called mini puberty. In boys, long-term testicular functions and sperm production are regulated with mini puberty and mini puberty contributes to masculinization of the brain. The role of mini puberty in female newborns is not known. Central hypogonadism, Turner syndrome and ovarian hyperstimulation in preterm babies may be diagnosed with evaluation of mini puberty. In this article, mini puberty and related problems were reviewed and the importance of this issue was emphasized.
Keywords: Mini puberty, newborn, interpretation
Gonadotropins which are at high levels in the middle of the fetal life are considerably low in the cord blood and estrogen is measured to be high. The reason for this is the inhibitory effect of increased estrogen levels in the circulation arising from the placenta on gonadotropin releasing hormone (GnRH) pulse generator and pituitary gonadotropins (1, 2). In male fetuses, testosterone levels probably contribute additionally to inhibition (3). Gonadotropin inhibition discontinues with placental seperation, the hypothalamo-pituitary-gonadal axis is activated and the hormonal changes which occur are identified as mini puberty (2, 4). The activation of the hypothalamo-pituitary-gonadal axis reach a peak level approximately in the 6–8th week after delivery. In this period, the levels of sex steroids are similar to early-middle pubertal levels, but their peripheral effects are not realized (4).
In boys, the level of luteinizing hormone (LH) increases approximately 10-fold in the minutes following delivery and the level of tesosterone increases. This state lasts approximately for 12 hours (5, 6). The increase in follicle stimulating hormone (FSH) and LH continues in the second week after delivery, reaches a peak in the 4–10th week, decreases in the 6th month to the prepubertal levels. The level of testosterone increased by release of luteinizing hormone reaches a peak at around the third month and decreases to the prepubertal levels in the 6–9th months (1, 7, 8). Similarly, the levels of 17-hydroxyprogesteron and androstenodione which are increased with testosterone decrease with reduction of the level of LH (2). After delivery, an increase in the number of Leydig cells starts. This increase reaches a peak in the third month. Afterwards, reduction and apopytosis occur in fetal and infantile Leydig cells (9–11). Inhibin-B is a glycoprotein belonging to the family of transforming growth factor beta (TGF-β) and is released from Sertoli cells in the prepubertal period. It is considered an indicator of sertoli cell function in prepubertal male children, but it does not reflect the number and function of germ cells. Inhibin-B acts as a regulator of release of FSH (1). Inhibin-B can be measured in the cord blood, it increases after delivery, reaches a peak in the 4–12th month (exceeds the male adult level). It is at a considerably low level at the age of 3–9 years and increases again with the initiation of the puberty (3, 7). Serum inhibin-B levels are low in cases of hypogonadotropic hypogonadism (1, 12).
Anti-Mullerian hormone (AMH) is a glycoprotein released from the sertoli cells starting from the fetal period. It is found in the cord blood and its level increases in the first 30 months after delivery. It reaches a peak in the 6th month, starts to decrease in the childhood and decreases to considerably low levels in the pubertal period (13, 14). The level of AMH decreases in parallel to increased testosterone in the puberty.
In boys, both inhibin-B and AMH levels give an idea about the presence and function of Sertoli cells in the months following delivery and in the prepubertal period (1). In cases of bilateral criptorchidism, human chorionic gonadotropin hormone stimulation test can be performed to determine the presence and function of the testicles. However, it should be kept in mind that inhibin-B level may be normal and AMH may be low in “persistent Mullerian duct” syndrome, if there is AMH receptor unresponsiveness (1).
Conclusively, the testicular volume increases with elongation of the seminiferous tubules (6-fold until the age of one) with mini puberty which starts in the neonatal period and continues until the middle infancy (11, 15). It has been found that the mass of the Sertoli cells increases to constitute 85–95% of the seminefous tubules (16). It is thought that transient scrotal hairing which can be observed in the first year of life in boys is related with the physiological testosterone level which increases in the process of mini puberty (Figure 1) (17–19). The increase in the number and proliferation of Sertoli cells is determinative for spermatogenetic functions (20). The total number of germ cells increases approximately up to 100 days (50–150) (21). This increased mitotic activity allows the genocytes (non-primary reproductive cells) to transform into adult spermatogoniae which are considered stem cells for spermatogenesis between the 30th and 80th days in boys with increased gonadotropins and testosterone level (2, 20, 22). After the 6th month after delivery, the spermatogonia decrease in number in parallel to decreased gonadotropins. Activity in the GnRH pulse generator which lasts from the neonatal period to the middle infancy (mini puberty) is important for criptorchidism and the spermatogenic function and fertility in advanced years (20, 23–27). In addition, it is thought that increased level of testosterone provides masculinization of the brain an determines cognitive functions, sexual behavior and sexual orientation in relation (4, 28). In this period, the testicles increase from 0.5 mL to 1.5 mL, while penile growht is more insignificant. Lack of contribution to the penile growth and absence of other peripheral effects are explained by relative androgen insensitivity in the neonatal period and the first months of life (4). Similarly, acceleration in the bone age starts after the 6th month after delivery in cases of congenital adrenal hyperplasia (29). In contrast to some views, it has been proposed that increased testosterone after delivery has no effect on masculine type of psychosexual development based on the information that subjects with Kallman syndrome can be masculinized by administration of pubertal testosterone (2). One of the interesting points is the fact that no increase in LH and testosterone is observed after delivery in cases of complete androgene insensitivity in contrast to partial androgene insensitivity (3, 30).
Figure 1.
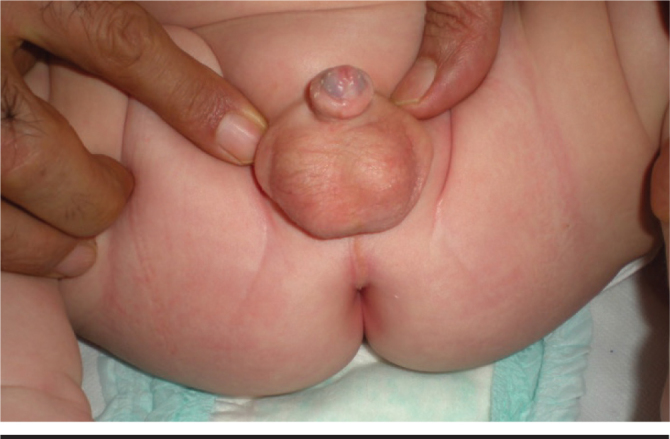
Scrotal hair growth
(from the archive of Erciyes University Medical Faculty, Department of Neanology)
The FSH level in the circulation in girls is high in the middle period of pregnancy, decreases at delivery and increases again with activation of the hypothalamic-pituitary-gonadal axis. Production of AMH is limited in fetal ovaries. In female babies, transformation to estradiole increases with production of LH androstenodione which increases moderately after delivery and with increased FSH afterwards. The level of FSH is higher in girls. The hormones excluding follicle stimulating hormone decrease from the 6th month after delivery (31). Increased FSH in girls continues until the age of 2–4 years. In girls, the level of estradiol is high in the 2–4th months after delivery (the levels may fluctuate) (31). Inhibin-B reaches a peak in the first four months and is maintained at the prepubertal level until the age of 2 years. In premature babies, increased FSH in the postnatal period is more exaggerated. Folliculogenesis and increase in AMH starts with increased FSH. However, this is observed later in preterm and near term babies compared to term babies (32). This explains increased FSH levels. In contrast to boys, it is not exactly known which superiorities mini puberty provides for girls. In a study conducted with girls aged approximately three months, inhibin-B was found to be 82 pg/mL, FSH was found to be 3.8 IU/L, LH was found to be 0.07 IU/L, estradiol was found to be 31 pM and SHBG was found to be 137 nM (32). FSH was found to be higher than 4.5 IU/L in 38% of the girls. In preterm babies, estradiol and inhibin-B were found to be higher. In addition, estradiol was higher in babies with intra-uterine growth retardation. Anti-Mullerian hormone and inhibin-B are high in the follicular phase and interpreted as ovarian function with counting of the antral follicles by ultrasonography.
Evaluation of mini puberty
The external genitalia are examined carefully after delivery. In male newborns, the penile lenght and testicular volume are measured with Prader orchimeter and US and hypospadias, criptorchidism, transverse testicular ectopy and inguinal hernia are noted. In girls and in babies whose external appearances are compatible with female gender, the labia majora, minora, hymen and urethra are examined. Gonad palpation is performed inside the labia or inguinal channel. Pigmentation in the genital region and nipples and other malformations are explored in both genders. Approximately after two months when mini puberty appears, FSH, LH, testosterone, estradiol, MAH, inhibin-B are measured and uterus-ovarian US is performed (Table 1, 2) (33). In term male babies, the level of testosterone increases to 60–400 ng/dL between the 20th and 60th days and decreases to the prepubertal level after the 7th month (<3–10 ng/dL). In girls, the level of estradiol is high at delivery and decreases in the first week. It increases to 5–50 pg/mL between the 30th and 60th days. Afterwards, it decreases to the prepubertal level (below 15 pg/mL) (34). The uterus and ovaries are evaluated ultrasonographically in female babies. The dimensions of the uterus are increased due to the hormones crossing from the mother through the placenta (35, 36). The body of the uterus is more prominent compared to the cervix, the height of the uterus is 35 mm and the highest width is approximately 14 mm. The endometrium is apperent and echogenic. A small amount of fluid is found in the endometrial space in approximately 25% of the babies. The dimensions of the ovary in female newborns are larger compared to the childhood (approximately 1 mL). The size of the ovary is measured to be 1 mL until the age of one and 0.67 mL in the second year (37). In 84% of the babies, follicles the diameters of which do not exceed 1 cm are found from the neonatal period to the age of 2 years. The same finding is observed with a rate of 68% in girls aged between 2 and 6 years (38).
Table 1.
Gonadotropin levels in female newborn babies (RAI)
| Days | LH (mIU/L) | FSH (mIU/L) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Median | Mean | SD | Median | |
| 1–5 | 0.48 | 0.66 | 0.20 | 2 | 1.37 | 1.80 |
| 06–10 | 0.45 | 0.33 | 0.30 | 2.44 | 2.52 | 1.40 |
| 11–15 | 1.8 | 1.28 | 1.60 | 8.16 | 4.27 | 8.95 |
| 16–20 | 1.03 | 1.39 | 0.35 | 1.62 | 1.05 | 1.90 |
| 21–25 | 0.46 | 0.25 | 0.50 | 7.07 | 5.92 | 3.90 |
| 26–28 | 2.75 | 2.39 | 2.80 | 9.74 | 9.89 | 6.15 |
LH: luteinizing hormone; FSH: follicle stimulating hormone; SD: standard deviation
Table 2.
Gonadotropin levels in male newborn babies (RAI)
| Days | LH (mIU/L) | FSH (mIU/L) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | SD | Median | Mean | SD | Median | |
| 1–5 | 0.39 | 0.48 | 0.20 | 0.96 | 0.60 | 0.85 |
| 06–10 | 2.31 | 2.29 | 1.50 | 2.91 | 4.38 | 1.40 |
| 11–15 | 3.55 | 2.84 | 2.90 | 3.71 | 2.69 | 3 |
| 16–20 | 4.13 | 2.76 | 3.65 | 2.63 | 1.45 | 2.15 |
| 21–25 | 2.86 | 1.51 | 2.70 | 2.50 | 1.5 | 2.10 |
| 26–28 | 2.22 | 2.37 | 1.40 | 2.25 | 0.81 | 2.40 |
LH: luteinizing hormone; FSH: follicle stimulating hormone; SD: standard deviation
Problems related with mini puberty
If there is hypogonadotropic hypogonadism in boys, decreased FSH, testosterone and inhibin-B is found in addition to micropenis (strecthed penis <2.5 cm) and criptorchidism; LH may be inmeasurably low or may show low amplitude pulses. A blunted response is obtained in the gonadotropin releasing hormone test. Hypogonadotropism may be found solely in the spectrum of Kallmann syndrome, DAX-1 mutation, multiple pituitary hormone deficiency. With this objective, pituitary and cranial magnetic resonance imaging, pituitary hormone tests and genetic tests may be performed. A picture of hypergonadotropism is observed in cases of gonadal agenesis (2). Gonadotropin treatment starting from the newborn period and allowance of testicular function by mini puberty created are recommended in cases of congenital hypogonadotropic hypogonadism (39).
The most important cause of the picture of hypogonadism is Turner syndrome. In babies with Turner syndrome, low birth weight, edema on hands and feet, weblike neck, inverted and hyperteloric nipples and pigmented nevi are noted (Figure 2). Aort coarctation and horseshoe kidney may be found. The uterus is hypoplasic and streak ovaries are observed on ultrasonography. A level of FSH below 40 is diagnostic, but it may not always be increased (40). Luteinizing hormone is normal and estradiol is low.
Excessive ovarian stimulation sydrome in preterm babies: This syndrome was reported by Sedin et al. (41) in 1985 for the first time in four preterm babies with a gestational age of 24–28 weeks. Edema in the vulva, hypogastric area and thigh was was noted in the postconceptional 36–39th weeks (Figure 3). The investigations performed revealed high gonadotropin levels in addition to multiple cysts in the ovaries on ultrasonography. Afterwards, nine cases were published by Starzyk et al. (42). In addition to the same laboratory and clinical findings, solitary or multiple ovarian cysts with a mean diameter of 21 mm were found. Increase in gonadotropins was observed with GnRH test in a portion of the cases. Breast growht may be added to the picture in some cases (43). Solitary or monthly vaginal bleeding may occur in cases with a severe clinical course (44, 45). The pathophysiology of this picture which is observed in preterm babies is not known fully. It is thought that lack of maturation of the hypothalamo-pituitary-gonadal axis and lack of complete development of negative feedback mechanisms with discontinuation of placental steroids are involved (42, 46). It has been proposed that edema observed in the subjects is related with vascular endothelial growth factor released from the theca and granulosa cells (43, 47). It has been reported that fetal ovarian hyperstimulation may occur rarely (47). In a fetus with a gestational age of 35 weeks, macrosomy, placental thickenning, polyhydramniosis, large ovaries (right 17.8, left 16.3 mL), 2 ovarian cysts with large septae and abdominal distention were observed and the clinical picture was associated with the increased level of beta-hCG found in the mother.
Figure 2.
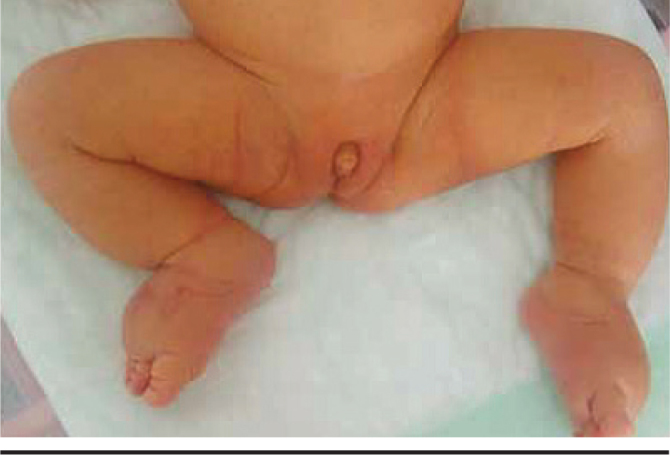
Karyotype 45 x=, edema in the dorsal part of the feet, hypoplasic uterus, ovaries could not be visualized, FSH 49.47, LH 4.43 mIU/mL
(from the archive of Erciyes University Medical Faculty, Department of Neanology)
Figure 3.
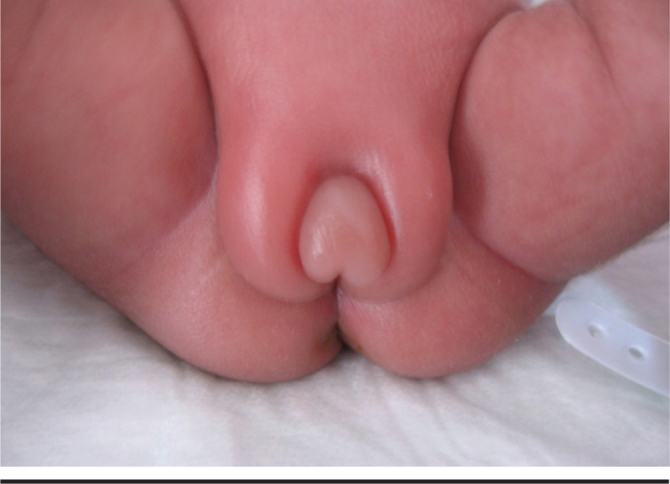
Severe edema in the vulva, upper thigh and lower abdomen in a case of overstimulation of the overies
(from the archive of Erciyes University Medical Faculty, Department of Neanology)
In the diagnosis, increased FSH, LH, estradiol, increased dimensions of the ovaries and uterus and presence of ovarian cysts are directive in addition to the clinical picture.
The subjects should be followed up with clinical picture, FSH, LH, estradiol levels and ovarian ultrasonography. Ovarian cysts should be expected to become smaller gradually. Hence, reduction in the cysts are observed in the first month in 50% of the cases and at the end of the second month in 25% of the cases. The cysts may persist for longer than three months in 10% of the cases (42). Drainage by aspiration may be necessary because of the risk of tortion in solitary or multiple cysts with a diameter larger than 4–5 cm which do not get smaller (41). In severe cases where clinical regression is not observed, intracranial medroxyprogesteron acetate may be administered at a dose of 150 mg/m2 with the aim of decreasing the synthesis of ovarian estradiol (42).
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept - S.K., O.B, Design - S.K., O.B., Supervision - S.K., O.B.; Funding - S.K., O.B.; Analysis and/or Interpretation - S.K., O.B.; Literature review - S.K., O.B.; Writer - S.K., O.B.; Critical Review -S.K., O.B.; Other - S.K., O.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Grumbach MM, Kaplan SL. The neuroendocrinology of human puberty: an ontogenic perspective. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Baltimore: Williams-Wilkins; 1990. pp. 1–62. [Google Scholar]
- 2.Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90:3122–7. doi: 10.1210/jc.2004-2465. http://dx.doi.org/10.1210/jc.2004-2465. [DOI] [PubMed] [Google Scholar]
- 3.Quigley CA. Editorial: The postnatal gonadotropin and sex steroid surge-insights from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2002;87:24–8. doi: 10.1210/jcem.87.1.8265. http://dx.doi.org/10.1210/jcem.87.1.8265. [DOI] [PubMed] [Google Scholar]
- 4.Saeger P. Definition, etiology, and evaluation of precocious puberty. Up To Date 2012.
- 5.Corbier P, Dehennin L, Castanier M, Mebazaa A, Edwards DA, Roffi J. Sexdifferences in serum luteinizing hormone and testosterone in the human neonateduring the first few hours after birth. J Clin Endocrinol Metab. 1990;71:1344–8. doi: 10.1210/jcem-71-5-1344. http://dx.doi.org/10.1210/jcem-71-5-1344. [DOI] [PubMed] [Google Scholar]
- 6.de Zegher F, Devlieger H, Veldhuis JD. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res. 1992;32:605–7. doi: 10.1203/00006450-199211000-00025. http://dx.doi.org/10.1203/00006450-199211000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Forest MG. Pituitary gonadotropins and sex steroid secretion during the first two years of life. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Baltimore: Williams-Wilkins; 1990. pp. 451–77. [Google Scholar]
- 8.Andersson AM, Toppari J, Haavisto AM, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–81. doi: 10.1210/jcem.83.2.4603. http://dx.doi.org/10.1210/jc.83.2.675. [DOI] [PubMed] [Google Scholar]
- 9.Nistal M, Paniagua R, Regadera J, Santamarìa L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res. 1986;246:229–36. doi: 10.1007/BF00215884. http://dx.doi.org/10.1007/BF00215884. [DOI] [PubMed] [Google Scholar]
- 10.Lejeune H, Habert R, Saez JM. Origin, proliferation and differentiation of Leydig cells. J Mol Endocrinol. 1998;20:1–25. doi: 10.1677/jme.0.0200001. http://dx.doi.org/10.1677/jme.0.0200001. [DOI] [PubMed] [Google Scholar]
- 11.Chemes HE. Infancy is not a quiescent period of testicular development. Int J Androl. 2001;24:2–7. doi: 10.1046/j.1365-2605.2001.00260.x. http://dx.doi.org/10.1046/j.1365-2605.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Main KM, Schmidt IM, Toppari J, Skakkebaek NE. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. Eur J Endocrinol. 2002;146:75–9. doi: 10.1530/eje.0.1460075. http://dx.doi.org/10.1530/eje.0.1460075. [DOI] [PubMed] [Google Scholar]
- 13.Rey RA, Belville C, Nihoul-Fékété C, et al. Evaluation of gonadal function in 107 intersex patients by means of serum antimüllerian hormone measurement. J Clin Endocrinol Metab. 1999;84:627–31. doi: 10.1210/jcem.84.2.5507. http://dx.doi.org/10.1210/jc.84.2.627. [DOI] [PubMed] [Google Scholar]
- 14.Lee MM, Misra M, Donahoe PK, MacLaughlin DT. MIS/AMH in the assessment of cryptorchidism and intersex conditions. Mol Cell Endocrinol. 2003;211:91–8. doi: 10.1016/j.mce.2003.09.014. http://dx.doi.org/10.1016/j.mce.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Müller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys whosuffered from sudden death. Int J Androl. 1983;6:143–56. doi: 10.1111/j.1365-2605.1983.tb00333.x. http://dx.doi.org/10.1111/j.1365-2605.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 16.Cortes D, Müller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589–96. doi: 10.1111/j.1365-2605.1987.tb00358.x. http://dx.doi.org/10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 17.Diamond FB, Jr, Shulman DI, Root AW. Scrotal hair in infancy. J Pediatr. 1989;114:999–1001. doi: 10.1016/s0022-3476(89)80448-2. http://dx.doi.org/10.1016/S0022-3476(89)80448-2. [DOI] [PubMed] [Google Scholar]
- 18.Slyper AH, Esterly NB. Nonprogressive scrotal hair growth in two infants. Pediatr Dermatol. 1993;10:34–5. doi: 10.1111/j.1525-1470.1993.tb00009.x. http://dx.doi.org/10.1111/j.1525-1470.1993.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 19.Janus D, Wojcik M, Tyrawa K, Starzyk J. Transient isolated scrotal hair development in infancy. Clin Pediatr (Phila) 2013;52:628–32. doi: 10.1177/0009922813480845. http://dx.doi.org/10.1177/0009922813480845. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. http://dx.doi.org/10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 21.Müller J, Skakkebaek NE. Fluctuations in the number of germ cells during the late foetal and early postnatal periods in boys. Acta Endocrinol (Copenh) 1984;105:271–4. doi: 10.1530/acta.0.1050271. [DOI] [PubMed] [Google Scholar]
- 22.Hadziselimovic F, Hadziselimovic NO, Demougin P, Krey G, Oakeley EJ. Deficient expression of genes involved in the endogenous defense system againts transposons in cryptorchid boys with impaired mini-puberty. Sex Dev. 2011;5:287–93. doi: 10.1159/000335188. http://dx.doi.org/10.1159/000335188. [DOI] [PubMed] [Google Scholar]
- 23.Berensztein EB, Sciara MI, Rivarola MA, Belgorosky A. Apoptosis and proliferation of human testicular somatic and germ cells during prepuberty: high rate of testicular growth in newborns mediated by decreased apoptosis. J Clin Endocrinol Metab. 2002;87:5113–8. doi: 10.1210/jc.2002-020032. http://dx.doi.org/10.1210/jc.2002-020032. [DOI] [PubMed] [Google Scholar]
- 24.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF., Jr Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4128–36. doi: 10.1210/jc.2002-020518. http://dx.doi.org/10.1210/jc.2002-020518. [DOI] [PubMed] [Google Scholar]
- 25.Sharpe RM, Fraser HM, Brougham MF, et al. Role of the neonatal period of pituitary-testicular activity in germ cell proliferation and differentiation in the primate testis. Hum Reprod. 2003;18:2110–7. doi: 10.1093/humrep/deg413. http://dx.doi.org/10.1093/humrep/deg413. [DOI] [PubMed] [Google Scholar]
- 26.Hadziselimovic F, Zivkovic D, Bica DT, Emmons LR. The impotance of mini-puberty for fertility in cryptorchidism. J Urol. 2005;174:1536–9. doi: 10.1097/01.ju.0000181506.97839.b0. http://dx.doi.org/10.1097/01.ju.0000181506.97839.b0. [DOI] [PubMed] [Google Scholar]
- 27.Zivkovic D, Hadziselimovic F. Development of Sertoli cells during mini-puberty in normal and cryptochid testes. Urol Int. 2009;82:89–91. doi: 10.1159/000176032. http://dx.doi.org/10.1159/000176032. [DOI] [PubMed] [Google Scholar]
- 28.Lewis K, Lee PA. Endocrinology of male puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16:5–9. doi: 10.1097/MED.0b013e32832029be. http://dx.doi.org/10.1097/MED.0b013e32832029be. [DOI] [PubMed] [Google Scholar]
- 29.Kuhns LR, Finnstrom O. New standarts of ossification of the newborn. Radiology. 1976;119:655–60. doi: 10.1148/119.3.655. http://dx.doi.org/10.1148/119.3.655. [DOI] [PubMed] [Google Scholar]
- 30.Bouvattier C, Carel JC, Lecointre C, et al. Postnatal changes of T, LH, and FSH in 46,XY infants with mutations in the AR gene. J Clin Endocrinol Metab. 2002;87:29–32. doi: 10.1210/jcem.87.1.7923. http://dx.doi.org/10.1210/jcem.87.1.7923. [DOI] [PubMed] [Google Scholar]
- 31.Kuuri-Hanninen T, Kallio S, Seuri R, et al. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96:3432–9. doi: 10.1210/jc.2011-1502. http://dx.doi.org/10.1210/jc.2011-1502. [DOI] [PubMed] [Google Scholar]
- 32.Chellakooty M, Schmidt IM, Haavisto AM, et al. Inhibin A, inhibin B, follcle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in healthy infant girls. J Clin Endocrinol Metab. 2003;88:3515–20. doi: 10.1210/jc.2002-021468. http://dx.doi.org/10.1210/jc.2002-021468. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H, Schwarz HP. Serum concenrations of LH and FSH in the healthy newborn. Eur J Endocrinol. 2000;143:213–5. doi: 10.1530/eje.0.1430213. http://dx.doi.org/10.1530/eje.0.1430213. [DOI] [PubMed] [Google Scholar]
- 34. ESOTERIX Laboratory Services, Endocrinology Expected Values, 2009.
- 35.Nussbaum AR, Sanders RC, Jones MD. Neonatal uterine morphology as seen on real-time US. Radiology. 1986;160:641–3. doi: 10.1148/radiology.160.3.3526401. http://dx.doi.org/10.1148/radiology.160.3.3526401. [DOI] [PubMed] [Google Scholar]
- 36.Hata K, Nishigaki A, Makihara K, Takamiya O, Hata T, Kitao M. Ultrasonicevaluation of the normal uterus in the neonate. J Perinat Med. 1989;17:313–7. doi: 10.1515/jpme.1989.17.4.313. http://dx.doi.org/10.1515/jpme.1989.17.4.313. [DOI] [PubMed] [Google Scholar]
- 37.Cohen HL, Shapiro MA, Mandel FS, Shapiro ML. Normal ovaries in neonates and infants: a sonographic study of 77 patients 1 day to 24 months old. AJR Am J Roentgenol. 1993;160:583–6. doi: 10.2214/ajr.160.3.8430559. http://dx.doi.org/10.2214/ajr.160.3.8430559. [DOI] [PubMed] [Google Scholar]
- 38.Cohen HL, Eisenberg P, Mandel F, Haller JO. Ovarian cysts are common in premenarchal girls: a sonographic study of 101 children 2–12 years old. AJR Am J Roentgenol. 1992;159:89–91. doi: 10.2214/ajr.159.1.1609728. http://dx.doi.org/10.2214/ajr.159.1.1609728. [DOI] [PubMed] [Google Scholar]
- 39.Bouvattier C, Maione L, Bouligand J, Dodé C, Guiochon-Mantel A, Young J. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2011;8:172–82. doi: 10.1038/nrendo.2011.164. http://dx.doi.org/10.1038/nrendo.2011.164. [DOI] [PubMed] [Google Scholar]
- 40.Heinrichs C, Bourdoux P, Saussez C, Vis HL, Bourguignon JP. Blood spot follicle-stimulating hormone during early postnatal life in normal girls and Turner’s syndrome. J Clin Endocrinol Metab. 1994;78:978–81. doi: 10.1210/jcem.78.4.8157730. http://dx.doi.org/10.1210/jcem.78.4.8157730. [DOI] [PubMed] [Google Scholar]
- 41.Sedin G, Bergquist C, Lindgren G. Ovarian hyperstimulation syndrome in preterm infants. Pediatr Res. 1985;19:548–52. doi: 10.1203/00006450-198506000-00009. http://dx.doi.org/10.1203/00006450-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Starzyk J, Wojcik M, Wojtys J, Tomasik P, Mitkowska Z, Pietrzyk JJ. Ovarian hyperstimulation syndrome in newborns- a case presentation and literature review. Horm Res. 2009;71:60–4. doi: 10.1159/000173743. http://dx.doi.org/10.1159/000173743. [DOI] [PubMed] [Google Scholar]
- 43.Marinkovic M, Rasmussen M, Jones K. Feminizing changes in a prematurely infant. Clin Pediatr. 2010;49:188–9. doi: 10.1177/0009922809337624. http://dx.doi.org/10.1177/0009922809337624. [DOI] [PubMed] [Google Scholar]
- 44.Jackson K, Babar G, Ugrasbul F. Extremly minipuberty presenting with vaginal bleeding in a month-old preterm girl. Horm Res. 2013;80:431. [Google Scholar]
- 45.Altuntas N, Turkyılmaz C, Yuce O, et al. Preterm ovarian hyperstimulation syndrome presented with vaginal bleeding: a case report. J Pediatr Endocrinol Metab. 2014;27:355–8. doi: 10.1515/jpem-2013-0166. http://dx.doi.org/10.1515/jpem-2013-0166. [DOI] [PubMed] [Google Scholar]
- 46.Eichalal U, Schenker JG. Pathophysiology of ovarian hyperstimulation syndrome-views and ideas. Hum Reprod. 1997;12:1129–37. doi: 10.1093/humrep/12.6.1129. http://dx.doi.org/10.1093/humrep/12.6.1129. [DOI] [PubMed] [Google Scholar]
- 47.Berezowski AT, Machado JC, Mendes MC, Maura MD, Duarte G, Cunha SP. Prenatal diagnosis of fetal ovarian hyperstimulation. Ultrasound Obstet Gynecol. 2001;17:259–62. doi: 10.1046/j.1469-0705.2001.00349.x. http://dx.doi.org/10.1046/j.1469-0705.2001.00349.x. [DOI] [PubMed] [Google Scholar]


