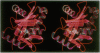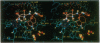Abstract
The structure of the metalloproteinase and hemorrhagic toxin atrolysin C form d (EC 3.4.24.42), from the venom of the western diamondback rattlesnake Crotalus atrox, has been determined to atomic resolution by x-ray crystallographic methods. This study illuminates the nature of inhibitor binding with natural (< Glu-Asn-Trp, where < Glu is pyroglutamic acid) and synthetic (SCH 47890) ligands. The primary specificity pocket is exceptionally deep; the nature of inhibitor and productive substrate binding is discussed. Insights gained from the study of these complexes facilitate the design of potential drugs to treat diseases where matrix metalloproteinases have been implicated, e.g., arthritis and tumor metastasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baramova E. N., Shannon J. D., Bjarnason J. B., Gonias S. L., Fox J. W. Interaction of hemorrhagic metalloproteinases with human alpha 2-macroglobulin. Biochemistry. 1990 Jan 30;29(4):1069–1074. doi: 10.1021/bi00456a032. [DOI] [PubMed] [Google Scholar]
- Beszant B., Bird J., Gaster L. M., Harper G. P., Hughes I., Karran E. H., Markwell R. E., Miles-Williams A. J., Smith S. A. Synthesis of novel modified dipeptide inhibitors of human collagenase: beta-mercapto carboxylic acid derivatives. J Med Chem. 1993 Dec 10;36(25):4030–4039. doi: 10.1021/jm00077a006. [DOI] [PubMed] [Google Scholar]
- Bjarnason J. B., Tu A. T. Hemorrhagic toxins from Western diamondback rattlesnake (Crotalus atrox) venom: isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry. 1978 Aug 8;17(16):3395–3404. doi: 10.1021/bi00609a033. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Rüth F. X., Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. 1993 Sep 27;331(1-2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Bode W., Reinemer P., Huber R., Kleine T., Schnierer S., Tschesche H. The X-ray crystal structure of the catalytic domain of human neutrophil collagenase inhibited by a substrate analogue reveals the essentials for catalysis and specificity. EMBO J. 1994 Mar 15;13(6):1263–1269. doi: 10.1002/j.1460-2075.1994.tb06378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkakoti N., Winkler F. K., Williams D. H., D'Arcy A., Broadhurst M. J., Brown P. A., Johnson W. H., Murray E. J. Structure of the catalytic domain of human fibroblast collagenase complexed with an inhibitor. Nat Struct Biol. 1994 Feb;1(2):106–110. doi: 10.1038/nsb0294-106. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Davies B., Brown P. D., East N., Crimmin M. J., Balkwill F. R. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993 May 1;53(9):2087–2091. [PubMed] [Google Scholar]
- Dixon M. M., Matthews B. W. Is gamma-chymotrypsin a tetrapeptide acyl-enzyme adduct of alpha-chymotrypsin? Biochemistry. 1989 Aug 22;28(17):7033–7038. doi: 10.1021/bi00443a038. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Campbell R., Beggerly L., Bjarnason J. B. Substrate specificities and inhibition of two hemorrhagic zinc proteases Ht-c and Ht-d from Crotalus atrox venom. Eur J Biochem. 1986 Apr 1;156(1):65–72. doi: 10.1111/j.1432-1033.1986.tb09549.x. [DOI] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff D. L., Jenny T. F., Knecht L. J., Benner S. A. A secondary structure prediction of the hemorrhagic metalloprotease family. Biochem Biophys Res Commun. 1993 Jul 15;194(1):560–565. doi: 10.1006/bbrc.1993.1856. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Kress L. F., Bode W. First structure of a snake venom metalloproteinase: a prototype for matrix metalloproteinases/collagenases. EMBO J. 1993 Nov;12(11):4151–4157. doi: 10.1002/j.1460-2075.1993.tb06099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Stöcker W., Huber R., Zwilling R., Bode W. Refined 1.8 A X-ray crystal structure of astacin, a zinc-endopeptidase from the crayfish Astacus astacus L. Structure determination, refinement, molecular structure and comparison with thermolysin. J Mol Biol. 1993 Feb 20;229(4):945–968. doi: 10.1006/jmbi.1993.1098. [DOI] [PubMed] [Google Scholar]
- Gooley P. R., Johnson B. A., Marcy A. I., Cuca G. C., Salowe S. P., Hagmann W. K., Esser C. K., Springer J. P. Secondary structure and zinc ligation of human recombinant short-form stromelysin by multidimensional heteronuclear NMR. Biochemistry. 1993 Dec 7;32(48):13098–13108. doi: 10.1021/bi00211a020. [DOI] [PubMed] [Google Scholar]
- Grobelny D., Poncz L., Galardy R. E. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992 Aug 11;31(31):7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- Gross J., Harper E., Harris E. D., McCroskery P. A., Highberger J. H., Corbett C., Kang A. H. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem Biophys Res Commun. 1974 Nov 27;61(2):605–612. doi: 10.1016/0006-291x(74)91000-6. [DOI] [PubMed] [Google Scholar]
- Hite L. A., Jia L. G., Bjarnason J. B., Fox J. W. cDNA sequences for four snake venom metalloproteinases: structure, classification, and their relationship to mammalian reproductive proteins. Arch Biochem Biophys. 1994 Jan;308(1):182–191. doi: 10.1006/abbi.1994.1026. [DOI] [PubMed] [Google Scholar]
- Kato H., Suzuki T. Bradykinin-potentiating peptides from the venom of Agkistrodon halys blomhoffi. Isolation of five bradykinin potentiators and the amino acid sequences of two of them, potentiators B and C. Biochemistry. 1971 Mar 16;10(6):972–980. doi: 10.1021/bi00782a007. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Abe S., Robey P. G., Martin G. R. Preferential digestion of basement membrane collagen by an enzyme derived from a metastatic murine tumor. Proc Natl Acad Sci U S A. 1979 May;76(5):2268–2272. doi: 10.1073/pnas.76.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Robey P. G., Abe S. Partial purification and characterization of a neutral protease which cleaves type IV collagen. Biochemistry. 1981 Jan 6;20(1):100–104. doi: 10.1021/bi00504a017. [DOI] [PubMed] [Google Scholar]
- Meyer E. Internal water molecules and H-bonding in biological macromolecules: a review of structural features with functional implications. Protein Sci. 1992 Dec;1(12):1543–1562. doi: 10.1002/pro.5560011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Allan J. A., Willenbrock F., Cockett M. I., O'Connell J. P., Docherty A. J. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992 May 15;267(14):9612–9618. [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- Muthukumaran G., Blumberg B., Kurkinen M. The complete primary structure for the alpha 1-chain of mouse collagen IV. Differential evolution of collagen IV domains. J Biol Chem. 1989 Apr 15;264(11):6310–6317. [PubMed] [Google Scholar]
- Niedzwiecki L., Teahan J., Harrison R. K., Stein R. L. Substrate specificity of the human matrix metalloproteinase stromelysin and the development of continuous fluorometric assays. Biochemistry. 1992 Dec 22;31(50):12618–12623. doi: 10.1021/bi00165a011. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977 Apr 22;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- Ownby C. L., Bjarnason J., Tu A. T. Hemorrhagic toxins from rattlesnake (Crotalus atrox) venom. Pathogenesis of hemorrhage induced by three purified toxins. Am J Pathol. 1978 Oct;93(1):201–218. [PMC free article] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Robeva A., Politi V., Shannon J. D., Bjarnason J. B., Fox J. W. Synthetic and endogenous inhibitors of snake venom metalloproteinases. Biomed Biochim Acta. 1991;50(4-6):769–773. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seltzer J. L., Akers K. T., Weingarten H., Grant G. A., McCourt D. W., Eisen A. Z. Cleavage specificity of human skin type IV collagenase (gelatinase). Identification of cleavage sites in type I gelatin, with confirmation using synthetic peptides. J Biol Chem. 1990 Nov 25;265(33):20409–20413. [PubMed] [Google Scholar]
- Shannon J. D., Baramova E. N., Bjarnason J. B., Fox J. W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J Biol Chem. 1989 Jul 15;264(20):11575–11583. [PubMed] [Google Scholar]
- Swanson S. M. Core tracing: depicting connections between features in electron density. Acta Crystallogr D Biol Crystallogr. 1994 Sep 1;50(Pt 5):695–708. doi: 10.1107/S0907444994002398. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wolfsberg T. G., Bazan J. F., Blobel C. P., Myles D. G., Primakoff P., White J. M. The precursor region of a protein active in sperm-egg fusion contains a metalloprotease and a disintegrin domain: structural, functional, and evolutionary implications. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10783–10787. doi: 10.1073/pnas.90.22.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Byrne M. H., Stacey A., Goldring M. B., Birkhead J. R., Jaenisch R., Krane S. M. Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Mochizuki T., Smeets H., Antignac C., Laurila P., de Paepe A., Tryggvason K., Reeders S. T. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science. 1993 Aug 27;261(5125):1167–1169. doi: 10.1126/science.8356449. [DOI] [PubMed] [Google Scholar]