Abstract
Aim:
In this study, it was aimed to evaluate the clinical, antropometric and laboratory findings of female patients diagnosed with central precocious puberty and to determine the laboratory value with the best diagnostic accuracy in the diagnosis of central precocious puberty.
Materials and Method:
Female patients whose breast development began before the age of 8 years were included in the study. The data of the patients were obtained by retrospectively examining file records. The chronogical age, age at the time of onset of the complaint, antropometric variables, bone age and hormonal tests were recorded. The patients whose bone age/chronological age ratio was >1 and in whom pubertal response was obtained to gonodotropin releasing hormone stimulation test were considered central precocious puberty and the patients who did not meet these criteria were considered premature thelarche. Receiver operating charecteristic curve (ROC) analysis was performed to determine the diagnostic accuracy of the laboratory variables.
Results:
Fifty one patients with idiopathic central precocious puberty and 36 patients with premature thelarche were included in the study. In the patients with central precocious puberty, the height standard deviation score, bone age and bone age/chronological age ratio were found to be significantly higher compared to the patients with premature thelarche. The basal luteinizing hormone, basal follicle stimulating hormone, basal luteinizing hormone/follicle stimulating hormone, peak luteinizing hormone, peak follicle stimulating hormone and peak luteinizing hormone/follicle stimulating hormone values were found to be significantly higher in the patients with central precocious puberty. When the cut-off value for the peak luteinizing hormone/follicle stimulating hormone ratio was taken as >0.24, the sensitivity was found to be 100% and specificity was found to be 84%. When the cut-off value for the basal follicle stimulating hormone was taken as >1.9 IU/L, the sensitivity was found to be 71% and specificity was found to be 68%. When the cut-off value for the basal luteinizing hormone was taken as >0.1 IU/L, the sensitivity was found to be 71% and specificity was found to be 64%.
Conclusions:
In female children, a peak luteinizing hormone/follicle stimulating hormone ratio of >0.24 can be used in the diagnosis of central precocious puberty. However, the findings should be assessed in association with the clinical and antropometric variables.
Keywords: Bone age, central precocious puberty, GnRH stimulation test, premature thelarche
Introduction
Puberty is the period when pulsatile release of the gonodotropin releasing hormone (GnRH) starts as a result of activation of hypothalamo-pituitary-gonadal axis (HPG) and the secondary sex characteristics develop (1, 2). Central precocious puberty (CPP) is defined as development of secondary sex characteristics in relation with activation of the HPG axis before the age of 8 years in girls and before the age of 9 years in boys (1–3). Although the incidence of central precocious puberty is not known exactly, it ranges between 1/5 000 and 1/10 000 (3).
Premature thelarche (PT) is defined as isolated breast development in the absence of the other clinical findings of puberty freqently in the first three years of life. Premature thelarche is considered as a variant of normal development and not evaluated to be pathological (2, 3). In individuals with premature thelarche, the growth rate is normal in contrast to CPP, the bone age is not advanced and the basal gonadotropin and estradiol values are at prepubertal levels (4). The cause of premature thelarche is not known exactly and its prevalence is 4.7%. Thirteen % of the cases of premature thelarche may progress to CPP (2, 4). The clinical findings, bone age and basal and stimulated gonodotropin levels should be evaluated carefully.
The basal and stimulated luteinizing hormone (LH) values are assistive tests which show activation of the HPG axis and enable differentiation of CPP cases from cases of PT. Currently, the GnRH stimulation test is considered gold standard in differentiation of CPP from PT. However, the most important disadvantages of this test include high cost and its time-consuming property (5). Therefore, cut-off values giving the best sensitivity and specificity in differentiation of CPP cases from PT cases have been tried to be determined in many studies by evaluating basal and stimulated LH levels using different measurement methods (6–12).
In this study, (i) it was aimed to compared the clinical, antropometric and laboratory findings of the patients who presented to the pediatric endocrinology outpatient clinic with premature breast development and diagnosed with CPP and PT and (ii) to determine the laboratory test which has the best sensitivity and specificity value in the diagnosis of CPP.
Material and Methods
This study includes retrospective examination of file records of the patients who presented to the Pediatric Endocrinology Outpatient Clinic between 2005 and 2014 because of early (<8 years) breast development. The peak FSH and LH values of the patients whose age at presentation (PA), age at the time of onset of the complaint, puberty stage at presentation (Tanner’s stage), body weight (kg), height (cm), body mass index (BMI), body mass index-standard deviation score (BMI-SDS), bone age (BA), baseline and GnRH stimulated follicle stimulating hormone (FSH) and LH measurements were recorded.
It was learned from the file records that the follicle stimulating hormone and LH values were measured by immunchemiluminescence (ICMA) method, bone age was evaluated by Greulich and Pyle atlas (13) and pubertal staging was made according to Marshall and Tanner (14). Gonodotropin relasing hormone test was performed by taking blood samples at the 30th, 60th, 90th and 120th minutes following intravenous administration of 100 µg/m2 (maximum 100 µg) LHRH (LHRH Ferring ampul, Ferring İlaç San. ve Tic. Ltd.). Height was measured using Harpenden stadiometer with a measurement sensitivity of 0,1 cm (Holtain Limited, Crymych, Dyfed, U.K) and the body weight was measured using SECA (name of the device) (GMBH & CO KG Hamburg, Germany) with a measurement sensitivity of 0.1 kg. The body mass index was calculated by dividing the body weight to the square of the height in meters. The body weight SDS, height SDS and BMI SDS values were obtained using the CDC data. The subjects with a body mass percentile between the 85th and 95th and >the 95th according to 2000 CDC were considered as overweight and obese respectively (15).
The subjects who had a BA/CA ration of >1, a peak LH value of >5 IU/L on the GnRH test (16) or a basal LH value of >1.1 IU/L (7) combined with isolated and/or axillary hair accompanied by breast development were considered CPP. The subjects who had isolated breast development, BA≤CA, a peak LH value of <5 IU/L on the GnRH test and who had no progression in the pubertal findings and bone age at the end of at least a one-year follow-up period were considered PT. The subjects were divided into three groups according to the age at presentation as ≤3 years, 3–7 years and 7–8 years.
The subjects who were found to have lacking data in the file records, cases of peripheral precocious puberty and the patients who were found to have organic pathology on brain magnetic resonance imaging were excluded from the study.
Statistical analysis
Statistical analysis was performed using SPPS 21.0 (SPSS Inc., Chicago, IL, USA) program. All data were expressed as mean±standard deviation (SD). The homogeneous distribution of the data was evaluated using the Kolmogorov-Smirnov test. The student’s t-test and Mann-Whitney U test were used in comparison of the groups. The chi-square test was used in comparison of the group percentages. A p value of <0.05 was considered statistically significant. In cases of idiopathic CPP, the receiver operating characteristic curve (ROC) analysis was used in calculation of the cut-off value for the laboratory test which had the best sensitivity and specificity.
Results
The file data of 87 female subjects (51 idiopathic CPP, 36 PT) who met the study inclusion criteria were recorded. The age at presentation and the age of onset of the complaints were older in the patients with central precocious puberty compared to the patients with PT, but the difference was not significant (7.12±1.18 years and 6.59±1.47 years, p=0.068; 6.46±1.17 years and 6.21±1.65 years, p=0.402, respectively). Fourteen (27.5%) of the patients who were diagnosed with central precocious puberty were in the 3–7 year age group and 37 (72.5%) were in the 7–8 year age group. Two (5.6%) of the patients who were diagnosed with isolated PT were in the <3 year age group, 15 (41.7%) were in the 3–7 year age group and 19 (52.8%) were in the 7–8 year age group. No difference was found between the groups in terms of age distribution (p=0.067). When the patients included in the study were evaluated in terms of being overweight and obese at presentation, it was found that 12 subjects (21%) in the CPP group and 3 subjects (5.8%) in the PT group were overweight and 4 subjects (11.1%) in the CPP group were obese, whereas no subject in the PT group was obese.
Bilateral breast development was present in 60.7% of the subjects (n=31) with central precocious puberty and in 44.4% of the subjects (n=16) with PT. The difference was not statistically significant (p=0.191). At presentation, 48.6% (n=34) of the subjects who were diagnosed with CPP were considered Tanner stage II, 29.4% (n=15) were considered Tanner stage III and 3.9% (n=2) were considered Tanner stage IV, whereas all PT patients were considered Tanner stage II. When the groups were compared in terms of antropometric data and BMI SDS, values were found to be high in CPP patients, but the difference was not statistically significant (0.41±1.12 and 0.22±0.85, respectively, p=0.403). In contrast, the height SDS, BA, BA/CA ratio were found to be statistically significantly higher in the CPP group compared to the PT group (p<0.05). When the groups were compared in terms of the laboratory data, the basal LH, FSH, and FSH/LH ratio, peak LH, FSH and LH/FSH ratio were found to be signififcantly high in CPP group (p<0.05) (Table 1). When ROC analysis was performed to determine the best sensitivity and specificity value in the diagnosis of central precocious puberty, it was found that the variables which gave the best sensitivity and specificity were the peak LH/FSH ratio (AUC=0.962, p=<0.001), basal FSH (AUC=0.763, p<0.001) and basal LH (AUC=0.705, p=0.007) (Table 2 and Figure 1).
Table 1.
Clinical, antropometric and laboratory properties of the subjects with CPP and PT
| CPP (n=51) | PT (n=36) | p | |
|---|---|---|---|
| Age at presentation (years) | 7.12±1.18 | 6.59±1.47 | 0.068a |
| Age of onset of complaints (years) | 6.46±1.17 | 6.21±1.65 | 0.402a |
| Bilateral breast development (%) | 31 (60%) | 16 (44%) | 0.191a |
| Basal LH | 0.94±1.10 | 0.18±0.43 | <0.001b |
| Basal FSH | 3.33±1.78 | 1.56±0.81 | <0.001a |
| Basal LH/FSH ratio | 0.28±0.31 | 0.16±0.43 | 0.001b |
| Peak LH | 13.54±12.26 | 2.07±1.15 | <0.001a |
| Peak FSH | 14.46±5.15 | 11.51±5.71 | 0.041a |
| Peak LH/FSH ratio | 1.11±1.01 | 0.19±0.09 | <0.001b |
| BMI SDS | 0.41±1.12 | 0.22±0.85 | 0.403a |
| Height SDS | 1.03±1.07 | 0.50±0.82 | 0.008a |
| Bone age | 8.81±1.97 | 7.11±1.63 | <0.001a |
| Bone age/chronological age (years) | 1.24±0.25 | 1.05±0.19 | <0.001a |
| Target height SDS | −0.45±0.90 | −0.62±0.69 | 0.374b |
Student T test;
Mann-Whitney U test
FSH: follicle stimulatin hormone; LH: luteinizing hormone; PT: premature telarche; CPP: central precocious puberty; SDS: Standard deviation score; BMI: body mass index
Table 2.
ROC analysis results of the laboratory variables in the diagnosis of CPP
| Variables | Area | Standard error | p | 95% confidence interval
|
|
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Basal LH (IU/L) | 0.705 | 0.070 | 0.007 | 0.567 | 0.842 |
| Basal FSH (IU/L) | 0.763 | 0.061 | 0.001 | 0.644 | 0.882 |
| Peak FSH (IU/L) | 0.674 | 0.073 | 0.023 | 0.531 | 0.816 |
| Basal LH/FSH | 0.605 | 0.078 | 0.170 | 0.451 | 0.758 |
| Peak LH/FSH | 0.962 | 0.022 | <0.001 | 0.920 | 1.000 |
FSH: follicle stimulatin hormone; LH: luteinizing hormone; ROC: receiver opertating characteristic; CPP: central precocious puberty
Figure 1.
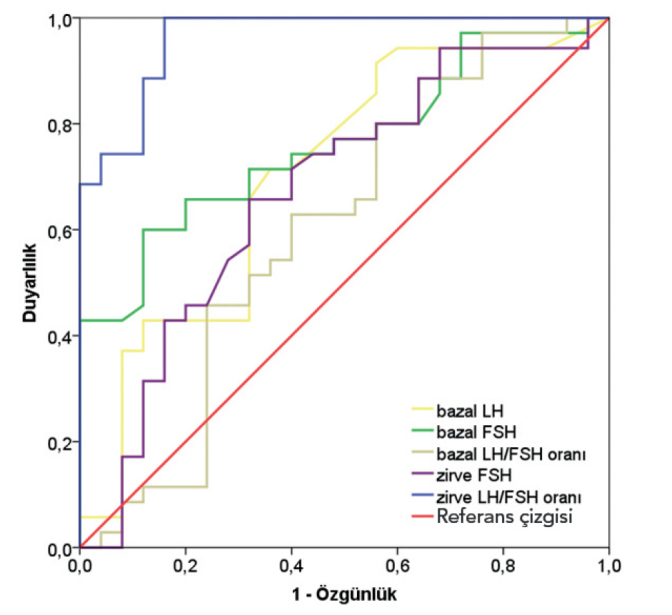
Inventory of ROC analysis of the laboratory variables used in the diagnosis of CPP
The best sensitivity (100%) and specificity (84%) value for the peak LH/FSH ratio was found to be 0.24, the best sensitivity (71%) and specificity (64%) value for basal LH was found to be 0.1 IU/L, the best sensitivity (71%) and specificity (68%) value for basal FSH was found to be 1,9 and the best sensitivity (80%) and specificity (40%) value for peak FSH was found to be >9.1 IU/L. The basal LH value was found to be >0.1 IU/L in 80.4% of the central precocious puberty group and in 27.8% of the PT group. When the cut-off value for basal LH was considered >0.3 IU/L, the sensitivity was found to be 42% and specificity was found to be 88% for the diagnosis of CPP. The variables giving the best sensitivity and specificity value according to the ROC curve are summarized in Table 3.
Table 3.
Limit values which give the best sensitivity and specificity in the diagnosis of CPP
| Sensitivity (%) | Specificity (%) | |
|---|---|---|
| Basal LH | ||
| >0.1 IU/L | 71 | 64 |
| >0.3 IU/L | 42 | 88 |
| Basal FSH | ||
| >1.9 IU/L | 71 | 68 |
| Peak FSH | ||
| >9.1 IU/L | 80 | 40 |
| Basal LH/FSH | ||
| >0.075 | 60 | 60 |
| Peak LH/FSH ratio | ||
| >0.24 | 100 | 84 |
| >0.33 | 80 | 88 |
FSH: follicle stimulating hormone; LH: luteinizing hormone; CPP: central precocious puberty
When the correlation of the basal LH with the peak LH stimulated by GnRH was evaluated, a significant positive correlation was found (r=0.382, p=0.020).
Discussion
CPP patients presenting with early breast development should be differentiated from PT patients in order to plan treatment timely and provide sufficient height gain. With this objective, many laboratory tests, growth follow-up, bone age and imaging methods have been utilized until the present time. However, no diagnositic method which would definitely differentiate these two conditions from each other has been found yet.
Antropometric variables are important variables in differentiating CPP and PT cases. In CPP cases, advanced bone age, increased growth rate and body weight are significant findings which are expected because of the effects of the sex hormones. Studies have found that 25–27% of the CPP patients are obese at the time of diagnosis (17, 18) and 42,8% become obese in the last one year (18). Kılıç et al. (19) found the bone age, height SDS, weight SDS, BMI and BMI-SDS values to be significantly higher in CPP patients compared to the PT group. In this study, we found the BMI-SDS value to be 2-fold higher in the CPP group compared to the PT group which was compatible with the literature, but the difference was not significant. In addition, the rates of overweight and obesity were found to be higher in the CPP group (21%, 5.8%, respectively) compared to the PT group (11.1% and 0%, respectively). These findings suggest that antropometric variables are significant clinical findings to support and differentiate the diagnosis when evaluating the CPP and PT cases.
In girls, CPP is frequently idiopathic and the mean age at the time of diagnosis is higher in cases of idiopathic CPP compared to the cases with organic cause (20). In a large-scale study in which girls with a diagnosis of CPP were evaluated (493 female patients), the mean age at the time of onset of the complaints was found to be 6.68±1.35 years and the age at the time of diagnosis was found to be 7.55±1.44 years (18). In our study, the age at the time of diagnosis (7.12±1.18 years) and the age at the time of onset of the complaints (6.46±1.17 years) were found to be close to the literature data in idiopathic CPP cases. Cicternino et al. (20) reorted that 59.6% of the CPP patients were diagnosed between the ages of 7 and 8 years, whereas organic CPP patients were diagnosed earlier (<4 years) (20). When Giabicani et al. (18) classified idiopathic CPP cases by age groups, it was found that the age at the time of diagnosis was <3 year in 2.2%, 2–6 years in 15.4%, 6–7 years in 22.5% and 7–8 years in 59.8%. Prete et al. (17) found that the age at the time of diagnosis was <3 years in 2%, 3–7 years in 38% and 7–8 years in 67% in the patients diagnosed with idiopathic CPP. In this study, we found no significant difference when we compared the ages at presentation and at the time of diagnosis between the CPP and PT groups. In addition, we found that 72.5% of the patients with idiopathic CPP and 52.8% of the patients with PT were diagnosed between the ages of 7 and 8 years when we evaluated the patients in terms of age distribution. We thought that exclusion of organic CPP cases was a cause of the fact that the rate of CPP was higher in the 7–8 year age group.
Many tests with screening and diagnostic objective are being used in assessment of early activation of the HPG axis in cases of central precocious puberty. Although it is emphasized that the basal LH value measured randomly is a sensitive screening test in demonstrating the pubertal status, its diagnostic rate is low. Lee et al. (6) emphasized that the basal LH value was not a reliable test in the diagnosis of CPP by demostrating that pubertal response was obtained against the GnRH test in 55.6% of the patients who presented with findings of precocious puberty and whose basal LH values were <0.1 IU/L. However, many studies have proposed that the basal LH value can be used in the differential diagnosis of CPP and PT (6, 7, 10). When the basal LH was taken as >0.1 IU/L (ICMA), the sensitivity for CPP was found to be 56.4–94.7% and the specificity was found to be 64–88.4% (8, 10, 21). Suh et al. (22) found the sensitivity to be 87.8% and the specificty to be 20.9%, when they took the basal LH value as >0.22 IU/L (immunoenzymatic). In another study, the sensitivity for CPP was found to be 69,2% and the specificity was found to be 50,5%, when the basal LH value was taken as >1,1 IU/L (immunoradiometric) (7). In the study of Neeley et al. (8) in which 49 patients who were diagnosed with CPP were evaluated, it was found that the diagnostic value of the FSH level stimulated by GnRH was low, but the basal LH level measured by a third generation measurement method (ICMA) was more reliable. In the same study, a strongly positive correlation was shown between LH stimulated with GnRH and the basal LH (r=0.79) (8). In our study, the basal LH value was found to be significantly higher in the CPP group compared to the PT group and one of the variables which were found to be significant by the ROC curve was the basal LH value. In our study which was compatible with the literature, the sensitivity was found to be 71% and the specificity was found to be 64% when the basal LH value was taken as >0.1 IU/L. In addition, a significantly positive correlation was found between the peak LH value and the basal LH value also in our study and the basal LH value was found to be >0.1 IU/L in 80.4% of the patients diagnosed with CPP, whereas it was found to be <0.1 IU/L in 72.2% of the PT patients. These findings support the information that the basal LH value can be used as a screening test in the diagnosis of CPP. These results show that taking the basal LH value measured randomly (third generation measurement method) as >0.1 IU/L can be used a reliable screening test in accordance with some other studies in the literature.
In the literature, controversial results and different cutoff values have been reported in relation with basal LH/ FSH and peak LH/FSH ratios in the differential diagnosis of CPP and PT. Lee et al. (6) recommended that the basal LH and basal LH/FSH ratio should be evaluated primarily in screening and differential diagnosis of CPP and the GnRH test should be performed in case of clinical suspicion. In the same study, the sensitivity was found to be 54.4% and the specificity was found to be 93.7% in the diagnosis of CPP, when the basal LH/FSH ratio was taken as >0.04. In another study, the sensitivity was found to be 75% and the specificity was found to be 82% in the diagnosis of CPP, when the basal LH/FSH was taken as >0.2 (11). In our study, the basal LH/FSH ratio was found to be significantly higher in the CPP group compared to the PT group, but the ROC analysis revealed that the basal LH/FSH ratio was not distinctive. The peak LH/FSH ratio stimulated by GnRH has been reported to be one of the most reliable variables in the diagnosis of central precocious puberty (8). In one study, a peak LH/FSH ratio stimulated by GnRH of >0.66 (radioimmunoassay) in girls with CPP had a sensitivity of 96% and a specificity of 100% (23). In two different studies, a cut-off value of >1.0 for the peak LH/FSH value (radioimmunoassay) was proposed in differentiating the CPP and PT groups (9, 24). In more sensitive hormone analysis methods (IFMA), it was emphesized that taking the peak LH/FSH ratio as >0.3 was a significant parameter for CPP (12, 21). In our study, the peak LH/FSH ratio was found to be the variable which yielded the best sensitivity and specificty in the diagnosis of CPP. When the peak LH/FSH ratio was taken as >0.24, the sensitivity for CPP was found to be 100% and the specificity was found to be 84% and when the peak LH/FSH ratio was taken as >0.33, the sensitivity for CPP was found to be 80% and the specificity was found to be 88%.
In the literature, it has been reported that the basal FSH value and the FSH value stimulated by GnRH were not reliable and distinctive in the diagnosis of CPP. Pasternak et al. (10) reported a sensitivity of 76% and a specificity of 73% for the basal FSH value (>2.25 IU/L) in the diagnosis of CPP. In our study, the sensitivity was found to be 71% and the specificity was found to be 68% when the basal FSH value was taken as >1.9 IU/L. When the peak FSH value was taken as >9.1 IU/L, the sensitivity was found to be 80% and the specificity was found to be 40%. Although the basal and peak FSH values were found to be significant variables in the ROC analysis in our study, their low specificities suggest that they can not be reliably used in practice in the diagnosis of CPP.
Conclusively, the variable which yielded the best sensitivity (100%) and specificity (84%) in the diagnosis of CPP as a result of the ROC analysis was found to be the peak LH/FSH ratio in our study (cut-off value for the peak LH/FSH ratio: >0.24). In girls presenting with early breast development, the cut-off value of >0.24 for the peak LH/FSH ratio can be used in the diagnosis of CPP. However, it should be kept in mind that laboratory findings are variebles which support clinical findings and should be evaluated together with clinical findings.
Footnotes
Ethics Committee Approval: Due to the retrospective nature of this study, ethics committee approval was waived.
Informed Consent: Due to the retrospective nature of this study, informed consent was waived.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.A., E.B.; Design - A.A., G.Ç.; Supervision - E.B., A.A.; Funding - A.A., G.Ç.; Materials - G.Ç., P.E.; Data Collection and/or Processing - G.Ç., P.E.; Analysis and/or Interpretation - A.A., G.Ç.; Literature Review - A.A., G.Ç.; Writer - G.Ç., A.A.; Critical Review - E.B., A.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. http://dx.doi.org/10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 2.Kletter GB, Klein KO, Wong YY. A Pediatrician’s guide to central precocious puberty. Clin Pediatr (Phila) 2014 doi: 10.1177/0009922814541807. [DOI] [PubMed] [Google Scholar]
- 3.Partsch CJ, Sippell WG. Treatment of central precocious puberty. Best Pract Res Clin Endocrinol Metab. 2002;16:165–89. doi: 10.1053/beem.2002.0188. http://dx.doi.org/10.1053/beem.2002.0188. [DOI] [PubMed] [Google Scholar]
- 4.Pasquino AM, Pucarelli I, Passeri F, Segni M, Mancini MA, Municchi G. Progression of premature thelarche to central precocious puberty. J Pediatr. 1995;126:11–4. doi: 10.1016/s0022-3476(95)70492-2. http://dx.doi.org/10.1016/S0022-3476(95)70492-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Kee SJ, Seo JY, Yang EM, Chae HJ, Kim CJ. Gonadotropin-releasing hormone stimulation test for precocious puberty. Korean J Lab Med. 2011;31:244–9. doi: 10.3343/kjlm.2011.31.4.244. http://dx.doi.org/10.3343/kjlm.2011.31.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DS, Ryoo NY, Lee SH, Kim S, Kim JH. Basal luteinizing hormone and follicular stimulating hormone: is it sufficient for the diagnosis of precocious puberty in girls? Ann Pediatr Endocrinol Metab. 2013;18:196–201. doi: 10.6065/apem.2013.18.4.196. http://dx.doi.org/10.6065/apem.2013.18.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HS, Park HK, Ko JH, Kim YJ, Hwang JS. Utility of Basal luteinizing hormone levels for detecting central precocious puberty in girls. Horm Metab Res. 2012;44:851–4. doi: 10.1055/s-0032-1321905. http://dx.doi.org/10.1055/s-0032-1321905. [DOI] [PubMed] [Google Scholar]
- 8.Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. 1995;127:47–52. doi: 10.1016/s0022-3476(95)70255-5. http://dx.doi.org/10.1016/S0022-3476(95)70255-5. [DOI] [PubMed] [Google Scholar]
- 9.Partsch CJ, Hummelink R, Lorenzen F, Sippell WG. [The significance and characteristics of the LHRH test in diagnosing precocious puberty development in girls: the stimulated LH/FSH quotient differentiates between central precocious puberty and premature thelarche] Manatsschr Kinderheiled. 1989;137:284–8. [PubMed] [Google Scholar]
- 10.Pasternak Y, Friger M, Loewenthal N, Haim A, Hershkovitz E. The utility of basal serum LH in prediction of central precocious puberty in girls. Eur J Endocrinol. 2012;166:295–9. doi: 10.1530/EJE-11-0720. http://dx.doi.org/10.1530/EJE-11-0720. [DOI] [PubMed] [Google Scholar]
- 11.Supornsilchai V, Hiranrat P, Wacharasindhu S, Srivuthana S, Aroonparkmongkol S. Basal luteinizing hormone/follicle stimulating hormone ratio in diagnosis of central precocious puberty. J Med Assoc Thai. 2003;86(Suppl 2):S145–151. [PubMed] [Google Scholar]
- 12.Eckert KL, Wilson DM, Bachrach LK, et al. A single-sample, subcutaneous gonadotropin-releasing hormone test for central precocious puberty. Pediatrics. 1996;97:517–9. [PubMed] [Google Scholar]
- 13.Greulich WW, S P. Radiologic atlas of skeletal development of the hand and wrist. 2 ed. Standford (CA): Stanford University Press; 1959. [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Childh. 1969;44:291–303. doi: 10.1136/adc.44.235.291. http://dx.doi.org/10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 16.Carel JC, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–62. doi: 10.1542/peds.2008-1783. http://dx.doi.org/10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 17.Prete G, Couto-Silva AC, Trivin C, Brauner R. Idiopathic central precocious puberty in girls: presentation factors. BMC Pediatr. 2008;8:27. doi: 10.1186/1471-2431-8-27. http://dx.doi.org/10.1186/1471-2431-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giabicani E, Allali S, Durand A, Sommet J, Couto-Silva AC, Brauner R. Presentation of 493 consecutive girls with idiopathic central precocious puberty: a single-center study. PloS one. 2013;8:e70931. doi: 10.1371/journal.pone.0070931. http://dx.doi.org/10.1371/journal.pone.0070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilic A, Durmus MS, Unuvar E, et al. Clinical and laboratory characteristics of children referred for early puberty: preponderance in 7–8 years of age. J Clin Res Pediatr Endocrinol. 2012;4:208–212. doi: 10.4274/Jcrpe.736. http://dx.doi.org/10.4274/Jcrpe.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cisternino M, Arrigo T, Pasquino AM, et al. Etiology and age incidence of precocious puberty in girls: a multicentric study. J Pediatr Endocrinol Metab. 2000;13(Suppl 1):695–701. doi: 10.1515/jpem.2000.13.s1.695. http://dx.doi.org/10.1515/JPEM.2000.13.S1.695. [DOI] [PubMed] [Google Scholar]
- 21.Lee PA. Laboratory monitoring of children with precocious puberty. Arch Pediatr Adolesc Med. 1994;148:369–76. doi: 10.1001/archpedi.1994.02170040035006. http://dx.doi.org/10.1001/archpedi.1994.02170040035006. [DOI] [PubMed] [Google Scholar]
- 22.Suh J, Choi MH, Kwon AR, et al. Factors that predict a positive response on gonadotropin-releasing hormone stimulation test for diagnosing central precocious puberty in girls. Ann Pediatr Endocrinol Metab. 2013;18:202–7. doi: 10.6065/apem.2013.18.4.202. http://dx.doi.org/10.6065/apem.2013.18.4.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71:1251–8. doi: 10.1210/jcem-71-5-1251. http://dx.doi.org/10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 24.Pelzer V, Von Ditfurth M, Wendel U. [Diagnostic differentiation between precocious puberty and premature thelarche using ultrasonography and the stimulated LH/FSH quotient] Geburtshilfe Frauenheilkd. 1990;50:964–8. doi: 10.1055/s-2008-1026400. http://dx.doi.org/10.1055/s-2008-1026400. [DOI] [PubMed] [Google Scholar]


